Abstract
Amyotrophic Lateral Sclerosis (ALS) is a fast-progressive neurodegenerative disease leading to progressive physical immobility with usually normal or mild cognitive and/or behavioural involvement. Many patients are relatively young, instructed, sensitive to new technologies, and professionally active when developing the first symptoms. Older patients usually require more time, encouragement, reinforcement and a closer support but, nevertheless, selecting user-friendly devices, provided earlier in the course of the disease, and engaging motivated carers may overcome many technological barriers. ALS may be considered a model for neurodegenerative diseases to further develop and test new technologies. From multidisciplinary teleconsults to telemonitoring of the respiratory function, telemedicine has the potentiality to embrace other fields, including nutrition, physical mobility, and the interaction with the environment. Brain-computer interfaces and eye tracking expanded the field of augmentative and alternative communication in ALS but their potentialities go beyond communication, to cognition and robotics. Virtual reality and different forms of artificial intelligence present further interesting possibilities that deserve to be investigated. COVID-19 pandemic is an unprecedented opportunity to speed up the development and implementation of new technologies in clinical practice, improving the daily living of both ALS patients and carers.
The present work reviews the current technologies for ALS patients already in place or being under evaluation with published publications, prompted by the COVID-19 pandemic.
Keywords: Amyotrophic lateral sclerosis, Telemedicine, Brain-computer interfaces, Eye-tracking, Virtual reality, Artificial intelligence, Robotics, COVID-19
Abbreviations: ADL, Activities of daily life; AI, Artificial intelligence; ALS, Amyotrophic Lateral Sclerosis; ALSFRS-R, Revised ALS functional rating scale; AAC, Augmentative and alternative communication; AR, Augmented Reality; BCI, Brain-computer interfaces; ECAS, Edinburgh Cognitive and Behavioural ALS Screen; EQoL-5D, European quality of life questionnaire; ENCALS, European Network for the Cure of ALS; ET, Eye tracking; EU, European Union; FVC, Forced vital capacity; GDPR, General Data Privacy Regulation; HAD scale, Hospital Anxiety and Depression scale; MIP, Maximal inspiratory pressure; ML, Machine Learning; QoL, Quality of life; LL, Lower limb; MIE, Mechanical insufflator-exsuflattor; NEALS, Northeast ALS Consortium; NIV, Non-invasive ventilation; PLS, Primary lateral sclerosis; pt, patient; UL, Upper limb; VR, Virtual Reality; WHO, World Health Organization
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disease in which death occurs mainly due to respiratory insufficiency and respiratory infections. ALS patients are followed every 1.5–3 months by multidisciplinary teams, interval usually extended for slow progressors. Extra visits may be needed for respiratory, nutritional and psychological support [1].
Contrary to patients suffering a specific event in a specific moment, neurological or not, as a cerebrovascular injury or a fracture, in whom the natural course of the disease, assisted by co-adjuvant therapies including rehabilitation, progresses frequently from a considerable initial functional impact to higher levels of independence, ALS patients face the opposite track. From fully independent, patients with initial lower limb (LL) weakness need progressive assistance with gait and balance until being unable to climb stars, walk or maintain posture. Ankle-foot orthosis assist patients with drop foot but progressive disto-proximal LL weakness will require a cane/ walker, until wheelchair and bed confinement ensue. Upper limb (UL) involvement initially limits fine finger-hand motor skills, as needed for handling a needle. Other activities of daily life (ADL) are progressively affected as holding a pen and write, using the cutlery and self-feeding, grabbing a glass and drinking, using the computer or the cell-phone. From hand-wrist orthosis to assist on different UL activities and positioning, many simple adapted devices facilitate ADL, including adapted cutlery with enlarged handles, sock aid sliders and button hook devices. Bulbar involvement leads to progressive language and communication deterioration with subsequent non-verbal compensation by writing and gestures, if and until UL functionality persists. Alphabet tables traditionally assist in communication, blinking the patients when the carer identifies the correct letter in a previous selected line to construct sentences. Drinking as well as chewing and swallowing food are also progressively impaired, as happens with the respiratory function, leading to total dependence of alternative nutritional and respiratory support, including nasogastric intubation or gastrostomy, and non-invasive (NIV)/ invasive ventilation and mechanical insufflation-exsufflation (MIE). Neck and trunk weakness have a negative impact in walking, standing, sitting, balance, UL activities, maintaining social interaction including communication, as well as eating and breathing, ameliorated by orthosis.
ALS patients and carers have, nevertheless, already gained considerable independence as compared to other neurological diseases, regaining control of remote communication and operational manoeuvres by using domotics, augmentative and assistive communication (AAC) with eye-tracking (ET) and brain computer interfaces (BCI) support. The specificity of the ALS clinical impairment with loss of any motor initiative was one of the compelling reasons for ALS patients with preserved intellectual functions trying to maintain themselves active, creative, and even developing business plans. Every ALS Center has several examples of locked-in patients capable of communicating and maintaining perfectly organized and efficient business. Young, instructed and professionally active patients are more sensitive to new technologies. Older patients, with lower education levels and not previously acquainted with new technologies, face considerable difficulties, requiring more time, encouragement, reinforcement and a closer support. AAC is a good example. Nevertheless, the earlier the adaptation, in earlier phases of the disease, the higher the compliance [[2], [3], [4]]. More motivated patients, with younger motivated carers [5] and selecting user-friendly devices [4] are associated with better outcomes.
The COVID-19 pandemic has rushed the need to remotely continue providing the best care to patients, therefore diminishing the risk for nosocomial infection during hospital visits. Its full impact is yet to be determined. It will surely provide the best benchmark for the fast implementation and development of emerging technologies in which telemedicine will have a central role. Telemedicine is particularly relevant for ALS patients with mobility limitations, especially as disease progresses, and for those living far from the tertiary ALS centres. Time, costs, and all the traveling logistics necessary to attend in-clinic visits, including carer absence from work and traveling with wheelchairs, respiratory and nutritional support, would be redundant. ALS patients are more than ready to be further fostered in the endeavour due to COVID-19.
We review the current technologies for ALS patients already in place or under evaluation, prompted by the COVID-19 pandemic.
2. Definition of emergent technologies and telemedicine
The Information and Telecommunications Revolution, also known as Digital Revolution or Third Industrial Revolution, was the most impacting technological revolution in the Human History so far, through the development and implementation of the Internet [6]. “Emerging technology” is defined as a “radically novel and relatively fast growing technology characterised by a certain degree of coherence persisting over time and with the potential to exert a considerable impact on the socio-economic domain(s) which is observed in terms of the composition of actors, institutions and patterns of interactions among those, along with the associated knowledge production processes” [7]. The development of new hardware/ software opened new opportunities in all fields, including in Medicine and Clinical Research, from robotics and BCI to artificial intelligence (AI).
Telemedicine is an open and constant evolving science incorporating new information and communication technological advancements to adapt and respond to the changing health care needs of the societies. The word” telemedicine” is used since the 1970s, initially meaning” healing at a distance” [8] and defined by the World Health Organization (WHO) as “The delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of health care providers, all in the interests of advancing the health of individuals and their communities” [9]. Home tele-management for chronic disease surveillance is of main concern for the European Community and the “vision for Europe 2020” calls for the implementation of telemedicine collaborative arrangements. In high income countries, it is mostly focused on diagnosis and therapeutics but it can be as wide as including evaluation, assessment, monitoring, prevention, intervention, supervision, education, consultation, and coaching [10], with the potentiality to transform health care delivery by shifting it from hospitals/ clinics to home care [11].
3. Already implemented or ongoing studies on emergent technologies and telemedicine in ALS
3.1. Telemedicine
3.1.1. Multidisciplinary consults
Multidisciplinary ALS consults have been sparsely done through telemedicine, either by live videoconferences on real-time, synchronous [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]] or though the store-and-forward method, asynchronous [25]. Videoconferences connect the multidisciplinary ALS teams at the tertiary centres to the patients at home, or to the local community hospitals/ health centres [12,21,22].
Two Australian tertiary hospitals established regular videoconferences with the local hospitals or community health services assisting ALS patients living far from the hospitals, thereby replacing the regular multidisciplinary in-clinic 3-mo consults [12]. Videoconferences were considered feasible, although respiratory and palliative concerns arose [12]. Feasibility, even in advanced stages, was also reported by the tele-visit program as a supplement of the regular visits developed by the Massachusetts General Hospital ALS clinic [13]. Patients lived 211 miles from the clinic (median) and the consults involved the contact with a physician and a nurse. Frequent addressed issues were medication management (89%), discussion of goals of care (74%), research (55%) and equipment use (50%). However, acute care issues were rarely discussed [13]. Adjusted cost-savings to medical usefulness of those video tele-visits as compared to in-clinic visits were further addressed [14], representing, per visit, $997 for the patients (in-clinic visit $1116; video tele-visit adjusted for medical usefulness $119) and $327 for the institution (in-clinic visit $799; video tele-visit adjusted for medical usefulness $472). Patients and providers rated medical usefulness modestly lower for video tele-visits, despite following the American Academy of Neurology Guidelines [26], as compared to in-clinic visits. On the contrary, a study conducted by the Veterans Affairs ALS Center in Ohio addressing outcomes, disease progression, malnutrition and survival, reported that videoconsult multidisciplinary care provided by physicians, nurses and other health providers when needed, had the same quality and similar clinical outcomes as in-clinic visits [15]. The latter group had previously used direct mobile videoconsults with patients or interaction through platforms with healthcare providers at the hospital in close connection with the ALS team referring economic and time benefits [22]. High levels of satisfaction were reported in a prospective study addressing the feasibility and satisfaction of ALS patients, carers and healthcare providers with multidisciplinary tele-visits, conducted by the Penn State Health Hershey ALS Center [16]. Despite negative aspects (video/audio problems; no physical examination; no body language cues; more impersonal, less emotional connection; lack of privacy in individual conversations between the patient/ carer and the team), benefits included continuing providing care, understanding home dynamics of patients/ carers dynamics, no travel requirements, time savings in addition to less fatigue and stress for the patients, who were more comfortable and talkative [16]. Two modalities of care were further assessed - full multidisciplinary visits with the ALS team on ALS clinic days or 1-on-1 visits with individual ALS healthcare providers on other days [17]. The tele-visit modality was preferred by the patients living farthest, also the ones having poorer physical and respiratory function and more likely to use NIV [17]. Despite patient satisfaction, good acceptance and reduced time burden, psychological and emotion issues have also been identified as needing to be discussed with face-to-face contact. Therefore, teleconsults (through a platform with access to a chat room and videoconsults) were considered to be an addition to the face-to-face consult and not to replace them [20].
In Sheffield, questionnaires on the condition of patients and carers weekly collected by the Tele-health in Motor Neuron Disease (TiM) were evaluated by a nurse from the multidisciplinary ALS team, who could, afterwards, clarify the information by phoning the patient/ carer, expedite clinic appointments or establish a liaison with the team. Recordings included weight and balance, the revised ALS functional rating scale (ALSFRS-R), pain and saliva assessments, quality of life (QoL) scales, the Hospital Anxiety and Depression (HAD) scale, in addition to the carers' strain, depression and anxiety [18,19]. Reported potential benefits included improved communication and care coordination, reassurance, identification of complications and being an alternative or addition to clinic, although a friendlier algorithm to deal with clinical alerts was needed [18,19].
A store-and-forward method [25] by having nurse home-visits video-recorded and later discussed by the multidisciplinary team was considered to be feasible and gathering a good team satisfaction. The less positive points included lack of physical examination and being time-consuming to review the videos, write recommendations and further discuss the plan with the patient, in addition to the necessary technological, financial and ethical support and considerations as well as the time required to train the nurse [25].
Multidisciplinary consults via telemedicine due to COVID-19 pandemic have been started in multiple centers worldwide. In Italy, 32 patients with motor neuron disease (29 with ALS, 4 of whom with fronto-temporal dementia) have been followed with a structured questionnaire collecting demographics, clinical information and functional scale (ALSFRS-R) but also information addressing possible signs/symptoms of COVID-19 infection [23]. The questionnaire was reported to be feasible and the team was able to detect respiratory deterioration at distance, including respiratory decompensation requiring urgent hospitalization. No patients were diagnosed with COVID-19. In the satisfaction survey via email or WhatsApp, 90% of the patients or carers were satisfied with the teleconsult (70% very satisfied), 85% felt as being talking to the neurologist face-to-face, and 90% were interested in continuing the the follow-up visits through teleconsult, which could overcome social isolation, “abandoned” feelings during the illness as well as economic and time constraints associated to in-hospital visits [23]. Telephone and/or videoconsults has also been reported to be implemented/ ongoing in ALS centres integrating ALS consortiums/ networks, namely the Northeast ALS Consortium (NEALS) [24] and the European Network for the Cure of ALS (ENCALS) during the COVID-19 (unpublished data).
3.1.2. Respiratory support
Initial works on telemedicine in ALS focused on home monitoring of the respiratory function when NIV was required. At the ALS Clinical Center in Lisbon, home telemonitoring was considered to be feasible and safe [27], decreasing the need for NIV parameter adjustments until achieving compliance and during afterwards monitoring. It increased survival, while decreasing health care costs as determined by the number of in-office and emergency room visits and hospital admissions [28,29]. The authors used NIV devices with an integrated software, with internet access by TCP/IP connection and a modem station, allowing for real-time bidirectional tele-medical assistance, with immediate exchange of the ventilator data as well as parameter adjustments at distance. Home tele-monitoring and tele-assistance of home ventilated patients, not only with ALS but also with other neuromuscular disorders, were also effective by using a modem and a phone line connection to acquire patients' symptomatology and data from pulse oximeters at the Pulmonology Rehabilitation Center, IRCCS, in Brescia [[30], [31], [32]]. The number of hospitalizations and costs decreased, which was also reported when telemedicine was used to determine the need, on-demand, for MIE [33,34]. In both the Portuguese and Italian groups, telemedicine relied on a central control station, run by rehabilitation physicians in the Portuguese setting and by nurses with close connection to pulmonologists in the Italian group. Both groups developed specific red flag alerts to call for a rapid medical response when needed. Hazenberg et al. (2014) followed patients with neuromuscular disorders using telemedicine (telephone calls in addition to data transmission from ventilator, nocturnal pulse oximeters and capnography) not only for monitoring NIV follow-up but also for its initiation, which was safe, feasible and cost-effective, with improvements in blood gas and in QoL not inferior to hospital initiation [35]. Transmission of data from spirometry was also feasible with high acceptability by patients, carers and respiratory therapists, showing forced vital capacity (FVC) and maximal inspiratory pressure (MIP) a strong correlation between remote and the standard assessments [36]. Recently, data from a self-reported questionnaire together with weekly ventilator and oximetry monitoring facilitated the maintenance of ventilation and SpO2 levels despite ALS progression [37]. In patients with Duchenne Muscle Dystrophy [38] and Facio-Scapulo-Humeral Muscle Dystrophy [38,39] tele-monitoring for home ventilatory support through videoconferencing with a multidisciplinary team and tele-monitoring of cardiorespiratory variables (oxygen saturation, heart rate, blood pressure and electrocardiogram) was feasible, user-friendly, efficient and reduced the need for hospital admissions. Weaning a patient with Duchenne Muscle Dystrophy off the ventilator using telemedicine to connect the in-patient visits by a nurse with a specialist at distance was also successful [40]. According to the statement of the European Respiratory Society Telemonitoring of Ventilator-Dependent Patients Task Force [41], more evidence is needed for the applicability and efficacy of telemedicine in ventilated-dependent patients. However, its developments (including hardware, software and cloud platforms) are likely to change future home NIV management [41,42]. Despite improving healthcare access, especially in rural/ remote areas, reducing standard services in health systems of developed countries is a concern [41].
3.1.3. Physiotherapy
Pinto et al. (2012) established a home respiratory training program with compliance assessed every week by telephone calls and review of the every-day charts filled-in by the ALS patients [43]. In-clinic visits at month 4 and 8 assured the clinical assessments. The protocol was feasible and safe, with high compliance. Braga et al. (2018) monitored a home–tailored personalized exercise program, in a treadmill or walking outside. Heart rate and pulse oxygen saturation during the exercise were transmitted via Bluetooth from a non-intrusive biosensor to a mobile phone, wireless connected to a digital platform, being the data directly available for analyses [44]. Telephone calls and email contacts with the multidisciplinary team were provided if necessary. The exercise monitored at distance through telemedicine was safe, feasible, with good compliance [44].
Controlling physical activity overtime in ALS is feasible by the remote use of accelerometers, showing good compliance (93%), with results strongly correlated with ALSFRS-R, and low variability [45]. It has also been successfully assessed at-home through accelerometers and an ECG sensor in addition to in-hospital evaluations every 3-months, also including speech recording in the latter setting [46]. Although comfortable and well tolerable, sensors can be associated to skin and subcutaneous lesions, specially dermatitis [46].
The Penn State Health Hershey ALS Center is assessing movement in relationship to falls in ALS at home through UL and LL wearable devices as well as a pendant monitor assessing general activity [17]. Developing an automated fall detection algorithm is planned [17].
3.1.4. Nutritional support
A randomized open-label standard of care controlled trial compared nutritional counselling in ALS through an app to provide tailored nutrition recommendations as compared to counselling by a physician/ nurse (standard of care) and to in-person counselling provided by a dietician (in-person) [47]. App recordings were done biweekly for dietary intake and weight or monthly if in-person. The app nutritional counselling was feasible, safe, but not superior to the in-person or standard of care at 6-mo follow-up [47]. On the contrary, another group studied self-reporting through an app of weight (weekly) in addition to daily well-being and monthly functional status and considered it easy, helpful and not burdensome, with an 83% adherence, adding value to the in-clinic visits and providing additional personalized feedback [48].
3.1.5. Telephone calls, emails, apps, and clinical trials
Surprisingly, immediate telephone calls to the healthcare team or emails are not a frequent resource for getting medical care, as represented respectively by 27% and 17% in a clinical survey carried out in the United Kingdom and Australia involving ALS patients [[49], [50], [51]]. The exponential growth of telephone apps and different platforms have, however, been frequently used. Physicians can monitor, at distance, patient's symptoms, functional status through functional scales, and medication. It also engages and empowers patients in their clinical status and medical decisions and directly connects the patients one-another, as exemplified by the PatientsLikeMe platform [52]. Defining automatic alerts to track clinical changes could allow the health teams to rapidly act upon them [18].
The need to promote patient recruitment in ALS clinical trials, at distance, decreasing the burden on patients/ carers, drove the Barrow Neurological Institute and the Beth Israel Deaconess Medical Centers to develop the ALS AT HOME web portal [53]. Patients were recruited all over US, enrolled, consented and trained entirely at distance with online videos. The data from handgrip dynamometry, actigraphy, spirometry, electrical impedance myography, ALSFRS-R and patient reported experience measures, as well as speech trough a different application, were recorded and analysed [53].
3.2. Other emerging technologies
3.2.1. Augmentative and alternative communication and cognitive assessment through Eye tracking and Brain-Computer Interfaces
Augmentative and alternative communication (AAC) systems complement (augment) and substitute (alternative) verbal communication. Touch screen mobile phones and ipads, with software incorporating symbols/ words, frequently used words/ phrases, text prediction and synthesized speech, improve communication and QoL in ALS patients when introduced in early stages of the disease [4].
Eye tracking (ET) and BCIs allow ALS patients and others with severe motor involvement, particularly locked-in syndrome, to live, communicate and control the surrounding with software, electronic or electromechanical systems through brain activity, without muscle contribution. Controlling computers or wheelchairs and switching on-off electrical devices is thereby possible. In ALS, studies in ET and BCIs focus on its applicability in AAC and, lately, on the administration of neuro-cognitive tests/ tasks.
ET applicability for AAC in ALS patients addressed internet surfing, e-mailing, phone-calls and social networking, with good performance [54,55]. In regular ET users, in late-stage ALS with tetraplegia and anarthria, ET increased communication abilities, QoL and patients' satisfaction [56]. Not only ET for AAC improves Qol but it diminishes significantly caregivers' burden [57]. QoL in locked-in state ALS patients is maintained on the cost of the QoL of their next of kin, as assessed by ET [58], with well-being usually underestimated by carers [59].
Assessing cognition in non-verbal, unable to write ALS patients led to the development of neuropsychological assessments to be performed through ET. Keller et al. (2015) showed that performance accuracy in Raven's coloured progressive matrices was significantly correlated, as verified for d2-test only in controls but not in ALS [60]. Results in paper or through ET were lower in patients and discriminative of cognitive impairment. In a second study [61], similar results were reported when comparing the paper and ET-version of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) in ALS and controls, with a 95% specificity for cognitive discrimination. A ET-based cognitive battery assessing language, attention, executive and social cognition abilities developed by Poletti et al. (2017), showed good levels of diagnostic accuracy and usability [62]. The same group [63] used an ET-based cognitive assessment to perform a verbal-motor free cognitive flexibility test (Arrows and Colours Cognitive Test) in addition to other cognitive measures, showing significant correlations between them. Lower prevalence of perseverative errors was observed and a successful discrimination between patients and controls, mainly in execution times, was recorded.
The development of eye-gaze fatigue and oculomotor impairment impairs ET's functional usage [55]. Oculomotor movements are usually considered spared in ALS. Nevertheless, patients can present ophthalmoparesis, specially at late stages of the disease in long-surviving cases. Defective persuit movements, saccadic movements, nystagmus and abnormal Bell phenomenon can also be present [64]. P300 evoked potentials, steady state visual evoked potentials, mu rhythm or slow cortical potentials have been addressed in BCI overpassing ET's ocular limitations.
McCane et al. (2014) showed that most severely disabled ALS patients could use the Wadsworth BCI (P300-based) home system [65]. Good BCI accuracy measures were achieved, despite disease severity, age, EEG montages or recording quality, with higher P300 amplitudes and more anterior located (fronto-centrally) in patients with good accuracies. Auditory and eyes-closed steady-state visual evoked potentials could be an alternative if visual impairment was not overcome by ptosis-glasses or eye-patch for diplopia. Using P300-based auditory BCI was feasible by using visual and/or auditory P300-based BCI to test controls and 3 patients in a 4-choice oddball paradigm (“yes” , “no”, “pass”, “end”) [66] although visual and visual plus auditory modality had higher accuracy levels and speed.
By using a P300-based matrix speller, Nijboer et al. (2008) showed that severely disabled patients could communicate by producing both cued and spontaneous text, with relatively stable performance over a 40-week period, as demonstrated by P300 amplitude and latency [67]. Both in late stages and in early and middle stages can ALS patients use a P300-BCI system for AAC, with a visual paradigm to choose between 4 icons representing basic needs [68]. In 1-year follow-up, BCI skills, including cognitive abilities, were maintained, despite disease progression. Patients' motivation was higher when higher immediate benefit was obtained and in the elderly [68].
In USA, 42 male ALS patients without verbal and/or written communication were recruited to assess home function reliability and extent of use of the Wadsworth BCI home system for communication, using email and audio/ video programs [69]. Telemedicine allowed for daily transmission of data on BCI use, including EEG activity, while home interviews and technical support were done at 3-month intervals. Fourteen patients (33%) completed the training and became BCI users. Eight completed the study. Death, rapid disease progression and decreased interest for BCI (in 6) conditioned study abandon. The system was reliable and useful. Patients' QoL did not decline overtime despite ALSFRS-R decay. According to most patients/ carers, BCI benefit exceeded burden. Rare technical problems were reported. Addressing speed, accuracy of selection (also dependent on carers' technical ability) and having more diverse computer applications were the suggested improvements while a dry-electrode technology, with easier usable caps, improved portability and telemetry would increase compliance and reduce its burden.
In Utrecht, subdural electrocorticographic electrodes (2 strips on the primary motor cortex, and two additional on the dorsolateral prefrontal cortex) were implanted in a 58-year-old late-stage ALS patient [70,71]. Home communication use was frequent after initial training, mainly replacing an ET in unfavourable light conditions, especially outdoors, with progressive less effort although both having high levels of satisfaction. A reliable long-term functional stability was reported, despite slight increase in impedance until month 5 and a slowly decline of the power in the high frequency band [71]. Performance was high consistently, increasingly steadily overtime.
Cognitive functions in late-stage ALS were assessed by Iversen et al. through a SCPs EEG-BCI system, requiring extensive initial training for controlling EEG components to move a cursor on a monitor [72]. A series of two-choice cognitive discriminative tasks and a matching-to-sample paradigm, assessing performance in discriminating numbers, letters and colours, and simple computations was addressed. In a second study [73], a conditional-associative learning task with arbitrary visual associations (signs, coloured disks, geometrical shapes) was tested. Good levels of accuracy in performance and good verbal instruction-task response were achieved.
The development of neurophysiological batteries for ALS patients in late stages has been also addressed. After studying the feasibility of applying a modified version of the Phonemic and Semantic Verbal Fluency test as well as psychological and usability questionnaires through BCI and ET [74], Poletti et al. 2016 [75] used BCI to apply a motor-verbal free neuropsychological battery, adapted from traditional neuropsychological tests (Token Test, Modified Card Sorting Test, Raven Coloured Progressive Matrices (Fig. 1 ), d2-Test) [75]. The battery correlated to the standard cognitive assessments, especially in the execution times, which was lower and discriminative in ALS than in controls. High rate of calibration accuracy, satisfactory levels of usability and sensitivity, independent from clinical factors (disease onset and clinical deterioration, using ALSFRS-R), and psychological factors (anxiety and depression) were observed. However, reliability could have been reduced by the length of time required for the administration of the tests and patients' cognitive effort.
Fig. 1.
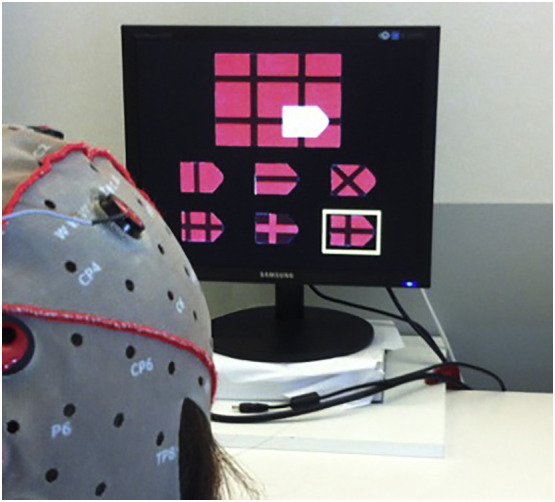
A BCI session - the BCI version of Raven's Coloured Progressive Matrices exemplifying the potential application of new technologies in providing tools for diagnosis and cognitive rehabilitation of ALS patients.
Courtesy of Barbara Poletti.
Few studies have directly compared ET with BCI. When comparing ET with P300 BCI, Pasqualotto et al. (2015) showed that the information transfer rate and System Usability Scale score were significantly higher for ET, with significant lower cognitive workload [76]. Therefore, ET seems more suitable when no ocular involvement limits it, which can be overcome by BCI. The comparison of electrooculography, ET and auditory BCI for AAC tested by a regular low-tech AAC user in locked-in stage, was feasible but none were considered an additional use, although auditory BCI was recognized as favouring independence from eye control, but more tiring [77].
3.2.2. Controlling the environment
A robot tele-operated by ALS patients with a joystick and buttons and able to move around obstacles, pick up objects (with different configurations and in various types of flooring) and deliver them back [78] was successfully tested with high overall satisfaction (6.7 out of 7). It was significantly easier to use than the patients' own hands, asking carers for help or using mechanical reaching devices.
Sparato et al. (2017) studied the performance of locked-in ALS patients as compared to controls in controlling a humanoid robot through P300-BCI, to reach, grasp and give a glass of water [79]. Three out of the 4 patients and all controls succeeded in the task, without differences in the number of correct commands, percentage of success and accuracy, which was easy to use and comfortable for all participants finishing the task.
In Seattle, an eye-controlled power wheelchair prototype for ALS patients, integrating software-hardware technology not dependent on preserved motor function or speech, rather only on oculomotor function, has been developed [80]. Twelve patients in different stages of the disease, without cognitive impairment, significant neck weakness, significant respiratory involvement or nystagmus, completed 3 trials of going forward and backward, stopping, turning and moving left and right. Eight performed the trials without errors. Errors were not related to clinical deterioration or glasses' usage although patients who committed errors were older. The rate of successful completion was 98.3% and the overall performance was excellent (4.6 out of 5), thus showing that the eye-controlled power wheelchair prototype was feasible, safe and with the potentiality of improving the mobility and independence in ALS.
3.2.3. Neurological evaluation using robots
Eleven ALS patients were studied in Colombia University with a robotic dynamic neck brace to characterize head motion with simultaneous surface recording of neck muscle activity [81]. The microcontroller of the robot was wireless synchronized with the electromyography receiver. Maximal neck movements in the 3 anatomical planes were repeated 5 times each, at self-selected speeds. Compared to controls, the head of ALS patients failed forward quicker and required early activation of the extensor muscles to be decelerated. In addition, there was a longer recruitment of splenius capitis muscle in lateral bending and sternocleidomastoid muscles in axial rotation, probably causing excessive fatigue.
Simmatis et al. (2019) used the KINARM robot to quantify UL sensorimotor and cognitive involvement in 17 ALS patients [82]. Cognitive involvement was correlated to the MoCA and FAB tests and sensorimotor and proprioceptive impairments were identified.
3.2.4. Neurorehabilitation in ALS using robotics
Multi-functional robotics can not only be used for assisting the patients but also for their assessment, training and reassessment overtime, possible by using the same robot. Robotic rehabilitation in ALS is giving its first steps. Contrary to other non-progressive diseases that begin as an acute event, the required support by the robot in ALS patients would be minimal at early stages of the disease, when the intensity and frequency of the training can be higher. The progressive physical weakness in ALS requires higher support provided by the robot and successive adjustments in the intensity and frequency of the training (decrease effort). More complex robots and software will provide a wider range of programs, relevant for motor learning as it is task-specific. Associating visual and auditory biofeedback, as provided by softwares with task-specific exercises/ games, increase the efficacy of the training. In a recent study, a 2-mo specific task-oriented right-UL exoskeleton training (Armeo® Power®, Hocoma® AG, Switzerland) combined with conventional physiotherapy for the training of a 69-year-old woman with flail-arm syndrome, improved right UL strength as compared to the initially stronger left UL [83].
Further information on other emergent technologies in Table 1 .
Table 1.
Other emerging technologies being tested in ALS.
| Technology | Nr subjects | ALS staging | Study duration | Positive aspects | Negative aspects | Ref |
|---|---|---|---|---|---|---|
| Communication | ||||||
| Touch screen mobile phone/ ipad (symbols/ words, frequently used words/ phrases, text prediction, synthesized speech), internet | 26 bulbar-onset ALS pts. (27 recruited); 17 carers | Early stage | 7–10 months | Positive effects in performance and QoL for pts. and carers if " "users" as compared to "non-users" | Number of hours per day of use not assessed. | [4] |
| ET (ERICA) (face-to-face interaction, group communication, phone calls, email, internet surfing) |
15 ALS pts | Different stages | nd | Good performance in all but 1. | Impaired eyelid control limits usage; Oculomotor apraxia demands extra hours of training; Necessary environmental, positioning and calibration adjustments |
[54] |
| ET Questionnaire on its home usage |
35 ALS pts | Late-stage | One time questionnaire | Improvement in communication ability, QoL; patients' satisfaction | nd | [56] |
| ET (communication with relatives/carers, email, internet surfing, social networking) Questionnaire on its home usage |
30 ALS pts | Late-stage, non-demented | One time questionnaire | Valuable for those using it for 15 months (median). Daily usage: 300 min (100–720). Good performance |
Oculomotor impairment and eye-gaze fatigue may limit its functional usage | [55] |
| ET (communications with carers, email, internet surfing) |
20 ALS pts.; 20 carers | Late stage | nd | Improvement in QoL of pts. Decreased carers' burden (10pts.). | To bulky to move with wheelchair. Oculomotor impairment/ nystagmus limit usage; costs | [57] |
| ET (Questionaire on QoL) |
11 ALS pts. (30 screened); 9 next of kin | Late-stage | One time questionnaire | Feasible. Pts reported good QoL, at the cost of their next of kin QoL. Next of kin and pts.' QoL rated similarly by next of kin (different relevant identified areas) | nd | [58] |
| ET Blink response (Questionnaire on QoL and end-of-life preferences) |
19 ALS pts. (out of 25 respondents); 19 carers | Late-stage | One time questionnaire | 9 answered by ET, 3 by ET and eye-blink, 6 by eye-blink and 1 verbally. Only 1 regular ET-user and 1 previous user. Both feasible. Pts. reported good QoL and non-significant depression, underestimated by carers, and willingness to maintain life sustain measures | nd | [59] |
| BCI, non-invasive, visual and/or P300-based 4-choice oddball paradigm - attend to 1 stimulus; disregard the others. Stimuli: visual, auditory, or both) |
3 ALS pts.; 3 controls |
Different stages, but retaining communication | 6 weeks (10 sessions) | Feasible as a non-muscular communication device. Higher accuracies and speed in visual and visual+ auditory modalities | nd | [66] |
| BCI, non-invasive, P300-based matrix speller (copy-spelling; free-spelling) |
6 ALS pts. (8 recruited) | Late-stage | 40 weeks | Home stable performance in communication producing both cued and spontaneous text. | High required expert supervision and time for setup/ cleanup. Slow speed of online usage. Home electrical noise impacts the recordings. Algorithm did not discriminating desirable EEG characters in 1. | [67] |
| BCI, non-invasive, P-300 based (visual paradigm with 4 icons in the screen, representing basic needs) |
21 ALS pts, ; 5 assessed longitudinally | Early and middle stages | 12 months, initial training period | BCI skills, including cognitive abilities, maintained overtime | Higher motivation only if higher immediate benefit. | [68] |
| BCI, non-invasive, P300-based (Wadsworth BCI home system, 8- and 16-channel montage); telemedicine | 17 ALS pts. (25 recruited) | Late-stage | One time evaluation | Feasible. Good BCI accuracies, despite disease severity, age, EEG montages or recording quality. | No accuracy improvement by the 16-channel montage.. Ptosis, diplopia, nystagmus present in pts. with poor performance. Second evaluation suggested to control for fluctuations in responses. |
[65] |
| BCI, non-invasive, P-300 based (Wadsworth BCI home system, 8-channel); telemedicine | 27 ALS pts | Late-stage | 12–18 months, initial training period | Reliable. Useful. Benefits exceed burden. Pts.' QoL did not declien overtime. |
Rare technical problems | [69] |
| BCI, non-invasive, P300-based; ET, SeeTech Pro set,7 × 7 grid (copy-spelling task, online encyclopedia search task, playing 2 songs on a Web site) |
11 ALS pts. (2 with bulbar-onset), 1 pt. with Duchenne muscular dystrophy | Different stages, from Early to late-stages | 2 sessions for BCI and 2 for ET | ET: faster and more accurate, with higher information transfer rate, more satisfying device for pts., requiring less cognitive workload, and less time consuming BCI: more fatigue, probably affecting performance, related to disease duration (but not age) |
[76] | |
| EOG; ET; Auditory BCI. |
1 ALS pts | Late-stage | 3 sessions for EOG and BCI, 1 for ET | Cost, communication speed, carer’ burden: lower for ET, medium for EOG and high for BCI. BCI: is the only that allows muscle-independent communication |
[77] | |
| BCI, invasive (subdural electrocorticographic electrodes); ET |
1 ALS pt | Late-stage | 36 months | BCI high consistent performance, increasing steadily overtime, replacing ET in unfavourable light conditions. BCI with high levels of satisfaction, progressive less usage effort, reliable long-term functional stability |
BCI: Slight impedance increase until month 5; slowly decline of the power in the high frequency band. ET: Not adapted to all situations |
[70, ,71] |
| Cognition | ||||||
| ET (Eye Link 1000) BCI, non-invasive, P300-based (modified version of the Phonemic and Semantic Verbal Fluency test, psychological and usability questionnaires; |
1 bulbar-onset ALS pt.; 8 controls |
Late-stage | Evaluation sessions | ET perceived as more usable. No negative affective state or anxiety with BCI or ET but a small anxiety increase after BCI in controls. Exploratory use in the ALS pt. |
Good BCI calibration is critical. Performance influenced by type of virtual keyboard used [66]. Reliability could be reduced by prolonged time for administration and cognitive effort. |
[74] |
| ET (Raven's coloured progressive matrices, D2-test [60] and ECAS [61]. |
48 ALS pts.; 32 controls [60] 46 ALS pt., 50 controls [61]; |
Late stage | Evaluation sessions | Feasible. ET and paper versions with significant accuracy correlations in controls and ALS [60,61], but not significant for D2-test in ALS. Lower results in paper and ET in ALS [60]. Discrimination of cognitive impairment [60,61]. Similar results for ECAS in paper and ET [61]. |
nd |
[60, 61] |
| ET (Eye Link 1000) (cognitive battery addressing language, attention, executive, social cognition) |
21 ALS pts.; 21 controls |
Non-demented and non terminal-ill | Two sessions within a week for each subject | Feasible. Good levels of diagnostic accuracy and usability in both groups. Lower results in ALS for frontal, social cognition and verbal fluency domains. Disease involvement not impactingusage. |
Verbal fluency assessments by ET required further adjustments. Non evaluation of ALS pts. in late stages of disease. |
[62] |
| ET (Eye Link 1000) (Arrows and Colours Cognitive Test and other measures of cognitive functions) |
21 ALS pts.; 21 controls |
Non-demented and non terminal-ill | Two sessions within a week for each subject | Successful discrimination between pts.- controls, mainly in execution times. Lower prevalence of perseverative errors, than other error-types. Significant correlations between ACCT and other ET-based frontal-executive measures but limited correlations with paper cognitive tests. |
Non evaluation of ALS pts. in late stages of disease, including with major respiratory involvement. Small bulbar-onset pts. included and without major cognitive involvement. | [63] |
| BCI, non-invasive, SCPs EEG (2-choice cognitive discriminative tasks; odd/even numbers, consonants/vowels, nouns/verbs, large/small numbers, shapes and colours/ simple computations [72] and conditional-associative learning task with visual stimuli arbitrary associations (signs, shapes, colours) [73] |
2 ALS pts. [72] 1 ALS pts. [73] |
Late-stage | Training sessions and a evaluation session | Feasible. Good performance accuracy (near 90% in [72] and near 100% in [73]) and verbal instruction-task response. |
Ability to control the SCP of the EEG is a prerequisite skill, which may take weeks, and may not be learnt. Not useful for tasks based on recall or for choices among more than 2 stimuli. [73] | [72, [73] |
| BCI, non-invasive, P300-based (BCI-based neuropsychological assessment - Token test, d2 test, Raven's Coloured Progressive Matrices, Modified Card Sorting Test), standard cognitive screening tools, psychological questionnaires, usability questionnaire |
15 ALS pts.; 15 controls |
Non-demented and non terminal-ill | Evaluation sessions | Feasible. High calibration accuracy, usability satisfaction and sensitivity, independent from disease onset/ progression, anxiety/ depression or respiratory involvement. Correlations between standard and BCI-based assessment, mainly in execution times in ALS, which is useful to discriminate pts.- controls (lower processing speed in pts). |
Non evaluation of pts. in late stages of disease, including with major respiratory involvement. Small bulbar-onset pts. included and without major cognitive involvement. | [75] |
| Controlling the environment | ||||||
| Robot tele-operated using a joystick and buttons (task: move around obstacles, pick up and deliver objects) |
20 ALS pts. (21 recruited) | Different stages, non terminal-ill | One evaluation session | High overall satisfaction (6.7/7). Significantly easier to use than the pts.' own hands, asking family members or using mechanical reaching devices, probably more useful for more disabled pts |
Space and time needed.Fixed robot. Probable greater challenges in “real” environments.Difficult to control when delivering objects to pts | [78] |
| Humanoid robot P300-BCI (task: reach, grasp and give a glass of water) |
4 ALS pts.; 4 controls | Late-stage, non-demented | 3 sessions for each | Easy. Comfortable. Task completed in 3 pts. and all controls. Tested at home in 3 pts. No differences in number of correct commands, percentage of success and accuracy. |
Motivation impacts successful control of the robot. Unavaible data of the performance in non-controlled environments and at distance. | [79] |
| Power wheelchair prototype Eye control (task: going forward and backward, stopping, turning and moving left and right). |
12 ALS pts | Different stages. No cognitive impairment, significant neck paresis, significant respiratory involvement, or nystagmus | 3 trials | Feasible. Safe. Potential to improve mobility and independence in ALS. Trials without errors in 8. Rate of successful completion: 98.3%. Overrall performance: excellent (4.6/5) |
Pts who committed errors were older (not related to clinical deterioration or glasses' usage). | [80] |
| Neurological evaluation | ||||||
| Robotic dynamic neck brace Surface electromyography (characterize head function) |
11 ALS pts.; 10 controls |
Different stages | One-time assessment (3 motions in each plane, each done in a cycle, for 5 times at self- selected speeds) | Wearable. Comfortable, ff f Feasible to assess head drop and disease progression. Head in ALS: Fails forward quicker, requires early extensor muscle activation for deceleration. Longer recruitment of splenius capitis in lateral bending and sternocleidomastoid in axial rotation, with excessive fatigue. Muscle activation correlated to clinical functionality and FVC. |
One-size brace Short number of patients included. |
[81] |
| KINARM robot Virtual reality system for biofeedback (quantify UL sensorimotor and cognitive involvement) |
16 ALS pts., 1 PLS pt |
Different stages, frontotemporal dementia was not an exclusion criteria | One time assessment | Feasible to assess cognition, sensorimotor and proprioception. Cognitive involvement correlated to MoCA and FAB tests. Sensorimotor and proprioceptive impairments were identified. |
Pain or discomfort from the robot's seat or arm position) . 9 pts. unable to complete 1 or more tasks (fatigue, low motivation, not understanding the task). 12 pts. required harness for upright posture. |
[82] |
| Neurorehabilitation using robots | ||||||
| Exoskeleton training supported with virtual reality + conventional physiotherapy (task-oriented) |
1 pt. with flail-arm | Early-stage | 2 months | Improvement right UL strength as compared to the initially stronger left UL | No stablished protocols, no usual access to this rehabilitation devices. | [83] |
For Abbreviations, please see refer to Abbreviation section.
4. Present moment at the COVID-19 pandemic and future developments
In the context of a highly contagious virus, for which the world population had not previous contact with, and without vaccines or effective therapies, more than 3.9 billion people (half of the world population) have been home confined in more than 90 countries/ territories. The population was asked to seek medical help only in acute cases to prevent being infected at healthcare infrastructures, with special advice for high risk patients, as ALS patients [84]. Mask usage, home confinement and hand hygiene, in addition to the identification of a primary carer to coordinate and provide the care and an additional carer to cover for, if needed, were recommendations provided by a European group of experts for the ALS patients and their families/carers during this period [84]. Hospital contact was promoted in the presence of symptoms suggestive of COVID-19, de novo respiratory symptoms or clinical respiratory deterioration related to the progression of the disease as well as respiratory emergencies [84]. Medical conditions for when to physically contact the ALS team or the emergency room instead of relying on at-distance technologies are proposed (Table 2 ). Planning, creativity and adaptability are essential to maintain the clinical care to these patients.
Table 2.
Proposed conditions for when to seek physical contact with the medical personnel at health facilities vs when to maintain remote contact.
| Contact with medical personal at health facilities | |
| Neurological status | First consult, diagnostic EMG, exclusion image exams |
| Rapid clinical deterioration | |
| Intractable spasticity associated with pain | |
| Advanced life directives discussion and end-of-life interventions | |
| Respiratory status | De novo respiratory symptoms |
| Adaptation to NIV | |
| Intolerance to NIV | |
| Tracheostomy intervention | |
| Respiratory decompensation (respiratory infections) | |
| Respiratory emergencies (aspiration, pulmonary embolism) | |
| Bulbar/ Nutritional status | Frequent swallowing problems with coughing/ choking |
| Rapid weight loss | |
| Dehydration | |
| Gastrostomy intervention | |
| Adaptation to AAC | |
| Limb status | Venous thrombosis |
| Falls and fractures | |
| Adhesive capsulitis of the shoulder with pain | |
| Intractable spasticity associated with pain | |
| Other medical conditions | Fecaloma |
| Other acute medical conditions requiring interventive measures (diagnostic and/or therapeutic) | |
| Optimal remote visit in ALS | |
| Neurological status | Regular follow-up consults |
| Functional scales including ALSFRS-R, and QoL scales | |
| Therapeutic prescriptions | |
| Patient/carer support | |
| Respiratory status | Regular evaluation of the presence of respiratory symptoms/signs of respiratory involvement |
| Respiratory tests if available (nocturnal pulse oxymetry, home spirometry, peak expiratory flow) | |
| Regular NIV follow-up | |
| Bulbar/ Nutritional status | Regular evaluation of bulbar symptoms with impact on the nutritional status |
| Nutritional tests (weight recordings, caloric and hydric intake recordings, energy expendure) | |
| Regular follow-up on gastrostomy care | |
| Speech recordings | |
| AAC training | |
| Cognitive status | Regular assessments, including emotional lability and cognitive-behavioural involvement |
| Regular cognitive training | |
| Limb status | Regular assessment (mobility and posture including with sensors if available, fasciculations, cramps, muscle atrophy, retractions, skin lesions, limb oedema) |
| Regular evalution of maximal articular amplitudes performed actively | |
| Muscle strenght evaluation (subjectively as for localized weakness of the neck, hands, etc., and objectively if a home dynamometer is available) | |
| Other medical conditions | Regular checking on the stability of other medical conditions |
For Abbreviations, please refer to “Abbreviations” section.
Steps have already been done in the way to move forward, being the widespread of telemedicine crucial. It has been shown to be feasible and safe, with good compliance and with time and cost savings for both patients/ carers and healthcare systems. The creation of platforms for bidirectional transmission of encrypted data, synchronization of devices, storage and data analyses, accordingly to ethical and political policies across countries, is essential. The availability of hardware and software, with the necessary adaptabilities to the patients during the progression of the disease, and the training of patients/ carers is a limiting factor. Nevertheless, tele-consults via internet or even over the phone (if no internet/ hardware availability), more focused on symptomatology and functional assessments, including nutritional, respiratory and psychological functional assessments, is feasible from day 1. In the market, not-so expensive sensors or basic devices are already available, which can, wireless or not, be connected to smartphones, to measure different biological parameters, including cutaneous temperature, heart rate, blood pressure, peripheral oxygen saturation, FVC, body positioning and even non-invasive ventilators and EEG. Many patients have already access to high-tech AAC devices (through ipads or or computers with specific writing programs and synthesized voice). ET can be feasible through goggles and adapted neurophysiological batteries tests are being developed. The implementation and the broad usage of what has already been validated in ALS is necessary. The creation of tighter clinical and scientific bounds between different ALS centers highly recognized for their own specific competencies fundamental for the holistic care of ALS patients can surely boost the process, more orientated and with greater efficacy.
Robots are mainly used for scientific proposes, available only to a limited number of patients, under strict protocols. The costs of robotics represent the main limitation for its usage. However, exoskeletons are already being used in some neurorehabilitation clinics, either for UL and cognitive training (as the UL robots), or for balance and gait training (as the stationary, in a treadmill, or the over ground exoskeletons), with studies published mainly on stroke and spinal cord injury [85,86], as in virtual reality [87].
ALS patients represent a human population suffering from one of the most devastating diseases causing a progressive physical deterioration and leading to immobilization, while most maintain intact or mild-involved cognition. This, in pair with the fact that many patients are relatively young, instructed, professionally active when developing the first symptoms and sensitive to new technologies, offers the ideal field to the development of those technologies, thereby also offering a referral for other neurological diseases. Technologies used in video games and entertainment may have, in the near future, useful applications in telemedicine. Virtual (VR) and augmentative reality (AR) are already stablished, widespread on multiple devices and developing at a fast pace. VR fully immerses the user in a virtual world who interacts with it through wearable headset covering the field of vision with screens placed in front of the eyes. Through joysticks or other gadgets, virtual world interaction includes moving in virtual spaces, interacting with objects and people, having sensorial hand experience through gloves (for example feeling vibration), or connecting and browsing the internet. Positive effects were already shown in patients with different neurological disorders [88]. Contrary to VR, AR does not fully immerse the user in a virtual world but adds information to the real world. Filming an object with a smartphone triggers the object recognition software and adds information about it on the screen. Interactions with the information or other displayed things are also possible. AR is used in gaming and in other industries, for example to check if furniture fits physically and aesthetically into one's room. Other technologies offer great potentials by making objects “smart” without triggering actions by interacting in a virtual world. Hypersurface is a new technology combining vibration sensors to ML/ AI, transforming any object of any material, shape, and size in an intelligent object able to recognize physical interactions. In ALS, an array of gestures could be instantly recognized and trigger specific commands, ridding the patients of unnecessary keyboards, buttons, and touch screens. It may also quantify physical activity with placed referrals and may have unpredictable further applications to improve QoL in ALS patients thus deserving further consideration. Finally, AI is not one technology, but rather a collection of them with different processes and tasks. ML is a one of the most common forms of AI, a statistical technique for fitting models to data and to ‘learn’ by training models with data, with high importance to healthcare. The most common application of traditional ML in healthcare is precision medicine – predicting which treatment protocols are likely to succeed based on various patient attributes and the treatment context. A more complex form of ML is the neural network, available since the 1960s, well established in healthcare research and used for categorising applications like determining whether a patient will acquire a particular disease. The most complex forms of ML involve deep learning or neural network models with many levels of features or variables that predict outcomes and future developments. It may also predict outcomes of the individual ALS patients. In the form of ML, AI is the primary capability behind the development of precision medicine, widely agreed to be a sorely needed advance in care.
Clinical trials represent a challenge in the era of COVID-19, as the existing protocols, in already on-going studies, have to be adjusted and new clinical trials are being postponed. The development of a new patient assessment, home-centred, by telemedicine and with access to the new emergent technologies, including end-effectors, will allow for most evaluations to be performed. ML models/ AI will allow appraising candidate diagnostic, monitoring, and prognostic markers, as well as accurate patient stratification into well-defined prognostic categories, especially if large data sets of patients with exhaustive clinical characterization are available [89]. The identification of the most likely medications and dosages that would be most effective to administer to ALS patients with specific identified phenotypes would certainly open new opportunities and bring new hope to the ALS community. COVID-19 pandemia may result in an unprecedented need to reset data managements and the high predictable clinical evolution of the ALS patients according to scales of functional evaluation (ALSFRS-R, [90]) and stage progression (King's [91] and MitoS' [92] staging), already recorded for thousands of patients may represent a previous referral to better define the impact of a new agents (SARS-CoV-2) on the clinical evolution of a high predictable disease.
5. Legal issues
From the intersection of technology and medicine emerges the generation and processing of people-related data. Although no global regulation exists, in many jurisdictions there are limitations, sometimes particularly strict on how to handle and use this data. In some countries, health data is treated as personal data while, in others, as particular personal data. In the United States, in general, personal data is considered marketable. In Europe, as opposed to the previous directive (European Directive 95/46/EC) [93] in which the protection of personal data was regulated with specific limitations on health data processing, the General Data Privacy Regulation (GDPR), European Union (EU) Regulation n.2016/679 [94,95], has no specific regulation regarding health data, beyond specific references related to the application of certain standards or institutions.
“Health-related data” or “data concerning health” is the “personal data related to the physical or mental health of a natural person, including the provision of health care services, which reveal information about his or her health status” [94,95]. “Personal data concerning health should include all data pertaining to the health status of a data subject which reveal information relating to the past, current or future physical or mental health status of the data subject”. “This includes information about the natural person collected in the course of the registration for, or the provision of, health care services as referred to in Directive 2011/24/EU of the European Parliament and of the Council” [[94], [95], [96]] “to that natural person; a number, symbol or particular assigned to a natural person to uniquely identify the natural person for health purposes; information derived from the testing or examination of a body part or bodily substance, including from genetic data and biological samples; and any information on, for example, a disease, disability, disease risk, medical history, clinical treatment or the physiological or biomedical state of the data subject independent of its source, for example from a physician or other health professional, a hospital, a medical device or an in vitro diagnostic test.” [94,95]
“Health data” is “Sensitive data” and, therefore, worthy of specific protection regarding fundamental rights and freedoms. Genetic and biometric data is considered in sensitive data [94,95], the processing of which, like for health data, may be subject to additional conditions and/or limitations, freely maintained or introduced by individual European Member States. Further fragmentation of the legal European regimes can occur, with potential legal uncertainties for the scientific research. Nevertheless, if the processing of health data is necessary to pursue scientific research purposes, GDPR requires the data controller to take technical and organisational security measures to ensure data minimisation [94,95]. Minimum amount of data that may be lawfully processed is not indicated and does not expand the scope of scientific research to any type of activity carried out in the health sector. Researchers and organizations may carry out scientific research without seeking the consent of the interested party [94,95]. In some isolated cases, researchers could even transfer the data of the data subject abroad as the research purpose, to which society entrusts the improvement of knowledge, prevails [94,95]. Organizations processing sensitive data for scientific research purposes may be exempt from the restrictions on the processing of sensitive data. Processing, further than that for which the data were originally collected, is lawful but compatible with the GDPR if also carried out for scientific research purposes and other requirements are met, including appropriate safeguards for the data subjects [94,95]. Despite seeming comforting, doubts of interpretation regarding the suitability of the guarantees for the data subjects still persist, which must be provided by those carrying out the research, and regarding the very concept of scientific research, essentially left to the interpretation of the national regulation authorities, which are required to apply European and national law in each specific case.
Acknowledging the above obstacles, it is the intention of the Council of the EU German's presidency to improve pan-European access to and the exchange of health-related data, laying the groundwork for a transparent European healthcare data space providing legal security and to set efforts to draft a code of conduct for the use of healthcare data in line with data protection legislation [97].
Technology can be instrumental in reducing the legal interpretative uncertainty through anonymisation techniques. In some applications, current anonymization techniques for statistical databases exhibit significant limitations, related to the utility-privacy trade-off, the introduction of artefacts, and the vulnerability to correlation. Some “recolouring” techniques have been proposed in order to preserve the same statistical characteristics of the original data up to the second order, but making data not directly linked to records of single individuals [98]. The recolouring procedure considers the database as an instance of a random population and applies statistical signal processing methods to it. In response to a query, the technique estimates the covariance matrix of the original data and builds a linear transformation of the data, producing an output that has the same statistical characteristics of the original data [98].
Advanced life directives have been regulated in different countries. New technologies can overcome communication limitations in late stages of the disease, thus allowing for the patients to express their wills on one hand and, on the other, to perform cognitive–behavioural assessments that can influence their decisions [59,99].
6. Conclusions
ALS represents a model to further develop telemedicine and new technologies, being the COVID-19 pandemic an unexpected opportunity to speed up the process. Telemedicine has already been successfully implemented in some ALS centers, mostly to provide multidisciplinary care and specific respiratory monitoring. Being feasible, safe, with positive cost-benefit aspects, its wide implementation worldwide should be promoted. Parallel to telemedicine, new technologies especially projected to facilitate ALS patients and carers in communication, mobility, interaction and control of the environment, as well as cognitive assessment are ongoing and many already available for daily usage. Nevertheless, costs are usually a limiting factor, which can be overcome if more companies invest in their applicability in clinical settings and their usage is widely spread. Their control through telemedicine would provide a more planned and day-to-day adjustability to the patients' clinical needs in addition to advanced life decisions. Legal issues deserve more attention in the near future.
The advantage of a somehow predictable disease as ALS for what it concerns the clinical progression combined with the acquired familiarity with new devices offered by the modern technologies indicated ALS as a prototype for neurodegeneration to further address the issue of the most efficient care of progressively disabled patients in the modern era.
Declaration of Competing Interest
None
Acknowledgements
We are grateful to Dr. Barbara Poletti for providing Fig. 1.
References
- 1.EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis, Andersen P.M., Abrahams S., Borasio G.D., de Carvalho M., Chio A., Van Damme P., Hardiman O., Kollewe K., Morrison K.E., Petri S., Pradat P.F., Silani V., Tomik B., Wasner M., Weber M. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)- revised report of an EFNS task force. Eur. J. Neurol. 2012;19(3):360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee A., Bruening L. Methods of communication at end of life for the person with amyotrophic lateral sclerosis. Top. Lang. Disord. 2012;32:168–185. [Google Scholar]
- 3.Beukelman D, Fager S, Nordness A. Communication support for people with ALS. Neurol Res Int 2011;2011:714693. [DOI] [PMC free article] [PubMed]
- 4.Londral A., Pinto A., Pinto S., Azevedo L., de Carvalho M. Quality of life in amyotrophic lateral sclerosis patients and caregivers: impact of assistive communication from early stages. Muscle Nerve. 2015;52(6):933–941. doi: 10.1002/mus.24659. [DOI] [PubMed] [Google Scholar]
- 5.Ball L., Schardt K., Beukelman D. Primary communication facilitators. Augment Commun News. 2005;17:6–7. [Google Scholar]
- 6.Hodson R. Digital Revolution. Nature. 2018;563(7733):S131. doi: 10.1038/d41586-018-07500-z. [DOI] [PubMed] [Google Scholar]
- 7.Rotolo D., Hicks D., Martin B. What is an emerging Technology? Res. Policy. 2015;44(10):1827–1843. [Google Scholar]
- 8.Strehle E.M., Shabde N. One hundred years of telemedicine: does this new technology have a place in paediatrics? Arch. Dis. Child. 2006;91(12):956–959. doi: 10.1136/adc.2006.099622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . 1997. A health telematics policy in support of WHO’s Health-fos-all strategy for global health development: report of the WHO group consultation on health telematics. [Google Scholar]
- 10.Galea M.D. Telemedicine in rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2019;(30):473–483. doi: 10.1016/j.pmr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 11.WHO Telemedicine Opportunities and Developments in State Members. 2010. https://www.who.int/goe/publications/goe_telemedicine_2010.pdf
- 12.Henderson R.D., Hutchinson N., Douglas J.A., Douglas C. Telehealth for motor neurone disease. Med. J. Aust. 2014;201(1):31. doi: 10.5694/mja14.00170. [DOI] [PubMed] [Google Scholar]
- 13.van De Rijn M., Paganoni S., Levine-Weinberg M., Campbell K., Swartz Ellrodt A., Estrada J., et al. Experience with telemedicine in a multi-disciplinary ALS clinic. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:143–148. doi: 10.1080/21678421.2017.1392577. [DOI] [PubMed] [Google Scholar]
- 14.Paganoni S., Van de Rijn M., Drake K., Burke K., Doyle M., Ellrodt A.S., Nicholson K., Atassi N., De Marchi F., Babu S., Estrada J., Schwamm L.H., Berry J.D. Adjusted cost analyses of video televisits for the care of prople with amyotrophic lateral sclerosis. Muscle Nerve. 2019;60:147–154. doi: 10.1002/mus.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selkirk S.M., Washington M.O., McClellan F., Flynn B., Seton J.M., Strozewski R. Delivering tertiary Centre specialty care to ALS patients via telemedicine: a retrospective cohort analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:324–332. doi: 10.1080/21678421.2017.1313867. [DOI] [PubMed] [Google Scholar]
- 16.Geronimo A., Wright C., Morris A., Walsh S., Snyder B., Simmons Z. Incorporation of telehealth into a multidisciplinary ALS clinic: feasibility and acceptability. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:555–561. doi: 10.1080/21678421.2017.1338298. [DOI] [PubMed] [Google Scholar]
- 17.Haulman A, Geronimo A, Chahwala A, Simmons Z. The use of telehealth to enhance care in ALS and other neuromuscular disorders. Muscle Nerve 2020 ((epub aheand of print). [DOI] [PMC free article] [PubMed]
- 18.Hobson E.V., Baird W.O., Partridge R., Cooper C., Mawson S., Quinn A., Shaw P., Walsh T., Wolstenholme D., Mcdermott C.J. The TiM system: developing a novel telehealth service to improve access to specialist care in motor neurone disease using user-centered design. Amyotroph Lateral Scler and Frontotemporal Degener. 2018;19(5–6):351–361. doi: 10.1080/21678421.2018.1440408. [DOI] [PubMed] [Google Scholar]
- 19.Hobson E.V., Baird W.O., Bradburn M., Cooper C., Mawson S., Quinn A., Shaw P., Walsh T., Mcdermott C.J. Process evaluation and explotation of telehealth in motor neuron disease in a UK specialist Centre. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2018-028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijeweme-d’Hollosy W.O., Janssen E.P.F. Huis in’t veld RMHA, Spoelstra J, Vollenbroek-Hutten MMR, Hermens HJ. Tele-treatment of patients with amyotrophic lateral sclerosis (ALS) J. Telemed. Telecare. 2006;12(Suppl. 1):31–34. doi: 10.1258/135763306777978434. [DOI] [PubMed] [Google Scholar]
- 21.Kelsen L., McCoy S., Hoffman P., Patwa H. Comprehensive care and home telehealth to veterans with ALS. Amyotroph. Lateral Scler. 2013;14(Suppl. 2):64–83. [Google Scholar]
- 22.McClellan F., Washington M., Ruff R., Selkirk S.M. Early and innovative symptomatic care to improve quality of life of ALS patients at Cleveland VA ALS Center. J. Rehabil. Res. Dev. 2013;50:vii–xvi. doi: 10.1682/jrrd.2013.05.0107. [DOI] [PubMed] [Google Scholar]
- 23.Capozzo R, Zoccolella S, Musio M, Barone R, Accogli M, Logroscino G. Telemedicine is a useful tool to deliver care to patients with amyotrophic lateral sclerosis during COVID-19 pandemic: results from southern Italy. Amyotroph Lateral Scler and Frontotemporal Degener 2020 ((epub ahead of print)). [DOI] [PubMed]
- 24.Andrews JA, Berry JD, Baloh RH, Carberry N, Cudkowicz ME, Dedi B, Glass J, Maragakis NJ, Miller TM, Paganoni S, Rothstein JD, Shefner JM, Simmons Z, Weiss MD; Bedlack RS. Amyotrophic lateral sclerosis care and research in the United States during the COVID-19 pandemic: challenges and opportunities. Muscle Nerve 2020 ((epub ahead of print)). [DOI] [PMC free article] [PubMed]
- 25.Pulley M.T., Brittain R., Hodges W., Frazier C., Miller L., Matyjasik-Liggett M., et al. Multidisciplinary ALS telemedicine care: the store and forward method. Muscle Nerve. 2018;59:34–39. doi: 10.1002/mus.26170. [DOI] [PubMed] [Google Scholar]
- 26.Miller R.G., Brooks B.R., Swain-Eng R.J., et al. Quality improvement in neurology: amyotrophic lateral sclerosis quality measures: report of the quality measurement and reporting subcommittee of the Ameri- can academy of neurology. Neurology. 2013;81:2136–2140. doi: 10.1212/01.wnl.0000437305.37850.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes de Almeida JP, Pinto AC, Pereira J, Pinto S, de Carvalho M. Implementation of a wireless device for real-time Telemedical assistance of home-ventilated amyotrophic lateral sclerosis patients: a feasibility study. Telemed. J. E Health 2010;16(8):883–8. [DOI] [PubMed]
- 28.Pinto A., Almeida J.P., Pinto S., Pereira J., Oliveira A.G., de Carvalho M. Home telemonitoring of non-invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2010;81(11):1238–1242. doi: 10.1136/jnnp.2010.206680. [DOI] [PubMed] [Google Scholar]
- 29.Lopes de Almeida J.P., Pinto A., Pinto S., Ohana B., de Carvalho M. Economic cost of home-telemonitoring care for BiPAP-assisted ALS individuals. Amyotroph. Lateral Scler. 2012;13(6):533–537. doi: 10.3109/17482968.2012.703675. [DOI] [PubMed] [Google Scholar]
- 30.Vitacca M., Assoni G., Pizzocaro P., Guerra A., Marchina L., Scalvini S., Glisenti F., Spanevello A., Bianchi L., Barbano L., Giordano A., Balbi B. A pilot study of nurse-led, home monitoring for patients with chronic respiratory failure and with mechanical ventilation assistance. J. Telemed. Telecare. 2006;12:337–342. doi: 10.1258/135763306778682404. [DOI] [PubMed] [Google Scholar]
- 31.Vitacca M., Comini L., Tentorio M., Assoni G., Trainini D., Fiorenza D., Morini R., Bruletti G., Scalvini S. A pilot trial of telemedicine-assisted, integrated care for patients with advanced amyotrophic lateral sclerosis and their caregivers. J. Telemed. Telecare. 2010;16:83–88. doi: 10.1258/jtt.2009.090604. [DOI] [PubMed] [Google Scholar]
- 32.Vitacca M., Comini L., Assoni G., Fiorenza D., Gilè S., Bernocchi P., Scalvini S. Tele-assistance in patients with amyotrophic lateral sclerosis: long term activity and costs. Disabil Rehabil Assist Technol. 2012;7(6):494–500. doi: 10.3109/17483107.2011.652999. [DOI] [PubMed] [Google Scholar]
- 33.Vitacca M., Paneroni M., Trainini D., Bianchi L., Assoni G., Saleri M., Gilè S., Winck J.C., Gonçalves M.R. At home and on-demand mechanical cough assistance program for patients with ALS. Am J Phys Med Rehabil. 2010;89(5):401–406. doi: 10.1097/PHM.0b013e3181d89760. [DOI] [PubMed] [Google Scholar]
- 34.Garuti G., Bagatti E., Verucchi, Massobrio M., Spagnolatti L., Vezzani G., Lusuardi M. Pulmonary rehabilitation at home guided by telemonitoring and access to healthcare facilities for respiratory complications in patients with neuromuscular disease. Eur J Phys Rehabil Med. 2013;49:51–57. [PubMed] [Google Scholar]
- 35.Hazenberg A., Kerstjens H.A.M., Prins S.C.L., Vermeulen K.M., Wijkstra P.J. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respir. Med. 2014;108(9):1387–1395. doi: 10.1016/j.rmed.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Geronimo A., Simmons Z. Evaluation of remote pulmonary function testing in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:348–355. doi: 10.1080/21678421.2019.1587633. [DOI] [PubMed] [Google Scholar]
- 37.Ando H., Ashcroft-Kelso H., Halhead R., Young CAChakrabarti B., Levene P., Cousins R., Angus R.M. Incorporating self-reported questions for Telemonitoring to optimize Care of Patients with MND on noninvasive ventilation (MND OptNIVent) Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(5–6):336–347. doi: 10.1080/21678421.2019.1587630. [DOI] [PubMed] [Google Scholar]
- 38.Zamarrón C., Morete E., González F. Telemedicine system for the care of patients with neuromuscular disease and chronic respiratory failure. Arch. Med. Sci. 2014;27(10(5)):1047–1051. doi: 10.5114/aoms.2014.46223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portaro S., Calabrò R.S., Bramanti P., Silvestri G., Torrisi M., Conti-Nibali V., Caliri S., Lunetta C., Alagna B., Naro A., Bramanti A. Telemedicine for Facio-Scapulo-humeral muscular dystrophy: a multidisciplinary approach to improve quality of life and reduce hospitalization rate? Disabil Health J. 2018;11(2):306–309. doi: 10.1016/j.dhjo.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Unnikrishnan D, Raman D, Joshi D, Ajaikumar BS. Use of home telemedicine for critical illness rehabilitation: an Indian success story. BMJ Case Rep. (2019 21;12(2). [DOI] [PMC free article] [PubMed]
- 41.Ambrosino N., Vitacca M., Dreher M., Isetta V., Montserrat J.M., Tonia T., Turchetti G., Winck J.C., Burgos F., Kampelmacher M., Vagheggini G. ERS Tele-monitoring of ventilator-dependent patients task force. Tele-monitoring of ventilator-dependent patients: a European Respiratory Society statement. Eur. Respir. J. 2016;48(3):648–663. doi: 10.1183/13993003.01721-2015. [DOI] [PubMed] [Google Scholar]
- 42.Borel J.-C., Palot A., Patout M. Technological advances in home non-invasive ventilation monitoring: reliability of data and effect on patient outcomes. Respirology. 2019 Feb:10. doi: 10.1111/resp.13497. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 43.Pinto S., Swash M., de Carvalho M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2012;13(1):33–43. doi: 10.3109/17482968.2011.626052. [DOI] [PubMed] [Google Scholar]
- 44.Braga ACM, Pinto A, Pinto S, de Carvalho M. The role of moderate aerobic exercise as determined by cardiopulmonary exercise testing in ALS. Neurol Res Int 2018; 8218697. [DOI] [PMC free article] [PubMed]
- 45.van Eijk R.P.A., Bakers J.N.E., Bunte T.M., de Fockert A.J., Eijkemans M.J.C., van den Berg L.H. Accelerometry for remote monitoring of physical activity in amyotrophic lateral sclerosis: a longitudinal cohort study. J. Neurol. 2019;266:2387–2395. doi: 10.1007/s00415-019-09427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Gancedo L., Kelly M.L., Lavrov A., Parr J., Hart R., Marsden R., Turner M.R., Talbot K., Chiwera T., Shaw C.E., Al-Chalabi A. Objectively monitoring amyotrophic lateral sclerosis patient symptoms during clinical trials with sensors: observational study. JMIR Mhealth Uhealth. 2019;7(12) doi: 10.2196/13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wills A.M., Garry J., Hubbard J., Mezoian T., Breen C.T., Ortiz-Miller C., Nalipinski P., Sullivan S., Berry J.D., Cudkowicz M., Paganoni S., Chan J., Macklin E.A. Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS. BMC Neurol. 2019;19:104. doi: 10.1186/s12883-019-1330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helleman J., van Eenennaam R., Kruitwagen E.T., Kruithof W.J., Slappendel M.J., van Den Berg, Visser-Meily J.M.A., Beelen A. Telehealth as part of specialized ALS care: feasibility and user experiences with ALS home-monitoring and coaching. Amyotroph Lateral Scler Frontotemporal Degener. 2020:31;1–10. doi: 10.1080/21678421.2020.1718712. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 49.Hobson E.V., Fazal S., Shaw P.J., McDermott C.J. Anything that makes life’s journey better. Exploring the use of digital technology by people living with motor neurone disease. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):378–387. doi: 10.1080/21678421.2017.1288253. [DOI] [PubMed] [Google Scholar]
- 50.Mackenzie L., Bhuta P., Rusten K., Devine J., Love A., Communications Technology Waterson P. Motor neuron disease: an australian survey of people with motor neuron disease. JMIR Rehabil Assist Technol. 2016;25;3(1) doi: 10.2196/rehab.4017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James N., Power E., Hogden A., Vucic S. Patients’ perspectives of multidisciplinary home-based e-health service delivery for motor neurone disease. Disabil Rehabil Assist Technol. 2019 Oct;14(7):737–743. doi: 10.1080/17483107.2018.1499139. [DOI] [PubMed] [Google Scholar]
- 52.https://www.patientslikeme.com
- 53.Rutkove S.B., Qi K., Shelton K., Liss J., Berisha V., Shefner J.M. ALS longitudinal studies with frequent data collection at home: study design and baseline data. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:61–67. doi: 10.1080/21678421.2018.1541095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ball L.J., Nordness A., Fager S., et al. Eye-gaze access of AAC technology for persons with amyotrophic lateral sclerosis. J Med Speech Lang Pathol. 2010;18:11–23. [Google Scholar]
- 55.Spataro R., Ciriacono M., Manno C., La Bella V. The eye-tracking computer device for communication in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2014;130(1):40–45. doi: 10.1111/ane.12214. [DOI] [PubMed] [Google Scholar]
- 56.Caligari M., Godi M., Guglielmetti S., Franchignoni F., Nardone A. Eye tracking communication devices in amyotrophic lateral sclerosis: impact on disability and quality of life. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7–8):546–552. doi: 10.3109/21678421.2013.803576. [DOI] [PubMed] [Google Scholar]
- 57.Hwang C.S., Weng H.H., Wang L.F., Tsai C.H., Chang H.T. An eye-tracking assistive device improves the quality of life for ALS patients and reduces the caregivers’ burden. J. Mot. Behav. 2014;46(4):233–238. doi: 10.1080/00222895.2014.891970. [DOI] [PubMed] [Google Scholar]
- 58.Linse K., Rüger W., Joos M., Schmitz-Peiffer H., Storch A., Hermann A. Eye-tracking-based assessment suggests preserved well-being in locked-in patients. Ann. Neurol. 2017;81(2):310–315. doi: 10.1002/ana.24871. [DOI] [PubMed] [Google Scholar]
- 59.Kuzma-Kozakiewicz M., Andersen P.A., Ciecwierska K., Vázquez C., Helczyk O., Loose M., Uttner I., Ludolph A.C., Lulé D. An observational study on quality of life and preferences to sustain life in locked-in state. Neurology. 2019;93(10):e938–e945. doi: 10.1212/WNL.0000000000008064. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keller J., Gorges M., Horn H.T., Aho-Özhan H.E.A., Pinkhardt E.H., Uttner I., Kassubek J., Ludolph A.C., Lulé D. Eye-tracking controlled cognitive function tests in patients with amyotrophic lateral sclerosis: a controlled proof-of-principle study. J. Neurol. 2015;262(8):1918–1926. doi: 10.1007/s00415-015-7795-3. [DOI] [PubMed] [Google Scholar]
- 61.Keller J., Krimly A., Bauer L., Schulenburg S., Böhm S., Aho-Özhan H.E.A., Uttner I., Gorges M., Kassubek J., Pinkhardt E.H., Abrahams S., Ludolph A.C., Lulé D. A first approach to a neuropsychological screening tool using eye-tracking for bedside cognitive testing based on the Edinburgh cognitive and behavioural ALS screen. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):443–450. doi: 10.1080/21678421.2017.1313869. [DOI] [PubMed] [Google Scholar]
- 62.Poletti B., Carelli L., Solca F., Lafronza A., Pedroli E., Faini A., Ticozzi N., Ciammola A., Meriggi P., Cipresso P., Lulé D., Ludolph A.C., Riva G., Silani V. An eye-tracker controlled cognitive battery: overcoming verbal-motor limitations in ALS. J. Neurol. 2017;264(6):1136–1145. doi: 10.1007/s00415-017-8506-z. [DOI] [PubMed] [Google Scholar]
- 63.Poletti B., Carelli L., Faini A., Solca F., Meriggi P., Lafronza A., Ciringione L., Pedroli E., Ticozzi N., Ciammola A., Cipresso P., Riva G., Silani V. The arrows and Colors cognitive test (ACCT): a new verbal-motor free cognitive measure for executive functions in ALS. PLoS One. 2018;9(13):8. doi: 10.1371/journal.pone.0200953. e0200953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma R., Hicks S., Berna C.M., Kennard C., Talbot K., Turner M.R. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch. Neurol. 2011;68(7):857–861. doi: 10.1001/archneurol.2011.130. [DOI] [PubMed] [Google Scholar]
- 65.McCane L.M., Sellers E.W., McFarland D.J., Mak J.N., Carmack C.S., Zeitlin D., Wolpaw J.R., Vaughan T.M. Brain computer interface (BCI) evaluation in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014 Jun;15(3–4):207–215. doi: 10.3109/21678421.2013.865750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sellers E.W., Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin. Neurophysiol. 2006;117:538–548. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Nijboer F., Sellers E.W., Mellinger J., Jordan M.A., Matuz T., Furdea A., Halder S., Mochty U., Krusienski D.J., Vaughan T.M., Wolpaw J.R., Birbaumer N., Kübler A. A P300-based brain-computer Interface for people with amyotrophic lateral sclerosis. Neurophysiol. 2008;119(8):1909–1916. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silvoni S., Volpato C., Cavinato M., Marchetti M., Priftis K., Merico A., Tonin P., Koutsikos K., Beverina F., Piccione F. Based brain–computer Interface communication: evaluation and follow-up in amyotrophic lateral sclerosis. Front. Neurosci. 2009;3:60. doi: 10.3389/neuro.20.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolpaw J.R., Bedlack R.S., Reda D.J., Ringer R.J., Banks P.G., Vaughan T.M., Heckman S.M., McCane L.M., Carmack C.S., Winden S., McFarland D.J., Sellers E.W., Shi H., Paine T., Higgins D.S., Lo A.C., Patwa H.S., Hill K.J., Huang G.D., Ruff R.L. Independent home use of a brain computer interface by people with amyotrophic lateral sclerosis. Neurology. 2018;91(3):e258–e267. doi: 10.1212/WNL.0000000000005812. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vansteensel M.J., Pels E.G., Bleichner M.G., Branco M.P., Denison T., Freudenburg Z.V., et al. Fully implanted brain-computer interface in a locked-in patient with ALS NEJMoa1608085. N. Engl. J. Med. 2016;375:21. doi: 10.1056/NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pels E. Stability of a chronic implanted brain-computer Interface in late-stage amyotrophic lateral sclerosis. Clin. Neurophysiol. 2019;130(10):1798–1803. doi: 10.1016/j.clinph.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iversen I.H., Ghanayim N., Kubler A., Neumann N., Birbaumer N., Kaiser J. A brain-computer interface tool to assess cognitive functions in completely paralyzed patients with amyotrophic lateral sclerosis. Clin. Neurophysiol. 2008;119:2214–2223. doi: 10.1016/j.clinph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Iversen IH, Ghanayim N, A. Kubler A, Neumann N, Birbaumer N, Kaiser J. Conditional associative learning examined in a paralyzed patient with amyotrophic lateral sclerosis using brain-computer interface technology. Behav. Brain Funct.. 2008b;4:53. [DOI] [PMC free article] [PubMed]
- 74.Cipresso P., Meriggi P., Carelli L., et al. Cognitive assessment of executive functions using brain computer interface and eye-tracking. ICST Transactions on Ambient Systems. 2013;13(01–06) [Google Scholar]
- 75.Poletti B., Carelli L., Solca F., et al. Cognitive assessment in amyotrophic lateral sclerosis by means of P300-brain computer Interface: a preliminary study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2016;17(7–8):473–481. doi: 10.1080/21678421.2016.1181182. [DOI] [PubMed] [Google Scholar]
- 76.Pasqualotto E., Matuz T., Federici S., Ruf C.A., Bartl M., Belardinelli M.O., Birbaumer N., Halder S. Usability and workload of access Technology for People with Severe Motor Impairment: a comparison of brain-computer interfacing and eye tracking. Neurorehabil. Neural Repair. 2015;29(10):950–957. doi: 10.1177/1545968315575611. [DOI] [PubMed] [Google Scholar]
- 77.Käthner I., Andrea Kübler A., Sebastian Halder S. Comparison of eye tracking, electrooculography and an auditory brain-computer interface for binary communication: a case study with a participant in the locked-in state. J Neuroeng Rehabil. 2015;12 doi: 10.1186/s12984-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King C.-H., Chen T.L., Fan Z., Glass J.D., Kemp C.C. Dusty: an assistive Mobile manipulator that retrieves dropped objects for people with motor impairments. Disabil Rehabil Assist Technol. 2012;7(2):168–179. doi: 10.3109/17483107.2011.615374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spataro R., Chella A., Allison B.M., Sorbello R., Guger C., La Bella V. Reaching and Grasping a Glass of Water by Locked-In ALS Patients through a BCI-Controlled Humanoid Robot. Front. Hum. Neurosci. 2017;11:68. doi: 10.3389/fnhum.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elliott M.A., Malvar H., Maassel L.L., Campbell J., Sophy N., Rifley J., M2 | Duffield, Crawford J., Cox E.J., Scanlan J.M. Eye-controlled, power wheelchair performs well for ALS patients. Muscle Nerve. 2019;60(5):513–519. doi: 10.1002/mus.26655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H., Chang B.-C., Andrews J., Mitsumoto H., Agrawal S. A robotic neck brace to characterize head-neck motion and muscle electromyography in subjects with amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2019;6(9):1671–1680. doi: 10.1002/acn3.50864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmatis L., Atallah G., Scott S.H., Taylor S. The feasibility of using robotic Technology to quantify sensory, motor, and cognitive impairments associated with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1–2):43–52. doi: 10.1080/21678421.2018.1550515. [DOI] [PubMed] [Google Scholar]
- 83.Portaro S., Cimino V., Accorinti M., Pidalà A., Naro A., Calabrò R.S. A promising tool for flail arm in amyotrophic lateral sclerosis rehabilitation: a case report. Eur J Phys Rehabil Med. 2019;55(4):515–518. doi: 10.23736/S1973-9087.18.05249-8. [DOI] [PubMed] [Google Scholar]
- 84.https://www.eanpages.org/2020/04/15/amyotrophic-lateral-sclerosis-and-covid-19-recommendations-to-patients-and-caregivers/
- 85.Calabrò R.S., Cacciola A., Bertè F., et al. Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now? Neurol. Sci. 2016;37(4):503–514. doi: 10.1007/s10072-016-2474-4. [DOI] [PubMed] [Google Scholar]
- 86.Bertani R., Melegari C., De Cola M.C., et al. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurol. Sci. 2017;38:1561–1569. doi: 10.1007/s10072-017-2995-5. [DOI] [PubMed] [Google Scholar]
- 87.Calabrò R.S., Naro A., Russo M., et al. The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial. J Neuroeng Rehabil. 2017;14 doi: 10.1186/s12984-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Massetti T., da Silva T.D., Crocetta T.B., Guarnieri R., de Freitas B.L., Lopes P.B., Watson S., Tonks J., de Mello Monteiro C.B. The clinical utility if virtual reality in Neurorehabilitation: a systematic review. J Cent Nerv Syst Dis. 2018;10 doi: 10.1177/1179573518813541. 1179573518813541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grollemund V., Pradat P.-F., Querin G., Delbot F., Le Chat G., Pradat-Peyre J.-F., Bede P. Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front. Neurosci. 2019;13:135. doi: 10.3389/fnins.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., Nakanishi A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function.BDNF ALS study group (phase III) Neurol. Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. 31. [DOI] [PubMed] [Google Scholar]
- 91.Roche J.C., Rojas-Garcia R., Scott K.M., Scotton W., Ellis C.E., Burman R., Wijesekera L., Turner M.R., Nigel Leigh P., Shaw C.E., Al-Chalabi A. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012;135(3):847–852. doi: 10.1093/brain/awr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chio A., Hammond E.R., Mora G., Bonito V., Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2015;86:38–44. doi: 10.1136/jnnp-2013-306589. [DOI] [PubMed] [Google Scholar]
- 93.https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31995L0046
- 94.https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
- 95.https://gdpr-info.eu
- 96.Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross-border healthcare (OJ L) 88, 42011, 45).
- 97.https://www.eu2020.de/blob/2360248/978a43ce17c65efa8f506c2a484c8f2c/pdf-programm-en-data.pdf
- 98.D’Acquisto G., Mazzoccoli A., Ciminelli F., Naldi M. Privacy through Data Recolouring. Proceedings of the Annual Privacy Forum. 2020 [Google Scholar]
- 99.Poletti B1, Carelli L, Lunetta C, Ticozzi N, Silani V. Advance care planning and mental capacity in ALS: a current challenge for an unsolved matter. Neurol. Sci. (epub ahead of print)). [DOI] [PubMed]


