Abstract
PURPOSE
The high-risk stratum of Children’s Oncology Group Study AALL1131 was designed to test the hypothesis that postinduction CNS prophylaxis with intrathecal triple therapy (ITT) including methotrexate, hydrocortisone, and cytarabine would improve the postinduction 5-year disease-free survival (DFS) compared with intrathecal methotrexate (IT MTX), when given on a modified augmented Berlin-Frankfurt-Münster backbone.
PATIENTS AND METHODS
Children with newly diagnosed National Cancer Institute (NCI) high-risk B-cell acute lymphoblastic leukemia (HR B-ALL) or NCI standard-risk B-ALL with defined minimal residual disease thresholds during induction were randomly assigned to receive postinduction IT MTX or ITT. Patients with CNS3-status disease were not eligible. Postinduction IT therapy was given for a total of 21 to 26 doses. Neurocognitive assessments were performed during therapy and during 1 year off therapy.
RESULTS
Random assignment was closed to accrual in March 2018 after a futility boundary had been crossed, concluding that ITT could not be shown to be superior to IT MTX. The 5-year postinduction DFS and overall survival rates (± SE) of children randomly assigned to IT MTX versus ITT were 93.2% ± 2.1% v 90.6% ± 2.3% (P = .85), and 96.3% ± 1.5% v 96.7% ± 1.4% (P = .77), respectively. There were no differences in the cumulative incidence of isolated bone marrow relapse, isolated CNS relapse, or combined bone marrow and CNS relapse rates, or in toxicities observed for patients receiving IT MTX compared with ITT. There were no significant differences in neurocognitive outcomes for patients receiving IT MTX compared with ITT.
CONCLUSION
Postinduction CNS prophylaxis with ITT did not improve 5-year DFS for children with HR B-ALL. The standard of care for CNS prophylaxis for children with B-ALL and no overt CNS involvement remains IT MTX.
INTRODUCTION
Survival rates for children and young adults diagnosed with B-cell acute lymphoblastic leukemia (B-ALL) have continued to improve, now reaching 88% to 90%.1-3 Although survival has improved, 34% of patients whose disease relapses have a CNS component.4
CONTEXT
Key Objective
The high risk stratum of AALL1131 was designed to test the hypothesis that post-induction CNS prophylaxis with intrathecal triple therapy including methotrexate, hydrocortisone, and cytarabine would improve the post-induction 5-year disease free survival compared to intrathecal methotrexate, when given on a modified augmented Berlin-Frankfurt-Münster backbone.
Knowledge Generated
Post-induction CNS prophylaxis with intrathecal triple therapy does not improve 5-year disease free survival for children with high-risk B-cell acute lymphoblastic leukemia.
Relevance
The standard of care for CNS prophylaxis for children with B-cell acute lymphoblastic leukemia and no overt CNS involvement remains intrathecal methotrexate.
Standard intrathecal therapeutic approaches to CNS prophylaxis have included intrathecal methotrexate (IT MTX)2,5 and intrathecal triple therapy (ITT) with methotrexate, hydrocortisone, and cytarabine.6,7 Although both approaches have decreased CNS relapse rates, a randomized comparison assessing relapse, survival, and toxicities in newly diagnosed patients has only been accomplished for patients with standard-risk B-ALL who received less intensive systemic therapy while participating in the Children’s Cancer Group (CCG) 1952 study.8 This study demonstrated that although CNS relapse rates were lower in patients receiving ITT, relapses in marrow were higher, and overall survival (OS) was lower compared with those receiving IT MTX. Grade 3-4 CNS toxicities were similar between the two groups, with the exception of an increased rate of CNS toxicities during a second delayed intensification phase in patients receiving ITT. Backbone therapy in CCG 1952 did not include intravenous methotrexate or intensive consolidation, so relevance to contemporary regimens used to treat high-risk (HR) B-ALL is unclear.
The HR stratum of the Children’s Oncology Group (COG) AALL1131 study was designed to evaluate if ITT, when administered on a modified augmented Berlin-Frankfurt-Münster (MBFM) backbone, would further increase disease-free survival (DFS) and decrease CNS relapse rate without increasing neurologic toxicities or decreasing cognitive function compared with IT MTX alone in patients with HR B-ALL.
PATIENTS AND METHODS
Patients
AALL1131 was a phase III clinical trial for children and young adults > 1 to < 31 years of age with newly diagnosed National Cancer Institute (NCI) HR B-ALL. Eligible patients were (1) 1 to 9 years of age with a WBC count ≥ 50,000/µL, (2) ≥ 10 to < 31 years of age with any WBC count, (3) > 1 to < 31 years of age with CNS3 leukemia or testicular leukemia, or (4) < 10 years of age and had received steroid pretreatment without obtaining a pre-steroid WBC count. CNS3 was defined as ≥ 5/μL WBCs with blasts seen on cytospin of the CSF and/or clinical signs of CNS leukemia.
At the end of induction, patients were risk stratified into three groups: HR, very high risk, or, after an amendment in August 2016, Philadelphia (Ph) chromosome–like (Ph-like) B-ALL. Patients with Down syndrome entered a separate single-arm stratum at time of initial diagnosis. Patients completing induction in AALL1131 were eligible for random assignment in the HR stratum if they were < 13 years of age at diagnosis without adverse prognostic features (ie, CNS3, intrachromosomal amplification of chromosome 21, KMT2A [MLL] rearrangement, or hypodiploidy [< 44 chromosomes and/or DNA index < 0.81]) and had a day 29 bone marrow (BM) minimal residual disease (MRD) < 0.01%. In addition, patients initially risk classified as having NCI standard-risk disease and enrolled in AALL0932 (ClinicalTrials.gov identifier: NCT01190930) were eligible at the end of induction to be randomly assigned in the HR stratum of AALL1131 if they lacked favorable cytogenetics (ie, ETV6-RUNX1 fusion or trisomy of chromosomes 4 and 10) and had a day 8 peripheral blood MRD ≥ 1% and a day 29 BM MRD < 0.01%, or had favorable cytogenetics and a day 29 BM MRD ≥ 0.01%. Patients with Ph+ ALL were not eligible for random assignment and were removed from protocol therapy at the end of induction. Toxicities were graded using the NCI Common Terminology Criteria for Adverse Events version 5.0.
At the end of induction, patients enrolled in AALL1131 and aged 6 to 11 years at the time of diagnosis (amended in October 2017 to include children up to age 12 years at diagnosis) were also eligible to enroll in the embedded AALL1131 correlative study, Longitudinal Computerized Assessment of Neurocognitive Functioning. The objectives of the neurocognitive study were to determine if the prevalence of cognitive deficits at 1 year off therapy is significantly higher than prevalence in the normative population and determine if there are significant declines in neurocognitive functioning over time, with clinically meaningful decrease in mean scores apparent at 1 year off therapy. Eligible patients were English, French, or Spanish speaking, without a history of neurodevelopmental disorder before diagnosis, and without significant visual impairment that would prevent computer use and recognition of the visual test stimuli. Results of the neurocognitive study presented in this report are a post hoc analysis limited to those patients in the HR stratum of AALL1131.
AALL1131 was approved by the NCI and by institutional review boards at the COG member institutions before patient enrollment. Informed consent was obtained from parents or guardians according to Department of Health and Human Services guidelines.
Treatment
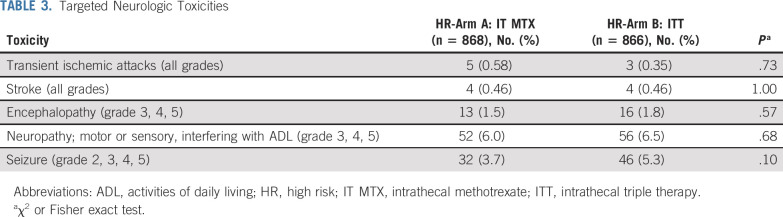
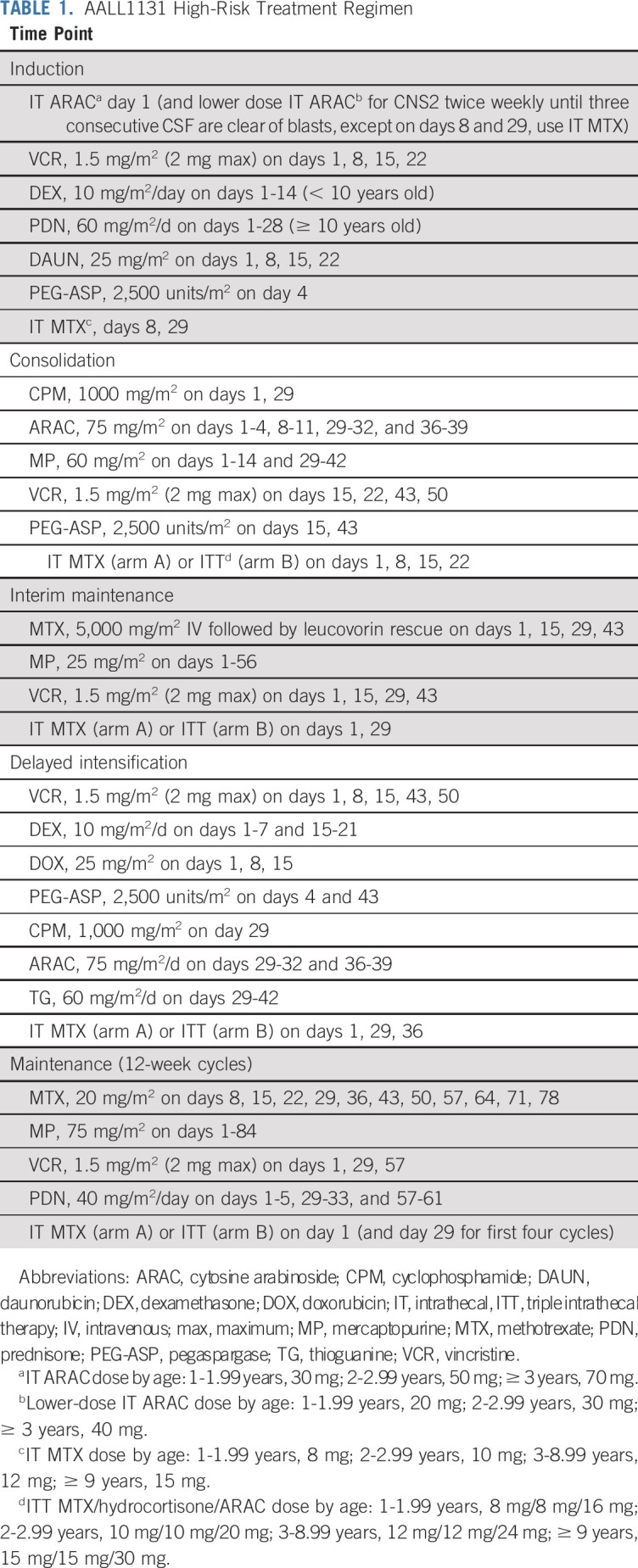
Patients enrolled in AALL1131 received a standard four-drug induction including 14 days of treatment with dexamethasone if < 10 years old and 28 days of prednisone if ≥ 10 years, followed by multiagent chemotherapy on a MBFM backbone (Table 1). Patients identified as having CNS2 disease at diagnosis (ie, < 5/μL WBCs with blasts seen on cytospin of CSF or traumatic spinal tap with > 5/μL WBCs and cytospin positive for blasts but negative by the Steinherz/Bleyer algorithm9 [CSF WBC count divided by CSF RBC count > 2 multiplied by (blood WBC count divided by blood RBC count)]) initially received identical IT chemotherapy as those with CNS1 disease (< 5/μL WBCs without blasts seen on cytospin of CSF). The protocol was amended in June 2016 for patients with CNS2 disease to receive twice weekly IT therapy during induction until three consecutive lumbar punctures exhibited CNS1 status, after an analysis of COG AALL0232 and AALL0331 that demonstrated an inferior outcome and increased risk of CNS relapse for these patients.10 Patients with a postinduction risk assignment of HR B-ALL were randomized 1:1 postinduction to arm A, using IT MTX, or arm B, using ITT for CNS prophylaxis. Patients received a total of 21 to 26 doses of IT therapy postinduction (consolidation, n = 4; interim maintenance, n = 2; delayed intensification, n = 3; and maintenance, n = 12 for girls and n = 16 for boys). The remainder of postinduction therapy was identical for patients in these two treatment arms. Total duration of treatment of girls and boys was 2 years and 3 years, respectively, from the start of interim maintenance. In addition to routine reporting, specific neurologic adverse event reporting for the HR stratum included: (1) transient ischemic attack (all grades), (2) stroke (all grades), (3) encephalopathy (grades 3, 4, 5), (4) motor and/or sensory neuropathy interfering with activities of daily living (grades 3, 4, 5), and (5) seizure (grades 2, 3, 4, 5).
TABLE 1.
AALL1131 High-Risk Treatment Regimen
Patients enrolled in the Longitudinal, Computerized Assessment of Neurocognitive Functioning completed a 25- to 30-minute computerized cognitive evaluation, Cogstate11-13 (www.cogstate.com); targeting visual learning (One-Card Learning); reaction time/processing speed (detection); visual attention (identification); executive functioning (Groton Maze Learning Task); and working memory (one-back task) administered by a trained provider in the clinic. Cogstate tasks were designed to minimize practice effects that can occur when neurocognitive tests are administered multiple times, and these tasks have been used previously in studies of both healthy and medically ill children.12,14-21 Parents completed a 10- to 15-minute questionnaire, the Behavior Rating Inventory of Executive Functioning (BRIEF),22,23 a parent-reported measure of executive functioning at five (for girls) or six (for boys) time points from consolidation through 1 year off therapy. Results from the first four time points (consolidation, and end of maintenance courses 2, 4, and 6) are included in this report.
AALL1131 opened to accrual in February 2012. In March 2018, the HR arms were permanently closed after interim monitoring revealed that a futility monitoring boundary was crossed, signifying that ITT could not be shown to be superior to IT MTX. Subsequently, the protocol was amended to have all previously enrolled patients receive IT MTX for CNS prophylaxis for the remainder of protocol therapy.
Statistical Analysis
Patients initially enrolled in AALL0932 and AALL1131 who received an end of induction risk assignment of HR were eligible for postinduction treatment random assignments for the HR stratum of this study. The HR randomization was powered (83.7%) to detect an improvement in 5-year DFS from 90% to 94% (hazard ratio [HR], 0.587; n = 136 total expected DFS events) between IT MTX– and ITT-based regimens, using a two-sided log-rank test (α = 5%), with a total of 1,825 patients and 3 years of minimum follow-up. DFS was defined as time from postinduction random assignment to first event (ie, death in remission, relapse, or second malignant neoplasm) or date of last contact for those who remained event free. Survival rates were estimated using the method of Kaplan-Meier24 with standard errors of Peto et al.25
Interim monitoring for efficacy used an αt2 spending function and futility monitoring was based on the method of Anderson and High,26 with the first interim analysis scheduled when 20% of the expected DFS events were observed. Prospective interim monitoring rules for efficacy and futility were included where futility would be determined for a one-sided P ≥ .7664. The futility monitoring boundary was crossed (n = 78 total observed DFS events) and the randomization was closed early in March 2018, with a total of 1,734 patients being randomly assigned to treatment. At the time of analyses for this report, there was a total of 92 observed DFS events. Cumulative incidence was computed using the cumulative incidence function for competing risks, and comparisons were made using the K-sample test.27 Comparison of proportions between the two arms used a χ2 test or Fisher exact test. Finally, analysis of covariance procedures were also performed to evaluate differences in neurocognitive outcomes as a function of treatment randomization for three Cogstate tasks (detection, identification, and one-back speed) and two BRIEF indexes (Metacognition and Behavior Regulation). In each model, scores from the first assessment were added as a control to compare outcomes of randomization group (IT MTX or ITT) at the end of maintenance course 6. A P < .05 (two-sided test) was considered significant for all comparisons. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC). Graphics were generated with R, version 3.4.4 (http://www.r-project.org).
RESULTS
Outcome of the Randomization
From February 27, 2012, until March 19, 2018, 1,734 eligible, evaluable patients with HR-ALL were randomly assigned to treatment postinduction (Table 2). Study data current as of September 30, 2018, are included in this report. Patient characteristics were similar in the two groups. Among the 1,734 patients, 868 were randomly assigned to arm A with IT MTX and 866 were randomly assigned to arm B with ITT (Fig 1). Five-year DFS and OS rates (± SE) were 93.2% ± 2.1% v 90.6% ± 2.3% (P = .85), and 96.3% ± 1.5% v 96.7% ± 1.4% (P = .77) for patients receiving IT MTX versus ITT, respectively (Fig 2A and 2B). The corresponding estimated HR for the DFS analysis was 0.803 (95% CI, 0.532 to 1.211) for IT MTX versus ITT.
TABLE 2.
Patient Characteristics
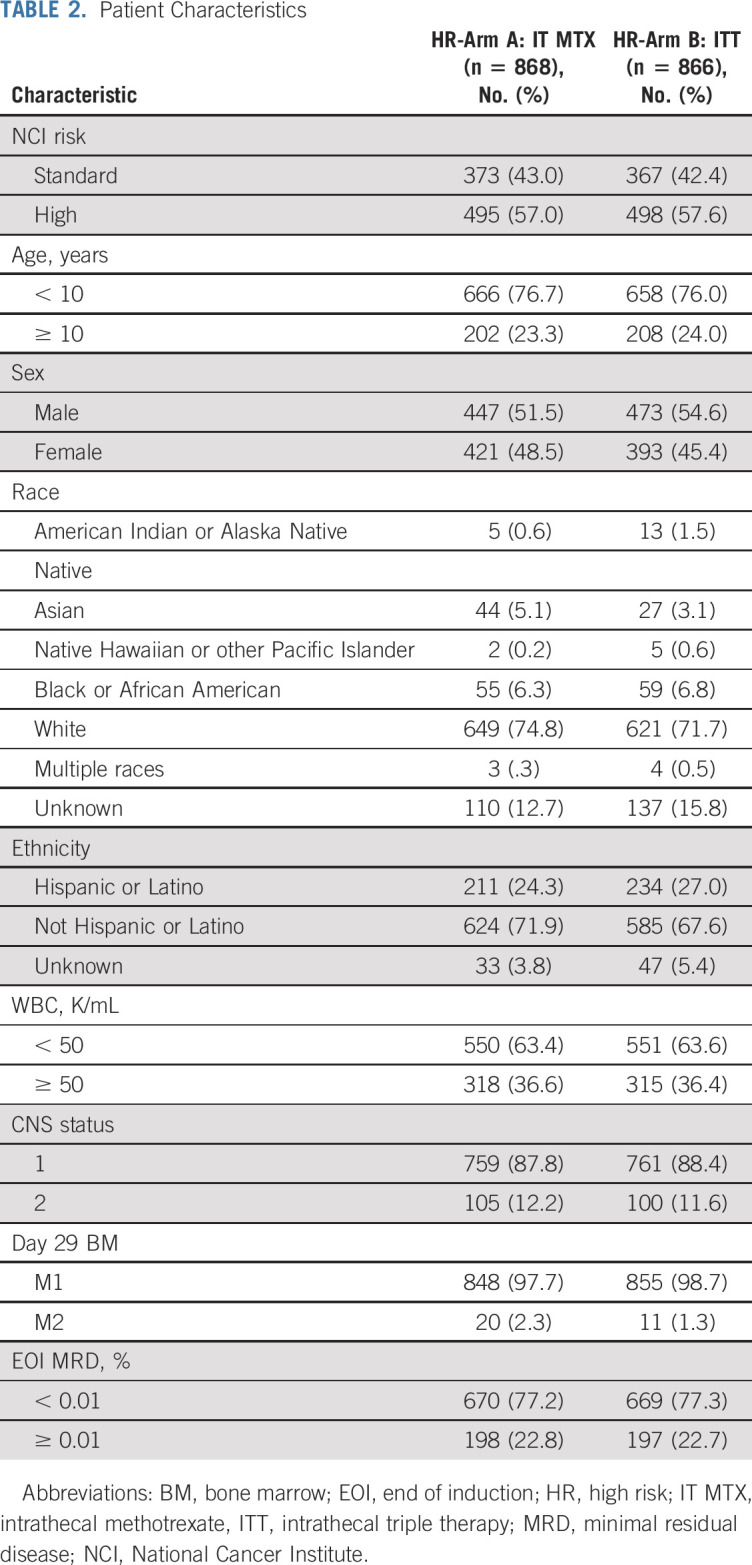
FIG 1.
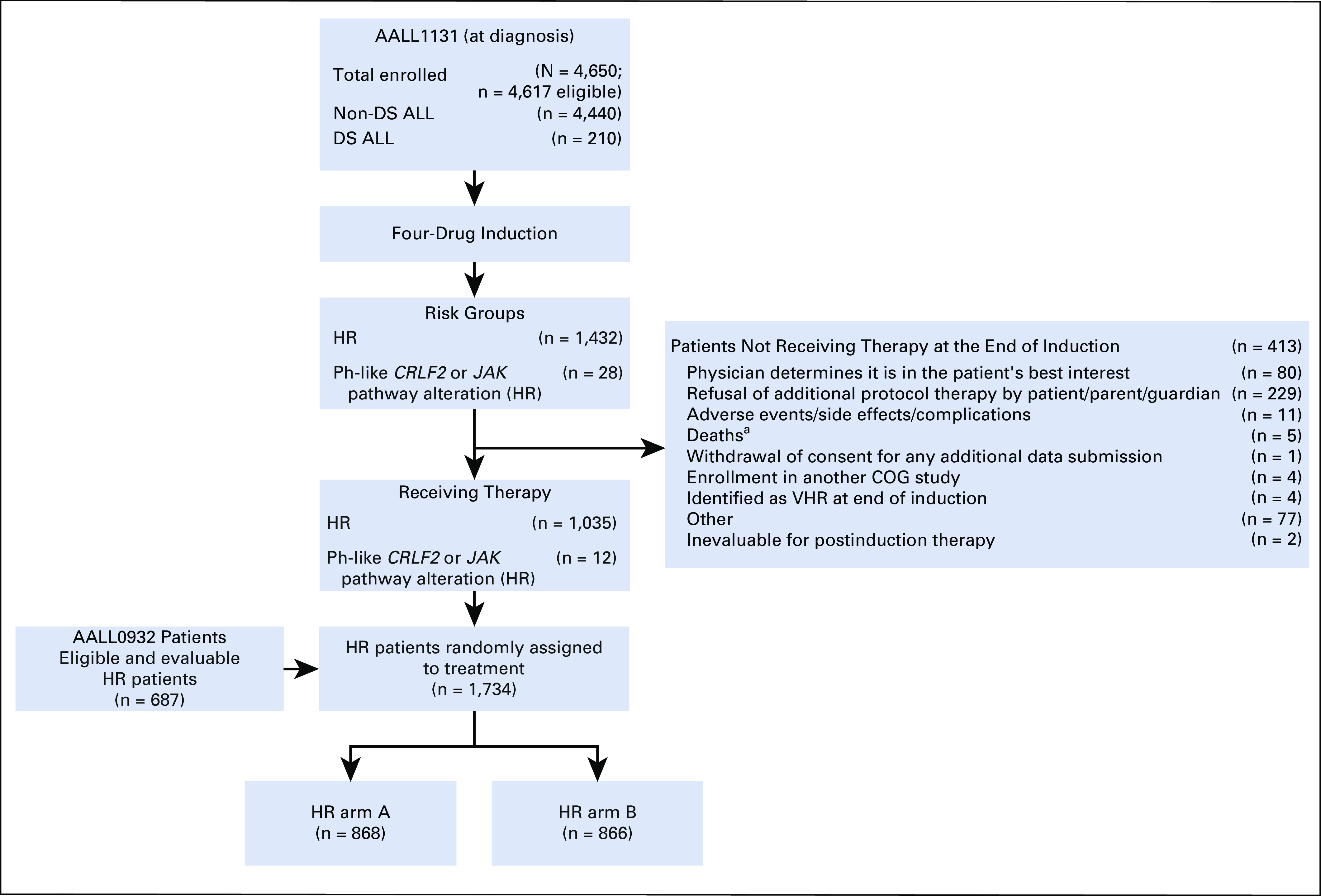
CONSORT diagram for selecting study patients with high-risk (HR) B-cell acute lymphoblastic leukemia (B-ALL). (a) Causes of induction death: n = 3 due to protocol treatment, n = 1 due to cardiac arrest, n = 1 patient in whom a pancreatic pseudocyst developed, which continued to grow; hemorrhaging occurred, followed by hypotension and respiratory failure. COG, Children’s Oncology Group; DS, Down syndrome; Ph, Philadelphia.
FIG 2.
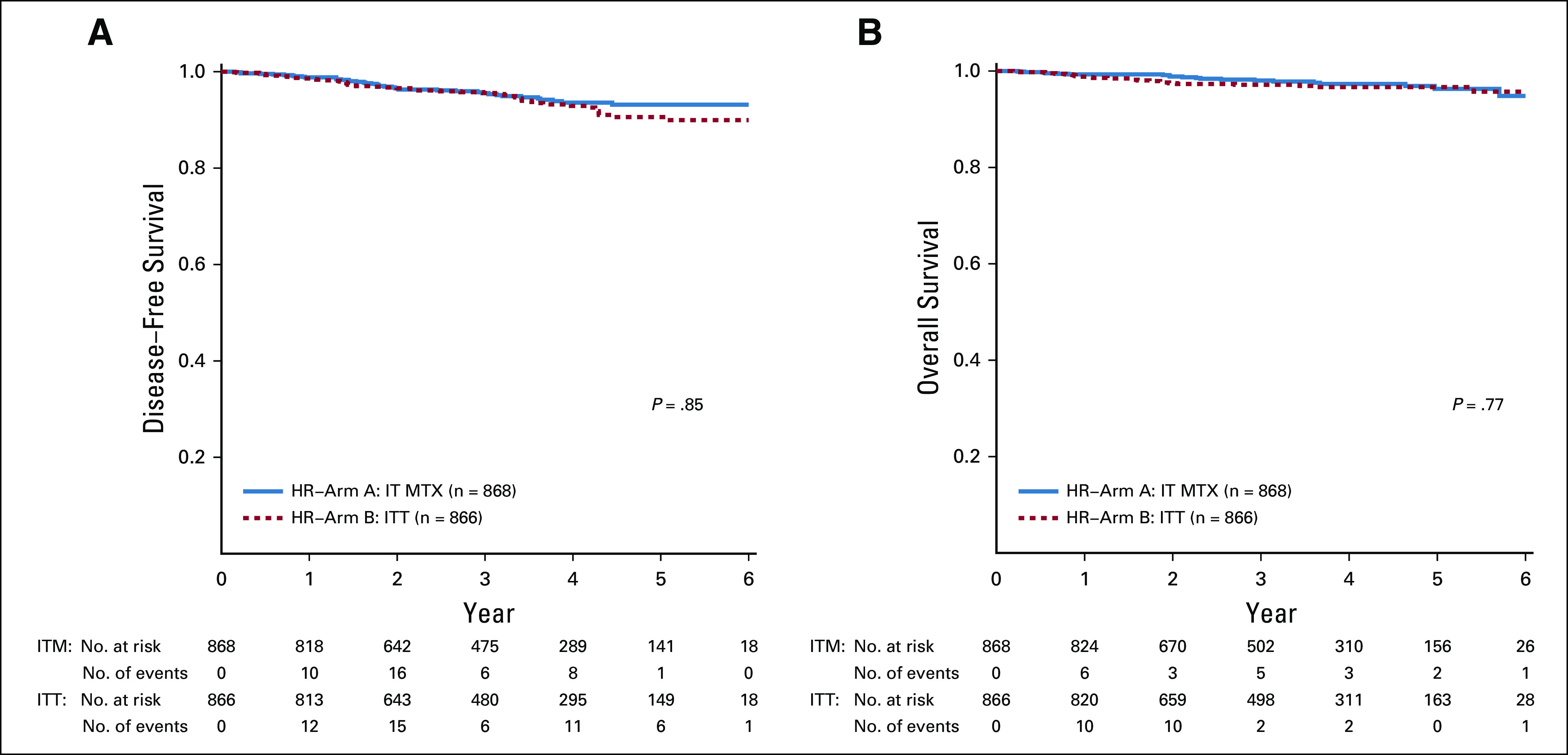
Comparison of survival rates for intrathecal MTX versus ITT. (A) Five-year disease-free survival (± SE): 93.2% ± 2.1% and 90.6% ± 2.3% (P = .85); (B) 5-year overall survival (± SE): 96.3% ± 1.5% and 96.7% ± 1.4% (P = .77). HR, high risk; ITT, intrathecal triple therapy; MTX, methotrexate.
The 5-year cumulative incidence rates (CIRs) of relapse (± SE) were 5.6% ± 1.1% v 7.0% ± 1.3% for IT MTX versus ITT (P = .97). The CIRs (± SE) of isolated BM relapse (2.5% ± 0.8% and 5.2% ± 1.2%; P = .08), isolated CNS relapse (1.7% ± 0.5% and 1.0% ± 0.4%; P = .15), and combined BM and CNS relapse (1.1% ± 0.5% and 0.2% ± 0.2%; P = .06) were not different between the two randomized arms (Fig 3A-3C). Isolated CNS relapse accounted for 37.5% (n = 12 of 32) and 18.9% (n = 6 of 32) of all relapses in patients receiving IT MTX and ITT. The 5-year CIRs (± SE) of second malignant neoplasms were 0% and 0.7% ± 0.4% (P = .03) in patients receiving IT MTX and ITT, respectively, and although these values are statistically significant, the clinical significance is unknown.
FIG 3.
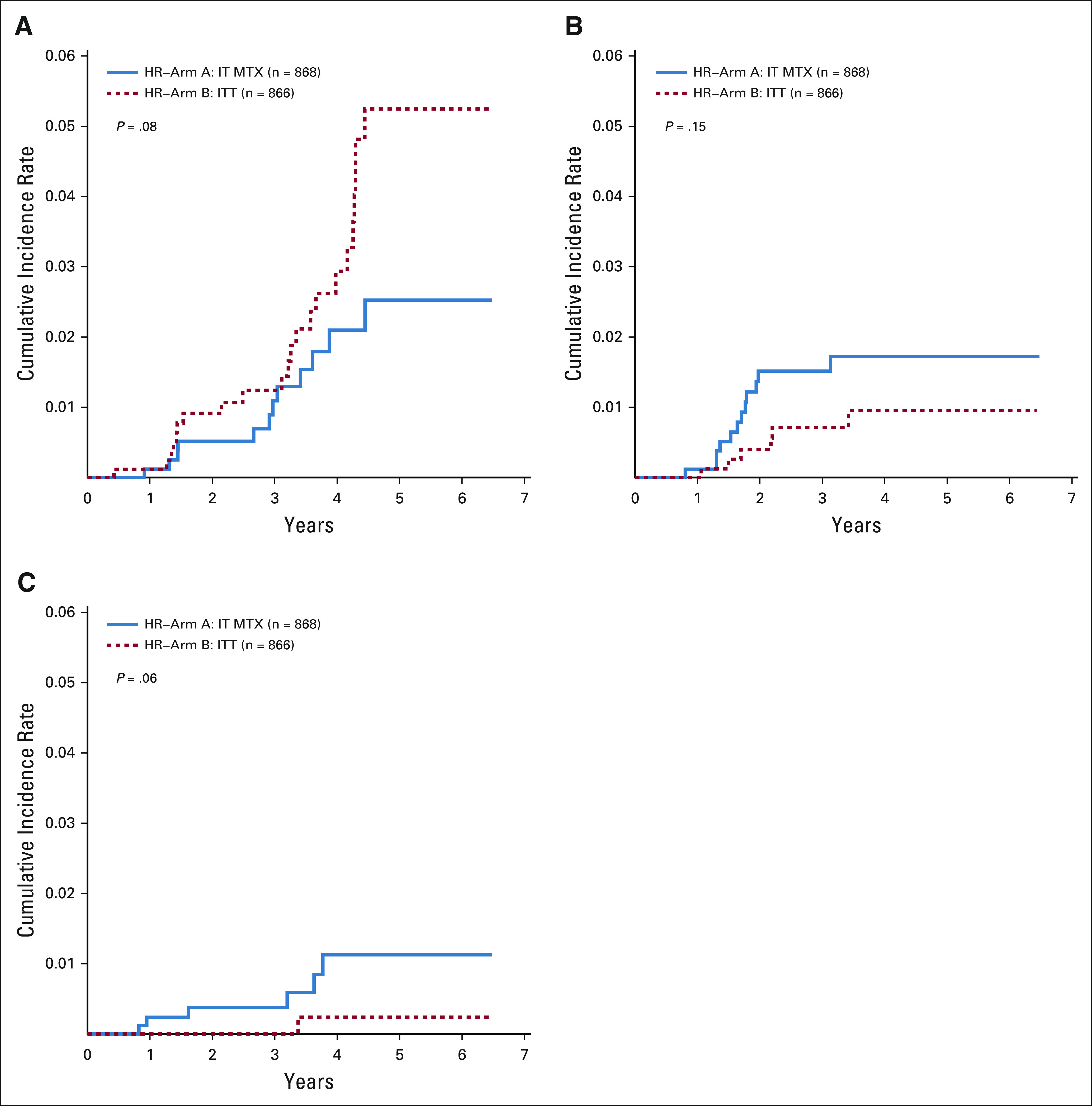
Comparison of cumulative incidence of relapse for IT methotrexate versus ITT. (A) Five-year cumulative incidence (± SE) of isolated bone marrow relapse was 2.5% ± 0.8% and 5.2% ± 1.2%, respectively (P = .08); (B) 5-year cumulative incidence of isolated CNS relapse were 1.7% ± 0.5% and 1.0% ± 0.4%, respectively (P = .15); and (C) 5-year cumulative incidence of combined bone marrow and CNS relapse were 1.1% ± 0.5% and 0.2% ± 0.2% (P = .06). IT MTX, intrathecal methotrexate; ITT, intrathecal triple therapy.
Neurologic Toxicities and Neurocognitive Outcomes
Postinduction targeted neurologic toxicities including transient ischemic attack (all grades), stroke (all grades), encephalopathy (grades 3, 4, 5), motor and/or sensory neuropathy interfering with activities of daily living, and seizure (grades 2, 3, 4, 5) were not different between the groups (Table 3).
TABLE 3.
Targeted Neurologic Toxicities
A total of 479 patients consented to the embedded neurocognitive study: HR stratum (n = 351), very high risk stratum (n = 126), Ph-like (ABL-class fusion) stratum (n = 2). Sixty-seven percent of participants eligible for the neurocognitive study in the HR stratum consented to participate (n = 351) and are the subject of this report; of these, 179 (51.0%) were randomly assigned to IT MTX and 172 (49.0%) were randomly assigned to ITT. Enrolled participants in the HR stratum who contributed any data (n = 297) were an average of 9.4 years old at diagnosis (standard deviation, 1.8) and 53.5% were boys (n = 159).
Across the first four assessments included in the current analyses (ie, end of consolidation, end of maintenance courses 2, 4 and 6), mean Cogstate scores were uniformly within the average range for reaction time/processing speed (mean z-score range, −0.78 to −0.49), visual attention (z-score range, −0.83 to −0.34), and working memory (z-score range, −0.22 to 0.00). Controlling for performance at the end of consolidation (time 1), there were no significant differences in reaction time (IT MTX mean detection z-score, −0.75 v ITT mean, −0.81; P = .40), visual attention (IT MTX mean identification z-score, −0.93 v ITT mean, −0.72; P = .08), or working memory scores (IT MTX mean one-back speed z-score, −0.08 v ITT mean z-score, −0.01; P = .28) at the end of maintenance course 6 (time 4). In addition, rates of abnormal performance (ie, ≤ 1.5 SDs below the mean) on Cogstate tasks did not significantly differ between groups for any of the three tasks (χ2 P ≥ .09 for all; Fig 4), although rates of abnormal scores for reaction time and visual attention were higher across both groups compared with the standardization sample at each time point (reaction time range, 19.0% to 25.7% v 8.4% expected; visual attention range, 19.0% to 30.7% v 7.7% expected; all P < .001).
FIG 4.
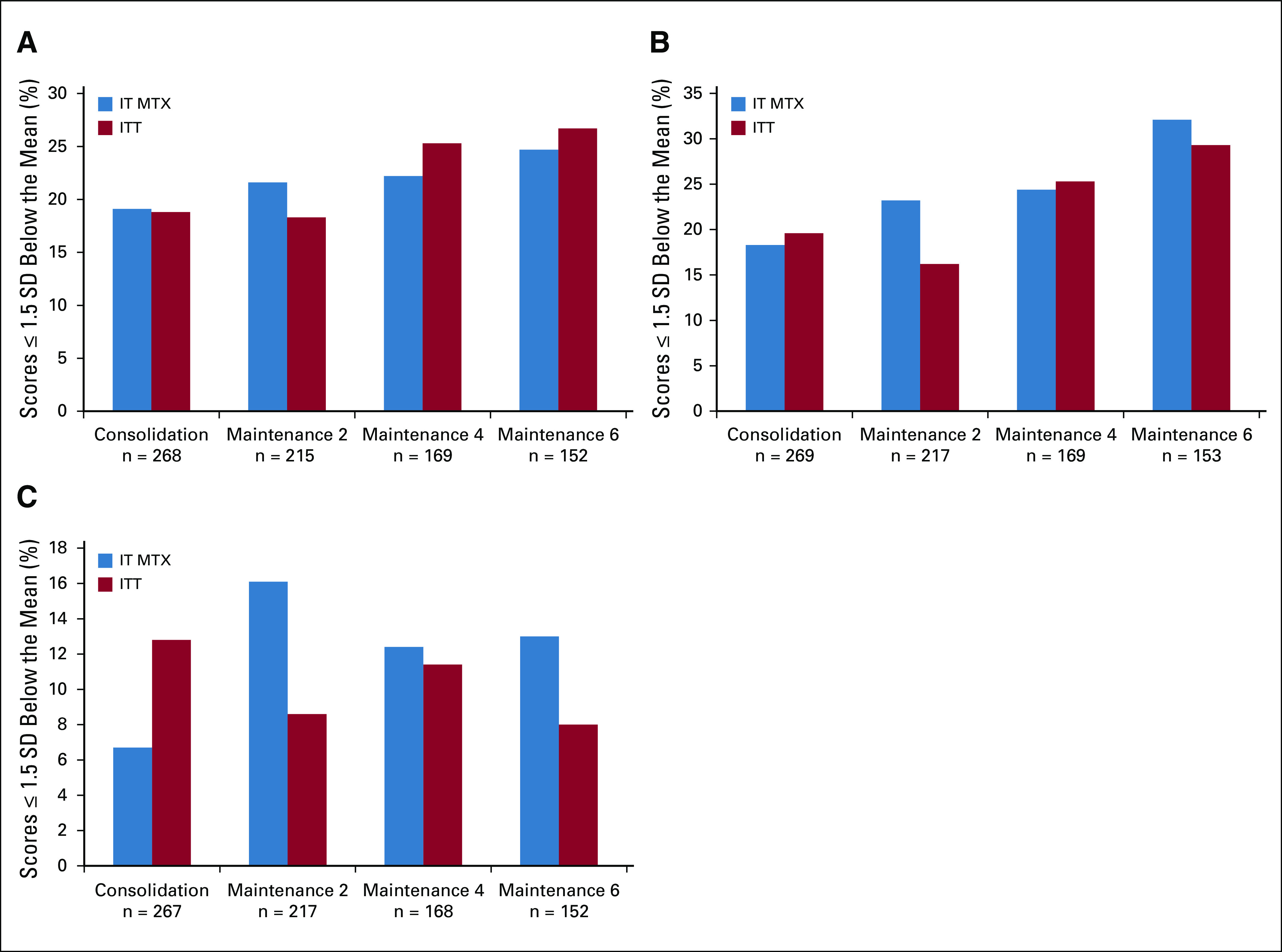
Comparison of Cogstate scores for IT MTX versus ITT. (A) Percentage of abnormal reaction time/processing speed scores over time by treatment arm. (B) Percentage of abnormal visual attention scores over time by treatment arm. (C) Percentage of abnormal working memory scores over time by treatment arm. Note: All results reflect all data collected as of September 30, 2018; many participants had not yet reached the end of maintenance courses 4 and 6. χ2 statistics comparing rates of impairment as a function of treatment group were nonsignificant for all time points. IT MTX, intrathecal methotrexate; ITT, intrathecal triple therapy; SD, standard deviation.
With regard to parent-reported cognitive and behavioral functioning on the BRIEF, there were no significant differences in parent ratings of participants’ behavioral regulation (mean Behavior Regulation Index T-score, 53.3 v 52.9 for IT MTX and ITT, respectively; P = .28) or metacognition (mean Metacognition Index T-score, 51.0 v 52.2 for IT MTX and ITT, respectively; P = .76) at the end of maintenance course 6 as a function of treatment, controlling for end of consolidation ratings. There were also no significant group differences in rates of abnormal scores (ie, ≥ 1.5 SD above the mean) on parent-reported BRIEF outcomes (χ2 P ≥ .16 for all). Although the mean parent ratings for the group of participants were within normal limits at each time point, the number of participants whose parents rated them as having clinically elevated problems on the BRIEF was higher than expected (Behavioral Regulation Index range, 14.3% to 18.5% v 8.8% expected; all P < .001; Metacognition Index range, 12.5% to 17.3% v 9.7% expected; P < .05 for all but consolidation).
DISCUSSION
Although survival has improved for newly diagnosed patients with HR B-ALL, relapse involving the CNS remains a significant issue. Isolated CNS relapse accounted for 24% of relapses in patients with HR B-ALL (n = 61 of 252) who were treated in the CCG-1961 study.28 Subsequently, COG AALL0232 sought to improve event-free survival (EFS) and provide better control of CNS disease with the addition of high-dose methotrexate (HD-MTX) compared with Capizzi MTX during interim maintenance. The addition of HD-MTX resulted in a significant improvement in EFS due to decreased rates of both marrow and CNS relapse, but isolated CNS relapse still accounted for 26% of relapses (n = 83 of 319) occurring in the MTX regimens.29 There was no difference when restricting the analysis to patients who received HD-MTX, with isolated CNS relapse accounting for 25% of all relapses (n = 34 of 136).
In CCG-1952, newly diagnosed children with SR ALL were randomly assigned to IT MTX versus ITT. Although isolated CNS relapse rates were higher in patients receiving IT MTX versus ITT (6-year CIR [±SE], 5.9% ± 1.2% and 3.4% ± 1.0%, respectively; P = .004), more BM relapses occurred in the patients receiving ITT, resulting in equivalent 6-year EFS (±SE) of 82.5% ± 1.8% and 80.7% ± 1.9%, respectively (P = .3).8 Paradoxically, perhaps due to inferior salvage rates after BM relapse, 6-year OS was higher for patients receiving IT MTX versus ITT (94.4% ± 1.1% and 90.3% ± 1.5%; P = .01).
In AALL1131, patients with HR B-ALL, without CNS3 disease, were randomly assigned to receive IT MTX versus ITT on the more intensive MBFM backbone that included the intensive COG consolidation and four courses of high-dose MTX. Contrary to results from CCG-1952, there were no differences in isolated CNS relapse rates, marrow relapse rates, and 5-year DFS or OS between the two arms in AALL1131. The increased intensity of therapy on the MBFM backbone may have obviated the improved CNS control with ITT observed with the less intensive CCG 1952 backbone. Isolated CNS relapse accounted for 28% of all relapses (n = 18 of 64) in the AALL1131 HR stratum, indicating that control of CNS disease continues to be a concern in upfront studies for B-ALL.
CNS prophylaxis with IT chemotherapy is an essential component of multiagent therapy for children and young adults with B-ALL. The most commonly used regimens include IT MTX or ITT.2,5-7 Acute neurotoxicities associated with IT therapy may include arachnoiditis, seizures, paraplegia, hemiplegia, dysarthria, stroke, and encephalopathy.6,30-33 Excessive neurotoxicity was noted in one study using ITT,32,34 but the neurotoxicity was attributable to prolonged systemic MTX exposure in the absence of leucovorin. The current study, randomly assigning patients to IT MTX versus ITT in patients with HR B-ALL, demonstrated no difference in rates of neurotoxicity between the two arms.
There were also no significant differences in neurocognitive outcomes between patients randomly assigned to IT MTX versus ITT, assessed approximately 2 years postdiagnosis via computerized cognitive tasks and parent ratings of executive functioning. This is largely consistent with results reported by Kadan-Lottick et al,35 who conducted neurocognitive evaluations almost 6 years postdiagnosis with 171 children who were previously randomly assigned to IT MTX or ITT in CCG 1952. In that study, children treated with IT MTX had significantly higher rates of impairment than those treated with ITT on traditional tasks of processing speed (19.5% v 6.9%), but there were no other significant group differences. Current results showed no significant differences in reaction time after controlling for functioning early in treatment, and no differences in parent-rated problems with behavioral regulation or metacognition skills, which include ratings of task efficiency. Longer follow-up will be needed to assess cognitive differences over time.
The AALL1131 HR stratum was designed to determine if ITT would improve the 5-year DFS compared with IT MTX and be tolerable in newly diagnosed children with HR B-ALL without CNS3 disease. Although neurotoxicity was not increased with ITT, the 5-year DFS was not improved with ITT, and relapses were no different in patients receiving ITT versus IT MTX. IT MTX remains the standard of care for CNS prophylaxis for this patient population.
SUPPORT
Supported by the National Health Institute (Grants no. U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173, and U10 CA180899) and by St. Baldrick’s Foundation.
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Wanda L. Salzer, Meenakshi Devidas, Lia Gore, Joanne M. Hilden, Eric Larsen, Michael J. Borowitz, Naomi Winick, William L. Carroll, Elizabeth A. Raetz, Mignon L. Loh, Stephen P. Hunger
Administrative support: Stephen P. Hunger
Provision of study material or patients: Yunfeng Dai, Michael J. Burke, Lia Gore, William L. Carroll
Collection and assembly of data: Wanda L. Salzer, Michael J. Burke, Meenakshi Devidas, Kristina K. Hardy, Lia Gore, Joanne M. Hilden, Eric Larsen, Karen R. Rabin, Patrick A. Zweidler-McKay, Michael J. Borowitz, Brent Wood, Nyla A. Heerema, Andrew J. Carroll, William L. Carroll, Stephen P. Hunger
Data analysis and interpretation: Wanda L. Salzer, Michael J. Burke, Meenakshi Devidas, Yunfeng Dai, Kristina K. Hardy, John A. Kairalla, Lia Gore, Joanne M. Hilden, Eric Larsen, Karen R. Rabin, Michael J. Borowitz, Brent Wood, Naomi Winick, William L. Carroll, Elizabeth A. Raetz, Mignon L. Loh, Stephen P. Hunger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Intrathecal Triple Therapy Versus Intrathecal Methotrexate on Disease-Free Survival for High-Risk B-Lymphoblastic Leukemia: Children’s Oncology Group Study AALL1131
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael J. Burke
Honoraria: Jazz Pharmaceuticals, Amgen, Shire
Consulting or Advisory Role: Amgen
Speakers' Bureau: Jazz Pharmaceuticals, Shire, Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Shire, Amgen
Meenakshi Devidas
Honoraria: PSI, Novartis
Kristina K. Hardy
Employment: Bayer Pharmaceuticals (I)
Honoraria: Bayer Pharmaceuticals (I)
Speakers' Bureau: Bayer Pharmaceuticals (I)
Travel, Accommodations, Expenses: Bayer Pharmaceuticals (I)
John A. Kairalla
Stock and Other Ownership Interests: Johnson & Johnson, Sophiris Bio
Lia Gore
Employment: Anchiano Therapeutics (I)
Leadership: Anchiano Therapeutics (I), Vedantra (I)
Stock and Other Ownership Interests: Amgen, Sanofi, Celgene, Clovis Oncology, Anchiano Therapeutics (I), Blueprint Medicines (I), Mirati Therapeutics (I), OnKure
Honoraria: Amgen, Roche
Consulting or Advisory Role: Celgene, Novartis, Amgen
Consulting or Advisory Role: Roche
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of magnetic resonance spectroscopy for leukemia.
Travel, Accommodations, Expenses: Roche
Patrick A. Zweidler-McKay
Employment: Immunogen
Stock and Other Ownership Interests: ImmunoGen
Patents, Royalties, Other Intellectual Property: Patent applications submitted, no royalties
Michael J. Borowitz
Consulting or Advisory Role: Amgen
Research Funding: Becton Dickinson
Travel, Accommodations, Expenses: Beckman Coulter
Brent Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Astellas Pharma
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Biosight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis
Travel, Accommodations, Expenses: Amgen
William L. Carroll
Other Relationship: Amgen
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: Celgene
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck (I), Amgen (I), Pfizer (I)
Honoraria: Amgen
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the Children’s Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: Results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108:1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherlow JM, Sather H, Steinherz P, et al. Craniospinal irradiation for acute lymphoblastic leukemia with central nervous system disease at diagnosis: A report from the Children’s Cancer Group. Int J Radiat Oncol Biol Phys. 1996;36:19–27. doi: 10.1016/s0360-3016(96)00272-6. [DOI] [PubMed] [Google Scholar]
- 10.Winick N, Devidas M, Chen S, et al. Impact of initial CSF findings on outcome among patients with National Cancer Institute standard- and high-risk B-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2017;35:2527–2534. doi: 10.1200/JCO.2016.71.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen NC, Kingma A, Schuitema A, et al. Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. J Clin Oncol. 2008;26:3025–3030. doi: 10.1200/JCO.2007.12.4149. [DOI] [PubMed] [Google Scholar]
- 12.Sands SA, Harel BT, Savone M, et al. Feasibility of baseline neurocognitive assessment using Cogstate during the first month of therapy for childhood leukemia. Support Care Cancer. 2017;25:449–457. doi: 10.1007/s00520-016-3422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 14.Ballard JC. Computerized assessment of sustained attention: Interactive effects of task demand, noise, and anxiety. J Clin Exp Neuropsychol. 1996;18:864–882. doi: 10.1080/01688639608408308. [DOI] [PubMed] [Google Scholar]
- 15.Betts J, McKay J, Maruff P, et al. The development of sustained attention in children: The effect of age and task load. Child Neuropsychol. 2006;12:205–221. doi: 10.1080/09297040500488522. [DOI] [PubMed] [Google Scholar]
- 16.Falleti MG, Maruff P, Collie A, et al. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–1112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- 17.Snyder AM, Maruff P, Pietrzak RH, et al. Effect of treatment with stimulant medication on nonverbal executive function and visuomotor speed in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2008;14:211–226. doi: 10.1080/09297040701220005. [DOI] [PubMed] [Google Scholar]
- 18.Collie A, Makdissi M, Maruff P, et al. Cognition in the days following concussion: Comparison of symptomatic versus asymptomatic athletes. J Neurol Neurosurg Psychiatry. 2006;77:241–245. doi: 10.1136/jnnp.2005.073155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cysique LA, Maruff P, Darby D, et al. The assessment of cognitive function in advanced HIV-1 infection and AIDS dementia complex using a new computerised cognitive test battery. Arch Clin Neuropsychol. 2006;21:185–194. doi: 10.1016/j.acn.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Bangirana P, Giordani B, John CC, et al. Immediate neuropsychological and behavioral benefits of computerized cognitive rehabilitation in Ugandan pediatric cerebral malaria survivors. J Dev Behav Pediatr. 2009;30:310–318. doi: 10.1097/DBP.0b013e3181b0f01b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harel BT, Pietrzak RH, Snyder PJ, et al. The development of associate learning in school age children. PLoS One. 2014;9:e101750. doi: 10.1371/journal.pone.0101750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1076/chin.6.3.235.3152. Baron IS: Test review: Behavior rating inventory of executive function. Child Neuropsychol 6:235-238, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Viola A, Balsamo L, Neglia JP, et al. The Behavior Rating Inventory of Executive Function (BRIEF) to identify pediatric acute lymphoblastic leukemia (ALL) survivors at risk for neurocognitive impairment. J Pediatr Hematol Oncol. 2017;39:174–178. doi: 10.1097/MPH.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JR, High R. Alternatives to the standard Fleming, Harrington, and O’Brien futility boundary. Clin Trials. 2011;8:270–276. doi: 10.1177/1740774511401636. [DOI] [PubMed] [Google Scholar]
- 27.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 28.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochs JJ. Neurotoxicity due to central nervous system therapy for childhood leukemia. Am J Pediatr Hematol Oncol. 1989;11:93–105. doi: 10.1097/00043426-198921000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winick NJ, Bowman WP, Kamen BA, et al. Unexpected acute neurologic toxicity in the treatment of children with acute lymphoblastic leukemia. J Natl Cancer Inst. 1992;84:252–256. doi: 10.1093/jnci/84.4.252. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: An association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 34.Winick N, Shuster JJ, Bowman WP, et al. Intensive oral methotrexate protects against lymphoid marrow relapse in childhood B-precursor acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2803–2811. doi: 10.1200/JCO.1996.14.10.2803. [DOI] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Brouwers P, Breiger D, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]