Abstract
The prevalence and progression of non-alcoholic fatty liver disease (NAFLD) is mediated via several factors correlating with hepatic necroinflammation (adipokines/cytokines). This study was performed to analyze the level of inflammatory markers according to the presence of NAFLD and to identify related nutritional factors. A total of 80 adults were classified into 2 groups (healthy and NAFLD), and their body composition, blood tests, and eating habits were evaluated. In addition, inflammatory markers (adiponectin, high-sensitivity C-reactive protein [CRP], and tumor necrosis factor-alpha [TNF-α]), nutrient intake status, and dietary quality were compared. The quality of diet was assessed according to the nutrient adequacy ratio and the mean adequacy ratio (MAR). The NAFLD group had a higher body mass index (p < 0.001) than the healthy group and also carried significantly higher CRP levels (p < 0.001) but lower adiponectin (p = 0.001). TNF-α levels increased significantly with fatty liver grade (p = 0.023). The NAFLD group showed significantly higher intake of energy, carbohydrates, iron, sodium, vitamin A and saturated fatty acids, but significantly lower intake of zinc and vitamin E than the healthy group. The MAR values were slightly higher in the NAFLD group but without any significant difference. The levels of adiponectin and vitamin E showed a significant inverse correlation (p < 0.05). Nutritional management of NAFLD patients is important, and the intake of antioxidant and anti-inflammatory nutrients such as zinc and vitamin E should be emphasized.
Keywords: Non-alcoholic fatty liver disease, Adipokines, Adiponectin, Nutrients, Vitamin E
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is defined as the excessive accumulation of triglycerides within the liver cells [1]. Accumulation of fat induces oxidative stress, peroxidation, cytokine response or other secondary damages, which may lead to non-alcoholic steatohepatitis or cirrhosis [2]. The high prevalence of NAFLD in metabolic disorders such as hypertension, dyslipidemia, abdominal obesity and type II diabetes is related to insulin resistance. Insulin resistance is an important mechanism underlying NAFLD [3]. Recently, the roles of various inflammatory markers have been reported. High-sensitivity C-reactive protein (hs-CRP), an acute phase protein, is an inflammatory marker that leads to endothelial cell damage, which has also been associated with NAFLD [4]. Even when corrected for various pathological traits of NAFLD, hs-CRP is still an independent risk factor for NAFLD [5].
Adiponectin is secreted by adipose tissue. As the amount of adipose tissue in the body increases, the amount of adiponectin in the blood decreases [6]. Therefore, the level of adiponectin in blood is inversely correlated with the fat content and insulin resistance of the liver tissue [7]. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine, and the concentrations of which are increased in patients with NAFLD. The levels of TNF-α and TNF-α type 1 receptor increase in the liver tissue of NAFLD patients, coupled with the increased fibrosis in the liver tissue [8]. Similarly, the abnormal expression of various adipokines can be used to explain the relationship between metabolic and liver disorders.
The liver is an essential organ in the human digestive system. Patients with a defective liver often present with nutritional imbalance because the liver plays a significant role in metabolism and nutritional balance [9]. The risk factors for NAFLD include lack of exercise and westernized diet; however, the progression of a fatty liver disease can be slowed by combining weight control with healthy diet [10]. Preventative factors include a healthy intake of nuts, ω-3 fatty acids and antioxidant vitamins. The higher the intake of carbohydrates and simple sugars, the higher the NAFLD [11,12]. Micronutrient deficiencies also play a key role in fatty liver disease, and especially zinc, copper, iron, selenium, magnesium, vitamins A, C, D, and E and carotenoids have antioxidant, antifibrotic and immunomodulatory effects [13]. NAFLD patients showed decreased levels of vitamin A and E in their blood. Therefore, vitamin E supplementation may be used to treat fatty liver [14]. In specific cases, Mediterranean diet significantly ameliorated fatty liver disease without weight loss, suggesting the need for a customized dietary plan for individual patients [15].
The relationship between NAFLD, nutrition and food has been investigated; however, the effect of nutrition must be elucidated in the future. Studies investigating the role of dietary intake in Korean patients with NAFLD and thus the correlation between NAFLD and inflammatory markers have yet to be reported. Therefore, this study analyzed the adipokine levels in NAFLD patients, determined their nutritional intake and quality of diet, and established the correlation between adipokines and nutrition from various angles.
MATERIALS AND METHODS
Subjects
The subjects of this study were healthy adults over the age of 30 years who visited the university hospital health screening examination center between April and September 2013. Subjects with a previous history of viral or toxic hepatitis, chronic liver disease, significant alcohol consumption, those currently on medications for fatty liver disease and those taking health supplements or trying folk remedies, were excluded. A total of 92 patients were recruited, but subjects with chronic liver disease (n = 5), examination refusal (n = 4), and missing data (n = 3) were excluded. All potential subjects were informed of the study objectives, and the study included 80 participants finally. The subjects were divided into the healthy and the NAFLD groups depending on their liver conditions. NAFLD has been diagnosed as a moderate fatty liver disease via abdominal ultrasound while consuming less than 140 g/week or 20 g/day of alcohol. The study protocol was approved by the Institutional Review Board (IRB) of Soonchunhyang University Bucheon Hospital (IRB No. SCHBC IRB 2013-08). Written informed consent was obtained from all patients. All the participants understood the study, and voluntarily agreed to participate in the study. The study protocol conformed to ethical guidelines of the World Medical Association Declaration of Helsinki.
Data collection
Parameters such as height, weight, body mass index (BMI) and the percentage of body fat were evaluated using the bio-electrical impedance fatness analyzer (Inbody 720; Bio-space, Seoul, Korea). The waist circumference was measured from the mid-point between the lowest rib and the highest part of the iliac spine, with the subject exhaling lightly. The hip circumference was considered as the largest part of the iliac bone. Both circumferences were measured twice, and the average value was used to calculate the waist/ hip ratio. The tests were conducted using blood samples drawn from venous blood in the upper arm after 12 hours of fasting. An automatic serum analyzer (Hitachi 7170; Hitachi Ltd., Tokyo, Japan) was used to conduct the basic blood tests and lipid analyses, while cytokines were measured using the automatic biochemical analyzer (Fuji DRI-CHEM 3500i; Fujifilm, Tokyo, Japan) and enzyme-linked immunosorbent assay test. The systolic and diastolic blood pressure levels were measured from the upper arm using an automatic blood pressure measurement tool, after 10 minutes of rest, and the presence of metabolic disorder was diagnosed based on NCEP ATP III criteria [16].
Dietary habits were identified via a survey of meal frequency, skipping meals, regularity of mealtime, eating speed, snacking frequency, frequency of eating out, exercise, smoking and drinking habits. Dietary intake was measured based on a 24-hours recall method, which was used to survey a 3-day meal. The consumed food was analyzed using a Computer Aided Nutritional Analysis Program for Professionals 4.0 (CAN-Pro 4.0; The Korean Nutrition Society, Seoul, Korea) based on the intake of individual nutrients. The quality of the diet was assessed according to the nutrient adequacy ratio (NAR) and mean adequacy ratio (MAR). The NAR is based on the intake recommended by the 2015 Dietary Reference Intakes for Koreans as a reference value [17]. The nutrients identified were protein, calcium, phosphorous, iron, zinc, vitamin A, vitamin B1, vitamin B2, vitamin B6, niacin, vitamin C, and folic acid.
Definitions
The formula used to calculate NAR and MAR are as follows [18].
| NAR = nutrient intake/recommended nutrient intake |
| MAR = sum of NAR of nutrients/the number of nutrients |
The NAR calculated from the recommended nutrient intake represents the gender-specific and age-specific requirements of daily intake. A NAR close to 1 indicates that the amount of nutrient intake is close to the daily recommended intake. A value higher than 1 suggests that the intake exceeds the daily recommended intake, while a value less than 1 implies that the daily intake is not adequate to meet the daily recommended intake [18]. A fatty liver was diagnosed when the liver parenchymal echogenicity was higher than the kidney cortex. The severity of the fatty liver was graded as normal, mild, moderate, or severe according to echogenicity of the liver parenchyma [19]. The metabolic syndrome was defined by the presence of three or more of the following five risk factors: central obesity (men > 102 cm, women > 88 cm), triglycerides ≥ 150 mg/dL, low high-density lipoprotein (HDL) cholesterol (men < 40 mg/dL, women < 50 mg/dL), high blood pressure (≥ systolic 130 mmHg or ≥ diastolic 85 mmHg), and impaired fasting glucose (≥ 110 mg/dL) [20].
Statistical analyses
The clinical and nutrient-related factors of the subjects were summarized as means and standard deviations for continuous variables, and frequency and percentage (%) for categorical variables. The significant differences between the healthy and the NAFLD groups were identified by independent 2-sample t-test and Wilcoxon's rank-sum test for continuous data and Fisher's exact test for categorical data. Multiple linear regression analysis was also carried out to identify the risk factors for fatty liver disease. All the analyses were conducted using R (version 3.6.1; The R Foundation for Statistical Computing, Vienna, Austria) and the statistical significance was set at 0.05 based on the 2-sided test.
RESULTS
Demographic status of the subjects
The average age of the subjects was 47.80 years, and the average age of the NAFLD group was significantly higher than that of the healthy group (50.91 years vs. 45.72 years). The NAFLD group also had a higher percentage of male subjects (68.2% vs. 37.5%). The average BMI was 24.50 ± 3.45 kg/m2, which falls in the overweight category, whereas the NAFLD group had a BMI of 26.60 ± 3.53 kg/m2, which falls in the obese category. The BMI differed significantly between the 2 groups. The waist-hip ratio was also significantly higher in the NAFLD group. Subjects with metabolic disorders constituted 12.5% of the healthy group and 50.0% of the NAFLD group, and were significantly higher in the NAFLD group (Table 1).
Table 1. Demographic data of the subjects.
| Variable | Total (n = 80) | Healthy (n = 48) | NAFLD (n = 32) | p value | |
|---|---|---|---|---|---|
| Age (yr) | 47.80 ± 9.62 | 45.72 ± 10.13 | 50.91 ± 7.98 | 0.017 | |
| Sex | 0.012 | ||||
| Male | 40 (50.0) | 18 (37.5) | 22 (68.8) | ||
| Female | 40 (50.0) | 30 (62.5) | 10 (31.2) | ||
| BMI (kg/m2) | 24.50 ± 3.45 | 23.09 ± 2.60 | 26.60 ± 3.53 | < 0.001 | |
| Body fat (%) | 25.58 ± 6.41 | 24.85 ± 6.35 | 26.68 ± 6.44 | 0.211 | |
| WC (cm) | 85.75 ± 12.13 | 83.89 ± 6.70 | 88.53 ± 17.14 | 0.094 | |
| WHR | 0.90 ± 0.06 | 0.88 ± 0.05 | 0.94 ± 0.05 | < 0.011 | |
| SBP (mmHg) | 122.89 ± 16.75 | 118.00 ± 12.47 | 130.22 ± 19.68 | 0.003 | |
| DBP (mmHg) | 73.71 ± 11.43 | 71.46 ± 10.02 | 77.09 ± 12.71 | 0.030 | |
| Metabolic syndrome (yes) | 22 (27.5) | 6 (12.5) | 16 (50.0) | < 0.001 | |
Data represented as mean ± standard deviation and number (%). The p value by χ2 test (categorical variables) and independent t-test (continuous variables).
NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Comparative biochemistry of healthy and NAFLD groups
The laboratory test results of the subjects are shown in Table 2. The NAFLD group showed significantly higher levels of hemoglobin, hematocrit, glucose, aspartate aminotransferase, alanine aminotransferase (ALT), gamma glutamyl transferase, total cholesterol, triglyceride, and low-density lipoprotein (LDL) cholesterol, while the HDL cholesterol levels were significantly lower. In particular, the levels of blood glucose, ALT, triglyceride, and LDL cholesterol in the NAFLD group were higher than the normal range. Compared to a healthy group, the NAFLD group showed significantly higher CRP levels (p < 0.001) but lower adiponectin (p = 0.001) (Table 2).
Table 2. Biochemical data of the subjects.
| Variable | Normal range | Total (n = 80) | Healthy (n = 48) | NAFLD (n = 32) | p value |
|---|---|---|---|---|---|
| Hgb (g/dL) | 13–17 | 14.42 ± 1.45 | 14.07 ± 1.54 | 14.94 ± 1.19 | 0.008 |
| Hct (%) | 36–52 | 42.89 ± 3.61 | 42.00 ± 3.79 | 44.20 ± 2.92 | 0.007 |
| Albumin (g/dL) | 3.3–5.2 | 4.56 ± 0.24 | 4.54 ± 0.22 | 4.59 ± 0.27 | 0.409 |
| Glucose (mg/dL) | 60–108 | 102.28 ± 20.10 | 97.79 ± 15.40 | 109.00 ± 24.32 | 0.025 |
| AST (IU/L) | 5–40 | 25.38 ± 12.51 | 21.38 ± 8.04 | 31.41 ± 15.44 | 0.002 |
| ALT (IU/L) | 0–40 | 30.01 ± 24.50 | 21.59 ± 13.84 | 42.66 ± 31.06 | < 0.001 |
| ALP (IU/L) | 44–119 | 81.23 ± 46.53 | 80.55 ± 57.82 | 82.25 ± 21.26 | 0.874 |
| LDH (IU/L) | 219–480 | 310.41 ± 66.39 | 299.98 ± 71.73 | 326.06 ± 54.87 | 0.085 |
| γGT (IU/L) | 12–73 | 44.05 ± 37.38 | 31.48 ± 31.92 | 62.91 ± 37.48 | < 0.001 |
| Calcium (mg/dL) | 8.3–10 | 9.12 ± 0.38 | 9.11 ± 0.33 | 9.13 ± 0.45 | 0.841 |
| Phosphorous (mg/dL) | 2.5–4.5 | 3.67 ± 0.42 | 3.67 ± 0.44 | 3.67 ± 0.40 | 0.957 |
| Cholesterol (mg/dL) | 90–250 | 205.93 ± 43.07 | 197.33 ± 40.69 | 218.81 ± 43.94 | 0.028 |
| Triglyceride (mg/dL) | 0–200 | 153.50 ± 89.36 | 110.38 ± 68.81 | 218.19 ± 77.29 | < 0.001 |
| HDL-cholesterol (mg/dL) | 35–50 | 53.64 ± 12.27 | 56.63 ± 12.18 | 49.16 ± 11.14 | 0.007 |
| LDL-cholesterol (mg/dL) | 0–140 | 134.31 ± 41.24 | 124.48 ± 40.06 | 149.06 ± 39.08 | 0.008 |
| CRP (mg/dL) | 0–0.5 | 0.20 ± 0.17 | 0.12 ± 0.19 | 0.34 ± 0.22 | < 0.001 |
| Adiponectin (μg/mL) | 5–25 | 5.08 ± 3.81 | 7.16 ± 4.21 | 3.37 ± 2.28 | 0.001 |
| TNF-α (pg/mL) | 2.42 ± 1.98 | 2.13 ± 2.00 | 2.86 ± 1.90 | 0.109 |
Data represented as mean ± standard deviation and number (%). The p value by χ2 test (categorical variables) and independent t-test (continuous variables).
NAFLD, non-alcoholic fatty liver disease; Hgb, hemoglobin; Hct, hematocrit; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; γGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; TNF-α, tumor necrosis factor-alpha.
Healthy habits and eating behaviors of healthy group and NAFLD group
The dietary habits and lifestyles of the 2 groups are shown in Table 3: 34.5% of the total subjects ate 2 or fewer meals per day, with breakfast being the most frequently skipped meal; 73.7% had regular mealtimes, whereas 57.5% ate rapidly. One snack per day was the highest frequency of snacking, while 61.3% of the overall subjects never exercised. There were no significant differences in drinking frequency; and the highest frequency of eating out was once or twice a week. The healthy habits and eating behaviors of the 2 groups showed no significant differences, except for smoking, whereas the NAFLD group showed a significantly lower frequency of smoking (p < 0.007) (Table 3).
Table 3. Health habit and eating behavior of the subjects.
| Variable | Total (n = 80) | Healthy (n = 48) | NAFLD (n = 32) | p value | |
|---|---|---|---|---|---|
| Frequency of meal | 0.460 | ||||
| One time | 1 (1.3) | 1 (2.1) | 0 (0.0) | ||
| Twice | 27 (33.2) | 118 (37.5) | 9 (28.1) | ||
| Three times | 52 (65.0) | 29 (60.4) | 23 (71.9) | ||
| Skipping meal | 0.299 | ||||
| Breakfast | 18 (22.4) | 14 (29.2) | 4 (12.4) | ||
| Lunch | 7 (8.8) | 4 (8.3) | 3 (9.4) | ||
| Dinner | 3 (3.8) | 1 (2.1) | 2 (6.3) | ||
| None | 52 (65.0) | 29 (60.4) | 23 (71.9) | ||
| Regularity of mealtime | 0.381 | ||||
| Regular | 59 (73.7) | 33 (68.7) | 26 (81.3) | ||
| Irregular | 21 (26.3) | 15 (31.3) | 6 (18.7) | ||
| Eating speed | 0.534 | ||||
| Fast | 46 (57.5) | 30 (62.4) | 16 (50.0) | ||
| Normal | 28 (35.0) | 15 (31.3) | 13 (40.6) | ||
| Slow | 6 (7.5) | 3 (6.3) | 3 (9.4) | ||
| Frequency of snack | 0.196 | ||||
| 1 time/day | 38 (47.5) | 23 (47.9) | 15 (46.9) | ||
| 2 times/day | 10 (12.4) | 7 (14.6) | 3 (9.4) | ||
| 3 times/day | 21 (26.3) | 12 (25.0) | 9 (28.1) | ||
| ≥ 4 times/day | 4 (5.0) | 4 (8.3) | 0 (0.0) | ||
| None | 7 (8.8) | 2 (4.2) | 5 (15.6) | ||
| Exercise | 0.244 | ||||
| None | 49 (61.3) | 29 (60.4) | 20 (62.4) | ||
| 3–4 times/week | 21 (26.3) | 15 (31.3) | 6 (18.8) | ||
| Daily | 10 (12.4) | 4 (8.3) | 6 (18.8) | ||
| Smoking | 0.007 | ||||
| Nonsmoker | 48 (60.0) | 35 (72.9) | 13 (40.6) | ||
| Smoker at past | 14 (17.5) | 4 (8.3) | 10 (31.3) | ||
| Smoker | 18 (22.5) | 9 (18.8) | 9 (28.1) | ||
| Drinking | 0.831 | ||||
| Nondrinker | 31 (38.8) | 19 (39.6) | 12 (37.5) | ||
| Drinker at past | 8 (10.0) | 4 (8.3) | 4 (12.5) | ||
| Drinker | 41 (51.2) | 25 (52.1) | 16 (50.0) | ||
| Frequency of eating out | 0.124 | ||||
| Daily | 5 (6.3) | 3 (6.3) | 2 (6.3) | ||
| ≥ 4 times/week | 23 (28.7) | 18 (37.5) | 5 (15.6) | ||
| 1–2 times/week | 34 (42.5) | 20 (41.6) | 14 (43.7) | ||
| 1–2 times/month | 17 (21.2) | 7 (14.6) | 10 (31.3) | ||
| None | 1 (1.3) | 0 (0.0) | 1 (3.1) | ||
Data represented as number (%). The p value by χ2 test (categorical variables).
NAFLD, non-alcoholic fatty liver disease.
Comparison of nutrient intake and dietary quality between healthy and NAFLD groups
The results of nutrient intake and dietary quality of the healthy and the NAFLD groups are shown in Tables 4 and 5. Compared to the healthy group, the NAFLD group showed significantly higher intakes of energy, carbohydrate, iron, sodium, vitamin A and saturated fatty acids, while the intake of zinc and vitamin E was significantly lower. The dietary quality analysis of nutritional intake was based on gender and age. NAR showed that the NAFLD group showed significantly higher levels of iron and vitamin A intake, while the intake of zinc was significantly lower. MAR values were slightly higher in the NAFLD group: 1.24 ± 0.32 compared to 1.18 ± 0.29 in the healthy group with no significant difference between the 2 groups. However, when the nutrients that do not meet the daily intake recommendations were analyzed, the healthy group showed a lack of calcium, vitamin A, vitamin B2 and folic acid, while the NAFLD group was deficient in calcium, zinc, vitamin B2 and folic acid (Tables 4 and 5).
Table 4. Nutrients intake of the subjects.
| Variable | Total (n = 80) | Healthy (n = 48) | NAFLD (n = 32) | p value |
|---|---|---|---|---|
| Energy (kcal) | 1,897.44 ± 408.38 | 1,820.00 ± 357.03 | 2,028.61 ± 449.59 | 0.018 |
| Protein (g) | 81.42 ± 23.37 | 77.60 ± 20.14 | 87.15 ± 26.84 | 0.074 |
| Fat (g) | 50.94 ± 19.10 | 49.8 ± 18.2 | 52.6 ± 20.5 | 0.520 |
| Carbohydrate (g) | 273.46 ± 65.94 | 258.85 ± 62.04 | 295.37 ± 66.46 | 0.014 |
| Fiber (g) | 22.10 ± 7.52 | 21.90 ± 7.70 | 22.41 ± 7.32 | 0.766 |
| Calcium (mg) | 576.28 ± 231.08 | 577.72 ± 264.80 | 574.1 ± 172.5 | 0.946 |
| Phosphorous (mg) | 1,133.90 ± 324.34 | 1,096.94 ± 339.12 | 1,189.34 ± 297.38 | 0.214 |
| Iron (mg) | 15.41 ± 5.21 | 14.14 ± 4.45 | 17.32 ± 5.74 | 0.007 |
| Sodium (mg) | 5,435.70 ± 1,851.07 | 4,868.42 ± 1,840.64 | 6,286.63 ± 1,532.85 | 0.001 |
| Potassium (mg) | 2,984.46 ± 976.88 | 2,827.25 ± 983.61 | 3,220.27 ± 932.41 | 0.078 |
| Zinc (mg) | 10.89 ± 6.89 | 12.43 ± 8.30 | 8.58 ± 2.73 | 0.014 |
| Vitamin A (μgRE) | 817.89 ± 423.52 | 721.91 ± 389.82 | 961.85 ± 437.09 | 0.012 |
| Retinol (μg) | 99.44 ± 976.88 | 105.70 ± 136.24 | 90.04 ± 63.09 | 0.546 |
| β Carotene (μg) | 3,665.69 ± 2,242.78 | 3,648.34 ± 2,189.41 | 3,691.72 ± 2,355.79 | 0.933 |
| Vitamin B1 (mg) | 1.19 ± 0.38 | 1.14 ± 0.38 | 1.24 ± 0.38 | 0.289 |
| Vitamin B2 (mg) | 1.13 ± 0.40 | 1.08 ± 0.42 | 1.21 ± 0.35 | 0.139 |
| Vitamin B6 (mg) | 2.35 ± 0.90 | 2.44 ± 0.93 | 2.21 ± 0.85 | 0.276 |
| Niacin (mgNE) | 17.56 ± 6.14 | 16.71 ± 5.26 | 18.83 ± 7.16 | 0.130 |
| Vitamin C (mg) | 109.68 ± 79.29 | 106.07 ± 76.18 | 115.08 ± 84.69 | 0.621 |
| Folic acid (μg) | 283.33 ± 121.50 | 291.09 ± 134.83 | 271.67 ± 99.11 | 0.487 |
| Vitamin E (mg α-TE) | 14.98 ± 6.93 | 16.37 ± 7.55 | 12.89 ± 5.32 | 0.027 |
| Cholesterol (mg) | 330.68 ± 187.34 | 299.48 ± 168.48 | 377.47 ± 206.48 | 0.068 |
| Total fatty acids (g) | 29.86 ± 16.73 | 27.64 ± 15.42 | 33.18 ± 18.27 | 0.147 |
| SFA (g) | 9.76 ± 6.22 | 8.21 ± 4.48 | 12.08 ± 7.69 | 0.013 |
| MUFA (g) | 11.36 ± 8.00 | 10.30 ± 7.38 | 12.94 ± 8.69 | 0.148 |
| PUFA (g) | 8.95 ± 4.92 | 9.15 ± 5.07 | 8.65 ± 4.76 | 0.656 |
Data represented as mean ± standard deviation. The p value by independent t-test (continuous variables).
NAFLD, non-alcoholic fatty liver disease; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Table 5. Diet quality of the subjects.
| Variable | Total (n = 80) | Healthy (n = 48) | NAFLD (n = 32) | p value | |
|---|---|---|---|---|---|
| NAR | |||||
| Protein | 1.48 ± 0.43 | 1.41 ± 0.37 | 1.58 ± 0.49 | 0.074 | |
| Calcium | 0.77 ± 0.31 | 0.77 ± 0.35 | 0.77 ± 0.23 | 0.946 | |
| Phosphorous | 1.62 ± 0.46 | 1.57 ± 0.48 | 1.70 ± 0.42 | 0.216 | |
| Iron | 1.54 ± 0.52 | 1.41 ± 0.44 | 1.73 ± 0.57 | 0.007 | |
| Zinc | 1.21 ± 0.77 | 1.39 ± 0.92 | 0.95 ± 0.30 | 0.004 | |
| Vitamin A | 1.09 ± 0.56 | 0.96 ± 0.52 | 1.28 ± 0.58 | 0.015 | |
| Vitamin B1 | 1.19 ± 0.38 | 1.15 ± 0.39 | 1.25 ± 0.38 | 0.289 | |
| Vitamin B2 | 0.87 ± 0.31 | 0.83 ± 0.32 | 0.93 ± 0.27 | 0.136 | |
| Vitamin B6 | 1.81 ± 0.70 | 1.88 ± 0.72 | 1.70 ± 0.66 | 0.279 | |
| Niacin | 1.10 ± 0.38 | 1.04 ± 0.33 | 1.18 ± 0.45 | 0.133 | |
| Vitamin C | 1.10 ± 0.79 | 1.06 ± 0.76 | 1.15 ± 0.85 | 0.618 | |
| Folic acid | 0.71 ± 0.31 | 0.73 ± 0.34 | 0.67 ± 0.27 | 0.389 | |
| MAR | 1.21 ± 0.38 | 1.18 ± 0.29 | 1.24 ± 0.32 | 0.398 | |
Data represented as mean ± standard deviation. The p value by independent t-test (continuous variables).
NAFLD, non-alcoholic fatty liver disease; NAR, nutrient adequacy ratio; MAR, mean adequacy ratio.
Correlation between inflammatory markers and vitamin E level
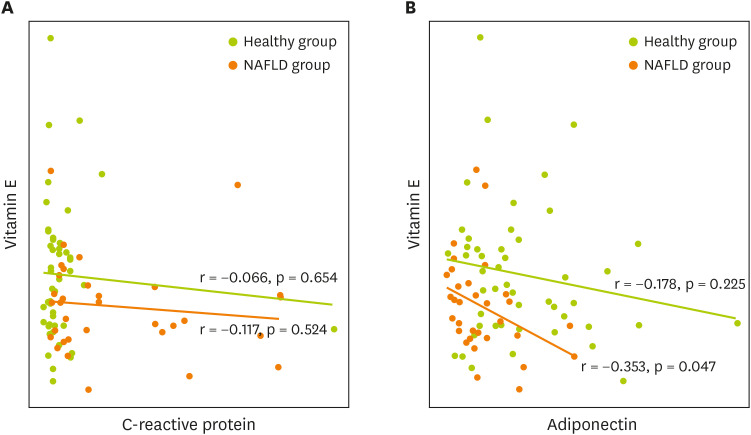
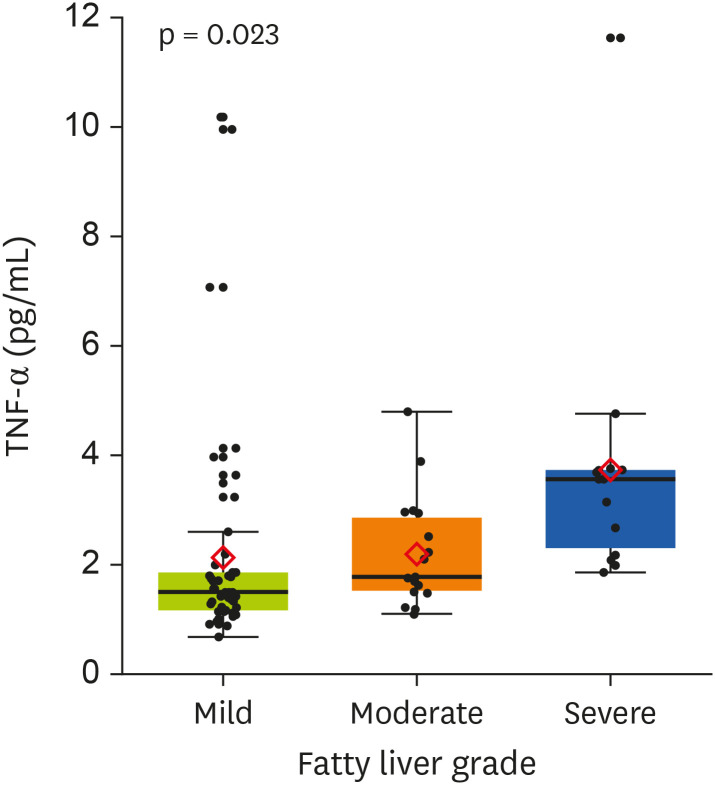
The nutritional factors associated with inflammatory markers that showed a significant difference in the nutrient intake between the healthy and the NAFLD groups were identified. As mentioned earlier, the CRP levels in the fatty liver group were significantly higher than in the healthy group. Comparing TNF-α according to fatty liver grade, TNF-α level was 2.17 ± 0.86 in mild grade, 3.01 ± 1.04 in moderate grade, and 5.50 ± 0.92 in severe grade. In addition, the TNF-α levels increased significantly with the fatty liver grade (p = 0.023). A significant inverse correlation was found between adiponectin and vitamin E (p < 0.05) (Figures 1 and 2).
Figure 1. Correlation of adipokines and vitamin E in the subjects.
(A) Correlation of C-reactive protein and vitamin E, (B) Correlation of adiponectin and vitamin E.
Figure 2. Differences in the level of TNF-α by fatty liver grade.
TNF-α, tumor necrosis factor-alpha.
Factors affecting fatty liver disease development
Univariate analysis was conducted to confirm the factors related to NAFLD occurrence. Variables identified in this analysis were further subjected to multivariate analysis. The results showed that age (odds ratio [OR], 1.09; 95% confidence interval [CI], 1.01–1.19), CRP (OR, 1.55; 95% CI, 1.21–2.26) and metabolic disorders (OR, 4.36; 95% CI, 1.24–16.84) significantly correlated with the occurrence of fatty liver. The Hosmer-Lemeshow goodness-of-fit test for the final model yielded a value of 5.801 (p = 0.67) which indicated a non-significant differences between observed and predicted occurrences (Table 6).
Table 6. Logistic regression analysis for NAFLD.
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| Demographic factor | |||||
| Age | 1.07 (1.01–1.14) | 0.023 | 1.09 (1.01–1.19) | 0.042 | |
| Male | 3.67 (1.45–9.79) | 0.007 | 2.45 (0.58–13.34) | 0.267 | |
| Inflammatory marker | |||||
| CRP | 1.55 (1.2–2.29) | 0.006 | 1.55 (1.21–2.26) | 0.003 | |
| Adiponectin | 0.73 (0.58–0.88) | 0.002 | 0.78 (0.55–1.03) | 0.119 | |
| TNF-α | 1.21 (0.96–1.61) | 0.131 | |||
| Lifestyle-related factor | |||||
| Obesity | 3.93 (1.55–10.46) | 0.005 | |||
| Metabolic syndrome | 7.00 (2.43–22.56) | 0.001 | 4.36 (1.24–16.84) | 0.025 | |
| Regularity | 1.97 (0.69–6.17) | 0.217 | |||
| Exercise | 0.92 (0.36–2.29) | 0.851 | |||
| Smoking | 1.70 (0.58–4.95) | 0.328 | |||
| Drinking | 0.92 (0.37–2.26) | 0.855 | |||
Goodness-of-fit: Hosmer-Lemeshow statistic = 5.801, DF = 8, p = 0.67.
NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; CRP, C-reactive protein; TNF-α, tumor necrosis factor-alpha.
DISCUSSION
In this study, we found that inflammation was closely related not only with the occurrence of fatty liver disease, but also its severity, which is probably related to inadequate micronutrient intake such as vitamin E or zinc.
NAFLD which accounts for a high proportion of chronic liver disease, is increasing in frequency in Korea due to the westernized diet, imbalance nutrition and lifestyle changes. Males are known to be more susceptible to NAFLD and the disease is closely related with metabolic disorders and visceral obesity [21]. The results of this study also showed a higher number of males in the NAFLD group, with higher percentage of body fat and abdominal obesity and higher frequency of metabolic disorders. The prevalence of NAFLD increases to 58% in overweight people (BMI ≥ 25 kg/m2), rising to 74%–98% in obese people (BMI ≥ 30 kg/m2), who underwent bariatric surgery [22]. The role of intestinal microbiota has recently been identified as the leading cause of obese NAFLD, but factors other than endocrine disease such as excessive energy intake, imbalanced diet and sedentary lifestyles are implicated in obesity and metabolic disorders, which leads to a stronger correlation between NAFLD and obesity.
The accumulation of fat in the liver occurs due to abnormal fatty acid metabolism, including not only synthesis but hepatic uptake of free fatty acids and triglyceride export [23,24]. These changes may lead to inflammation, oxidative stress and even abnormal adipokine synthesis, which has implications for downstream signaling pathways [25]. The analyses of subjects showed that the NAFLD group had higher triglyceride and LDL cholesterol levels, but lower HDL cholesterol. Thus, various strategies including weight management, diet therapy, exercise and medication should be implemented for NAFLD patients with abnormal lipid metabolism.
The pathogenesis of NAFLD is strongly mediated via dysregulation of adipokines and cytokines [26]. Adiponectin is hepatoprotective and increases the viability of hepatic cells and hepatic insulin sensitivity, and attenuates liver inflammation and fibrosis [27]. However, the effect is only transient and may be affected or altered by genetic or external factors, which requires customized treatment strategies targeting adipokines in the NAFLD [28]. In this study, the NAFLD group showed a normal range of adipokines, but significantly lower levels of adiponectin and higher hs-CRP, which underscored the need to validate the interactions between various factors.
The subjects' lifestyles were also compared. The NAFLD group had similar dietary habits compared to the healthy group but showed lower proportion of subjects who exercised and a higher percentage of smoking subjects. The management of NAFLD recommends that lifestyle management is the primary option, with medications playing only a supplementary role as needed [29]. In this context, diet therapy may help to improve insulin resistance and hepatic steatosis in NAFLD patients [30]. Exercise may decrease hepatic steatosis, and the cohort study reported that adequate strength exercises improved liver enzymes and insulin resistance independent of weight loss [31].
A study investigating the dietary habits and actions of NAFLD patients identified excessive intakes of energy, carbohydrate, and fat but lack of necessary vitamin and mineral intake as key risk factors [32]. This study also identified higher levels of energy, carbohydrate, sodium and saturated fatty acid intake and lower zinc and vitamin E intake in the NAFLD group compared to the healthy group, suggesting a similar trend. A recent study proposed that excessive intake of simple sugars such as sucrose and fructose is the main risk factor for NAFLD. The average daily intake of calories and soft drinks by the NAFLD patients was twice as high as that of non-NAFLD subjects [33]. Excessive intake of carbohydrates activates sterol regulatory element-binding protein-1c, which leads to the synthesis of enzymes needed for fatty acid production, which is also related to obesity [34]. Intake of an abundance of carbohydrates leads to a rapid increase in the serum glucose level and response hypoglycemia, resulting in feelings of empty stomach, increased appetite and overeating [35]. This study failed to identify the causative food behind increased carbohydrate intake, but it did identify the need to control the carbohydrate and fat intake of NAFLD patients.
The increased oxidative stress due to vitamin E deficiency is considered as one of the key factors in the onset and development of NAFLD, and vitamin E is a key therapeutic agent [36]. Progression from NAFLD to nonalcoholic steatohepatitis is influenced by oxidative stress. The fibrosis scores of NAFLD patients are inversely correlated with total antioxidant status, which points to the importance of antioxidants [37]. Well-known antioxidant nutrients include vitamins E, C, and A, zinc and selenium. In this study, the NAFLD group had a significantly lower intake of vitamin E and zinc, while vitamin E showed an inverse correlation with adiponectin among all adipokines. Adiponectin, an endogenous adipokine, increases insulin sensitivity via PPARγ-mediated upregulation. Since vitamin E increases the expression of adiponectin, it improves insulin resistance in the body [38]. Vitamin E is also implicated in the expression of genes that regulate the broad cellular functions such as cell cycle, inflammation and cell adhesion. It is a fat-soluble vitamin with antioxidant property, and is present in high concentrations in fish, some oils and nuts as well as green vegetables [39]. NAFLD patients need more vitamin E in an effort to combat oxidative stress; however, it was evident that these patients actually suffer from insufficient intake. Therefore, management strategies must include ways to meet the daily recommended intake of vitamin E.
The study limitations include a relatively small number of patients in a retrospective study, and the possibility of recall bias. Despite the small sample size, this study measured the adipokine levels in NAFLD patients and established their correlation with nutrient levels. In contrast, large-scale nutrition studies often excluded key nutritional details, whereas our study presented more comprehensive nutritional information.
CONCLUSION
Our study showed that a balanced diet rich in antioxidant and anti-inflammatory nutrients is more effective dietary management for NAFLD patients. In addition, NAFLD patients must be managed via a multidisciplinary approach closely monitoring their weight, blood lipid levels and other factors that affect metabolic status, which underscores the need for further studies in the field of diet and nutrition.
Footnotes
Funding: This work was supported by the Soonchunhyang University Research Fund.
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 3.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 4.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 5.Targher G. Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol. 2006;45:879–881. doi: 10.1016/j.jhep.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 7.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 8.Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V, Younossi ZM. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 9.Rusu E, Enache G, Jinga M, Dragut R, Nan R, Popescu H, Parpala C, Homentcovschi C, Nitescu M, Stoian M, Costache A, Posea M, Rusu F, Jinga V, Mischianu D, Radulian G. Medical nutrition therapy in non-alcoholic fatty liver disease--a review of literature. J Med Life. 2015;8:258–262. [PMC free article] [PubMed] [Google Scholar]
- 10.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11:677. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Fernández-Galilea M, Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez JA, Moreno-Aliaga MJ. Oxidative stress and non-alcoholic fatty liver disease: effects of omega-3 fatty acid supplementation. Nutrients. 2019;11:872. doi: 10.3390/nu11040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickett-Blakely O, Young K, Carr RM. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol. 2018;6:451–462. doi: 10.1016/j.jcmgh.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN) Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, De Lorenzo A. Diet and non-alcoholic fatty liver disease: the Mediterranean way. Int J Environ Res Public Health. 2019;16:3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappagoda CT. Perspectives: NCEP III-A call to arms! Prev Cardiol. 2001;4:132–134. doi: 10.1111/j.1520-037x.2001.00928.x. [DOI] [PubMed] [Google Scholar]
- 17.Paik HY. Dietary Reference Intakes for Koreans (KDRIs) Asia Pac J Clin Nutr. 2008;17(Suppl 2):416–419. [PubMed] [Google Scholar]
- 18.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc. 1996;96:785–791. doi: 10.1016/S0002-8223(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23:290–301. doi: 10.3350/cmh.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 21.Schwenger KJP, Bolzon CM, Li C, Allard JP. Non-alcoholic fatty liver disease and obesity: the role of the gut bacteria. Eur J Nutr. 2019;58:1771–1784. doi: 10.1007/s00394-018-1844-5. [DOI] [PubMed] [Google Scholar]
- 22.Duarte SMB, Stefano JT, Miele L, Ponziani FR, Souza-Basqueira M, Okada LSRR, de Barros Costa FG, Toda K, Mazo DFC, Sabino EC, Carrilho FJ, Gasbarrini A, Oliveira CP. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: a prospective pilot study. Nutr Metab Cardiovasc Dis. 2018;28:369–384. doi: 10.1016/j.numecd.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Almeda-Valdes P, Altamirano-Barrera A, Méndez-Sánchez N. Insights in non-alcoholic fatty liver disease pathophysiology with lipidomic analyses. Ann Hepatol. 2015;14:567–569. [PubMed] [Google Scholar]
- 24.Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol. 2016;22:1664–1673. doi: 10.3748/wjg.v22.i4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43:509–518. doi: 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- 27.Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010;12:365–383. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 28.Musso G, Gambino R, Pacini G, De Michieli F, Cassader M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009;89:558–567. doi: 10.3945/ajcn.2008.26720. [DOI] [PubMed] [Google Scholar]
- 29.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Chao S, Konerman M, Zhang W, Rao H, Wu E, Lin A, Wei L, Lok AS. Survey of nonalcoholic fatty liver disease knowledge, nutrition, and physical activity patterns among the general public in Beijing, China. Dig Dis Sci. 2019;64:3480–3488. doi: 10.1007/s10620-019-05709-0. [DOI] [PubMed] [Google Scholar]
- 31.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 32.Yasutake K, Kohjima M, Kotoh K, Nakashima M, Nakamuta M, Enjoji M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1756–1767. doi: 10.3748/wjg.v20.i7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki T, Nakamori A, Sasaki E, Wada S, Ezaki O. Fish oil prevents sucrose-induced fatty liver but exacerbates high-safflower oil-induced fatty liver in ddy mice. Hepatology. 2007;46:1779–1790. doi: 10.1002/hep.21934. [DOI] [PubMed] [Google Scholar]
- 35.Melanson KJ, Westerterp-Plantenga MS, Saris WH, Smith FJ, Campfield LA. Blood glucose patterns and appetite in time-blinded humans: carbohydrate versus fat. Am J Physiol. 1999;277:R337–R345. doi: 10.1152/ajpregu.1999.277.2.R337. [DOI] [PubMed] [Google Scholar]
- 36.Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- 37.Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed] [Google Scholar]
- 38.Gray B, Swick J, Ronnenberg AG. Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr Rev. 2011;69:155–161. doi: 10.1111/j.1753-4887.2011.00377.x. [DOI] [PubMed] [Google Scholar]
- 39.Shi L, Liu ZW, Li Y, Gong C, Zhang H, Song LJ, Huang CY, Li M. The prevalence of nonalcoholic fatty liver disease and its association with lifestyle/dietary habits among university faculty and staff in Chengdu. Biomed Environ Sci. 2012;25:383–391. doi: 10.3967/0895-3988.2012.04.002. [DOI] [PubMed] [Google Scholar]