Abstract
Objective
We aimed to examine the dynamics of serum Wisteria floribunda agglutinin-positive human Mac-2-binding protein glycosylation isomer (M2BPGi) in patients with acute liver injury.
Methods
Serum M2BPGi levels at the time of the diagnosis (n=77) and normalization of the serum alanine aminotransferase (ALT) level (n=26) were examined retrospectively. The difference in the serum M2BPGi level according to the etiology, and the correlations with other laboratory parameters were evaluated.
Results
The serum M2BPGi level at the time of the diagnosis was increased in 59 of 77 patients [2.3 cutoff index (COI); range, 0.31-11.1 COI] and was significantly decreased at the time of serum ALT normalization (0.68 COI; range, 0.15-1.87 COI; p<0.0001). The serum M2BPGi level was positively correlated with the duration for which serum ALT normalization was achieved (n=46, Spearman rho=0.53, p<0.0001). A multivariate analysis identified total bilirubin (T-bil), albumin, ALT, alkaline phosphatase, and etiology (e.g., drug-induced liver injury or etiology unknown) as independent factors for increased serum M2BPGi. In patients with infectious mononucleosis, the serum M2BPGi level was higher relative to the degree of increase of serum ALT or T-bil levels in comparison to other etiologies.
Conclusion
The serum M2BPGi level in patients with acute liver injury reflects the magnitude and duration of liver injury. However, it should be noted that the degree of increase of serum M2BPGi in patients with acute liver injury may differ according to the etiology.
Keywords: Wisteria floribunda agglutinin-positive human Mac-2-binding protein, WFA+-M2BP, M2BPGi, acute liver injury
Introduction
Wisteria floribunda agglutinin-positive human Mac-2-binding protein assays based on a M2BP glycan isomer (M2BPGi) are used to assess liver fibrosis in patients with viral hepatitis (1-3), non-alcoholic fatty liver disease (4), primary biliary cholangitis (5), and autoimmune hepatitis (6). The cutoff serum M2BPGi levels differ among patients stratified according to the etiology of chronic liver injury, even in those with the same stage of fibrosis (7).
The serum M2BPGi level has been reported to transiently increase in the presence of acute liver injury (8). However, the clinical significance and mechanism of the increase in the serum M2BPGi level in patients with acute liver injury are not well known. Acute liver injury may be caused by viral infection, autoimmune liver disease, and drug-induced liver injury (DILI) (9), and the mechanism of hepatocyte injury (e.g., host immune response (10,11), cytopathic effect, and intrinsic or idiosyncratic hepatotoxicity), varies according to the etiology (12). Thus, the mechanism underlying the increased serum M2BPGi level may also differ according to the etiology of acute liver injury.
In this study, we analyzed the transition of the serum M2BPGi level, and the relationship between this value and other clinical parameters or the pathogenesis.
Materials and Methods
Patients
One hundred patients were diagnosed with acute liver injury at the Kanazawa Medical Center between December 1999 and August 2017. Among these patients, 23 were excluded from this retrospective analysis due to the lack of stored serum samples. The remaining 77 patients [male, n=28; female, n=49; median age, 37.5 years (range, 11-93 years)] were enrolled in this study (Table 1). Among these patients, 57 patients underwent conservative medical treatment, such as rest and fluid therapy, while 22 patients received medical care, such as antiviral or steroid therapy. Two patients (2.5%) were diagnosed with acute liver failure based on ≤40% prothrombin (PT) activity. Two patients died from acute liver failure; no patients underwent liver transplantation.
Table 1.
Characteristics and Treatment of 77 Patients with Acute Liver Injury.
| Total (n=77) |
Autoimmune liver disease (n=5) | Infectious mononucleosis (n=17) | DILI (n=20) |
HBV (n=9) |
HAV (n=9) |
Etiology unknown (n=17) | |
|---|---|---|---|---|---|---|---|
| Age (year) | 37.5 (11-93) | 47 (11-69) | 29 (19-54) | 57 (17-73) | 41 (25-93) | 36 (23-55) | 26 (15-71) |
| Sex (M/F) | 28/49 | 0/5 | 7/10 | 8/12 | 4/5 | 4/5 | 5/12 |
| Treatment | |||||||
| Conservative medical treatment | 57 | 0 | 16 | 11 | 6 | 9 | 15 |
| Intensive medical care | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anti-viral therapy | 4 | 0 | 1 | 0 | 3 | 0 | 0 |
| Steroid therapy | 12 | 5 | 0 | 6 | 1 | 0 | 0 |
| Others | 5 | 0 | 0 | 4 | 0 | 0 | 1 |
| Clinical outcome | |||||||
| Recovery | 75 | 5 | 17 | 19 | 8 | 9 | 17 |
| Death | 2 | 0 | 0 | 1 | 1 | 0 | 0 |
Conservative medical treatment means to treat with rest and complemental liquid. DILI: drug-induced liver injury, HBV: hepatitis B virus, HAV: hepatitis A virus
Written informed consent was obtained from the patients or responsible family members prior to enrollment, and this study was approved by the Institutional Review Board of Kanazawa Medical Center Hospital. All study protocols conformed to the ethical guidelines of the 2013 Declaration of Helsinki.
Acute liver injury
Acute liver injury has various etiologies, among which the magnitude of the ALT level increase differs (13). In this study, patients with no history of liver injury and whose ALT level transiently increased by ≥42 U/L were diagnosed with acute liver injury. To analyze liver injuries due to various etiologies, including mild cases, an abnormal ALT value was defined as ≥42 U/L, which is the upper limit of the normal range in our laboratory.
The etiologies of acute liver injury included autoimmune liver disease [autoimmune hepatitis (AIH; n=4) and AIH-primary biliary cholangitis overlap syndrome (n=1)], hepatitis viral infection [hepatitis A virus (HAV; n=9); hepatitis B virus (HBV; n=9)], non-hepatitis viral infection [infectious mononucleosis (n=17) (Epstein-Barr virus (EBV) and cytomegalovirus (CMV))], DILI (n=20), and etiology unknown (n=17). All patients with autoimmune liver disease were diagnosed by liver biopsy. They were in the acute hepatitis phase, as indicated by the absence of histological findings of chronic liver disease, such as fibrous expansion of the portal area (14,15). Patients with acute liver injury caused by alcohol consumption were excluded from this study, due to the possibility of additional effects of increasing M2BPGi due to mild fibrosis in a background of chronic liver disease (16), which might have led to the overestimation of the serum M2BPGi level.
We evaluated the serum M2BPGi level according to the etiology of acute liver injury, as well as the correlations between the serum M2BPGi level and other laboratory parameters at the time of the diagnosis.
Determination of the serum M2BPGi level after serum ALT normalization
Twenty-six patients had stored serum samples that were obtained at the time of the diagnosis and after normalization of serum ALT. The etiologies of acute liver injury in these patients were autoimmune liver disease (n=4), infectious mononucleosis (n=4), DILI (n=6), HBV (n=3), HAV (n=5), and etiology unknown (n=4). The median follow-up period was 59 days (range, 14-176 days). This period is not necessarily the number of days until serum ALT normalization was first confirmed. The serum M2BPGi level at the time of the diagnosis and after serum ALT normalization was determined.
Forty-six patients could be followed up until serum ALT normalization. This period is defined as the shortest period (in days) for which serum ALT normalization was confirmed. Among these patients, those whose serum M2BPGi level was not measured at the time of serum ALT normalization were also included. We evaluated the correlation between the serum M2BPGi level at the time of the diagnosis and the time to ALT normalization in these patients who received conservative treatment. The etiology of acute liver injury in these patients was autoimmune liver disease (n=1), infectious mononucleosis (n=11), DILI (n=9), HBV (n=5), HAV (n=8), and etiology unknown (n=12). The median follow-up period was 42 days (range, 15-123 days).
Measurement of serum M2BPGi
We measured the serum M2BPGi levels using an HISCL M2BPGi kit (Sysmex, Kobe, Japan) and a chemiluminescence enzyme immunoassay instrument (HISCL; Sysmex) (1). The measured Wisteria floribunda agglutinin-conjugated M2BPGi values were indexed as follows: Cutoff index (COI)=[(M2BPGi)sample-(M2BPGi)NC]÷[(M2BPGi)PC-(M2BPGi)NC], where (M2BPGi)sample is the M2BPGi count, PC is the positive control, and NC is the negative control. The PC was a calibration solution standardized to yield a COI value of 1.0 (7).
Statistical analysis
Data are presented as the median and interquartile range. Differences were evaluated by the Mann-Whitney U test and Wilcoxon signed-rank test. P values of <0.05 were considered to indicate statistical significance. Correlations were evaluated by calculating Spearman's rank-correlation coefficient (r). A step-wise multiple regression analysis was performed. Each etiology included in the analysis (autoimmune liver disease, infectious mononucleosis, HAV/HBV, DILI, etiology unknown) was classified using dummy variables with HAV/HBV as a reference value. The statistical analyses were performed using the Prism v. 5 (GraphPad Software, San Diego, USA) and SPSS (SPSS, Chicago, USA) software programs.
Results
Serum M2BPGi levels at the time of the diagnosis and after serum ALT normalization
The laboratory data are shown in Table 2. The degree of hepatocellular injury varied according to the etiology. The median serum M2BPGi level was 2.3 (range, 0.31-11.1) COI. The serum M2BPGi level differed significantly according to the etiology of acute liver injury (p<0.001), particularly between autoimmune liver disease and etiology unknown, and HBV and etiology unknown (Fig. 1a).
Table 2.
Laboratory Parameters of 77 Patients with Acute Liver Injury.
| Total (n=77) |
Autoimmune liver disease (n=5) |
Infectious mononucleosis (n=17) |
DILI (n=20) |
HBV (n=9) |
HAV (n=9) |
Etiology unknown (n=17) |
p value | |
|---|---|---|---|---|---|---|---|---|
| WBC (×103/mm3) | 5.9 (2.1-18.9) |
5.9 (3.5-6.9) |
8.8 (3-18.9) |
5.6 (2.9-9.7) |
6.5 (4.7-8.3) |
3.9 (2.9-6.1) |
5.7 (2.1-11.1) |
p<0.001 |
| Hb (g/dL) | 13.7 (9.6-17) |
12.9 (10.7-14.9) |
13.6 (11.8-16.4) |
13.2 (10.8-17) |
14.7 (10.7-16.2) |
14.3 (11.7-16.6) |
13.8 (9.6-15.5) |
n.s. |
| Plt (×103/mm3) | 205 (56-460) |
217 (196-301) |
194 (70-460) |
205 (89-314) |
206 (56-369) |
143 (116-233) |
226 (116-372) |
n.s. |
| AST (U/L) | 539 (52-11,609) |
844 (351-2,046) |
204 (52-661) |
603 (184-2,123) |
1,124 (465-3,205) |
1,126 (535-8,451) |
324 (98-11,609) |
p<0.0001 |
| ALT (U/L) | 784 (116-8,386) |
848 (569-2,385) |
297 (117-547) |
877 (146-2,258) |
2,290 (1,343-3,830) |
2,163 (797-8,363) |
515 (116-8,010) |
p<0.0001 |
| GGT (U/L) | 195 (20-1,296) |
127 (40-335) |
144 (37-334) |
197 (29-1,296) |
195 (39-611) |
354 (164-789) |
229 (20-1,074) |
p<0.05 |
| ALP (U/L) | 501 (142-1,373) |
580 (208-1,021) |
566 (206-1,191) |
450 (178-1,373) |
530 (279-753) |
456 (333-732) |
429 (142-1,316) |
n.s. |
| LDH (U/L) | 538 (151-10,604) |
436 (296-534) |
575 (337-1,648) |
492 (270-1,227) |
520 (434-1,677) |
698 (506-1,218) |
344 (151-10,604) |
n.s. |
| T-bil (mg/dL) | 1.6 (0.3-14.2) |
9.4 (0.9-14.2) |
0.8 (0.5-3.5) |
1.8 (0.5-11.9) |
3 (0.8-8) |
3.6 (0.7-7.6) |
1.2 (0.3-8.7) |
p<0.001 |
| Alb (g/dL) | 4.2 (2.7-5.2) |
3.9 (3.6-4.2) |
4.1 (3.2-4.9) |
4.2 (2.9-4.9) |
4.3 (2.7-4.7) |
4 (3.3-4.7) |
4.2 (3-5.2) |
n.s. |
| PT (%) | 93.6 (22-130) |
84 (70-115) |
97 (67-130) |
96 (30-130) |
91 (47-115) |
70 (34-105) |
97 (22-130) |
n.s. |
| IgG (mg/dL) | 1,288 (656-2,344) |
1,970 (948-2,344) |
1,274 (780-2,114) |
1,310 (656-2,168) |
1,380 (715-2,320) |
1,450 (1,010-2,040) |
1,027 (870-1,380) |
p<0.05 |
| IgM (mg/dL) | 168 (24-811) |
155 (69-303) |
300 (82-811) |
83 (24-341) |
193 (88-234) |
475 (190-798) |
114 (52-218) |
p<0.0001 |
| IgA (mg/dL) | 220 (79-539) |
187 (159-212) |
224 (130-421) |
244 (156-427) |
196 (123-389) |
253 (120-539) |
191 (79-364) |
n.s. |
P values for Kruskal-Wallis test. DILI: drug-induced liver injury, HBV: hepatitis B virus, HAV: hepatitis A virus, WBC: white blood cell, Hb: hemogurobin, ALT: alanine aminotransferase, AST: aspartate transaminase, GGT: γ-glutamyltransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, T-bil: total bilirubin, Alb: albumin, PT: prothrombin time, IgG: immunoglobulin G, IgM: immunoglobulin M, IgA: immunoglobulin A
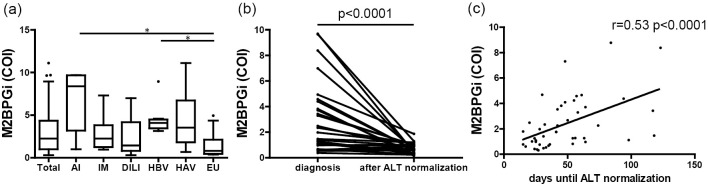
Figure 1.
(a) Serum M2BPGi levels in the overall study population and according to the etiology of acute liver injury. Differences were evaluated by the Kruskal-Wallis test, and significant differences were verified by Dunn’s multiple comparison test. *p<0.05. (b) The serum M2BPGi levels at the times of the diagnosis and at the normalization of ALT in 26 patients. The serum M2BPGi level was 2.2 (range, 0.38-9.7) COI at the time of the diagnosis and 0.68 (range, 0.15-1.87) COI at the time of serum ALT normalization (p<0.0001). Differences between the two groups were evaluated by Wilcoxon’s signed-rank test. (c) The correlation between the serum M2BPGi level and the time to serum ALT normalization in 46 patients. The serum M2BPGi level (r=0.53, p<0.0001) was positively correlated with the time to serum ALT normalization. Correlations were evaluated by calculating Spearman’s rank-correlation coefficient (r). AI: autoimmune liver disease, IM: infectious mononucleosis, DILI: drug-induced liver injury, HBV: hepatitis B virus, HAV: hepatitis A virus, EU: etiology unknown, ALT: alanine aminotransferase, M2BPGi: Mac-2-binding protein glycosylation isomer
The serum M2BPGi level was significantly decreased after serum ALT normalization in comparison to the time of the diagnosis [0.68 (range, 0.15-1.87) COI and 2.2 (range, 0.38-9.7) COI, respectively; p<0.0001] (Fig. 1b).
Furthermore, the serum M2BPGi level at the diagnosis was positively correlated with the time to serum ALT normalization (r=0.53, p<0.0001) (Fig. 1c). The time to ALT normalization was correlated more strongly with the serum M2BPGi level than with the serum levels of ALT, lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), total bilirubin (T-bil), and albumin (Alb), or the serum PT activity.
In addition, the correlation between serum M2BPGi level at the time of the diagnosis and the days until serum ALT normalization was analyzed separately according to the etiology of liver injury, the degree of the serum T-bil level, and the degree of PT activity (Table 3). Only infectious mononucleosis showed a different correlation.
Table 3.
The Differences in Correlation Due to Factors between Serum M2BPGi Level at the Time of Diagnosis and the Days until Serum ALT Level Normalization.
| Patients | Spearmann | p value | ||||
|---|---|---|---|---|---|---|
| Etiology | ||||||
| Infectious mononucleosis | n=11 | r=0.0667 | p=0.865 | |||
| Drug-induced liver injury | n=9 | r=0.494 | p=0.178 | |||
| Virus hepatitis | n=13 | r=0.495 | p=0.086 | |||
| Etiology Unknown | n=12 | r=0.330 | p=0.295 | |||
| Degree of T-bil | ||||||
| T-bil<1.0 mg/dL | n=15 | r=0.131 | p=0.642 | |||
| 1.0 mg/dL<T-bil | n=28 | r=0.536 | p=0.0033 | |||
| Degree of PT | ||||||
| 70%<PT | n=7 | r=0.714 | p=0.0881 | |||
| PT>70% | n=34 | r=0.501 | p=0.0026 |
Some cases have missing data. T-bil: total bilirubin, PT: prothrombin time
Correlations between serum M2BPGi and other laboratory parameters
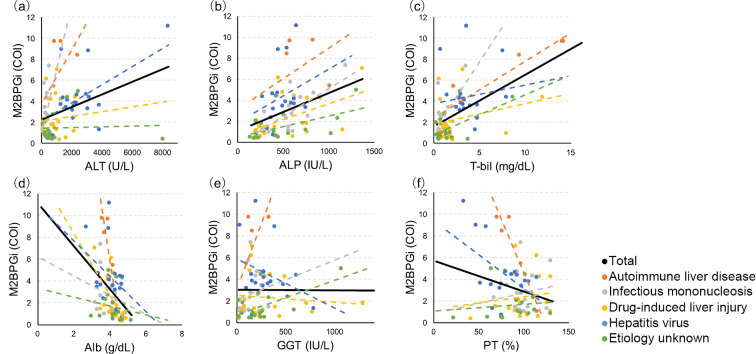
In the overall study population, the serum ALT (r=0.34, p<0.01), ALP (r=0.46, p<0.0001), and T-bil (r=0.49, p<0.0001) levels were positively correlated with the serum M2BPGi level (Fig. 2a-c), while the serum Alb level (r=-0.45, p<0.0001) was negatively correlated with the serum M2BPGi level (Fig. 2d). The serum M2BPGi level was not significantly correlated with the serum GGT level or the serum PT activity (Fig. 2e, f). The correlations of the serum M2BPGi level with the serum ALP, T-bil, and Alb levels did not differ according to the etiology (Fig. 2b-d). However, the correlation of the serum M2BPGi level with the serum ALT, GGT, and PT activity levels differed according to the etiology (Fig. 2a, e, f).
Figure 2.
Correlations between the serum level of M2BPGi and serum levels of ALT, ALP, T-bil, Alb, and GGT, as well as the serum PT activity. Black line, correlations for the overall study population; dotted lines, correlations according to the etiology. The serum M2BPGi level was significantly correlated with the serum ALT level in the overall study population (r=0.34, p<0.01), and this correlation differed according to etiology (a). The serum M2BPGi level was significantly correlated with the serum levels of ALP (r=0.46, p<0.0001), T-bil (r=0.49, p<0.0001), and Alb (r=-0.45, p<0.0001) in the overall study population and in each etiology group (b, c, d). The serum M2BPGi level was not correlated with the serum GGT level or PT activity in the overall study population, and the correlations differed according to the etiology of acute liver injury (e, f). Orange dotted line, autoimmune liver disease; gray dotted line, infectious mononucleosis; yellow dotted line, drug-induced liver injury (DILI); blue dotted line, hepatitis virus (hepatitis A virus/hepatitis B virus); green dotted line, etiology unknown. M2BPGi: Mac-2-binding protein glycosylation isomer, ALT: alanine aminotransferase, ALP: alkaline phosphatase, T-bil: total bilirubin, Alb: albumin, GGT: γ-glutamyltransferase, PT: prothrombin time
Factors contributing to the increase in serum M2BPGi
To determine the factors contributing to the increase in serum M2BPGi, a step-wise multiple regression analysis was performed using the following factors: age, sex, ALT, GGT, ALP, LDH, Alb, T-bil, PT, platelet count, and each etiologies of acute liver injury (autoimmune liver disease, infectious mononucleosis, HAV/HBV, DILI, and etiology unknown). All variables were included in the analysis, because it was confirmed (using the correlation matrix table) that there was no variable with |r|>0.9. Our results showed that independent variables, with the exception of age, sex, GGT, LDH, PT, platelet count, and etiologies such as autoimmune liver disease and infectious mononucleosis, remained in the final equation (Table 4). The serum T-bil level was most closely associated with the serum M2BPGi level (coefficient β, 0.418; p<0.001). Regarding the association between the serum M2BPGi level and the etiology of acute liver injury, etiology unknown and DILI did not contribute to elevation of M2BPGi in comparison to viral hepatitis, because HAV/HBV was used as a reference value in this analysis (etiology unknown, coefficient β, -0.259, p<0.01; DILI, -0.221, p<0.05).
Table 4.
Step-wise Multiple Regression Model to Identify Significant Independent Factors Affecting Serum M2BPGi Level in Acute Liver Injury.
| Final fitted model | Standardized coefficient β | p value | ||
|---|---|---|---|---|
| T-bil | 0.418 | p<0.001 | ||
| Alb | -0.262 | p<0.01 | ||
| ALT | 0.185 | p<0.05 | ||
| ALP | 0.196 | p<0.05 | ||
| Etiology unknown | -0.259 | p<0.01 | ||
| DILI | -0.221 | p<0.05 |
r2=0.57, ANOVA p<0.001. Durbin-Watson ratio was 2.064.
T-bil: total bilirubin, Alb: albumin, ALT: alanine aminotransferase, ALP: alkaline phosphatase, DILI: drug-induced liver injury
Serum M2BPGi levels in patients with infectious mononucleosis
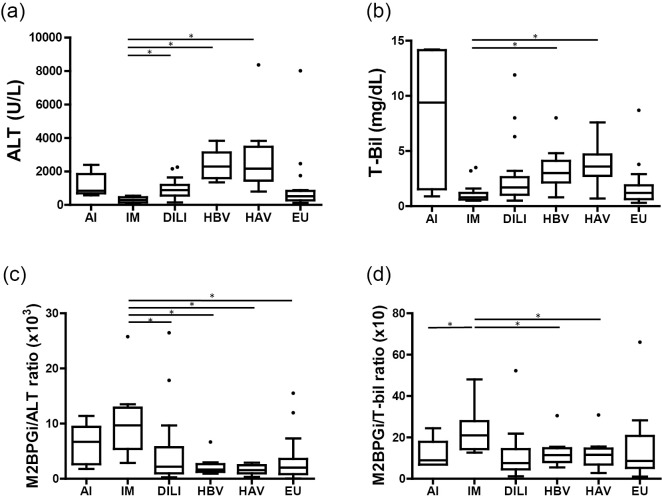
In patients with infectious mononucleosis, the degrees of increase in ALT or T-bil were the lowest in comparison to other etiologies (ALT, p<0.0001; T-bil, p<0.001) (Fig. 3a, b). On the other hand, the median serum M2BPGi level in infectious mononucleosis did not differ from the median value for all cases, suggesting that the serum M2BPGi levels in infectious mononucleosis were higher relative to the degree of increase in serum ALT or T-bil levels in comparison to other etiologies. Furthermore, the serum M2BPGi-to-ALT ratio in infectious mononucleosis was significantly higher in comparison to other etiologies (Fig. 3c) (p<0.0001). Similarly, the serum M2BPGi-to-T-bil ratio in infectious mononucleosis was significantly higher in comparison to other etiologies (p<0.01). In addition, with the exception of infectious mononucleosis, the serum M2BPGi-to-T-bil ratio of other etiologies did not differ to a statistically significant extent and the ratios were almost the same, suggesting the possibility that the nature of the elevation of M2BPGi in infectious mononucleosis might differ from other etiologies (Fig. 3d).
Figure 3.
(a) Serum ALT levels according to the etiology of acute liver injury. The serum ALT level differed significantly according to the etiology of acute liver injury (p<0.0001). In the infectious mononucleosis group, the serum ALT level was lowest and was significantly lower in comparison to the DILI, HBV, and HAV groups. (b) Serum T-bil levels according to the etiology of acute liver injury. The serum T-bil level differed significantly according to the etiology of acute liver injury (p<0.001). In the infectious mononucleosis group, the serum T-bil level was lowest and was significantly lower in comparison to the HBV and HAV groups. (c) The M2BPGi-to-ALT ratio according to the etiology of acute liver injury. This ratio differed significantly according to the etiology of acute liver injury (p<0.0001). In the infectious mononucleosis group, this ratio was highest and significantly higher in comparison to other etiologies, with the exception of autoimmune liver disease. (d) The M2BPGi-to-T-bil ratio according to the etiology of acute liver injury. This ratio differed significantly according to the etiology of acute liver injury (p<0.01). In the infectious mononucleosis group, this ratio was highest and was significantly higher in comparison to the autoimmune liver disease, HBV and HAV groups. Differences were evaluated by the Kruskal-Wallis test, and significant differences were verified by Dunn’s multiple comparison test. *p<0.05. AI: autoimmune liver disease, IM: infectious mononucleosis, DILI: drug-induced liver injury, HBV: hepatitis B virus, HAV: hepatitis A virus, EU: etiology unknown, ALT: alanine aminotransferase, M2BPGi: Mac-2-binding protein glycosylation isomer
Discussion
We investigated the serum M2BPGi levels in patients with acute liver injury. The serum M2BPGi level was transiently increased at the time of the diagnosis and significantly decreased after serum ALT normalization, consistent with a previous report (8). Furthermore, the serum M2BPGi level at the diagnosis was significantly correlated with the time to serum ALT normalization. The serum ALT level does not reflect the amount of liver cells that have already been injured but reflects the amount of liver cells that are being injured. Thus, the correlation between the serum M2BPGi level and the time required for the serum ALT level to normalize suggested that the serum M2BPGi level might predict the total amount of injured liver cells.
Our results suggest that the serum M2BPGi level might reflect the liver inflammation in acute liver injury, as reported previously (8), because the serum M2BPGi level significantly decreased with the improvement of liver inflammation. However, the correlation between the serum M2BPGi and ALT levels was weak and differed according to the etiology. This implied that the increase in the serum M2BPGi level might also reflect factors other than liver inflammation.
Our step-wise multiple regression model showed that etiology unknown and DILI did not contribute to the increase in the serum M2BPGi level in comparison to viral hepatitis. Etiology unknown and DILI have various mechanisms of liver injury in comparison to other etiologies. Thus, it is not easy to clarify the association between the increase in the serum M2BPGi level and etiology. However, the results of the multivariate analysis suggested that the etiology of acute liver injury might be considered as a factor other than liver inflammation that contributed to the increase in the serum M2BPGi level.
In patients with infectious mononucleosis, the serum M2BPGi level was higher relative to the degree of increase of the serum ALT or T-bil levels in comparison to other etiologies, and the correlation between the serum M2BPGi level at the time of the diagnosis and the time to serum ALT normalization differed from other etiologies (Table 3). These results suggest that the mechanism by which M2BPGi increases in infectious mononucleosis might differ from the mechanisms in other etiologies. In this study, the infectious mononucleosis patient population was typical (17,18), and the degree of liver injury in patients with infectious mononucleosis is usually mild in comparison to other etiologies of acute liver injury (19). Thus, the serum M2BPGi level in infectious mononucleosis might be higher relative to the degree of liver injury, and disease-specific factors might be involved in the mechanism by which M2BPGi increases in these patients. One possible factor is the activation of hepatic stellate cells, which are assumed to be M2BPGi producing cells (11). EBV or CMV, which are causative viruses of infectious mononucleosis, tend to specifically infect fibroblasts or epithelial cells in addition to B cells (20). Since hepatic stellate cells are a type of fibroblast, it is considered that these viruses might infect hepatic stellate cells, and the change in the cellular environment induced by infection might contribute to the increase in the serum M2BPGi level.
The reason why M2BPGi increases in acute liver injury is not well understood. However, it has been reported that hepatic stellate cells, which are assumed to be M2BPGi-producing cells (11), are activated during the recovery process in acute liver injury (21); this suggests an association with the transient increase in serum M2BPGi level in acute liver injury. The fact that the etiology in acute liver injury affects the increase in serum M2BPGi suggests the possibility that the mechanism of activation or secretion in M2BPGi secreting cells or M2BPGi secreting cells may differ according to the etiology. It is necessary to clarify these associations in a large cohort in the future.
Liver fibrosis, a highly conserved and coordinated wound-healing process, is a reaction aimed at maintaining organ integrity. The activation of the hepatic stellate cells plays a central role in this process. In a rat model of liver injury, it has been reported that the response of hepatic stellate cells in acute liver injury is terminated by transient activation, while in chronic liver injury hepatic stellate cells become myofibroblast-like and the response is sustained (22). Thus, it is a very interesting result that M2BPGi elevation is detected despite the different phenotypes of hepatic stellate cell activation in both acute and chronic liver injury. The fact that M2BPGi is also elevated in acute liver injury may indicate the potential to further classify M2BPGi subsets or elucidate the mechanisms of hepatic stellate cells activation and secretory functions.
The serum M2BPGi level was not significantly correlated with the serum PT activity, which is not in agreement with a previous report (8). This may be because 60% of the patients in the previous study had liver failure in comparison to 2.5% in our study. The number and type of injured liver cells may differ in patients with severe liver injury in comparison to mild liver injury, or the changes in the liver could depend on the severity of acute liver injury.
This study was associated with several limitations. First, the distribution of etiologies among the acute liver injury patients was uneven, and most patients had a preserved hepatic reserve, which may have biased the pathology assessment. Second, the correlations with the serum M2BPGi level were only evaluated based on clinical data, which may not have reflected the actual liver microenvironment. Even after ALT normalization, not all M2BPGi values were within the standard range (Fig. 1b). To accurately understand the dynamics of M2BPGi in acute liver injury, it is important to clarify the timing of M2BPGi measurement in the natural course of acute liver injury and to track the detailed time course of M2BPGi.
In conclusion, in patients with acute liver injury, the serum M2BPGi level reflects the magnitude and duration of liver injury. However, when measuring the serum M2BPGi levels in patients with acute liver injury, it should be noted that the degree of increase in serum M2BPGi may differ according to the etiology. In addition, an increase in serum M2BPGi due to transient liver injury suggests that it may sometimes be overestimated in the evaluation of fibrosis in chronic liver injury.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Kuno A, Ikehara Y, Tanaka Y, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 3: 1065, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamasaki K, Tateyama M, Abiru S, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 60: 1563-1570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou X, Zhu MY, Yu DM, et al. Serum WFA+-M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int 37: 35-44, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Abe M, Miyake T, Kuno A, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol 50: 776-784, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Umemura T, Joshita S, Sekiguchi T, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol 110: 857-864, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa H, Enomoto H, Iwata Y, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res 46: 613-621, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Shirabe K, Bekki Y, Gantumur D, et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol 53: 819-826, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Morio K, Imamura M, Daijo K, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increase in patients with acute liver injury. J Gastroenterol 52: 1252-1257, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Mochida S, Nakayama N, Ido A, et al. Revised criteria for classification of the etiologies of acute liver failure and late-onset hepatic failure in Japan: a report by the Intractable Hepato-biliary Disease Study Group of Japan in 2015. Hepatol Res 46: 369-371, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Das S, Jameel S. The biology and pathogenesis of hepatitis viruses. Current Science 89: 312-325, 2010. [Google Scholar]
- 11.Bekki Y, Yoshizumi T, Shimoda S, et al. Hepatic stellate cells secreting WFA+-M2BP: its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 32: 1387-1393, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Maddur H, Chalasani N. Idiosyncratic drug-induced liver injury; a clinical update. Curr Gastroenterol Rep 13: 65-71, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 46: 2050-2068, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: does it exist? A published work review. Hepatol Res 41: 498-504, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 59: 246-249, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol 47: 849-861, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunno A, Abe M, Yamada M, Murakami K. Clinical and histological features of cytomegalovirus hepatitis in previously healthy adults. Liver 17: 129-132, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Drebber U, Kasper HU, Krupacz J, et al. The role of Epstein-Barr virus in acute and chronic hepatitis. J Hepatol 44: 879-885, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Vallbracht A, Maier K, Stierhof YD, Wiedmann KH, Flehming B, Fleischer B. Liver-derived cytotoxic T cells in hepatitis A virus infection. J Infect Dis 160: 209-217, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol 76: 741-750, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Dechene A, Sowa JP, Gieseler RK, et al. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 52: 1008-1016, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K, Matsuzaki K. Differential regulation of TGF-β/Smad signaling in hepatic stellate cells between acute and chronic liver injury. Front Physiol 3: 53, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]