Abstract
Inadequate/excessive gestational weight gain (GWG) can cause adverse pregnancy outcomes and only few studies have described patterns of weight gain in Indian women. Also, studies pertaining to dietary intake throughout gestation are insufficient. This prospective cohort study was conducted to evaluate GWG and nutrient intake in all trimesters of pregnancy and investigate the relationship between themselves along with that of birth weight (BW). Our study was carried out in a population-based prospective birth cohort in Odisha, India. The 418 pregnant women were followed till delivery with measurements of maternal weight, weight gain throughout gestation, and BW. Macronutrients were assessed based on a 24-hour dietary recall method in each trimester. Women characterized by under-weight pre-pregnancy body mass index (BMI) were 16.20%, and a total of 6.45% did not comply with current weight gain recommendations. Particularly, overweight and obese women gained more weight than recommended. In a multivariate analysis GWG correlated significantly with BMI (p = 0.03), total calorie intake (p < 0.001) and fat intake (p < 0.001), while BW of newborns correlated significantly with adequacy of weight gain and fat intake (p < 0.001). Though measures are taken by health workers to record the weight but nutritional counseling is not being provided regularly. A high priority should be given to increase awareness among general population regarding the importance of diet in pregnancy and how to adhere to the balanced diet for optimal growth of child.
Keywords: Pregnancy, Gestational weight gain, Diet, Nutrition, Birth weight
INTRODUCTION
Monitoring mother's weight, height and gestational weight gain (GWG) has been a component of prenatal care for decades [1]. Inadequate/excessive GWG can cause adverse pregnancy outcomes but, only very few studies have been identified that described patterns of weight gain in Indian women [2]. According to United States Institute of Medicine (US-IOM) guidelines, recommended GWG for various groups of pregnant women is different as per body mass index (BMI) classes: 12.5–18 kg for underweight women (< 18.5 kg/m2), 11.5–16 kg for normal weight women (18.5–24.9 kg/m2), 7–11.5 kg for overweight women (25–29.9 kg/m2) and 5–9 kg for obese women (> 30 kg/m2) [3].
Women who are underweight/undernourished at the beginning of the pregnancy are unlikely to improve their nutritional status during pregnancy, and have higher occasions of underweight/still-births/mentally retarded/preterm deliveries [4,5,6,7,8]. In developing countries, the risks for malnourished newborns are even higher due to other existing issues like poor diet, frequent reproductive cycles, socio-economic constraints, etc. [9].
Despite high prevalence of low birth weight (LBW) (22.0%) in India as per National Family health Survey (NFHS)-3, the method to predict the same during pregnancy has always remained a debatable issue [10]. For provision of routine maternal health services, assessment of pregnant woman's health status is done by health workers mainly on the basis of BMI, GWG and Mid Upper Arm Circumference [11,12]. However questions have been raised regarding the validity of using only anthropometric indicators in predicting malnourishment among newborns with no consideration to dietary assessment [13].
GWG and birth weight (BW) of newborns is influenced by adherence to a balanced diet in pregnancy [14]. As suggested by National Institute of Nutrition (NIN), India, the energy demand in pregnancy increases by 350 kcal along with increased demand of macronutrients and micronutrients [15]. However, studies pertaining to dietary intake throughout gestation in Indian women are insufficient and those available were mostly cross-sectional in nature [16]. Owing to the fact that dietary behaviour has many implications on mother and child's weight, this prospective cohort study was conducted to evaluate GWG and nutrient intake in all trimesters of pregnancy and investigate the relationship between themselves along with that of BW.
MATERIALS AND METHODS
Design and recruitment
From July 2017 to June 2019, a prospective cohort study was conducted in Tangi Block, Khordha district of Odisha, India consisting of 440 pregnant women as study participants who were recruited in the 1st trimester and were followed up till the delivery of the baby. The study was conducted in all the 6 sectors under the block namely Tangi, Bhusandapur, Kuhudi, Badapokharia, Olasingh and Nirakarpur.
At 4 time points (once per trimester: 0 to 12 weeks; 13 to 26 weeks; 27 to 36 weeks and at delivery), the participants were visited for a detailed socio-demographic history (such as age, education, socio-economic status, occupation), morbidity profile, hemoglobin status, anthropometric measurements, nutrient intake and BW measurement within 2 days of delivery of newborn. The newborns were weighed and the value was cross-checked in the Mother and Child Protection card.
Data collection
Assessment of anthropometric data
The maternal weight and height—light clothing, no shoes—were measured using digital weighing scales and stadiometer to the nearest 0.1 kg and 0.1 cm respectively in each visit. The BMI was calculated as weight (kg)/height (m)2. GWG was compared with the recommendation of the US-IOM.
Assessment of nutrient intake
Information regarding nutrition was obtained by using a 24-hour dietary recall method in each trimester, i.e., for 3 visits. The amount of intake of each food was assessed by the Indian Food Composition Tables and analyzed in the Nutrify India Now application developed by NIN, India [17,18]. Recommendations for dietary intake of energy and macronutrients—carbohydrates, proteins and fat (visible and invisible)—during pregnancy were based on the Recommended Dietary Allowances (RDAs) for Indians suggested by NIN, India [15].
Statistical analysis
Continuous variables are presented as means (± standard deviations) while the categorical variables are shown as rates. Comparison between groups are performed using analysis of variance. The mean nutritional values (in all trimesters) are derived by patient-individual averaging. Associations of GWG and maternal factors were analyzed using multivariate linear regression models adjusted for other factors (p < 0.20) that may influence birth outcome. Using similar tests, associations of BW with adequacy of weight gain and other maternal factors were analyzed. Not normally distributed parameters were log-transformed and all the analysis are with 2-sided α = 0.05. All of the calculations were performed using SPSS software version 21 (IBM Corporation, Armonk, NY, USA).
Ethical approval
This study was approved for conduct by the Institute Ethics Committee, All India Institute of Medical Sciences (AIIMS) Bhubaneswar having number IEC/AIIMS BBSR/2017-18/7 and written informed consent was obtained from all the study participants.
RESULTS
A total of 418 pregnant women could be followed up till delivery of the child out of the 440 recruited initially with 5 having spontaneous abortion, 3 still-births and 14 being lost to follow-up. Table 1 highlights the characteristics between the 2 groups, in which no significant difference was found.
Table 1. Comparison of baseline characteristics of study participants with respect to those lost to follow-up.
| Characteristics | Participants (n = 418) | Lost to follow-up (n = 22) | p value | |
|---|---|---|---|---|
| Maternal age (yr) | 24.53 ± 3.76 | 23.98 ± 3.47 | 1.12 | |
| Maternal weight (kg) | 50.56 ± 9.01 | 51.01 ± 8.34 | 0.62 | |
| Maternal height (cm) | 151.50 ± 4.95 | 150.23 ± 2.56 | 1.23 | |
| Maternal BMI (kg/m2) | 21.89 ± 3.97 | 21.78 ± 2.98 | 1.43 | |
| Maternal MUAC (cm) | 25.83 ± 2.76 | 25.76 ± 1.98 | 0.82 | |
| Age at menarche (yr) | 11.56 ± 0.62 | 11.23 ± 1.23 | 0.19 | |
| Schooling (yr) | 8.62 ± 2.54 | 8.91 ± 1.23 | 0.32 | |
| Hemoglobin (g/dL) | 9.98 ± 1.43 | 10.01 ± 1.32 | 0.76 | |
| Occupation (%) | 0.67 | |||
| Homemaker | 99.28 | 100.00 | ||
| Self employed | 0.71 | 0.00 | ||
| Socio-economic status*(%) | 0.11 | |||
| Lower middle | 70.57 | 90.91 | ||
| Middle | 28.70 | 9.09 | ||
| Upper middle | 0.71 | 0.00 | ||
| Gravid status (%) | 0.13 | |||
| 1 | 47.36 | 68.18 | ||
| 2 | 43.30 | 22.72 | ||
| ≥ 3 | 10.76 | 9.09 | ||
Values are presented as mean ± standard deviation or percentage. The t-test has been used as the test of significance for the continuous variables. Fischer exact statistics has been applied to determine significant difference between mothers who were followed up and those lost to follow-up.
BMI, body mass index; MUAC, Mid Upper Arm Circumference.
*Udai-Pareek scale.
The study population mainly consisted of pregnant women between 20–25 years of age (59.1%) and 9.31% of them had teenage pregnancy. Almost all of them were home-makers in spite of 73.4% having an educational qualification of higher secondary and above. Most of them (70.57%) belonged to lower middle class family (Udai-Pareek scale) and nearly 79.31% of the pregnancies were unplanned of which 47% were primigravid. More than half were multigravid women (54.06%) of which 40.31% had their pregnancy within 1–2 years of last childbirth. The mean hemoglobin % was found to be 9.98. Only 4.11% reported thyroid disorders, however otherwise the morbidity profile of the study participants was insignificant.
The mean pregnancy week at which the study participants were recruited was 10.43 ± 1.47 wk. All of them were followed-up till delivery at mean pregnancy weeks of 23.30 ± 2.58 wk, 33.6 ± 1.90 wk and 38.22 ± 1.52 wk. There was no significant difference in terms of pregnancy weeks among the subgroups (based on BMI) during each of the follow-up visits.
Gestational weight gain
In the present study, 62.67% of the participants had a normal weight at time of recruitment, with 16.02% being underweight and 2.87% being obese. As outlined in Table 2, the mean weight was 50.97 kg and there was a significant difference in the mean weights between the subgroups of pregnant women. The mean weight gains varied among the subgroups significantly across all trimesters of pregnancy. GWG was found to be highest among underweight pregnant women (12.93 kg) and least was observed among the obese group (8.35 kg).
Table 2. Distribution of study participants based on follow-up visit and weight gain.
| Maternal parameters | Total (n = 418) | Under-weight (n = 67) | Normal weight (n = 262) | Overweight (n = 77) | Obese (n = 12) | p value | |
|---|---|---|---|---|---|---|---|
| Weight (at recruitment; kg) | 50.97 ± 9.16 | 41.74 ± 3.41 | 48.45 ± 4.14 | 63.49 ± 5.03 | 77.00 ± 5.29 | < 0.001* | |
| WG (kg) | |||||||
| 1st–2nd trimester | 5.38 ± 0.91 | 5.65 ± 0.76 | 5.77 ± 0.44 | 4.16 ± 0.68 | 3.21 ± 0.73 | < 0.001* | |
| 2nd–3rd trimester | 4.27 ± 0.75 | 4.64 ± 0.40 | 4.50 ± 0.45 | 3.31 ± 0.89 | 3.28 ± 0.63 | < 0.001* | |
| 3rd trimester–delivery | 3.27 ± 1.30 | 3.87 ± 1.35 | 3.42 ± 1.24 | 2.47 ± 1.01 | 1.85 ± 0.59 | < 0.001* | |
| GWG (kg) | 12.93 ± 2.29 | 14.17 ± 1.45 | 13.70 ± 1.48 | 9.95 ± 1.85 | 8.35 ± 1.47 | < 0.001* | |
The test of significance used to determine statistical difference among the groups was analysis of variance.
WG, weight gain; GWG, gestational weight gain.
*p < 0.05.
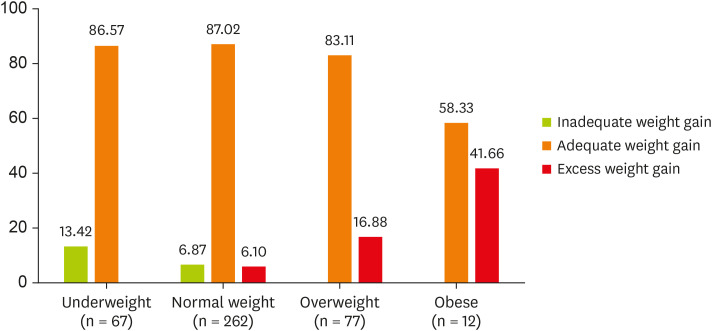
As per US-IOM guidelines for GWG, it was found that in all the subgroups, mostly an adequate weight gain was observed (Figure 1).
Figure 1. Distribution of weight gain pattern over different body mass index groups as per Institute of Medicine guidelines (in %) (n = 418).
Nutrient intake
As shown in Table 3, during gestation the average daily energy intake in our study population decreased from 1st trimester to 2nd and last trimester (p = 0.002 for decrease between 1st and last trimester). The mean carbohydrate intake also decreased from 239.32 g in the 1st trimester to 200.54 g in the 2nd trimester to 193.12 g in the last trimester non-significantly (p = 0.262). Protein intake decreased significantly from 1st trimester to 2nd (p = 0.037) and the 3rd trimester (p < 0.001 for decrease between 1st and 3rd trimester). With regard to the fat consumption daily intake decreased significantly over the trimesters (p = 0.002 for decrease between 1st and 3rd trimester).
Table 3. Mean nutrient intake (per day) of women in various trimesters.
| Nutrient intake | RDA | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| Total calorie intake (kcal) | 2,250 | 1,987.23 ± 512.34 | 1,700.98 ± 489.58 | 1,620.42 ± 300.92 |
| Carbohydrate (g) | 335 | 239.32 ± 65.12 | 200.54 ± 63.43 | 193.12 ± 60.48 |
| Protein (g) | 85 | 76.12 ± 14.45 | 68.45 ± 12.76 | 64.23 ± 16.75 |
| Fat (g) | 63 | 77.23 ± 20.12 | 68.78 ± 18.98 | 62.78 ± 19.32 |
RDA, Recommended Dietary Allowance [15].
When categories of energy intake were taken into account instead of mean values, 28.23% of the pregnant women reported a protein intake below the recommended allowance. Within the study population, 75.64% of the pregnant women reported a fat intake above the RDA. As to carbohydrate intake, 86.23% of the women were below the recommendation (Table 3).
Multivariate regression
Association between maternal factors with GWG
As presented in Table 4, each kcal of energy was significantly responsible for about 32 g of GWG while each gram of fat intake was associated with an increase of 270 g in GWG (p < 0.01). Also, maternal BMI at recruitment was found to have a significant correlation with GWG.
Table 4. Multivariate regression analysis between maternal factors and GWG and birth weight of newborn.
| Outcome variables | Parameters | B | SEM | t* | p value |
|---|---|---|---|---|---|
| GWG (kg) | Weight (kg) | −0.10 | 0.37 | −0.29 | 0.772 |
| BMI | −0.87 | 0.11 | −2.14 | 0.031† | |
| Hemoglobin | −0.34 | 0.52 | −0.71 | 0.486 | |
| Calorie intake (kcal) | 0.032 | 0.01 | 5.60 | < 0.001† | |
| Fat (g) | 0.27 | 0.07 | 3.37 | < 0.001† | |
| Birth weight (g) | BMI | 0.25 | 0.27 | 0.92 | 0.352 |
| Calorie intake (kcal) | 0.31 | 0.26 | 1.46 | 0.212 | |
| Fat (g) | 2.13 | 0.54 | 5.14 | < 0.001† | |
| Inadequate WG | −212.32 | 81.21 | −2.87 | < 0.001† | |
| Excess WG | 93.16 | 82.52 | 2.41 | 0.044† | |
| Adequate WG | 0‡ | - | - | - |
All the explanatory variables (maternal age, socio-demographic variables, mothers' weight, BMI, hemoglobin, WG and their nutrient intakes) entered the regression model and only those variables with p < 0.2 were included and the final model was determined.
GWG, gestational weight gain; SEM, standard error of mean; BMI, body mass index; WG, weight gain.
*Test statistic; †p < 0.05; ‡Regression coefficient.
Association between maternal factors with BW of newborns
Regression analysis showed that BW of newborns was affected in a significant manner by the amount of fat intake by mother, i.e., 1 g of fat intake was associated with an increase of 2.13 g (p < 0.01) in mean weight of newborns. It was also found that BW was significantly different between women with inadequate weight gain and excess weight gain. The weight was significantly lower (−212.32 g [p < 0.001]) in neonate whose mother had an inadequate weight gain in comparison to infants whose mother had adequate weight gain. On the other hand, the BW was found to be significantly higher (93.16 g [p = 0.04]) in neonates with mother having excess weight gain (Table 4).
DISCUSSION
One of the objectives of this study was to study the adequacy of weight gain in pregnant women and it was found that 85.41% of the participants had an adequate weight gain as per US-IOM guidelines. The mean GWG of all the weight groups was however found to be within normal limits and differed significantly from each other [3]. Moreover, it was observed that the weight gain was maximum in between 1st to 2nd trimester rather than in 3rd trimester which was not consistent with another study done in Germany by Diemert et al. [19]. The reason behind this might be the study population, dietary and cultural patterns of mothers as well as timing and methods used to collect data.
Almost 1 in every 2 women with obesity and 6.1% of women with a normal weight gained more than recommended, whereas about one-tenth of underweight women failed to gain adequately. Though studies by Koletzko et al. [20] and Fraser et al. [21] have reported that weight at beginning of pregnancy has a significant impact on pregnancy outcome rather than GWG, however the role of low GWG cannot be overlooked as it has been shown to be unfavourable in many studies [22,23]. A systematic review of outcomes of maternal weight gain found strong evidence to support the association between low GWG and LBW [24]. Another meta-analysis done in developing countries showed that low GWG (defined as < 11.5 to < 12.5 kg for normal or underweight women, respectively) was also associated with increased risk of LBW in developing countries. This would indicate that, on average, weekly weight gain of < 300 g would indicate high risk [25]. But there is no clear evidence of which weight gain cut-off is most sensitive to LBW.
Dietary habits before and during pregnancy have a potential influence on GWG and have an impact on both the health of mother and child. It has also been shown that in-utero programming of newborn's appetite and food preference many be influenced by maternal dietary habits [26,27]. Though NIN, India suggests a 350 kcal increase in energy for pregnant women, a detailed information regarding macronutrients and micronutrients content of the diet in different trimesters is limited. In this study, the mean energy intake was found to decrease significantly by 14% between 1st and 2nd trimester and by 18% between 1st and 3rd trimesters, which was much lesser than the recommended as 99.30% of the pregnant women were moderate workers (home-makers). Even though studies have shown that most women decrease their physical activity as pregnancy advances, still the decrease was beyond the recommended limit of 10% [10]. Similar decrease was also observed in case of macronutrient intake. The reason behind decrease in food intake might be due to fullness in abdomen and bloating sensation and being overly concerned about weight gain which was inferred upon by interviewing some of the study participants.
A significant association was found in this study between GWG and baseline BMI. Similar findings were established in other studies [28,29]. But this study could not establish any association with other socio-demographic factors like SES which is similar to deductions in another study conducted at Sudan by Elshibly et al. [30]. However in this study the initial maternal weight has been calculated at a mean of 10.43 weeks, which might not have reflected the exact GWG.
GWG was also found to be significantly associated with average calorie intake and fat intake, i.e., there existed a positive correlation. Though studies pertaining to effect of diet on GWG is limited but similar positive relation between diet and weight change has been focused in many studies [14,31,32].
Findings of this study revealed a significant association between maternal energy & fat intake and BW. Elias et al. [33] had confirmed the role of fatty acids like arachidonic and docosahexaenoic acid in growth of fetus. Mani et al. [34] had established the same in South Indian population. The findings also have been supported by other studies in Japan [35]. Though the mean GWG was found to have no significant association with that of BW, however, when the adequacy of BW was taken into account, a significant association was established. Though studies are insufficient which have measured the adequacy of weight gain but some have established significant association between GWG and BW (Rode et al. [28]) in Denmark.
Socio-demographic factors like maternal age, socio economic status, literacy and residence also play vital role in BW as mentioned in studies by Agorinya et al. [36] however, no such association could be established in the present study. Elshibly et al. [30] had also mentioned that social factors are not responsible for BW of a child.
Some of the limitations of the study which needs to be addressed are that the initial recruitment was done late in the first trimester, though it didn't differ significantly from pre-pregnancy weight (as inferred by patient recall). The nutrient intake was obtained based on 24-hour dietary recall method once in each trimester which might not have reflected the exact diet followed throughout the pregnancy. The categorization of nutrient intake based on BMI category and to observe its effect on GWG and BW couldn't be carried out as the sample size in each group wasn't large enough to deduct any significant inference. However, this study is a first-of-its-kind in establishing the relationship between adequacy of GWG and BW of newborns and as far as our knowledge is a first population-based study evaluating the dietary habits and its effect on weight gain in India.
CONCLUSION
This study contributes to the limited knowledge available on nutrient intake and GWG in pregnancy. It focuses on the need to develop guidelines about adequacy of weight gain in Indian population based on BMI by conducting further researches. It also shows that majority of the women do not follow the recommendations of energy and other macronutrients' and micronutrients' intake. Even though measures are taken by health workers to record the weight but nutritional counseling is not being provided regularly. This highlights the need to increase awareness among general population regarding the importance of diet in pregnancy and how to adhere to the same for optimal growth of child.
ACKNOWLEDGEMENTS
We would like to extend our heartfelt gratitude to the frontline health workers of Tangi Block for their support and help in smooth conduct of the study.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Barker DJ. Mothers, babies and disease in later life. 2nd ed. London: Churchill Livingstone; 1998. [Google Scholar]
- 2.Tang AM, Dong K, Deitchler M, Chung M, Maalouf-Manasseh Z, Tumilowicz A, Wanke C. Use of cutoffs for Mid-Upper Arms Circumference (MUAC) as an indicator or predictor of nutritional and health-related outcomes in adolescents and adults: a systematic review. Washington, D.C.: FHI 360/FANTA; 2013. [Google Scholar]
- 3.Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexamining the guidelines. Washington, D.C.: National Academics Press; 2009. [PubMed] [Google Scholar]
- 4.Han Z, Mulla S, Beyene J, Liao G, McDonald SD Knowledge Synthesis Group. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40:65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 5.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DR, Persson B, Hod M, Oats JJ HAPO Study Cooperative Research Group. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, Coory M, Gordon A, Ellwood D, McIntyre HD, Fretts R, Ezzati M. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 8.Slyvka Y, Zhang Y, Nowak FV. Epigenetic effects of paternal diet on offspring: emphasis on obesity. Endocrine. 2015;48:36–46. doi: 10.1007/s12020-014-0328-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16:1340–1353. doi: 10.1017/S1368980012004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Institute for Population Sciences and Macro International. National Family Health Survey (NFHS-3), 2005-06: India: volume I. Mumbai: IIPS; 2007. [Google Scholar]
- 11.Devaki G, Shobha R. Maternal anthropometry and low birth weight: a review. Biomed Pharmacol J. 2018;11:815–820. [Google Scholar]
- 12.Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013;5 doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honest H, Bachmann LM, Ngai C, Gupta JK, Kleijnen J, Khan KS. The accuracy of maternal anthropometry measurements as predictor for spontaneous preterm birth--a systematic review. Eur J Obstet Gynecol Reprod Biol. 2005;119:11–20. doi: 10.1016/j.ejogrb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Nahar S, Mascie-Taylor CG, Begum HA. Impact of targeted food supplementation on pregnancy weight gain and birth weight in rural Bangladesh: an assessment of the Bangladesh Integrated Nutrition Program (BINP) Public Health Nutr. 2009;12:1205–1212. doi: 10.1017/S1368980008003765. [DOI] [PubMed] [Google Scholar]
- 15.Krishnaswamy K, Sesikeran B. Dietary guidelines for Indians: a manual. 2nd ed. Hyderabad: ICMR-National Institute of Nutrition; 2011. [Google Scholar]
- 16.Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, van Dam RM, Chong MF. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr. 2019;10:685–695. doi: 10.1093/advances/nmy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian food composition tables. Hyderabad: ICMR-National Institute of Nutrition; 2017. [Google Scholar]
- 18.Nutrify India now. Hyderabad: National Institute of Nutrition; c2020. [cited 2020 June 2]. Available from https://www.nin.res.in. [Google Scholar]
- 19.Diemert A, Lezius S, Pagenkemper M, Hansen G, Drozdowska A, Hecher K, Arck P, Zyriax BC. Maternal nutrition, inadequate gestational weight gain and birth weight: results from a prospective birth cohort. BMC Pregnancy Childbirth. 2016;16:224. doi: 10.1186/s12884-016-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koletzko B, Bauer CP, Bung P, Cremer M, Flothkötter M, Hellmers C, Kersting M, Krawinkel M, Przyrembel H, Rasenack R, Schäfer T, Vetter K, Wahn U, Weissenborn A, Wöckel A. German national consensus recommendations on nutrition and lifestyle in pregnancy by the ‘Healthy Start - Young Family Network’. Ann Nutr Metab. 2013;63:311–322. doi: 10.1159/000358398. [DOI] [PubMed] [Google Scholar]
- 21.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, Ness A, Deanfield J, Hingorani A, Nelson SM, Smith GD, Lawlor DA. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, Brown TJ, Summerbell CD. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008;9:635–683. doi: 10.1111/j.1467-789X.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 23.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. Pregnancy and obesity: a review and agenda for future research. J Womens Health (Larchmt) 2006;15:720–733. doi: 10.1089/jwh.2006.15.720. [DOI] [PubMed] [Google Scholar]
- 24.Ricalde AE, Velásquez-Meléndez G, Tanaka AC, de Siqueira AA. Mid-upper arm circumference in pregnant women and its relation to birth weight. Rev Saude Publica. 1998;32:112–117. doi: 10.1590/s0034-89101998000200002. [DOI] [PubMed] [Google Scholar]
- 25.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201:339.e1–339.14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Brion MJ, Ness AR, Rogers I, Emmett P, Cribb V, Davey Smith G, Lawlor DA. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr. 2010;91:748–756. doi: 10.3945/ajcn.2009.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gugusheff JR, Ong ZY, Muhlhausler BS. The early origins of food preferences: targeting the critical windows of development. FASEB J. 2015;29:365–373. doi: 10.1096/fj.14-255976. [DOI] [PubMed] [Google Scholar]
- 28.Rode L, Hegaard HK, Kjaergaard H, Møller LF, Tabor A, Ottesen B. Association between maternal weight gain and birth weight. Obstet Gynecol. 2007;109:1309–1315. doi: 10.1097/01.AOG.0000266556.69952.de. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Sappenfield WM, Bish C, Salihu H, Goodman D, Bensyl DM. Assessment of the Institute of Medicine recommendations for weight gain during pregnancy: Florida, 2004-2007. Matern Child Health J. 2011;15:289–301. doi: 10.1007/s10995-010-0596-5. [DOI] [PubMed] [Google Scholar]
- 30.Elshibly EM, Schmalisch G. The effect of maternal anthropometric characteristics and social factors on gestational age and birth weight in Sudanese newborn infants. BMC Public Health. 2008;8:244. doi: 10.1186/1471-2458-8-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckland G, Bach A, Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev. 2008;9:582–593. doi: 10.1111/j.1467-789X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 33.Elias SL, Innis SM. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001;73:807–814. doi: 10.1093/ajcn/73.4.807. [DOI] [PubMed] [Google Scholar]
- 34.Mani I, Dwarkanath P, Thomas T, Thomas A, Kurpad AV. Maternal fat and fatty acid intake and birth outcomes in a South Indian population. Int J Epidemiol. 2016;45:523–531. doi: 10.1093/ije/dyw010. [DOI] [PubMed] [Google Scholar]
- 35.Nagata C, Iwasa S, Shiraki M, Sahashi Y, Shimizu H. Association of maternal fat and alcohol intake with maternal and umbilical hormone levels and birth weight. Cancer Sci. 2007;98:869–873. doi: 10.1111/j.1349-7006.2007.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agorinya IA, Kanmiki EW, Nonterah EA, Tediosi F, Akazili J, Welaga P, Azongo D, Oduro AR. Socio-demographic determinants of low birth weight: evidence from the Kassena-Nankana districts of the Upper East Region of Ghana. PLoS One. 2018;13:e0206207. doi: 10.1371/journal.pone.0206207. [DOI] [PMC free article] [PubMed] [Google Scholar]