Summary
In December 2019, an outbreak of pneumonia, which was named COVID-2019, emerged as a global health crisis. Scientists worldwide are engaged in attempts to elucidate the transmission and pathogenic mechanisms of the causative coronavirus. COVID-19 was declared a pandemic by the World Health Organization in March 2020, making it critical to track and review the state of research on COVID-19 to provide guidance for further investigations. Here, bibliometric and knowledge mapping analyses of studies on COVID-19 were performed, including more than 1,500 papers on COVID-19 available in the PubMed and China National Knowledge Infrastructure databases from January 1, 2020 to March 8, 2020. In this review, we found that because of the rapid response of researchers worldwide, the number of COVID-19-related publications showed a high growth trend in the first 10 days of February; among these, the largest number of studies originated in China, the country most affected by pandemic in its early stages. Our findings revealed that the epidemic situation and data accessibility of different research teams have caused obvious difference in emphases of the publications. Besides, there was an unprecedented level of close cooperation and information sharing within the global scientific community relative to previous coronavirus research. We combed and drew the knowledge map of the SARS-CoV-2 literature, explored early status of research on etiology, pathology, epidemiology, treatment, prevention, and control, and discussed knowledge gaps that remain to be urgently addressed. Future perspectives on treatment, prevention, and control are also presented to provide fundamental references for current and future coronavirus research.
Keywords: COVID-19, SARS-CoV-2, bibliometric analysis, research status, knowledge scape, knowledge map
Graphical Abstract

Public Summary
-
•
China initiated COVID-19-related research in considerable scope and depth at the early stage of the outbreak
-
•
Researchers all over the world have rapidly launched unprecedented joint research efforts
-
•
The knowledge map of SARS-CoV-2 is becoming increasingly comprehensive, and knowledge gaps to be filled have been identified
-
•
The next step is to consider other factors conducive to research innovation, such as public and private's cooperation, equitable health system
Introduction
In December 2019, a cluster of cases of pneumonia with unknown cause was detected in Wuhan City, Hubei Province of China. A novel coronavirus was identified as the causative virus by Chinese authorities on January 7, 2020. The Coronavirus Study Group of the International Committee on Taxonomy of Viruses designated the causative virus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease, which subsequently spread globally, was named coronavirus disease of 2019 (COVID-2019) by the World Health Organization (WHO). In March 2020, the WHO declared COVID-19 a pandemic.
Like other major epidemics caused by coronaviruses (such as SARS and MERS), COVID-19 is a substantial threat to public health, with severe economic implications. The COVID-19 pandemic calls for increased research on traceability analysis, transmission, diagnosis methods, epidemiology, treatment, prevention, and containment of the disease.1
In response to the COVID-19 pandemic, which the WHO declared the sixth Public Health Emergency of International Concern, researchers worldwide raced to characterize the virus through joint scientific research efforts. Around 60 key research organizations and journals, including BMJ, signed a joint statement pledging to “rapidly and openly” share research data and findings relevant to the outbreak. Medical journals, such as the Lancet, the New England Journal of Medicine, JAMA, BMJ, Springer Nature, National Medical Journal of China, and China Biotechnology set up a special hub to support the global response to COVID-19 by enabling fast and direct access to the latest available research, evidence, and data.2,3 As of March 8, 2020, more than 1,500 studies on COVID-19, covering topics, such as etiology, diagnosis, epidemiology, treatment, prognosis, nursing, prevention, and control, were available in PubMed and China National Knowledge Infrastructure (CNKI)databases.
In this review, the global literature related to COVID-19 were analyzed using bibliometric methods, citation analysis, and knowledge mapping methods. Studies reporting temporal patterns, main countries affected, and core subjects were identified, and the status and trends in COVID-19 research were explored from January 2020 to March 2020 to reveal the conceptual knowledge map in SARS-CoV-2. The results provide a reference for current and future coronavirus research and policymaking worldwide.
Results
Temporal Distribution
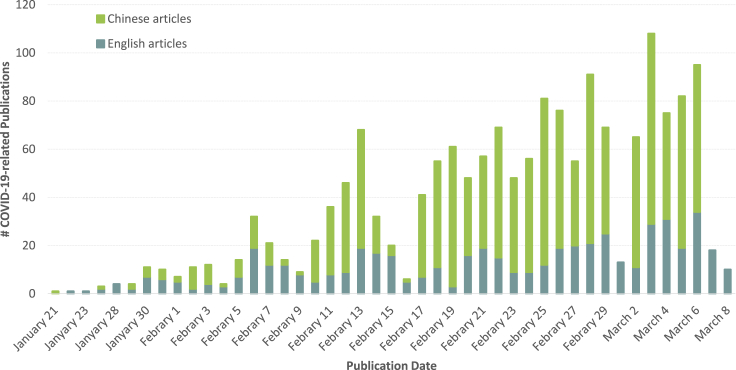
Since the outbreak and global spread of COVID-19, researchers worldwide have embarked on intense research efforts, and the number of publications on COVID-19 has shown an increasing trend, with fluctuations, over time. The first related study, which was published on January 22, 2020, discussed the transmission of COVID-19 across species.4 Before February 5, 2020, the number of daily publications increased by single digits, and thereafter by double digits. In terms of research topic, etiology, epidemiology, and prevention research were the focus of early published papers. As the outbreak progressed, the focus of publications shifted to diagnosis and prevention. There were relatively fewer studies on prognosis and nursing as of March 8, 2020.
The number of Chinese articles published was larger than that of English articles, especially after the middle of February, by more than 2.5 times. This may be because China was the most severely affected country in the early stages of the pandemic. The first article appearing in Chinese journals was published on January 21, 2020. In this article, Gao and other scholars from the School of Life Sciences of Nankai University discovered, through traceability analysis, that the horseshoe bat was the original host of the virus, and suggested that this virus may be a new variant of coronavirus that mutates quickly, with a wide host range and strong host adaptability.5 In terms of research subdomains, treatment and prevention were the focus of these Chinese articles (details in Figure 1).
Figure 1.
Temporal Distribution of COVID-19-Related Early Publications in 2020
Regional Distribution and Cooperation
Global Distribution
Regional distribution showed that the number of articles on COVID-19 is positively correlated with the severity in that particular country. China was one of the most severely affected countries in the early stages of the epidemic until late February; therefore, COVID-19-related studies were carried out rapidly. As a result, China (including Hong Kong, Macao, and Taiwan) ranked no. 1 with 353 published articles. Because of its firm foundations in medical and scientific research as well as its recent experience in the prevention and control of seasonal influenza, the US ranked second, with 97 published studies related to COVID-19. European countries, such as the UK (27 papers), Germany (15 papers) and Italy (15 papers) were among the countries with the highest number of publications focusing on the epidemiology of COVID-19.
The specific focus of COVID-19 studies has varied across different regions worldwide. China has mainly focused on the epidemiology of COVID-19. Patients in the early outbreak provided large samples of clinical characteristics and predictive spread of COVID-19. Research institutions on the Chinese mainland, such as Tongji Hospital, conducted numerous studies on the clinical characteristics and biochemical markers of COVID-19.6 A research foundation in Hong Kong, with 26 articles on SARS, rapidly initiated studies on the effective control of the spread of COVID-19. Research institutions in Hong Kong include universities (such as the University of Hong Kong, Hong Kong Polytechnic University) as well as hospitals (such as the Queen Mary Hospital), have focused on epidemiology as well as preventative and control measures for COVID-19.7,8 The Government of Macau has placed greater emphasis on studies related to the prevention and control (one paper) and nursing care (one paper), and has taken early, proactive measures against transmission of COVID-19. Furthermore, studies performed by hospitals, in Taiwan (nine papers) (such as the National Taiwan University College of Medicine and regional general hospitals), have mainly focused on routes of transmission and clinical characteristics of COVID-19.9
In the US, considerable studies on the epidemiology (30 papers), prevention and control, and etiology of COVID-19 have been published. For instance, the University of Washington has studied the impacts of travel restrictions on disease spread, the Mayo Clinic has investigated the establishment and rationality of prevention and control measures, and the University of Chicago has explored the pathological characteristics of SARS-CoV-2. Studies in the UK have mainly focused on the epidemiology and prevention and control of COVID-19; for example, University College London and the London School of Hygiene & Tropical Medicine have studied the effects of the disease on public health and the corresponding prevention and control measures to be undertaken at different phases of epidemic development. Research in Canada has focused on the epidemiology of COVID-19, such as the study from University of Toronto about the transmission dynamics and risk assessment. Japan has placed significant emphasis on epidemiological studies, with studies published by Hokkaido University and the Japan Science and Technology Agency describing the incubation period of SARS-CoV-2 and quarantine duration. Similarly, Germany has mainly focused on epidemiological studies; for example, Heidelberg University established a model to assess the epidemic potential of SARS-CoV-2 and predict its transmission dynamics and impact. Studies in Italy have placed a relatively higher focus on the epidemiological and etiological aspects of COVID-19. Scientists at the Lazzaro Spallanzani National Institute for Infectious Diseases have reported an unusual radiological presentation of COVID-19 using chest computed tomography (CT) scans. The Biomedical University of Rome has focused on investigating the pathogenesis and evolutionary pattern of SARS-CoV-2.
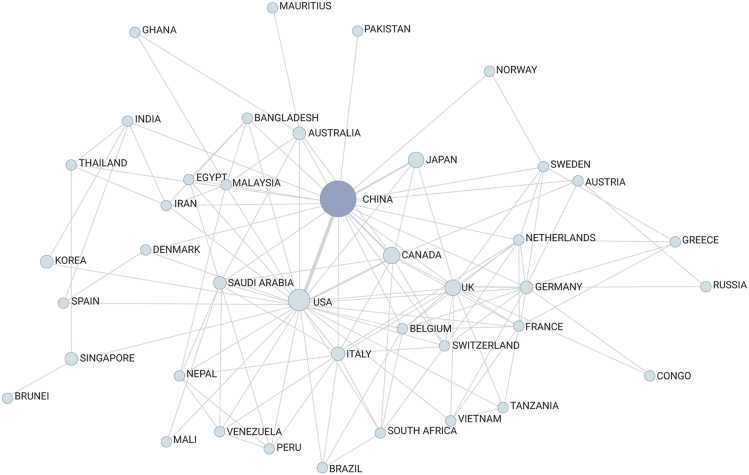
Journal publications on COVID-19 suggested that scientists from different countries worldwide have strengthened their research collaborations and launched joint research projects for the prevention and control of the epidemic. China has collaborated with 21 countries, including the US, Canada, and Japan, and published 80 related articles on the subject before March 8, dominating COVID-19 research. Furthermore, collaborative networks have been established among the US, Europe, and Southeast Asia as well as within European countries, which have jointly conducted studies on the virus and its pathogenic mechanism, as well as related clinical studies (Figure 2 and Table 1).
Figure 2.
Networks Formed by Collaborative Countries on COVID-19 Research.
The size of the nodes represent the number of papers. The lines between two nodes represent collaboration links, the intensity of which is proportional to the thickness of the line.
Table 1.
Number of Publications Resulting from Collaborations between China and Collaborative Countries, by Research Subdomain
| Etiology | Diagnosis | Epidemiology | Treatment | Prognosis | Nursing | Prevention and Control |
|---|---|---|---|---|---|---|
| US (9) | US (4) | US (11) | Japan (3) | Canada (1) | Canada (2) | US (8) |
| Japan (2) | UK (1) | Saudi Arabia (2) | US (1) | Australia (1) | Australia (2) | |
| Australia (1) | Germany (1) | Japan (2) | Italy (1) | Canada (2) | ||
| Austria (1) | Japan (1) | Italy (2) | Bangladesh (1) | |||
| Germany (1) | Switzerland (1) | Germany (2) | Sweden (1) | |||
| Saudi Arabia (1) | the Netherlands (1) | Canada (2) | Denmark (1) | |||
| Norway (1) | Thailand (1) | Iran (1) | ||||
| Sweden (1) | Malaysia (1) | |||||
| Malaysia (1) | Norway (1) | |||||
| India (1) | UK (1) | |||||
| Egypt (1) | ||||||
| Belgium (1) |
Epidemiological, etiological, and diagnostic studies (75.6%) predominate among research arising from collaborations between China and other countries across a range of research topics related to COVID-19, including predictive modeling (e.g., R0), infection mechanism, genome sequencing of the virus, origin of the virus, receptors, PCR techniques, and immunological detection methods. In addition, China and Japan have collaborated to conduct clinical studies on extracorporeal membrane oxygenation, noninvasive respiratory support, potential antiviral therapeutics, and clinical features of patients. Furthermore, China has launched joint research projects with the US, Australia, and Canada to study protective measures for postpartum women and newborns, the experience of handling the epidemic, and to undertake discussions on countermeasures.
Province-level Distribution
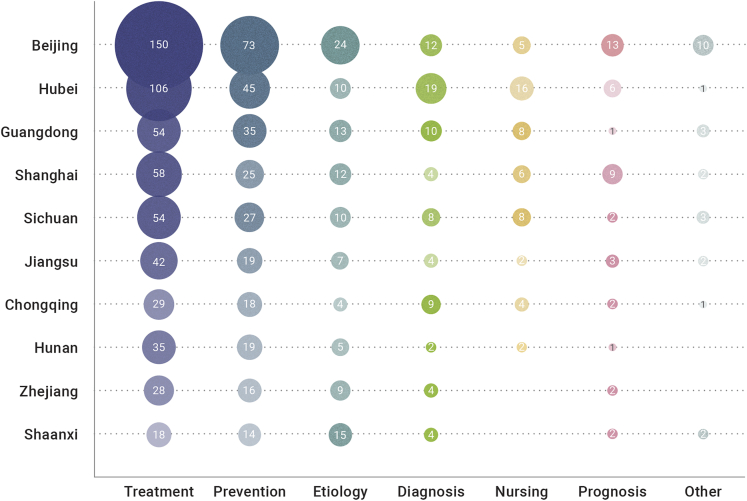
As of March 8, all 31 provincial-level entities in China have published COVID-19-related articles in Chinese journals. From the perspective of publication numbers, Beijing, Hubei, Guangdong, Shanghai, Sichuan, Jiangsu, Chongqing, Hunan, Zhejiang, and Shaanxi rank among the top 10. These cities and provinces also represented the seat of medical research power in China. Beijing, which has the strongest medical research capacity, ranked first with 256 papers, while Hubei, the region most severely affected by the epidemic in China, ranked second (177 papers). Furthermore, the numbers of COVID-19-related research papers in Guangdong (105 papers), Shanghai (100 papers), and Sichuan (100 papers) were also prominent. Research carried out in Beijing has covered all aspects of SARS-CoV-2, with a focus on treatment, prevention, etiology, and prognosis, ranking first in China in terms of number of publications. In addition to treatment and prevention, researchers in Hubei have focused on diagnosis of COVID-19 and nursing practice; research performed in Guangdong and Sichuan has mainly covered etiology, diagnosis, and nursing; studies in Shanghai have focused on etiology, prognosis, and nursing; publications from Jiangsu, Zhejiang, and Shaanxi mainly focus on etiology, while research in Chongqing has been centered around diagnosis (Figure 3).
Figure 3.
Distribution of Chinese Articles on COVID-19 across Major Cities and Provinces, by Research Subdomain (Number of Publications ≥50).
In terms of research subdomains, Chinese articles on the novel coronavirus have mainly focused on treatment and prevention, accounting for more than 85%. In addition to treatment and prevention, other research subdomains have been the focus of studies from different cities and provinces. The publication data also showed active collaborations on COVID-19 research across China. Among the around 1,000 retrieved research papers, the proportion of publications resulting from inter-provincial/city cooperation is 18%. From the perspective of research topic, about 20% of papers in the subdomains of treatment, prevention, and etiology resulted from inter-provincial/city research cooperation, while the corresponding percentages for studies on diagnosis and nursing were comparatively low, at about 13% and 6%, respectively. From the perspective of provinces and cities, about half of Beijing's papers were published in cooperation across provinces and cities, and almost all the provinces and cities established cooperative research relationships with Beijing. For Hubei, the number of inter-provincial collaborative papers was the second highest in China, and about 80% of institutions in other provinces and cities established joint research efforts with Hubei institutions. However, Hubei's inter-provincial collaborative rate was slightly lower. The numbers of publications resulting from inter-provincial collaboration in Jiangsu, Shanghai, and Guangdong were relatively similar. Among them, Jiangsu had the highest proportion of inter-provincial collaborative publications, exceeding 50%, and the corresponding percentages for Tianjin, Sichuan, and Liaoning were relatively close. However, their inter-provincial collaborative rates were quite different: Liaoning, over 70%; Tianjin, over 50%; and Sichuan, only about 22% (Table 2).
Table 2.
Publications on COVID-19 Resulting from Research Collaborations by Provinces and Cities in China
| Province/City | No. Chinese Papers | No. Inter-provincial Collaborative Papers | Ratio of Inter-provincial Collaborative Papers (%) |
|---|---|---|---|
| Beijing | 256 | 119 | 46.5 |
| Hubei | 177 | 53 | 29.9 |
| Jiangsu | 73 | 38 | 52.1 |
| Shanghai | 100 | 35 | 35.0 |
| Guangdong | 105 | 33 | 31.4 |
| Tianjin | 47 | 24 | 51.1 |
| Sichuan | 100 | 22 | 22.0 |
| Liaoning | 30 | 22 | 73.3 |
| Hunan | 55 | 18 | 32.7 |
| Shaanxi | 51 | 18 | 35.3 |
| Chongqing | 61 | 16 | 26.2 |
| Zhejiang | 51 | 13 | 25.5 |
| Shandong | 32 | 13 | 40.6 |
| Henan | 39 | 12 | 30.8 |
| Total | 1,002 | 184 | 18.4 |
Panoramic Analysis
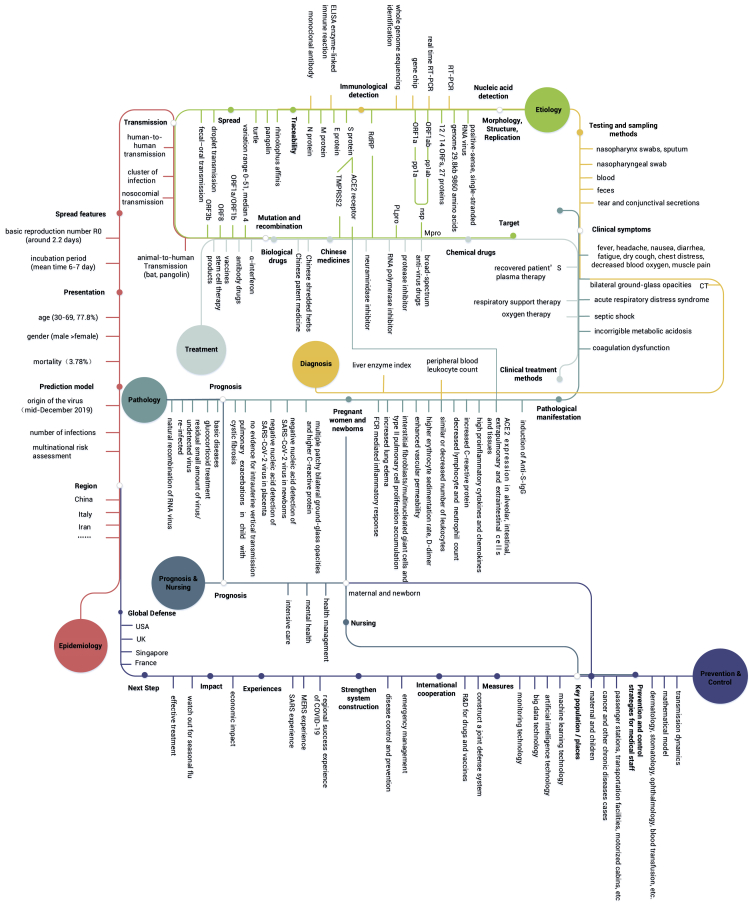
As of March 8, 2020, research on SARS-CoV-2 had covered the research subdomains of etiology, pathology, diagnosis, epidemiology, treatment, prevention and control, prognosis, and nursing, resulting in a huge knowledge map (Figure 4).
Figure 4.
SARS-CoV-2 Knowledge Scape.
Different colors represent different research subdomains. The gray hollow circle represents the intersection of key words. Different research subdomains will involve the overlap of key words, such as the "nuclear acid detection" in the topic "testing and sampling methods" and the "morphology, structure, replication" in "pathway." Therefore, the two research subdomains are anchored together at this node, represented by a hollow circle, with two lines pasted together.
Etiology
SARS-CoV-2 is the seventh coronavirus belonging to β-coronavirus genus that is capable of causing disease in humans.
Morphology and Structure
SARS-CoV-2 is a spherical, enveloped, positive-sense, single-stranded RNA coronavirus, with a characteristic crown-like appearance produced by the peplomers of the spike (S) glycoprotein radiating from the virus lipid envelope when observed under an electron microscope.10 Its genome contains ~29.8 kb nucleotides, annotated to possess 12 or 14 open reading frames (ORFs), and encodes 9,860 amino acids and 27 proteins (such as 3a, 3b, p6, 7a, 7b, 8b, 9b, and orf14).
Sequence analysis showed that the ORF regions, such as ORF1ab, ORF3b, and ORF8, are important functional domains that may be related to protein stability and nucleophilic effects, as well as to mutations subject to selection pressure (e.g., Nsp2 and NSP3, aa83-aa89).11,12 The spike glycoprotein on the virion surface mediates receptor recognition and membrane fusion. The conserved RNA binding domain in subunit S1 may be involved in the novel coronavirus's ability to cross species barriers imposed by receptor specificity. The C/N termini of the S1 subunit may also be responsible for direct interaction with the host receptor.
Traceability
Early epidemiological investigations have found that most COVID-19 patients in China had a history of travel to Wuhan, as well as a history of exposure to wildlife. The phylogenetic tree of coronaviruses suggested that the natural host of the novel coronavirus is the bat (Rhinolophus affinis),11,13 and that SARS-CoV-2 represents a recombination, on the S protein, between the bat coronavirus and an origin-unknown coronavirus. Turtles and pangolins were suggested to be potential intermediate hosts for SARS-CoV-2 based on analyses using the MEGA program.4
Spread
The transmission of SARS-CoV-2 between bats and intermediate hosts might be attributed to the contamination of food environments by animal droppings, restricted air flow in the environment, or the aggregation behavior of animals.14 Transmission between humans did not exclude the fecal-oral route, and the transmission intensity depended on the length of the incubation period and the importance of asymptomatic carriers with regard to disease transmission.15,16
Mutation and Recombination
The high replication rate and strong immunologic pressure in humans might be conducive to the adaptive evolution of the virus.17,18 As the virus continues spreading in the population, the possibility of occurrence of such mutations demanded attention to track the emergence of new strains for vaccine development and new clinical symptoms.19 Studies have estimated the median number of intra-host variants to be from 1 to 4, ranging between 0 and 51 in different samples.20 Further evidence confirmed the existence of potential recombination hotspots around gene ORF1a/ORF1b or region 11083-20953.21
Target
As a specific receptor that facilitates the entry of SARS-CoV-2 into target cells, ACE2 is a potential therapeutic target; the S protein (RNA binding domain and S2 subunits) of SARS-CoV-2 may also serve as an important antigenic target for neutralizing antibodies and vaccines. In addition, Mpro and RdRp, which are both involved in viral replication; TMPRSS2, which is essential for entry and viral spread of SARS-CoV-2 through interaction with the ACE2 receptor;22 and PLpro, which plays a major role in viral replication and innate immunity escape mechanisms, should be regarded as potential therapeutic targets.23
Pathology
Clinical Symptoms
Fever, diarrhea, fatigue, dry cough, asthma, dyspnea, headache, nausea, coarse crackles, hypoxemia, and muscle pain were the most common clinical symptoms in patients infected with SARS-CoV-2. The most common features of chest CT scans in patients with COVID-19 were bilateral ground-glass opacity,24,25 some showed crazy-paving pattern and prevalence of air bronchograms.26,27 As the disease progresses, chest CT images showed "white lung" in later stages.26, 27, 28, 29 The disease may then escalate rapidly in severity, with patients exhibiting acute respiratory distress syndrome, RNAemia consolidation mixed focus, septic shock, refractory metabolic acidosis, and coagulopathy, with poor prognosis.24,27,30,31 In some of the most severe cases, acute cardiac injury, secondary liver injury, hypoalbuminemia, bile duct injury, central nervous system injury, or systemic inflammatory response were observed26,32, 33, 34 (details in Figure 4).
Pathological Manifestation
COVID-19 patients with severe pneumonia might experience cytokine storms owing to systemic inflammatory responses. The factors causing the inflammatory storm, which may lead to liver damage, multiple organ failure, and even death, included (1) rapid viral replication and cellular damage;28,35 (2) renin-angiotensin system dysfunction caused by angiotensin-converting enzyme II (ACE2) downregulation and shedding; and (3) induction of anti-S-IgG.33,36 The former two were primary inflammatory reactions, while the latter were secondary inflammatory reactions. No thrombus was found in the umbilical cord blood vessels, and nucleated red blood cells were not observed in the blood vessels of the placental villi, and transaminase levels were normal in three infected pregnant women. Recent research, however, suggested possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn.37
Prognosis
Underlying diseases, such as diabetes mellitus and hypertension might affect prognosis in COVID-19 patients. Glucocorticoid treatment and impaired blood circulation might lead to the prevalence of viral residues in cells, which remain immunogenic and relapse after a period of time. False-negative nucleic acid test might indicate the possibility of a recurrence of COVID-19 pneumonia.38,39
Diagnosis
Virus Detection and Diagnosis Methods
There have been several methods for SARS-CoV-2 detection, which can mainly be classified into three categories: nucleic acid detection, immunological detection, and clinical feature identification. Among these, nucleic acid amplification detection methods, mainly including reverse transcriptase polymerase chain reaction (RT-PCR), real-time RT-PCR, diagnosis using gene chips, and whole-genome sequencing identification and classification10,40, 41, 42 have been reported most frequently. The nucleic acid detection method was first proposed by the Drosten research team. The study was publicized by WHO, and the scheme was recommended as a SARS-CoV-2 laboratory diagnostic technique. Immunological identification methods, which are reported to enable rapid and convenient viral detection, mainly include ELISA enzyme-linked immune assays and monoclonal antibody-based detection. Methods for evaluating clinical features include chest CT images, peripheral blood leukocyte counts, and determination of the liver enzyme index.27,43 In terms of testing and sampling methods, in addition to the traditionally used nasopharynx swabs, sputum, and other lower respiratory tract secretions, researchers also found that blood, tears and conjunctival secretion, feces, and other specimens are equally effective for detection of the virus.
Treatment
Chemical drugs and traditional Chinese medicines have been reported to be effective as anti-coronaviral drugs. In addition to broad-spectrum antiviral drugs, such as fabiravir and abidol, protease inhibitors (e.g., lopinavir, litonavir), RNA polymerase inhibitors (e.g., remdesivir), neuraminidase inhibitors (oseltamivir), and chloroquine phosphate have been shown to have therapeutic effects.44, 45, 46, 47 In addition, researchers have screened Chinese herbal medicines that directly inhibit SARS-CoV-2. Among biological drugs, there are many reports on the preventive and therapeutic potential of interferon alpha, antibody drugs, vaccines, stem cell therapies, and other products at the research and development stage.48,49 In regard to vaccines, more than 100 new research projects worldwide, and nearly 20 enterprises in China, are engaged in the discovery and development of an effective vaccine against COVID-19. In addition, clinical treatment methods include oxygen therapy, respiratory support therapy (such as mechanical ventilation), and the use of plasma from recovered patients.50, 51, 52
Epidemiology
Clinical Presentation
According to imaging data for 99 patients with SARS-CoV-2 pneumonia, 75% of the patients showed bilateral pneumonia, 14% showed multiple mottling and ground-glass opacity, and 1% had pneumothorax. The percentage of male patients was slightly higher than that of female patients, and the mild and moderate cases accounted for a larger proportion (80.9%). The majority of patients were 30–69 years (77.8%), and the proportion of children or newborns was low.53, 54, 55 Pediatric cases mainly resulted from family clusters. Compared with the adult patients, the clinical manifestations of pediatric cases were atypical and relatively mild.56,57
Transmission
The transmission characteristics of SARS-CoV-2 were similar to those of SARS-CoV and influenza viruses, highlighting the potential for global spread.58 As of April 17, 2020, data from WHO showed that 2,158,033 confirmed cases had been identified in at least 200 countries, areas or territories, compared with the total of 24,727 on March 8, 2020. Specifically, the worst-affected countries were the US, Spain, Italy, Germany, and China. The number of the deaths was 67,840, which exceeds the number for SARS in 2002 (8,273 confirmed, 775 deaths) and MERS in 2012 (1,139 confirmed, 431 deaths). On March 12, 2020, WHO Director-General, Dr Tedros Adhanom Ghebreyesus, declared the COVID-19 outbreak a global pandemic, marking a further escalation of this epidemic.
Risk
Countries with a higher risk of SARS-CoV-2 transmission were Thailand, Cambodia, Malaysia, Canada, and the US, based on the weekly simulated passengers' end-destination data for the period from January 1 to 31, 2020, derived from the online air travel dataset FLIRT.59 In Europe, the risk was concentrated among West European countries, such as the UK (39%), France (24%), and Germany (15%) before the implementation of the travel ban in Wuhan; this declined to 25% for the UK during the ban.60 The probability of travel of infectious COVID-19 cases from Wuhan to cities throughout China before the quarantine was expected to result in a COVID-19 risk of >50% in 130 cities (95% CI, 89–190) and >99% in the five largest metropolitan areas (Bazhong, Fushun, Laibin, Ziyang, and Chuxiong).61
R0
Studies on the basic reproduction number (R0), based on various epidemiological models, have been performed. The real-time changes in R0 were found to be an unstable trend, with large variances at the beginning of the outbreak, with a shift to a downward trend in late January that corresponded to the strengthening of the prevention and control strategies; this trend stabilized in late February, ranging from 1.4 to 4.1, and the estimated doubling time was 3.6 to 4.1 days.62 Specifically, R0 might be overestimated if asymptomatic cases have a shorter generation interval than symptomatic cases, or underestimated if this does not occur.63
Incubation Time
The incubation period was generally considered to be no more than 14 days, with the median ranging from 3 to 7 days. Other research on cluster cases showed that COVID-19 was still contagious during the incubation period.64 The median interval between primary and secondary cases was 2.6 days, which is much shorter for those with direct infection (5 days).15
Prevention and Control
SARS-CoV-2 infected cases usually have an incubation period with no symptoms; therefore, prevention and control of the infection requires cooperation between governments, medical, research, and manufacturing organizations. In the absence of effective antiviral drugs or vaccines, interventions, such as isolation, quarantine, and disinfection are the most effective procedures in controlling COVID-19.
Prevention and control measures implemented by the Chinese government include managing the source of infection, cutting off the routes of transmission, and protecting the susceptible population. In addition, Singapore, the US, the UK, and other countries have also taken active measures to contribute to global joint defense efforts. Main measures includes:
-
(1)
Advanced technologies have been applied in epidemic surveillance and control. Big-data technology, machine learning algorithms, and artificial intelligence technology were widely used in epidemic data analysis and fitting. These new applications can scientifically simulate the spread dynamics of the COVID-19 outbreak, monitor and manage data on urban health, apply new corrective measures to control spread of infection, and improve possible-COVID-19 case identifications.65
-
(2)
Daily prevention measures have been focused on key populations and areas. Pregnant women and children were the priority groups for COVID-19 prevention as pregnant women were considered an at-risk group while the low infection rates among children might be due to having more mild cases that are not reported. Related studies have reported clinical recommendations for diagnosis, prevention, and control among these groups.66 To prevent clustered infections, the government has strengthened the management and control of people's movements. Medical staff have designed remote diagnosis and treatment guidelines for cancer patients.67 In several studies, technologies and requirements for protection and disinfection in key places, such as passenger transport stations, transportation facilities, and mobile cabin hospitals, have been discussed. Nursing research focuses on the health management and mental health care of pregnant women, severe cases, and patients with chronic disease.
-
(3)
Protective measures for front-line workers have received widespread attention. Many studies are calling for interventions to protect the health of medical staff. In addition to front-line medical staff in infectious disease and respiratory departments, those in dermatology departments, dentistry departments, ophthalmology departments, blood transfusion departments, burn departments, radiology departments, and general surgery wards were all subject to increased risk of infection. In accordance with the specific clinical practice characteristics of these departments, studies designing precautions and control measures against the novel coronavirus aimed at decreasing the risk of nosocomial COVID-19 infection have been published.
-
(4)
Experiences have been shared to strengthen systems, including strengthening case isolation, tracing close contacts of identified cases, placing restrictions on affected areas to reduce movement and increase personal protection in China in the early stages of the COVID-19 outbreak.
-
(5)
Global strategies for joint prevention and control of COVID-19 transmission have been implemented. In addition to China, the US Health Community has reviewed and updated protocols developed for previous epidemics, rapidly expanded diagnostic testing, established protocols for emergency departments and urgent care centers, taken public health actions to slow the spread of the epidemic, and restricted temporary travel.68 The UK has announced that school closures and bans on mass gatherings would need to be considered.69 Singapore70 and France71 have taken measures, including setting up quarantine periods and tracing close contacts of confirmed cases.
Discussion
China Has Initiated COVID-19-Related Research in Considerable Scope and Depth
China has made great contributions to scientific progress related to the COVID-19 pandemic, responding rapidly and initiating critical research in considerable scope and depth. The early stages of the outbreak in China provided a large number of cases for scientific research on both the causative virus and treatment strategies. Chinese researchers first isolated the novel coronavirus from patients on January 7, 2020, and sequenced the viral genome on January 12, 2020. Furthermore, China shared its diagnosis and treatment strategies worldwide, actively participated in international scientific research cooperative efforts, and promoted the rapid dissemination of research results related to COVID-19. Since February 2020, the number of publications by Chinese researchers has increased dramatically on a daily basis. In view of the seriousness of the epidemic during the early stages, the focus of Chinese publications has been on treatment, prevention, and control, with the aim of applying the research findings to outbreak control in a timely manner in response to the call of the government. Among articles written in English, the collaboration between provinces and cities in China, as well as among medical communities, scientific research communities, and industrial communities was the focus of study, along with an emphasis on epidemiology, etiology, diagnosis, prevention, and control. These findings have been shared globally, representing unremitting efforts contributed to global joint research. In general, compared with the SARS epidemic in 2003, the scientific research capability of China has demonstrated great qualitative progress, promoting more virus-related research and yielding important results in a short time.72
Researchers all over the World Have Rapidly Launched Unprecedented Joint Research Efforts
The global research on COVID-19 is accelerating, and the international community has reached a consensus in strengthening scientific collaboration. As of March 8, 2020, at least 1,500 papers on COVID-19 have been published in English or Chinese. In addition to China, the US, Canada, and European countries also carried out scientific research on COVID-19 in the early stages of the pandemic. Regional distribution and related research topics are strongly relevant in the context of the epidemic situation and the need for data acquisition. The US focused on epidemiology, prevention, and control based on the epidemiological data for SARS-CoV-2 in this country, and previous experience of influenza prevention and control during the recent outbreak. European countries and Japan carried out epidemiological studies before the outbreak based on transmission data. Southeast Asian countries and the Middle East focused on epidemiology and control. Researchers around the world launched an unprecedented scale of joint research (26% global and about 18% inter-provincial in China) rapidly in response to WHO appeals; countries have, as a result, reached a consensus on strategic direction and cultivated scientific cooperation.73 China and the US have established cooperative networks with European and Southeast Asian countries, and achieved unprecedented and extensive communication and information sharing in research in the fields of etiology, epidemiology, and clinical studies, leading to research actions being implemented faster than in the SARS and MERS outbreaks. It is worth noting that, due to the limitations of data and clinical samples, the research disparities in different regions will affect the guidance of the global treatment, and more global clinical studies should be explored in the future for more suitable treatment guidance in different countries. For China, researchers should take full advantages of their early research (such as the early clinical samples, and the R&D of a recombinant protein-based vaccine), combined with other regional research advantages (such as the technologies of rapid detection of COVID-19 and nucleic acid vaccines in the US) and, based on the expansion of global clinical samples, promote international joint research.
The Knowledge Map of SARS-CoV-2 Is Becoming Increasingly Comprehensive, and Knowledge Gaps to Be Filled Have Been Identified
Considerable information was available to researchers all over the world within 2 months of the outbreak of COVID-19, making the contextual knowledge system for SARS-CoV-2 increasingly clear and forming a knowledge scape that includes etiology, epidemiology, diagnosis, treatment, prevention, and control. It should be noted that our understanding of novel coronavirus is still not comprehensive enough, and knowledge gaps still exist in subdomains such as the origin of the virus; modalities of transmission between animals and humans; surrogate markers for infectivity; spectrum of clinical disease; adequate animal models; supportive care interventions; immunotherapy; safe provision of care; psychological care; and ethical problems. It is imperative to establish timely and clear research routes and to coordinate and accelerate research aimed at filling the above-mentioned gaps to control the pandemic and prepare for future outbreaks involving other coronaviruses.
Improve R&D to Accelerate Research Innovation
The integration of research activities in the response to outbreaks has led to a prompt research response in terms of R&D activities on drug and vaccine development, and treatment. The next step is to consider other factors conducive to research innovation, such as fostering an innovative atmosphere in pharmaceutical R&D and focusing on research coordination to develop effective solutions. There is no approved drug therapy for SARS-CoV-2 or any other coronaviral infection at this time, as development efforts have been closely linked to free market dynamics. Thus, it is imperative to accelerate the improvement of innovative R&D of antiviral drugs. In the global market, public and private sectors must cooperate toward the development of a new regulatory category of broad-spectrum drugs for the treatment of coronaviral infections.74 For China in particular, acceleration of research aimed at the clinical isolation of dangerous pathogens is necessary, as well as improvement of the existing R&D facilities for the development of anti-coronaviral therapies. Advanced technologies have been applied in epidemic surveillance and control, and pandemic prevention and control strategies have been optimized in a timely manner. However, prevention and control measures taken by the international community have exposed shortcomings related to various aspects such as emergency reserve supplies as well as fundamental disease prevention and control systems in response to public health emergencies. It is suggested that the construction of advanced institutional mechanisms to reform the public health response in addition to an increased focus on coordination and cooperation among scientists, public health professionals, entrepreneurs, and government officials would be conducive to global joint defense against pandemics in the future. In addition to the above general suggestions, there are some other specific efforts that China must undertake. Regional differences in previous studies reflect the importance of developing an equitable health system and accelerating the construction of modern information systems to improve governmental coordination for effective pandemic preparedness and response.75, 76, 77, 78
Materials and Methods
The sources of literature used in this review were PubMed (for publications in English) and CNKI(for publications in Chinese), and studies published between January 1, 2020, and March 8, 2020, were retrieved. The search strategy was: TS = ((COVID-19) OR (2019-nCoV) OR (2019 novel coronavirus) OR (SARS-CoV-2) OR (Wuhan coronavirus) OR (Wuhan novel coronavirus) OR (COVID) OR (novel coronavirus) OR (Wuhan nCoV) OR (Wuhan coronavirus) OR (2019 novel CoV)) (Expressions with “Wuhan” were included in the search strategy to guarantee the comprehensiveness of the retrieval, as in the early stage of the outbreak of the COVID-19, certain scholars used “Wuhan” in their manuscripts before WHO gave the formal name. The authors insist that SARS-CoV-2 or COVID-19 should be used when referred to as the new coronavirus.) The source publications were Chinese articles in research journals, and English articles limited to article and review, excluding letters and commentary, and so forth. Temporal distribution, regional distribution, and organization distribution of the literature were characterized by bibliometric analysis and clustering classification. Visualization of data was performed in Excel 2013, Visio 2013, and UCINET v.6.186, and all figures were redesigned by Adobe Illustrator 2020 and Adobe Photoshop 2020.
Acknowledgments
This research received no external funding. English references with classifications were uploaded to our COVID-19 communication platform (http://ncov.scholarin.cn/explore). The Chinese references with the classification are available through Chinese Academic Journal (Network Edition): COVID-19 platform (http://cajn.cnki.net/gzbd/brief/Default.aspx).
Author Contributions
Y.G. designed the search strategy with input from T.Y and T.-C.M. Y.G., T.-C.M., and X.H. carried out the literature searches and screening, and any discrepancies were discussed with Y.G., T.-C.M., Y.-Y.X., R.Y., L.-J.G., S.-H.W., J.L., X.H., M.-L.Y., H.-G.L., and T.Y. Y.G. wrote the first draft of the review with input from T.-C.M., Y.-Y.X., R.Y., L.-J.G., S.-H.W., J.L., X.H., M.-L.Y., H.-G.L., and T.Y.
Declaration of Interests
The authors declare no conflict of interests.
Published: August 28, 2020
Contributor Information
Yue Gong, Email: gongy@mail.las.ac.cn.
Tao Yun, Email: yunt@cstec.org.cn.
References
- 1.Chen D.M., Zhao X.Q., Miao Y.G., Mao K.Y., Xiong Y. Analysis of the global coronavirus related research status and its enlightenment for the present and future. Chin. J. Clin. Med. 2020;27:1–12. [Google Scholar]
- 2.Zhu X.L., Huang C., Ma L.L., Zhang C., Gong Y., Zhao W.Y., et al. Research advances of novel coronavirus disease (COVID-19) China Biotechnol. 2020;40:38–50. [Google Scholar]
- 3.Gong Y., Shi Z.X., Chen J., Zhang Y.H., Zhao G.H. Current status of research on coronavirus. China Biotechnol. 2020;40:1–20. [Google Scholar]
- 4.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J.Y., Shi J.S., Qiu D.A., Liu C., Li X., Zhao Q., et al. Bioinformatics analysis of the 2019 novel coronavirus genome. Chin. J. Bioinform. 2020;1-10:96–102. [Google Scholar]
- 6.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Chin. J. Cardiovasc. Dis. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 7.Zhao S., Zhuang Z., Cao P., Ran J., Gao D., Lou Y., Yang L., Cai Y., Wang W., He D., et al. Quantifying the association between domestic travel and the exportation of novel coronavirus (2019-nCoV) cases from Wuhan, China in 2020: a correlational analysis. J. Travel Med. 2020;27:1–3. doi: 10.1093/jtm/taaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng V.C.C., Wong S.C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., Sridhar S., Chan J.F.W., Ho P.L., Yuen K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell. Host. Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalava K. First respiratory transmitted food borne outbreak? Int. J. Hyg. Environ. Health. 2020;226:113490. doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao W.J., Li L.M. Advances on presymptomatic or asymptomatic carrier transmission of COVID-19. Chin. J. Epidemiol. 2020;41:485–488. doi: 10.3760/cma.j.cn112338-20200228-00207. [DOI] [PubMed] [Google Scholar]
- 17.Lucas M., Karrer U., Lucas A., Klenerman P. Viral escape mechanisms—escapology taught by viruses. Int. J. Exp. Pathol. 2001;82:269–286. doi: 10.1046/j.1365-2613.2001.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berngruber T.W., Froissart R., Choisy M., Gandon S. Evolution of virulence in emerging epidemics. PLoS. Pathog. 2013;9:e1003209. doi: 10.1371/journal.ppat.1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheim J.O. A glimpse into the origins of genetic diversity in SARS-CoV-2. Clin. Infect. Dis. 2020;4:ciaa213. doi: 10.1093/cid/ciaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z.J., Xiao Y., Kang L., Ma W.T., Shi L.S., Li Z., et al. Genomic diversity of SARS-CoV-2 in coronavirus disease 2019 patients. Clin. Infect. Dis. 2020:ciaa203. doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi H. 2019 novel coronavirus is undergoing active recombination. Clin. Infect. Dis. 2020:ciaa219. doi: 10.1093/cid/ciaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Kang S.R., Tian R.H., Wang Y., Zhang X.Z., Li H.M., et al. CT characteristic appearances of patients with novel coronavirus pneumonia. Chin. J. Clin. Med. 2020;27:27–31. [Google Scholar]
- 26.Liu C., Jiang Z.C., Shao C.X., Zhang H.G., Yue H.M., Chen Z.H., et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Chin. J. Hepatol. 2020;28:148–152. doi: 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Guan G.W., Gao L., Wang J.W., Wen X.J., Mao T.H., Peng S.W., Zhang T., Chen X.M., Lu F.M. Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia. Chin. J. Hepatol. 2020;28:100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Feng S., Wang F., et al. Clinical diagnosis and treatment of critical patients with novel coronavirus pneumonia (report of 12 cases) Chin. J. Clin. Med. 2020;27:32–35. [Google Scholar]
- 30.Liu W., Tao Z.W., Lei W., Yuan M.L., Liu K., Zhou L., Wei S., Deng Y., Liu J., Liu H.G., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl.) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Yang Y., Zhang C., Huang F.M., Wang F.X., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life. Sci. 2020;50:258–269. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;15:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L.L., Wang W.J., Zhu Q.J., Yang L. Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy. Chin. J. Hepatol. 2020;28:97–99. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Xuan Z.X., Zhang G.B., Ye X.L., Huang P. Cause analysis of liver injury in patients infected by novel coronavirus and suggestion of drug monitoring. Chin. Pharmacol. Bull. 2020;36:19–20. [Google Scholar]
- 35.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;3:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Liu W. Puzzle of highly pathogenic human coronaviruses (2019-nCoV) Protein Cell. 2020;11:235–238. doi: 10.1007/s13238-020-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y., Nie X., Huang B.X. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Chin. J. Pathol. 2020;49:418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y., Ma Y., Zhang J., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy R.S., Raza K., Cooper M.S. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020;16:133–144. doi: 10.1038/s41584-020-0371-y. [DOI] [PubMed] [Google Scholar]
- 40.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y., Kang B.H., Kang M., Chung D.R., Yi G.S., Lee L.P., Jeong K.H. Nanoplasmonic on-chip PCR for rapid precision molecular diagnostics. ACS. Appl. Mater. Interfaces. 2020;12:12533–12540. doi: 10.1021/acsami.9b23591. [DOI] [PubMed] [Google Scholar]
- 42.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Milit. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiraki K., Daikoku T., Favipiravir An anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng X.W., Tao G., Zhang Y.W., Yang G.N., Huang P. Drug interaction monitoring of lopinavir/ritonavir in COVID-19 patients with cancer. Zhonghua Nei Ke Za Zhi. 2020;59:400–404. doi: 10.3760/cma.j.cn112138-20200219-00097. [DOI] [PubMed] [Google Scholar]
- 46.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 48.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Zhang X.R., Ju Z.Y., He W.H. Advances in the research of cytokine storm mechanism induced by corona virus disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36 doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 50.Li H., Wang Y.M., Xu J.Y., Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E002. doi: 10.3760/cma.j.issn.1001-0939.2020.0002. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W., Pan C., Song Q. We should pay close attention to some issues in the process of respiratory therapy of COVID-19. Med. J. Chin. PLA. 2020;45:229–233. [Google Scholar]
- 52.Yang X., Hou J. Current status of application of convalescent plasma in acute viral infectious diseases and its prospect in therapy of COVID-19. Chin. J. Biol. 2020;33:241–245. [Google Scholar]
- 53.Cheng J.L., Huang C., Zhang G.J., Liu D.W., Li P., Lu C.Y., Li J. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:327–331. doi: 10.3760/cma.j.cn112147-20200222-00148. [DOI] [PubMed] [Google Scholar]
- 54.Ki M., Task Force for -nCoV Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol. Health. 2020;42:e2020007. doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D., Ju X.L., Xie F., Lu Y., Li F.Y., Huang H.H., Fang X.L., Li Y.J., Wang J.Y., Yi B., et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58:269–274. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 57.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., et al. Epidemiologic features and clinical course of patients infected with SARS-Cov-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25:2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haider N., Yavlinsky A., Simons D., Osman A.Y., Ntoumi F., Zumla A., Kock R. Passengers' destinations from China: low risk of novel coronavirus (2019-nCoV) transmission into Africa and South America. Epidemiol. Infect. 2020;148:e41. doi: 10.1017/S0950268820000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pullano G., Pinotti F., Valdano E., Boëlle P.Y., Poletto C., Colizza V. Novel coronavirus (2019-nCoV) early-stage importation risk to Europe, January 2020. Euro Surveill. 2020;25:2000057. doi: 10.2807/1560-7917.ES.2020.25.4.2000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du Z., Wang L., Cauchemez S., Xu X., Wang X., Cowling B.J., Meyers L.A. Risk for transportation of 2019 novel coronavirus disease from Wuhan to other cities in China. Emerg. Infect. Dis. 2020;26:1049–1052. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai A., Bergna A., Acciarri C., Galli M., Zehender G. Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2. J. Med. Virol. 2020;92:675–679. doi: 10.1002/jmv.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park S.W., Cornforth D.M., Dushoff J., Weitz J.S. The time scale of asymptomatic transmission affects estimates of epidemic potential in the COVID-19 outbreak. MedRxiv. 2020;31 doi: 10.1101/2020.03.09.20033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu Y.Y., Wang S.Q., Wang X.L., Lu W.X., Qiao D., Li J.B., Gu Y.Y., Zeng Y., Chen Y., Bai W.Z., et al. Epidemiological analysis on a family cluster of COVID-19. Chin. J. Epidemiol. 2020;41:506–509. doi: 10.3760/cma.j.cn112338-20200221-00147. [DOI] [PubMed] [Google Scholar]
- 65.Perrella A., Carannante N., Berretta M., Rinaldi M., Maturo N., Rinaldi L. Novel coronavirus 2019 (SARS-CoV2): a global emergency that needs new approaches? Eur. Rev. Med. Pharmacol. Sci. 2020;24:2162–2164. doi: 10.26355/eurrev_202002_20396. [DOI] [PubMed] [Google Scholar]
- 66.Liang H., Acharya G. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet. Gynecol. Scand. 2020;99:439–442. doi: 10.1111/aogs.13836. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y., Liu H., Hu K., Wang M. Clinical management of lung cancer patients during the outbreak of 2019 novel coronavirus disease (COVID-19) Zhongguo Fei Ai Za Zhi. 2020;23:136–141. doi: 10.3779/j.issn.1009-3419.2020.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moberly T. Covid-19: school closures and bans on mass gatherings will need to be considered, says England's CMO. BMJ. 2020;368:m806. doi: 10.1136/bmj.m806. [DOI] [PubMed] [Google Scholar]
- 70.Wong J.E.L., Leo Y.S., Tan C.C. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020;20 doi: 10.1001/jama.2020.2467. [DOI] [PubMed] [Google Scholar]
- 71.Bernard Stoecklin S., Rolland P., Silue Y., Mailles A., Campese C., Simondon A., Mechain M., Meurice L., Nguyen M., Bassi C., et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25:2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gong Y., Liao Q.Y., YU Q.Q., Shi Z.X., Chen J., Zhang Y.H. A bibliometric analysis on coronavirus. China Biotechnol. 2020;40:21–37. [Google Scholar]
- 73.Stephen K.B. Coronavirus: three things all governments and their science advisers must do now. 24 March 2020. 2020. https://www.nature.com/articles/d41586-020-00888-7 [DOI] [PubMed]
- 74.Burley S.K. How to help the free market fight coronavirus. Nature. 2020;579:319–320. doi: 10.1038/d41586-020-00888-7. [DOI] [PubMed] [Google Scholar]
- 75.Wang X., Zhang X., He J. Challenges to the system of reserve medical supplies for public health emergencies: reflections on the outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic in China. Biosci. Trends. 2020;14:3–8. doi: 10.5582/bst.2020.01043. [DOI] [PubMed] [Google Scholar]
- 76.Wang M. The importance of strengthening the ability of fundamental disease prevention and control system from the perspective of the epidemic situation of COVID-19. Chin. J. Prev. Med. 2020;54:480–483. doi: 10.3760/cma.j.cn112150-20200220-00149. [DOI] [PubMed] [Google Scholar]
- 77.Cheng J.Q. Thoughts and suggestions on modern construction of disease prevention and control system. Chin. J. Prev. Med. 2020;54:1–5. doi: 10.3760/cma.j.cn112150-20200221-00151. [DOI] [PubMed] [Google Scholar]
- 78.Heymann D.L., Shindo N., WHO Scientific and Technical Advisory Group for Infectious Hazards COVID-19: What is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]