Abstract
Objective
Patients with suspected lung cancer often experience adverse side effects such as anxiety, depression, and a decreased appetite. These side effects influence the patients' quality of life and their ability to make decisions concerning appropriate treatment. This study examined the psychological status and quality of life of patients with suspected lung cancer before and after bronchoscopy treatment and evaluated the effect of mirtazapine prescribed to patients with depression.
Methods
To assess patient characteristics (e.g. age, gender, and medical history), a questionnaire including the Hospital Anxiety and Depression Scale - Japanese version and the Functional Assessment of Cancer Therapy-L was administered.
Patients
Forty-three patients admitted for bronchoscopy treatment between May 2017 and April 2018 were included.
Results
The results showed that patients with depression reported a worse quality of life than those without depression. Compared with no medication, the administration of mirtazapine alleviated depressive symptoms. Furthermore, the patients' depressive status was affected by their physical symptoms, including coughing, tightness of chest, and dyspnea.
Conclusion
Our results emphasize the importance of detecting depression in the early stages of a cancer diagnosis and have significant implications concerning pharmacological intervention in patients with cancer displaying signs of depression.
Keywords: bronchoscopy, depression, lung cancer, mirtazapine, quality of life
Introduction
The incidence of depression in patients with cancer has been proven to be very high. Depression in patients with cancer leads to a variety of adverse effects, including increased suicide rates, a decreased quality of life (QOL), and a worse disease prognosis (1,2). A previous study that utilized a large database reported that 12.4% of patients developed depression after a lung cancer diagnosis (3). Although numerous patients with lung cancer complain of symptoms such as anxiety, depression, and a decreased appetite, whether or not the depression was already present before the diagnosis is often unclear.
Understanding the mental status of patients in the early stages of a cancer diagnosis is crucial, especially as anxiety and depression may occur once the patient is informed of a possible cancer diagnosis. Furthermore, depression in patients with cancer may affect their judgment in deciding upon the appropriate treatment (4).
In the present study, we conducted a survey among patients with suspected lung cancer to assess the changes in their psychological status and QOL before and after bronchoscopy (a test used to confirm a lung cancer diagnosis). Various studies have been conducted on pharmacotherapy to alleviate depression in patients with cancer. However, no conclusive answers have been reached (5). Serotonin reuptake inhibitors (SSRIs) and serotonin-noradrenaline reuptake inhibitors (SNRIs) are widely used in the treatment of depression. However, mirtazapine, classified as a noradrenergic-specific serotonergic antidepressant, has also been used in recent years. Because mirtazapine offers rapid action and few side effects (such as nausea), it is typically prescribed to patients experiencing nausea during anticancer therapy.
In this study, we treated study subjects who had been judged to be depressed in the initial survey with mirtazapine and evaluated the effectiveness of this treatment.
Materials and Methods
Subjects
The study subjects were patients with a diagnosis of suspected lung cancer admitted for bronchoscopy at the Department of Pulmonary Medicine, Kyoto Prefectural University of Medicine between May 2017 and April 2018. We enrolled a total of 43 patients in this study and administered the Hospital Anxiety and Depression Scale - Japanese version (HADS) to screen for depression.
The eligibility criteria were men and women 24 to 75 years old at the time of the study who provided their informed consent.
The exclusion criteria were as follows: patients who were unable to respond to the questionnaire, patients with organic encephalopathy, patients with a history of psychotropic drug treatment within three months prior to the study, patients with severe hepatic or renal dysfunction, patients with a history of suicidal ideation or attempted suicide, patients receiving medication for heart disease, patients with glaucoma or increased intraocular pressure, patients receiving medication for dysuria, and patients who had been prescribed monoamine oxidase inhibitors within two weeks prior to the study.
Ethics
All procedures in this study were in accordance with the ethical standards of the institutional and national research committees and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Clinical Research Ethics Committee of the University Hospital, Kyoto Prefectural University of Medicine. Informed consent was obtained from all study participants.
Procedures
Patients provided their written consent to participate in the study to the attending physician or researcher of respiratory medicine on the day of admission. Patients who scored ≥11 on the HADS at the time of admission were considered to have depression. Those patients who wished to receive treatment were prescribed mirtazapine 15 mg orally, administered in 1 dose daily in the morning. A second survey was conducted at the first outpatient visit after bronchoscopy. The details of the questionnaires are listed below.
Attributes
Gender, age, medical history, concomitant medications.
Depression condition
The HADS questionnaire was used as the primary scale for assessing depression and anxiety symptoms in patients. This instrument is reliable and has been previously validated (6). It consists of 14 items: 7 items related to depression and 7 related to anxiety. Each item is scored from 0 to 3 points, with 0 indicating “not at all” and 3 indicating “all the time” or an equivalent measure. The subscales are calculated as the sum of the item scores, with higher scores indicating a lower well-being.
QOL
As a secondary assessment, we measured the patient QOL using a disease-specific measure for lung cancer: The Functional Assessment of Cancer Therapy - Lungs (FACT-L). This scale is one of the most widely used measures in large-scale clinical trials in the United States and Japan, and its reliability has been validated (7). It consists of seven items related to physical well-being, seven concerning social/family well-being, six concerning emotional well-being, seven concerning functional well-being, and seven concerning the lung cancer subscale.
Therapeutic intervention
Patients with a HADS score of ≥11 at admission were assigned to the depressed group and prescribed mirtazapine. Patients with a score of ≤10 were assigned to the non-depressed group and did not receive any medication.
Statistical analyses
Continuous data are presented as mean ± standard deviation (SD) and range and were compared using the Mann-Whitney U test. Categorical data are presented as counts and percentages and were compared using the chi-square test. The impact of lung cancer symptoms on the HADS score was evaluated by a multiple regression analysis. We performed the multivariate analysis based on the significant factors identified in the univariate analysis. All statistical analyses were performed using EZR for Windows, version 1.35 (Saitama Medical Center, Jichi Medical University, Japan). All p values <0.05 were considered statistically significant. This was an exploratory evaluation that only included data from participants, and there were no pre-determined hypotheses or power calculations.
Results
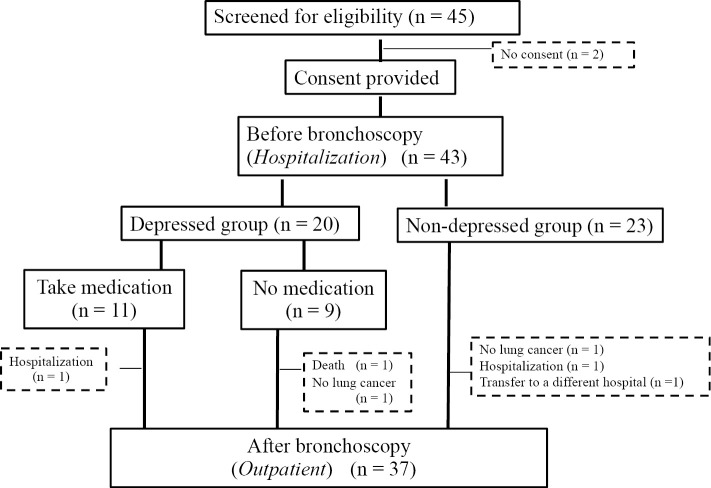
Twenty of the 43 patients had HADS scores of ≥11 and were thus considered to have depression. We administered mirtazapine to 11 of these patients who wished to receive treatment. A flow chart of the study subjects' participation in this study is shown in Fig. 1. Table 1 provides the patients' characteristics. Depression was observed in 46.5% (n=20) of the patients at the pre-bronchoscopy stage. There were no significant differences in the patient background characteristics between the depressed and non-depressed groups. There was, however, a tendency toward a greater incidence of depression among women than in men.
Figure 1.
Assignment of enrolled patients.
Table 1.
Patients’ Characteristics.
| Depressed group (n=20) | Non-depressed group (n=23) | p value | ||||
|---|---|---|---|---|---|---|
| Age: median (range) | 67.5 (42-75) | 68.0 (34-75) | 0.79 | |||
| Gender: male/female | 8/12 | 14/9 | 0.23 | |||
| Living together: yes/no | 17/3 | 20/3 | 1.00 | |||
| Using sleeping pills: yes/no | 2/18 | 3/20 | 1.00 | |||
| HADS score: median (range) | 17.0 (11-32) | 5.0 (0-10) | 0.00 |
HADS: Hospital Anxiety and Depression Scale - Japanese version
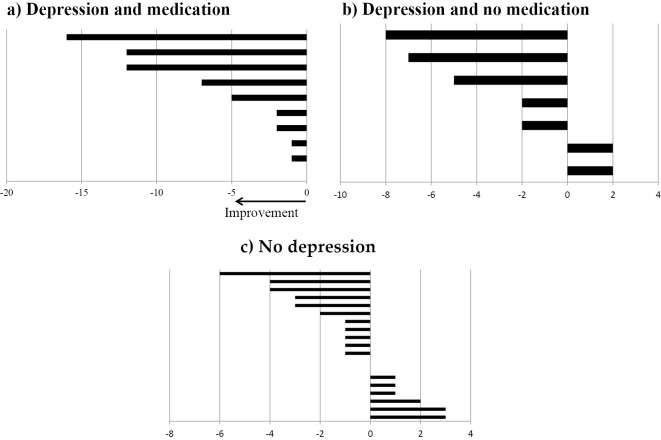
We also administered the FACT-L, which provided us with QOL scores for each patient. Table 2 shows the QOL scores of patients in the depressed and non-depressed groups. The results of the FACT-L assessment indicated significantly lower QOL scores in the depressed group in terms of physical, emotional, and functional QOL than in the non-depressed group. Multiple regression analyses were performed using seven FACT-L Lung Cancer subscales as independent variables to examine the symptoms affecting the HADS scores within the depressed group. The results suggested that coughing (p=0.002), respiratory comfort (p=0.001), and tightness in the chest (p=0.022) were significantly associated with the HADS scores and may have influenced the patients' depression scores (Fig. 2). Changes in the depressive state before and after bronchoscopy are shown in Fig. 3. All patients in the mirtazapine-treated group showed improvement in their HADS scores (mean change in the HADS score =5.8 points). Conversely, only five of the seven non-treatment patients showed improvement in their HADS scores, and the degree of improvement was lower than that in the treated group (mean change in the HADS score =2.8 points). The two remaining patients in the non-medicated group had worse HADS scores. Our results suggest that the interventions led to improvements in the HADS scores.
Table 2.
Comparison of the Quality of Life Scores for the Patients in the Depressed and Non-depressed Groups.
| FACT-L score | Depressed group (n=20) | Non-depressed group (n=23) | p value | |||
|---|---|---|---|---|---|---|
| Physical (PWB) | 21.5 (7-27) | 27.0 (19-28) | <0.01 | |||
| Social/Family (SWB) | 15.2 (3-28) | 16.0 (0-28) | 0.942 | |||
| Emotional (EWB) | 14.5 (3-24) | 20.0 (14-23) | <0.01 | |||
| Functional (FWB) | 16.0 (2-25) | 23.0 (14-28) | <0.01 | |||
| Lung cancer subscale | 17.5 (10-28) | 25.0 (14-28) | <0.01 | |||
| FACT-L Total score | 84.8 (54-116) | 111.0 (84-132) | <0.01 |
Values in parenthesis indicate the range of the scores.
EWB: emotional well-being, FACT-L: Functional Assessment of Cancer Therapy-L, FWB: functional well-being, PWB: physical well-being, SWB: social/family well-being
Figure 2.
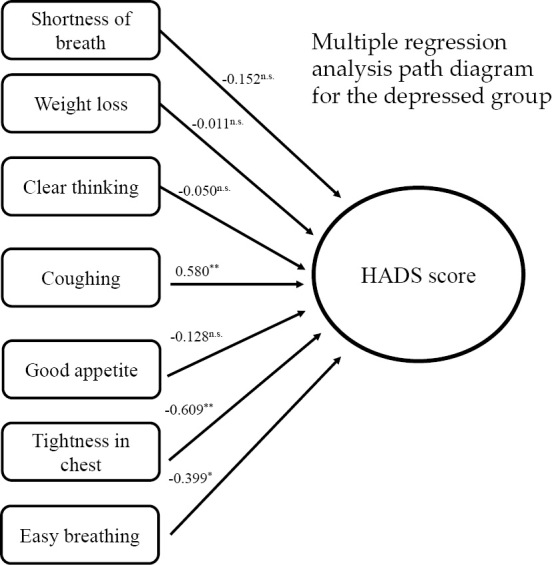
Effect of lung cancer subscale items on the HADS scores. *p<0.05; **p<0.001. HADS: Hospital Anxiety and Depression Scale-Japanese version, n.s.: non-significant
Figure 3.
Changes in the HADS scores at hospitalization and at the first outpatient visit after bronchoscopy. a) The HADS change after 2 weeks on mirtazapine. b) The HADS change after 2 weeks in the non-mirtazapine group, n=7. c) The HADS change in the non-depressed group (with or without mirtazapine), n=20. HADS: Hospital Anxiety and Depression Scale-Japanese version
Seven patients were found to be depressed at the start of the survey but refused mirtazapine medication. Among them, four had high HADS score in the second survey, and two chose to receive treatment. Both of the patients who received intervention showed improvement in their depression after surgery and have since discontinued mirtazapine. One of the two patients who refused mirtazapine medication again eventually showed worsening of depressive symptoms and, after a psychiatric liaison intervention, continued chemotherapy with antidepressants. The other patient has been treated surgically without antidepressants. Three patients who had an improved HADS score at the time of the second survey were considered to have bronchoscopy as a trigger for depression, as their depression had after bronchoscopy, although one patient had non-cancer lesions, and the other had surgically resectable early-stage lung cancer. One patient was treated with chemotherapy for advanced-stage lung cancer but was still able to be followed up without any liaison intervention thereafter.
Discussion
Bronchoscopy is an essential part of the process leading to the diagnosis of lung cancer, and a previous study found that patients undergoing bronchoscopy reported feeling anxious or depressed before the procedure (8). Furthermore, previous research has indicated that patients with lung cancer may be under mental stress from the early stages of the diagnosis (9). It has also been reported that assessing anxiety during the pre-bronchoscopy stage is important in terms of its impact on bronchoscopy performance, patient satisfaction with the procedure, and increased compliance with cancer treatment (10,11). Based on the above, this study aimed to determine the depression levels of patients scheduled to undergo bronchoscopy and the effect of mirtazapine treatment.
The results showed that nearly half of the patients reported symptoms of anxiety and depression after the lung cancer diagnosis but prior to bronchoscopy treatment. They also showed that patients with depression had significantly lower QOL scores than did those without. In the current study, 46.5% of patients already had depression when they were informed of their possible cancer diagnosis and the possible need for bronchoscopy. Several studies have reported that the attending physician's explanation concerning the patient's lung lesions influences the patient's anxiety (12,13), suggesting that more attention should be paid to the patient's mental state at the time of the explanation. Byrne et al. also reported that smokers and women with lung cancer were more likely to be anxious about their disease (14), while Walker et al. reported that colorectal and lung cancer were risk factors for comorbid depression in women (15).
The QOL of patients with cancer is multidimensional and consists of at least four aspects; physical (or symptom-related), social, functional, and emotional (or mental) QOL (16). This study assessed the QOL in patients with lung cancer using the FACT-L (17). The FACT-L is a 44-item self-reported instrument and consists of 2 parts: a 34-item measure of general health-related QOL and a 10-item measure emphasizing lung cancer symptoms (7). Results from this assessment revealed a significant decrease in the QOL in the depressed group compared with the non-depressed group. Differences in the QOL were observed mainly in physical symptoms, psychiatric symptoms, and activity status. Ostlund et al. (18) showed that emotional functioning and fatigue are important predictors of a declining QOL. Furthermore, depression itself is also a factor for a reduced QOL, with its incidence reported to be significantly higher in patients with lung cancer than in those with other types of cancer (19). The results of the present study suggest that depression in patients with lung cancer may be related to the patients' physical condition and activity situation and that depression negatively impacts the patient's QOL.
Various physical symptoms can be observed in patients with lung cancer. For example, Iyer et al. found that patients with advanced lung cancer reported symptoms such as fatigue (98%), anorexia (98%), respiratory disorders (94%), coughing (93%), and pain (90%). Furthermore, those with more severe symptoms had an even lower QOL than those with milder symptoms (20). Existing studies have reported that symptoms such as fatigue, pain, and dyspnea have the greatest impact on a reduced QOL (21). In the present study, cough, dyspnea, and chest tightness were shown to be associated with a reduced QOL in the depressed group, suggesting that lung cancer symptoms such as these may be associated with comorbid depression. There are several approaches to treating depression, both in patients with cancer and in general, including pharmacotherapy and psychotherapy. Even though previous studies have reported that some patients with cancer prefer psychotherapy to pharmacotherapy for treating depression (22,23), antidepressants and other medications are often used (5). Many people with cancer continue chemotherapy and hormone therapy for an extended period and therefore continue to display physical symptoms associated with the cancer itself and with its treatment. It is therefore preferable to administer antidepressants with few side effects. For example, newer antidepressants, such as SSRIs or SNRIs, are typically better suited for patients with cancer, as they have fewer side effects than conventional antidepressants. These medications have been confirmed to be effective against depression in patients with cancer (24). Nevertheless, it is important to note that antidepressants typically interact with the medications used for chemotherapy and hormonal therapy. Because many antineoplastic drugs catalyze the enzyme known as cytochrome P450 (CYP), the administration of escitalopram or mirtazapine-which has less potent CYP-inhibiting properties-is recommended (25,26).
In the present study, mirtazapine was administered to patients with depression. Our results showed an improvement in depression symptoms for all patients in the treatment group. Of the patients who received mirtazapine intervention, the HADS score was increased by an average of 3.8 points among those who underwent operations and by an average of 10 points among those who underwent chemotherapy. Temel et al. conducted a randomized controlled trial of early palliative versus usual care in non-small-cell lung cancer and reported that early palliative care significantly improved outcomes compared with usual care (27). Furthermore, that study showed that the group of patients who received early palliative care had less depression than the usual care group. The present findings suggest the need to provide care for depression at an earlier stage than at the start of lung cancer treatment.
When the study participants were told that lung cancer was suspected and bronchoscopy would be required, approximately half were found to already have depression, and the administration of mirtazapine to these patients resulted in an improvement in their depressive condition. These findings suggest that treatment with mirtazapine may help treat symptoms of depression in patients with lung cancer. Nevertheless, conclusions based on the results of our study should be drawn with caution, as there are some limitations. First, this study had a small sample size, which may have influenced the results. Furthermore, possible bias due to patient preference for intervention and disease stage differences cannot be excluded. Given these limitations, future studies with larger sample sizes and random treatment interventions are needed.
Informed consent was obtained from all individual participants included in the study.
Author's disclosure of potential Conflicts of Interest (COI).
Tadaaki Yamada: Research funding, Ono Pharmaceutical, Chugai Pharmaceutical and Takeda Pharmaceutical. Junji Uchino: Research funding, Eli Lilly Japan, AstraZeneca and Boehringer Ingelheim Japan. Koichi Takayama: Honoraria, AstraZeneca, Chugai-Roche, MSD-Merck, Eli Lilly, Boehringer-Ingelheim and Daiichi-Sankyo; Research funding, Chugai-Roche and Ono Pharmaceutical.
References
- 1.Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol) 26: 25-31, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs 16: 264-269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu K, Nakaya N, Saito-Nakaya K, et al. Clinical biopsychosocial risk factors for depression in lung cancer patients: a comprehensive analysis using data from the Lung Cancer Database Project. Ann Oncol 23: 1973-1979, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet 356: 1326-1327, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Ostuzzi G, Matcham F, Dauchy S, Barbu C, Hotopf M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69-77, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12: 199-220, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Poi PJ, Chuah SY, Srinivas P, Liam CK. Common fears of patients undergoing bronchoscopy. Eur Respir J 11: 1147-1149, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer 55: 215-224, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundbom LT, Bingefors K. The influence of symptoms of anxiety and depression on medication nonadherence and its causes: a population-based survey of prescription drug users in Sweden. Patient Prefer Adherence 7: 805-811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcántara C, Edmondson D, Moise N, Oyola D, Hiti D, Kronish IM. Anxiety sensitivity and medication nonadherence in patients with uncontrolled hypertension. J Psychosom Res 77: 283-286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slatore CG, Press N, Au DH, Curtis JR, Wiener RS, Ganzini L. What the heck is a “nodule”? A qualitative study of veterans with pulmonary nodules. Ann Am Thorac Soc 10: 330-335, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slatore CG, Golden SE, Ganzini L, Wiener RS, Au DH. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules. A cohort study. Ann Am Thorac Soc 12: 184-192, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 28: 917-925, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 1: 343-350, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer 44: 1793-1798, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Hollen PJ, Gralla RJ. Comparison of instruments for measuring quality of life in patients with lung cancer. Semin Oncol 23: 31-40, 1996. [PubMed] [Google Scholar]
- 18.Ostlund U, Wennman-Larsen A, Gustavsson P, Wengström Y. What symptom and functional dimensions can be predictors for global ratings of overall quality of life in lung cancer patients? Support Care Cancer 15: 1199-1205, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology 10: 19-28, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 81: 288-293, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Cooley ME. Quality of life in persons with non-small cell lung cancer: a concept analysis. Cancer Nurs 21: 151-161, 1998. [DOI] [PubMed] [Google Scholar]
- 22.McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders. J Clin Psychiatry 74: 595-602, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuyama T, Nakane Y, Endo C, et al. Mental health literacy in Japanese cancer patients: ability to recognize depression and preferences of treatments-comparison with Japanese lay public. Psychooncology 16: 834-842, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Torta R, Leombruni P, Borio R, Castelli L. Duloxetine for the treatment of mood disorder in cancer patients: a 12-week case-control clinical trial. Hum Psychopharmacol Clin Exp 26: 291-299, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Miguel C, Albuquerque E. Drug interaction in psycho-oncology: antidepressants and antineoplastics. Pharmacology 88: 333-339, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Henry NL, Stearns V, Flockhart DA, Hayes DF, Riba M. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry 165: 1251-1255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363: 733-742, 2010. [DOI] [PubMed] [Google Scholar]