Abstract
We herein report a 75-year-old man with non-small-cell lung cancer who developed tubulointerstitial nephritis due to pembrolizumab administration. He was successfully treated with atezolizumab following steroid administration. He was initially diagnosed with lung adenocarcinoma (T1bN3M1b, stage IV), with a programmed cell death-ligand 1 tumor proportion score of 25-49%. Although the tumor responded well to pembrolizumab, the drug was discontinued because of the diagnosis of tubulointerstitial nephritis on a renal biopsy. Tubulointerstitial nephritis was treated with 30 mg prednisolone, the dose of which was tapered to and maintained at 5 mg. Following lung cancer progression, atezolizumab was administered, and the tumor responded again. Its efficacy has been sustained for >15 months without recurrence of tubulointerstitial nephritis.
Keywords: immune checkpoint inhibitor, immune-related adverse event, tubulointerstitial nephritis, programmed death-ligand 1, atezolizumab
Introduction
Immune checkpoint inhibitors (ICIs) can lead to various immune-related adverse events (irAEs) such as interstitial lung disease, thyroid dysfunction, colitis, and type 1 diabetes mellitus. Among all irAEs, renal dysfunction is relatively rare (1). The recurrence rate of irAEs was 39-71.4% when ICIs were readministered to patients who discontinued the use of ICIs because of irAEs (2-5). There are few reports on the safety and efficacy of programmed cell death-ligand 1 antibody (anti-PD-L1) in patients who discontinued the use of programmed cell death-1 antibody (anti-PD-1) because of irAEs.
We herein report a patient with non-small-cell lung cancer (NSCLC) who developed tubulointerstitial nephritis due to anti-PD-1 (pembrolizumab), which was successfully treated with anti-PD-L1 (atezolizumab).
Case Report
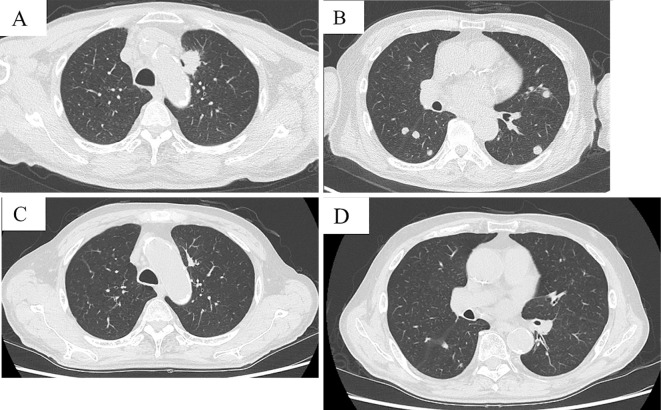
The patient was a 75-year-old man who visited our hospital with back pain. He was an ex-smoker (Brinkman index of 2,200) and had been exposed to asbestos. He was diagnosed with lung adenocarcinoma (T1bN3M1b, stage IV) with pulmonary, brain, pancreatic, and bone metastases. A molecular examination of the tumor revealed wild-type epidermal growth factor receptor gene but no rearrangement of the anaplastic lymphoma kinase fusion gene. Following stereotactic radiation therapy for brain metastasis, he received four cycles of carboplatin and pemetrexed. However, the disease progressed after three cycles of pemetrexed. Because the PD-L1 tumor proportion score according to PD-L1 IHC 22C3 pharmDx was 25-49%, pembrolizumab was administered as the second-line therapy. The tumor decreased significantly in size after four cycles of pembrolizumab (Fig. 1).
Figure 1.
Computed tomography before pembrolizumab administration (A, B) and after four cycles of pembrolizumab administration (C, D). The primary lesion size in the left upper lobes and pulmonary metastases was significantly reduced.
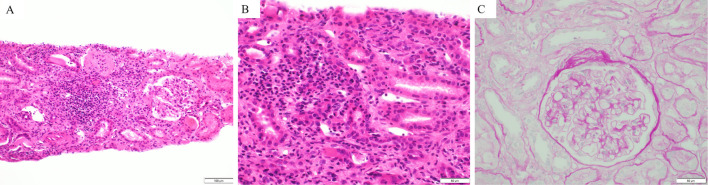
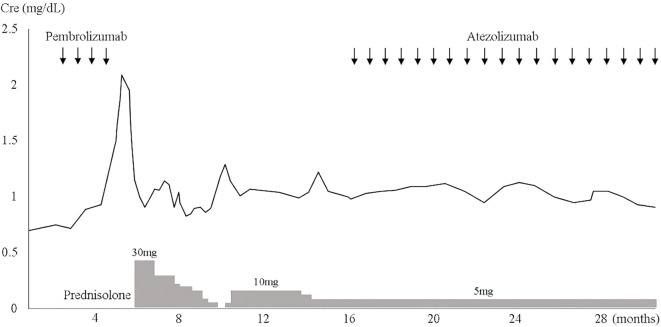
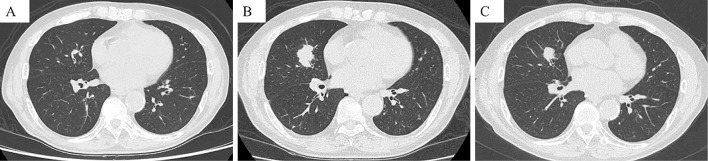
After 4 cycles of pembrolizumab, his serum creatinine level increased from 0.75 to 1.5 mg/dL. He also received rabeprazole sodium, a proton pump inhibitor (PPI), for 11 months. Serum antinuclear and antineutrophil cytoplasmic antibodies were negative. Negative results were obtained for urinary protein and fecal occult blood; microscopically, there were 1-4 white blood cells/high-power field. Urinary protein/creatinine ratio was 0.17 g/g creatinine. The N-Acetyl-beta-D-glucosaminidase activity and beta-2 microglobulin levels were 7.1 IU/L and 1,800 μg/L, respectively. The serum creatinine level worsened to 2.09 mg/dL [grade 2 acute kidney injury (AKI), as evaluated by the Common Terminology Criteria for Adverse Events version 4] despite pembrolizumab discontinuation. Thereafter, a renal biopsy was performed. The pathological specimen contained 10 glomeruli, only 1 of which showed global sclerosis. The other glomeruli were almost normal, without mesangial cell proliferation, basement membrane change, or endocapillary proliferation (Fig. 2). Glomerular deposition of immunoglobulin (Ig) G, IgA, IgM, C3, C4, C1q, or fibrinogen was not observed upon immunofluorescence staining (data not shown). Thus, tubulointerstitial nephritis was confirmed. Following prednisolone (30 mg) administration, renal function improved, and the dose of prednisolone was tapered. However, the serum creatinine level increased after prednisolone interruption; therefore, 5 mg prednisolone was readministered and maintained (Fig. 3). Because lung cancer progressed 11 months after the last pembrolizumab administration, atezolizumab was administered as the third-line therapy. With the maintenance of the prednisolone dose (5 mg), the serum creatinine level was maintained at <1.0 mg/dL; no other irAEs were observed. Lung cancer did not progress for >15 months after atezolizumab administration (Fig. 4).
Figure 2.
Hematoxylin and Eosin staining (A, B) and periodic acid-Schiff staining (C) of a right kidney specimen. Infiltration of numerous lymphocytes and histiocytes into the stroma and partly into the tubule (A, B) without a glomerular lesion (C) was observed.
Figure 3.
Clinical course of the patient. Cre: creatinine
Figure 4.
Computed tomography at the time of pembrolizumab discontinuation (A), before atezolizumab administration (B), and 14 months after atezolizumab administration (C). The pulmonary lesion size in the right middle lobes was significantly reduced after atezolizumab administration.
Discussion
We encountered a case of NSCLC with biopsy-proven tubulointerstitial nephritis due to pembrolizumab. He was successfully treated with atezolizumab under prednisolone maintenance.
In a pooled analysis of 3,695 patients treated with ICIs, the incidence of ICI-associated AKI was 2.2% for any grade and 0.7% for or grades ≥3. AKI was more common (any grade, 4.9%; above grade 3, 1.7%) in patients receiving combination therapy with ipilimumab and nivolumab than in those receiving ICI monotherapy (6). While the spectrum of renal irAEs in patients receiving ICIs is broad, the majority of patients are diagnosed with tubulointerstitial nephritis based on a biopsy specimen. Other drugs, such as PPIs and nonsteroidal anti-inflammatory drugs, can also lead to tubulointerstitial nephritis. In the present case, PPI use may have been associated with tubulointerstitial nephritis development due to ICI, as reported previously (7). Other renal irAEs include antinuclear cytoplasmic antibody-associated diseases, lupus nephritis, and podocytopathy (8-10). In 12 patients treated with pembrolizumab that caused AKI, 4 with acute interstitial nephritis (AIN) and 5 with acute tubular necrosis (ATN) were pathologically diagnosed (11). It is important to differentiate between cases of AIN and ATN because AIN, but not ATN, often requires corticosteroid therapy. It is difficult to differentiate AIN from ATN based on clinical features alone; therefore, a renal biopsy is required to determine the exact etiology of ICI-associated AKI.
A previous report noted a trend toward a higher recurrence rate following the development of a more severe initial irAE and toward more frequent recurrence in patients treated with corticosteroids following the initial irAEs (4). In the present case, because the serum creatinine level increased again after corticosteroid discontinuation, ICI was readministered with a low-dose corticosteroid, and an anticancer effect was achieved without the recurrence of any irAEs over a long period. While there are concerns that corticosteroid (particularly >10 mg prednisone) use may attenuate the effects of ICIs (12), it was recently reported that corticosteroid use for cancer-unrelated indications did not attenuate the effects of ICIs (13). In the present case, tubulointerstitial nephritis was not lethal and was well controlled with 5 mg prednisolone; therefore, we decided to readminister ICIs.
Some studies have shown that treatment outcomes were similar between the readministration and discontinuation cohorts among patients with NSCLC who showed an early objective response prior to the development of a severe irAE (3,5). We switched the ICI from pembrolizumab (an anti-PD-1) to atezolizumab (an anti-PD-L1). Anti-PD-L1 inhibits the PD-1/PD-L1 and B7.1/PD-L1 pathways, promoting the binding of CD28 expressed on T cells to B7.1 expressed on antigen-presenting cells (14,15). In the priming phase, anti-PD-L1 may also promote T-cell priming and activation more strongly than anti-PD-1 (14,15). Furthermore, in previous studies, patients were rechallenged with the ICIs used in the initial treatment that had induced irAEs (3,5). Switching from anti-PD-1 to anti-PD-L1 might be suitable in terms of an improved efficacy and safety.
In conclusion, we reported a patient with NSCLC who developed tubulointerstitial nephritis due to pembrolizumab administration but was successfully treated with atezolizumab administration. Anti-PD-L1 administration to patients who respond to anti-PD-1 but whose treatment is discontinued because of irAEs might be efficacious and safe under maintenance therapy with corticosteroids.
Author's disclosure of potential Conflicts of Interest (COI).
Nagio Takigawa: Honoraria, Chugai Pharmaceutical.
Acknowledgement
We thank Yasumasa Monobe, Department of Pathology, Kawasaki Medical School General Medical Center, for providing the pathological images.
References
- 1.Cortazar FB, Marrone KA, Troxell ML, et al. . Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638-647, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack MH, Betof A, Dearden H, et al. . Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29: 250-255, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santini FC, Rizvi H, Plodkowski AJ, et al. . Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6: 1093-1099, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonaggio A, Michot JM, Voisin AL, et al. . Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 5: 1310-1317, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouri A, Kaira K, Yamaguchi O, et al. . Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol 84: 873-880, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Sise ME, Seethapathy H, Reynolds KL. Diagnosis and management of immune checkpoint inhibitor-associated renal toxicity: illustrative case and review. Oncologist 24: 735-742, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287-291, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Fadel F, El Karoui K KB. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361: 211-212, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Lin N, Wang S, et al. . Minimal change disease associated with anti-PD1 immunotherapy: a case report. BMC Nephrol 19: 156, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Brom RR, Abdulahad WH, Rutgers A, et al. . Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology (Oxford) 55: 1143-1145, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Izzedine H, Mathian A, Champiat S, et al. . Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81-88, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbour KC, Mezquita L, Long N, et al. . Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36: 2872-2878, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 37: 1927-1934, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Hui E, Cheung J, Zhu J, et al. . T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355: 1428-1433, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol 45: 3567-3572, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]