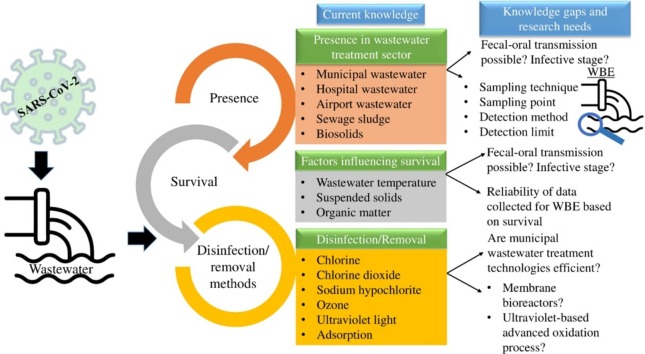
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Municipal wastewater, Hospital wastewater, Sewage, Disinfection
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the global pandemic coronavirus 2019 disease (COVID-19). The outbreak of COVID-19 as Public Health Emergency of International Concern is declared by World Health Organization on January 30, 2020. The known route of transmission is due to direct contact or via respiratory droplets. Recently, several studies reported SARS-CoV-2 ribonucleic acid (RNA) in wastewater treatment plant samples. The presence of SARS-CoV-2 RNA in wastewater may predict COVID-19 occurrence qualitatively and quantitatively. The concept is known as wastewater-based epidemiology (WBE) or sewage epidemiology. The present study reviewed the presence of coronavirus in wastewater and investigations relating to WBE development as a tool to detect COVID-19 community transmission. Few articles reported a correlation of SARS-CoV-2 RNA concentration in wastewater with the number of COVID-19 cases, whereas few reported higher prediction by wastewater surveillance than confirmed cases. The application of WBE is still in a preliminary stage but has the potential to indicate an early sign of transmission. The knowledge of persistence of coronavirus in municipal and hospital wastewater is needed for the application of WBE and to understand the chances of transmission. The studies reported more prolonged survival of coronavirus in low-temperature wastewater. Studies relating to the inactivation of coronavirus by disinfectants and removal of coronavirus are also presented. Research on the performance of the commonly adopted disinfection technologies in inactivating SARS-CoV-2 in municipal and hospital wastewater is required to reduce the risk associated with municipal and hospital wastewater.
1. Introduction
In the year of 2002 and 2003, in Guangdong province, China, the outbreak of severe acute respiratory syndrome (SARS) occurred [1]. After ten years, in 2012, emergence of Middle East respiratory syndrome coronavirus (MERS-CoV) occurred in Middle Eastern countries [2]. The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. China informed cases of pneumonia to World Health Organization (WHO) on December 2019 [4] and WHO declared the outbreak of COVID-19 as Public Health Emergency of International Concern on January 30, 2020 [5]. Thereafter, based on “alarming levels of spread and severity, and by the alarming levels of inaction”, a pandemic was declared by WHO on March 2020 [6]. Enveloped coronaviruses [7] (subfamily: Coronavirinae, family: Coronaviridae, order: Nidovirales) contain positive-sense, single-stranded ribonucleic acid (RNA). The four genera of the subfamily are Alpha-, Beta-, Gamma-, and Deltacoronavirus. The respiratory infection in humans is usually caused by Alpha- and Betacoronavirus [8]. SARS coronavirus, MERS coronavirus, and SARS-CoV-2 belong to Betacoronavirus genera [9,10].
The main route of transmission of COVID-19 is either by direct contact with infected person or via respiratory droplets [11]. However, for SARS, the wastewater plumbing system is believed to act as a potential route of transmission and caused the super spreading occurrence of SARS (342 cases in a 50-storied building) in Hong Kong due to the transportation of “virus-laden droplets” through empty U-bends of plumbing system [12]. The understanding of scientific knowledge related to COVID-19 and SARS-CoV-2 is rapidly changing. The presence of nucleic acid of SARS-CoV-2 has been reported in raw wastewater [13,14], sewage sample collected from hospital [15], and wastewater sample after secondary treatment [14]. The requirement of management of wastewater and fecal waste during the COVID-19 pandemic has been addressed in the interim guideline of WHO on Water, sanitation, hygiene, and waste management for the COVID-19 virus (April 23, 2020) [11].
Wastewater-based epidemiology (WBE) is a concept in which wastewater can be utilized as an indicator to understand the presence and scale of infection. WBE can provide an alarming and early indication about the presence of COVID-19 infected individuals in a city, town, and even in a housing complex. However, WBE is unable to detect specific infected individuals [16]. Currently, only 27 % of the global population is served by wastewater treatment facilities, which seriously undermine WBE’s applicability (reliance on access to wastewater treatment plants) [16].
Although there are few review articles describing persistence, occurrence, and detection methodology of coronavirus on water and wastewater [2,9,17], the present review is an attempt towards connecting the information available regarding occurrence and survival of coronavirus in wastewater with the booming concept of application of WBE for early detection of COVID-19 transmission. Besides, the information available on performance of wastewater treatment plants (WWTPs) in removing coronavirus and the importance of disinfection technology in inactivating coronavirus in hospital and municipal wastewater are also highlighted. Furthermore, the rapid progress of the field to fulfill the ever-increasing need also demands an updated review.
The review summarizes the information on the presence or occurrence of coronavirus (related to human health) in sewage, municipal wastewater, sludge sample (of treatment plant), biosolid, and hospital wastewater. The knowledge of the survival or persistence of coronavirus in sewage, municipal wastewater, and hospital wastewater are included to understand the chances of transmission which is also useful for the implementation of WBE. Furthermore, the inactivation of coronavirus in wastewater using disinfectants and removal methods are discussed. The important research needs to strengthen the knowledge of coronavirus in wastewater are highlighted.
2. Presence of coronavirus in wastewater and wastewater-based surveillance
Although there is a lack of studies on the presence of SARS-CoV-2 in wastewater, the detection of viral RNA (ribonucleic acid) in wastewater samples has gained considerable attention based on the idea of wastewater-based surveillance to detect community spread. As per the aim and scope of the present study, 34 works (eight research articles (Table 1 ) and 26 pre-print articles [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]) are included to understand the applicability of wastewater-based surveillance.
Table 1.
Presence of coronavirus in wastewater treatment sector.
| Virus studied | Sample type | Collection point | Important information and findings | Reference |
|---|---|---|---|---|
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Sewage | Hospital | Infectious SARS-CoV was not detected Nucleic acid of SARS-CoV was detected in sewage samples of both the hospitals before disinfection and in one hospital sample after disinfection |
Wang et al. [44] |
| Human coronavirus (HCoV) 229E and HKU1 | Biosolids | Wastewater treatment facility | Nine sequences of HCoV 229E and one sequence of HCoV HKU1 were identified | Bibby et al. [45] |
| HCoV HKU1 | Biosolids | Wastewater treatment facility | HCoV HKU1 was abundantly present (83 % occurrence observed) | Bibby and Peccia [46] |
| SARS-CoV-2 | Wastewater | Suburban pumping station and wastewater treatment plant | Out of 9 samples, two tested positive for N_Sarbeco assay but tested negative for NIID_2019-nCOV assay Among the two positive samples, one tested positive and one negative by direct extraction of RNA from electronegative membranes concentration method Among the two positive samples, one tested negative and one positive by ultrafiltration concentration method |
Ahmed et al. [13] |
| SARS-CoV-2 | Sewage | Sewage disinfection pool (hospital) | 3/3 and 1/1 sewage samples of inlets and outlets of preprocessing disinfection pool, and 0/1 sewage sample of final effluent of sewage disinfection pool were positive for nucleic acid of SARS-CoV-2 All the sewage samples came negative for viral culture Disinfection measures reduced hospital related virus infection risk |
Wang et al. [15] |
| SARS-CoV-2 | Influent, secondary and tertiary treated effluent | Wastewater treatment plant | 42 influent, 18 effluent (secondary treated), and 12 effluent (tertiary treated) samples from six wastewater treatment plants were tested and 35 out of 42, 2 out of 18, and 0 out of 12 came positive for SARS-CoV-2 RNA (ribonucleic acid) SARS-CoV-2 RNA positive wastewater samples were detected in three municipalities 12−16 days ahead of confirmed COVID-19 cases |
Randazzo et al. [14] |
| SARS-CoV-2 | Sewage | Wastewater treatment plant | 6 out of 12 wastewater samples tested positive for the presence of SARS-CoV-2 RNA A novel nested polymerase chain reaction assay for the screening of SARS-CoV-2 |
La Rosa et al. [47] |
| SARS-CoV-2 | Influent, secondary treated effluent | Wastewater treatment plant | SARS-CoV-2 RNA was found in 1 out of 5 secondary treated effluent samples, but none of the influent samples tested positive Higher limit of detection and lower filtration volume for influent samples could be the reason behind this discrepancy |
Haramoto et al. [48] |
Wang et al. [44] tested sewage samples collected from two hospitals receiving SARS (severe acute respiratory syndrome) patients. Both the raw sewage samples and sewage samples after the disinfection by chlorine were obtained. All samples were tested negative for the presence of infectious titer of SARS-CoV. However, RNA of SARS-CoV was found in sewage for both the hospitals before disinfection and only in one hospital after disinfection. Bibby et al. [45] found nine sequences of CoV 229E and one sequence of CoV HKU1 in class B biosolid samples (mixture of primary sedimentation residuals and activated sludge process residuals further treated by anaerobic digestion followed by dewatering) collected from a wastewater treatment facility (United States). Bibby and Peccia [46] reported abundance of coronavirus HKU1 in mesophilic anaerobic digester influent (primary + secondary sludge) and effluent (prior to dewatering) biosolid samples.
In an investigation conducted in Australia by Ahmed et al. [13], nine samples (composite grab samples) were collected, one was from suburban pumping station, and eight from two different WWTPs (influent). Out of nine samples, two (22.2 %) were tested positive (sampled from the same treatment plant but at different sampling events) and seven tested negative for SARS-CoV-2. However, for the two positive samples, they observed inconsistent results for different concentration methods used (one positive, one negative by direct extraction of RNA from electronegative membranes and one positive, one negative by ultrafiltration). The inconsistency in results was also observed for the two reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays (N_Sarbeco and NIID_2019-nCOV) used. Both the samples were tested positive by N_Sarbeco assay but tested negative by NIID_2019-nCOV assay. The study concluded that wastewater monitoring can help to control SARS-CoV-2 by providing an early indication of community spread even for asymptomatic individuals [13].
Wang et al. [15] investigated the occurrence of SARS-CoV-2 in sewage samples collected from a hospital to evaluate viral shedding in human feces. Three samples (total collected samples: 3) collected from inlet of preprocessing disinfection pool and one sample (total collected sample: 1) collected from outlet of preprocessing disinfection pool were tested positive for the presence of SARS-CoV-2 RNA. However, the sample collected from final outlet of sewage disinfection pool tested negative. To identify the viability, virus culture was conducted for all the RNA positive samples but came out negative [15]. Randazzo et al. [14] selected six treatment plants in the locality of low COVID-19 prevalence in the Iberian Peninsula to investigate the applicability of wastewater surveillance as an early signal of infection. Influent samples and samples after secondary and tertiary treatment were obtained in this regard. Among the samples collected, 35 out of 42 influent samples, 2 out of 18 secondary treated effluent samples, and 0 out of 12 tertiary treated effluent samples tested positive for the presence of SARS-CoV-2 RNA. The study concluded that wastewater surveillance can be used as a complementary tool to estimate the presence of COVID-19 cases. In Italy, La Rosa et al. [47] studied presence of SARS-CoV-2 in 12 influent sewage samples obtained from three WWTPs, out of which six samples were detected as positive. The study suggested that environmental surveillance may prove to be effective in detecting the extent of viral spread. Haramoto et al. [48] investigated presence of SARS-CoV-2 RNA in influent and secondary treated effluent of a WWTP in Japan. Although one out of five secondary treated effluent samples tested positive, none of the influent samples were positive. The discrepancy was probably due to higher limit of detection and lower filtration volume for influent samples.
A study is conducted by Green et al. [18] to evaluate the feasibility of combating COVID-19 spread by surveillance of SARS-CoV-2 in wastewater. Wastewater samples were obtained from influent pump stations, WWTPs, and interceptor lines located in Syracuse and Onondaga County, New York. The group attempted to develop a simple ultracentrifugation method for quantitative environmental surveillance of COVID-19 transmission. Furthermore, the ratio of log10(SARS-CoV-2):log10(cross-assembly phage or crAssphage) appeared to be correlated with the cumulative COVID-19 cases in Syracuse. The higher values of the ratio were observed in regions of higher cases [18]. In the United States, Peccia et al. [19] studied the presence of SARS-CoV-2 RNA in primary sewage sludge collected from the wastewater treatment facility, and RNA was detected in all the samples collected during the study period. They also observed that quantified RNA concentration was correlated (when adjusted for time lag) with the epidemiological curve (R2 value of 0.99) and the number of admissions in local hospital (R2 values of 0.99). Ampuero et al. [20] attempted to detect the circulation of SARS-CoV-2 by sewage surveillance of two WWTPs serving 85 % of the wastewater generated in Santiago, Chile. When the cases of COVID-19 were low, SARS-CoV-2 RNA was not detected in wastewater. However, as the number increased, SARS-CoV-2 genome copy number increased progressively correlating the number of confirmed cases in Santiago. Wu et al. [21] tracked SARS-CoV-2 dynamics using longitudinal wastewater analysis on a WWTP located at Massachusetts. Higher correlation was observed between viral titers and new clinical cases when a time lag of 4 days was considered which suggests that wastewater analysis may provide indications ahead of the clinical testing. They have also investigated several demographic variables affecting wastewater viral titers. Kaplan et al. [22] has also developed a SARS-CoV-2 epidemic model for the estimation of COVID-19 incidence based on the detection of SARS-CoV-2 RNA in municipal sewage sludge. The study also suggested that sewage sludge monitoring may lead the early detection of COVID-19 patients. With the objective of developing a statistical model to determine the infected population based on the viral load on wastewater, Vallejo et al. [23] established that natural logarithm of viral load obtained from WWTP fitted linearly (R2 = 0.851) with number of COVID-19 cases. Quadratic LOESS model also depicted higher R2 value of 0.88. While establishing the model, the authors considered several factors such as flow, viral load, temperature, rainfall, and humidity out of which only viral load was appeared to be significant. However, the other factors may appear as significant for different locations, even at different seasons. Trottier et al. [24] detected SARS-CoV-2 RNA in the influent of main WWTP of Montpellier, France during and post-lockdown. However, no temporal correlation was found as the RNA concentration was higher although there were few new cases post-lockdown.
Randazzo et al. [25] detected the presence of SARS-CoV-2 RNA in WWTPs samples even at the time of initial appearances of COVID-19 cases. The study also collected treated samples but did not observe the presence of viral RNA in nine samples tested. The study suggested wastewater surveillance as useful based on the signal of RT-qPCR assays, which increased continuously and reached a plateau faster than the confirmed cases [25]. Wu et al. [26] tested raw sewage samples (composite samples) collected from urban WWTP in Massachusetts. They observed SARS-CoV-2 viral titers at a significantly higher level than clinically confirmed cases using RT-qPCR assay [26]. The discrepancy in results was observed possibly due to the assumptions, for examples, no losses in viral titer during processing of samples, extraction of RNA, and due to degradation. For an accurate estimation, testing of individual stool samples may be required [26]. In France (Paris), Wurtzer et al. [27] investigated the occurrence of SARS-CoV-2 using RT-qPCR assay in samples obtained from three different WWTP inlets, and all the samples were tested positive. They tried to correlate the quantified of SARS-CoV-2 genomes in wastewater with carrier numbers and observed a positive correlation of genome units with the number of COVID-19 cases. They concluded that wastewater surveillance may provide an alternative and early tool for the identification of SARS-CoV-2 spread [27].
Medema et al. [28] investigated the presence of SARS-CoV-2 in domestic wastewater and airport wastewater at the beginning of the COVID-19 epidemic in the Netherlands and thus to understand the effectiveness of sewage surveillance to monitor the viral spread. Composite samples were taken from treatment plants, and no RNA of SARS-CoV-2 was identified in samples collected three weeks ahead of the first reported COVID-19 case. However, they detected SARS-CoV-2 in wastewater even when prevalence was low, which indicates the sensitivity of sewage surveillance [28]. In an investigation, in Istanbul, Turkey, samples were collected from two manholes and the inlet of seven WWTPs located at very serious, serious, moderate, and mild COVID-19 spots [29]. Five samples from treatment plants and two samples from manholes came out as positive for SARS-CoV-2 using RT-qPCR assay. The quantification of SARS-CoV-2 in wastewater may help in identifying areas with high risk and provide an early sign [29]. Kocamemi et al. [30] observed two positive cases of SARS-CoV-2 for primary sludge samples and seven positive cases for waste activated sludge samples collected from treatment plants located in Istanbul. Nemudryi et al. [31] obtained samples (pretreated) from the inlet of WWTP (Bozeman, Montana, United States) over a duration of 17 days to monitor increase or abatement of the spread of SARS-CoV-2 at the community level using RT-qPCR assay. SARS-CoV-2 was detected over the entire duration of the study but reduction was observed after the implementation of social isolation. Two sample collection methods (grab and composite sampling) were used, and composite sampling method was found to provide more reliable data [31]. Municipal wastewater samples (treated and untreated) and downstream river water samples were monitored by Rimoldi et al. [32] for the presence of SARS-CoV-2. Some raw samples tested positive, and all treated samples tested negative. Few river water samples also tested positive in real-time RT-PCR tests, possibly due to discharges without treatment or combined sewage overflows [32]. Bar-Or et al. [33] attempted to develop a virus concentration method using polyethylene glycol or alum. They have also collected raw sewage samples and WWTP samples from different locations in Israel, out of which several tested positive for SARS-CoV-2 using RT-qPCR assay.
In order to evaluate WBE as an early indication tool of COVID-19 spread, six municipal WWTPs were selected by Arora et al. [34] at Jaipur, India. Two sites were tested positive and four sites were tested negative and the areas served by the two positively tested WWTPs recorded increased number of patients soon. However, no positive results were found for treated wastewater of the two positively tested WWTPs where moving bed biofilm reactor and sequencing batch reactor were used for wastewater treatment confirming efficacy of the treatment process in removing viral particles. The applicability of WBE surveillance was also investigated by Kumar et al. [35] and for this purpose, a WWTP receiving effluent of a hospital treating COVID-19 patients located at Ahmedabad, Gujarat, India was selected. SARS-CoV-2 RNA was observed in the influent but not spotted in the effluent for samples collected on two different dates. The study suggested WBE as a potential tool for pandemic monitoring on the basis of higher viral loading in wastewater with increased number of COVID-19 patient. The reduction in viral RNA after upflow anaerobic sludge blanket and aeration pond treatment was also reported.
Hata et al. [36] evaluated the applicability of WBE in terms of detection limit by several PCR-based assays and they observed presence of SARS-CoV-2 in wastewater even when the number of cases per 100,000 was below 1. However, higher detection frequency was observed for cases greater than 10 per 100,000 population. The detection limit may change depending on the size of population served by WWTP. Although most of the studies on WBE are based on influent of WWTP, one recent study conducted by Balboa et al. [37] reported sludge thickener as a suitable monitoring/sampling spot for detecting SARS-CoV-2 due to the affinity of the enveloped virus towards biosolids. They have also investigated the presence of virus genetic material in the effluent of the WWTP and reported absence of SARS-CoV-2 RNA as they are retained by the sludge. However, after sludge treatment by thermal hydrolysis and anaerobic digestion, SARS-CoV-2 RNA was not detected in sludge leaving the plant. Curtis et al. [38] examined the difference between grab sampling and composite sampling in determining SARS-CoV-2 RNA concentration in wastewater and results showed good agreement between the grab and composite sampling. However, when the concentration data was used for viral load calculation and carrier prevalence estimation in a catchment population, discrepancy in results were obtained. The viral load and carrier prevalence estimation were underestimated when SARS-CoV-2 RNA concentration was determined using grab sampling technique. The variability in viral load and carrier prevalence estimates were also higher for grab samples.
In a study conducted by Chavarria-Miró et al. [39], SARS-CoV-2 RNA was detected in sewage sample collected from WWTP located in Barcelona, Spain 41 days ahead of the declaration of first COVID-19 case indicating the advantage of wastewater based surveillance for early detection of its emergence. Fongaro et al. [40] also reported that SARS-CoV-2 RNA was present in sewage collected from Florianopolis, Santa Catalina, Brazil much earlier than the first reported case. Based on wastewater analysis of Milan, Turin, and Bologna, La Rosa et al. [41] suggested that SARS-CoV-2 was present before the first clinically confirmed case due to fecal viral shedding by both symptomatic and asymptomatic carriers. Therefore, the monitoring through WBE can provide an early indication of emergence of COVID-19. Döhla et al. [42] observed 10 out of 66 wastewater samples as positive collected from toilets, showers, and washbasin of quarantined households. Sharif et al. [43] collected 78 wastewater samples (74 from polio surveillance sites, 3 from drains of affected area, 1 from drainage of quarantine center) out of which 21 samples were tested positive for the presence of SARS-CoV-2 RNA.
The studies related to coronavirus in wastewater and application of WBE for detection of infection, if any and also scale of infection within a community have highlighted several issues which requires special attention for successful implementation of WBE. The issues are explained point wise as (a) the several methods currently being used for SARS-CoV-2 detection produce inconsistent results (for example, positive result by one method and negative by another) [13], (b) variability in results were observed when grab and composite samples were compared, two investigations [31,38] suggested composite sampling technique to provide more reliable data, (c) although wastewater samples are collected in majority of the WBE related investigations, the study conducted by Balboa et al. [37] suggested WWTP sludge as more suitable due to the affinity of enveloped viruses towards solids, (d) the finding of no detection of SARS-CoV-2 due to low number of COVID-19 cases [20] expresses the necessity of research for finding detection limit and factors affecting detection limit, study conducted by Hata et al. [36] can provide some light in this regard, (e) although several investigations reported correlation being observed between SARS-CoV-2 in wastewater/sludge with confirmed COVID-19 cases, few reported lack of correlation [24,26]. Above all, WBE has the potential to detect infection within a community ahead of the onset of symptoms in COVID-19 positive individuals and even in case of asymptomatic individuals.
3. Persistence/survival of coronavirus in different types of wastewater
The articles describing the persistence/survival of coronavirus in different types of wastewater are presented in Table 2 . The survival of coronavirus depends upon the wastewater characteristics [49,50], type of virus [51,52], presence of suspended solids and organic matter [52], and temperature [[49], [50], [51], [52]].
Table 2.
Persistence/survival of coronavirus in different wastewater matrix.
| Virus studied | Wastewater type | Detection method | Study period | Important information and findings | References |
|---|---|---|---|---|---|
| Severe acute respiratory syndrome-associated coronavirus (SARS-CoV), BJ01 | Hospital wastewater, domestic sewage | Culture method, reverse transcription polymerase chain reaction | 14 days | SARS-CoV persisted for two days at 20 °C in hospital wastewater and domestic sewage SARS-CoV persisted for ≥14 days at 4 °C in hospital wastewater and domestic sewage |
Wang et al. [49] |
| Feline infectious peritonitis virus (FIPV) (ATCC-990) (an enteric feline coronavirus) | Wastewater treatment plant primary and secondary (activated sludge) effluent | Cell culture | 21 days | 99 and 99.9 % decrease in virus titer obtained in 1.6 and 2.4 days for primary effluent (filtered) at 23 °C 99 and 99.9 % decrease in virus titer obtained in 1.71 and 2.56 days for primary effluent (unfiltered) at 23 °C 99 and 99.9 % decrease in virus titer obtained in 1.62 and 2.42 days for secondary effluent (unfiltered) at 23 °C |
Gundy et al. [52] |
| Human coronavirus 229E (HCoV) (ATCC-740) | Wastewater treatment plant primary and secondary (activated sludge) effluent | Cell culture | 21 days | 99 and 99.9 % decrease in virus titer obtained in 1.57 and 2.35 days for primary effluent (filtered) at 23 °C 99 and 99.9 % decrease in virus titer obtained in 2.36 and 3.54 days for primary effluent (unfiltered) at 23 °C 99 and 99.9 % decrease in virus titer obtained in 1.85 and 2.77 days for secondary effluent (unfiltered) at 23 °C |
Gundy et al. [52] |
| Transmissible gastroenteritis (TGEV) (surrogate coronavirus) | Pasteurized settled sewage | Cell culture | 35 days for 4 °C and 21 days for 23−25 °C | Infectivity of TGEV decreased by −1.5 log10 per week at 25 °C and −0.3 log10 per week at 4 °C 99 % reduction in infectious titer was obtained in 9 days at 25 °C (the reduction followed first-order kinetics) 99 % reduction in infectious titer was obtained in 49 days at 4 °C (predicted) 99.9 % reduction in infectious titer was obtained in 14 days at 25 °C (predicted) (the reduction followed first-order kinetics) 99.9 % reduction in infectious titer was obtained in 73 days at 4 °C (predicted) |
Casanova et al. [51] |
| Mouse hepatitis (MHV) (surrogate coronavirus) | Pasteurized settled sewage | Cell culture | 35 days for 4 °C and 21 days for 23−25 °C | Infectivity of MHV decreased by −2 log10 per week at 25 °C and −0.2 log10 per week at 4 °C 99 % reduction in infectious titer was obtained in 7 days at 25 °C 99 % reduction in infectious titer was obtained in 70 days at 4 °C (predicted) 99.9 % reduction in infectious titer was obtained in 10 days at 25 °C (predicted) 99.9 % reduction in infectious titer was obtained in 105 days at 4 °C (predicted) |
Casanova et al. [51] |
| Murine hepatitis virus (MHV) (A59 strain), genus: coronavirus | Raw municipal wastewater | Cell culture | 48 h | For unpasteurized wastewater, T90 (time to reach 90% inactivation) was estimated as 13 ± 1 h at 25 °C For unpasteurized wastewater, T90 was estimated as 36 ± 5 h at 10 °C For pasteurized wastewater, T90 was estimated as 19 ± 8 h at 25 °C For pasteurized wastewater T90 was estimated as 149 ± 103 h at 10 °C Inactivation of MHV at both the temperature followed first-order kinetics for both pasteurized and unpasteurized wastewater |
Ye et al. [50] |
Wang et al. [49] investigated the survival of SARS-CoV in hospital wastewater and domestic sewage. They observed a significant effect of wastewater temperature on the survival of SARS-CoV. SARS-CoV survived for two days at the higher temperature of 20 °C, whereas, at the lower temperature of 4 °C, SARS-CoV persisted for ≥14 days in both hospital wastewater and domestic sewage. The study also hypothesized (based on the results on urine and phosphate-buffered saline) that the persistence of the virus will be higher in fluids containing salts as it helps to maintain osmotic pressure. The study suggested that the persistence of SARS-CoV is dependent on temperature, and the probability of survival of SARS-CoV is greater in wastewater with relatively low temperature [49].
The persistence of human CoV 229E (HCoV) and feline infectious peritonitis virus (FIPV) (an enteric feline coronavirus) in primary (filtered and unfiltered) and secondary effluent (unfiltered) of a WWTP was studied by Gundy et al. [52]. At 23 °C, 99 and 99.9 % decrease in virus titer for HCoV in filtered and unfiltered primary effluent took 1.57 and 2.35 days and 2.36 and 3.54 days, respectively. For secondary effluent, 99 and 99.9 % decrease in virus titer was obtained in 1.85 and 2.77 days at 23 °C. HCoV and FIPV survival was higher in unfiltered primary effluent than filtered effluent, which indicates that organic matter and suspended solids present in wastewater may protect and shield the virus [52]. At the same time, the settlement of solids from wastewater may also help in reduction. The tendency to adhere to solids is exerted by the hydrophobic nature of the envelope of coronavirus [52]. The above explanation is also supported by the higher persistence of HCoV in primary effluent in comparison with secondary effluent (both unfiltered).
The study on the persistence of two surrogate coronaviruses (transmissible gastroenteritis (TGEV) and mouse hepatitis (MHV)) was conducted by Casanova et al. [51]. They observed that these two viruses stayed infectious in pasteurized settled sewage for days to weeks at both the temperature studied (25 °C and 4 °C) (Table 2). The temperature showed a great influence on viral survival in settled sewage. At 25 °C, the time required for 99.9 % reduction in TGEV and MHV was 14 and 10 days, respectively, whereas the required time drastically increased to 73 and 105 days (predicted), respectively, at 4 °C. The pH of the settled sewage did not affect the viral infectivity much. They have also reported greater virus inactivation in pasteurized settled sewage in comparison with reagent grade water.
Ye et al. [50] investigated the persistence of murine hepatitis virus (MHV) (genus: coronavirus) in raw municipal wastewater (after screening and grit removal) at 10 °C and 25 °C (typical winter and summer temperature of wastewater). The time required for 90 % inactivation of MHV for unpasteurized wastewater was 13 ± 1 h for wastewater at 25 °C, whereas, the required time for 90 % inactivation increased to 36 ± 5 h at 10 °C. However, the time required for pasteurized wastewater was higher, 19 ± 8 h at 25 °C and 149 ± 103 h at 10 °C. The study anticipated that the long enough survival period of the model enveloped virus is of concern for stormwater overflow occurrence, wastewater treatment facilities, and wastewater intrusion event.
In this section, the survival of different kind of viruses related to coronavirus have been discussed. Although studies focused on survival of specifically SARS-CoV-2 in wastewater would be of particular interest, the persistence study of SARS-CoV-2 in wastewater is still rare. However, the methodology and approach described here can be adopted for future studies dealing with persistence of SARS-CoV-2 in wastewater. It is appeared that persistence of coronavirus in wastewater with low temperature may be much higher than wastewater with high temperature. In addition, the presence of organic matter, suspended solids may significantly influence the survival of coronavirus. Research focused on survival or persistence of SARS-CoV-2 in wastewater depending on temperature, presence of organic matter and suspended solids is the basic requirement to increase the reliability of the data used for WBE and also to understand the chances of transmission.
4. Inactivation by disinfectants and removal of coronavirus
Understanding the decay of SARS-CoV-2 during disinfection or removal by other treatment technologies will help in taking proper control measures to eliminate chances of transmission. The findings of three research articles (Table 3 ) [49,53,54], and one pre-print article [55] are summarized in this section.
Table 3.
Inactivation by disinfectants and removal process of coronavirus (human health related).
| Inactivation/removal process | Virus removed | Coronavirus detection method | Important information and findings | Reference |
|---|---|---|---|---|
| Disinfection by chlorine and chlorine dioxide | Severe acute respiratory syndrome-associated coronavirus (SARS-CoV), BJ01 | Culture method, reverse transcription polymerase chain reaction | Chlorine is found as more effective disinfectant than chlorine dioxide for inactivation of SARS-CoV 10 mg/L of chlorine resulted in 100 % inactivation of SARS-CoV (101.6TCID50a/mL) in 10 min, whereas, Escherichia Coli (4.6 × 105 cfub/L) and bacteriophage f2 (1.9 × 105 pfuc/L) were not inactivated completely 40 mg/L of chlorine resulted in 100 % inactivation of SARS-CoV (101.75TCID50a/mL) and 100% of Escherichia Coli (5.5 × 105 cfub/L) in 10 min, whereas, f2 phase (2.9 × 105 pfuc/L) was not inactivated completely |
Wang et al. [44] |
| Adsorption by Nano/microspheres of N-(2- hydroxypropyl)-3-trimethyl chitosan (HTCC-NS/MS) | Human coronavirus NL63 (HCoV-NL63) | Cell culture, reverse transcription quantitative polymerase chain reaction (RT-qPCR) | 10 mg/500 μl high-HTCCNS/MS in the samples yielded ∼415-fold decrease in TCID50a which corresponds to 99.7% reduction in the virus titer Desorption by 2 M NaCl solution resulted significant virus recovery HCoV-NL63 removal efficiency depends on adsorbent surface’s degree of cationization and is higher for more cationic HTCC-NS/MS The decrease in TCID50a was correlated with the amount of the adsorbent used |
Ciejka et al. [53] |
| Disinfection by sodium hypochlorite | Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) |
RT-qPCR | Although free chlorine of 6.5 mg/L was detected after 1.5 h contact time with 800 g/m3 of sodium hypochlorite disinfection of hospital sewage, SARS-CoV-2 ribonucleic acid (RNA) was again detected after 12 h of addition and free chlorine was not detectable The phenomenon may be observed due to release of SARS-CoV-2 viral RNA in septic tank that were embedded in stool particles For complete disinfection, 6700 g/m3 of sodium hypochlorite was used. As a result, free chlorine concentration ranged between 21−25 mg/L after 12 h addition and trichlormethane, tribromomethane, bromodichloromethane, and dibromochloromethane were formed |
Zhang et al. [54] |
TCID50 represents 50 % tissue culture infective dose.
cfu represents colony-forming unit.
pfu represents plaque-forming unit.
The disinfection of SARS-CoV in wastewater was analyzed by Wang et al. [49] using chlorine and chlorine dioxide. Chlorine concentration of 10 mg/L was able to inactivate SARS-CoV by 100 % in 10 min contact time, and residual chlorine was obtained as 0.4 mg/L. At the same conditions, inactivation rates of Escherichia Coli (E. Coli) and bacteriophage f2 were 14.29 and 18.32 %, respectively. Chlorine dioxide concentration of 40 mg/L resulted in 100 % inactivation of SARS-CoV in 5 min, and free residual chlorine was measured as 17.59 mg/L. Although the same dose of chlorine dioxide inactivated E. Coli by −100 %, only 23.46 % inactivation rate was found for bacteriophage f2 [49]. Ciejka et al. [53] attempted adsorptive removal of Human coronavirus (HCoV) NL63 and HCoV OC43 using Nano/microspheres of N-(2-hydroxypropyl)-3-trimethyl chitosan (HTCC-NS/MS). Although the adsorbent (10 mg/500 μl high-HTCCNS/MS in the viral samples) yielded ∼415-fold (i.e., 99.7 % reduction in virus titer) decrease in TCID50 (50 % tissue culture infective dose) and Log removal value of ∼3.1 (i.e., 99.92 %) for HCoV-NL63, it was ineffective in removing HCoV−OC43. The study also showed that HCoV-NL63 removal efficiency would be higher for more cationic HTCC-NS/MS [53].
Zhang et al. [54] investigated the presence of SARS-CoV-2 viral RNA by RT-qPCR in influent (after preliminary disinfection) and effluent (before discharge into drainage pipeline) of septic tanks of a hospital in China. Although the presence of SARS-CoV-2 viral RNA was not found in the influent sample, the effluent of septic tank tested positive for the presence of viral RNA, even after 2nd stage of disinfection with 800 g/m3 of sodium hypochlorite. This was possibly due to protection provided by suspended solids and organic compounds by embedding viruses during disinfection, which later on gets released into the aqueous phase [54]. The disinfection with 800 g/m3 of sodium hypochlorite resulted in free chlorine >6.5 mg/L after 1.5 h contact, but the presence of viral RNA was identified after 12 h of addition. The increase in disinfectant concentration to 6700 g/m3 resulted in free chlorine of 21−25 mg/L after 12 h of addition, and disinfection by-products, i.e., trichloromethane, tribromomethane, bromodichloromethane, and dibromochloromethane concentrations were detected in the range of 182–482, 0.6–3.1, 1.3–8.9 and ND (not detectable)-1.2 μg/L, respectively. Zhang et al. [55] studied wastewater samples of three hospitals located in China for the detection of SARS-CoV-2 viral RNA by RT-qPCR. The wastewater treatment sector of the first investigated hospital comprised of a series of treatment technologies (adjusting tank-bioreactor-secondary sedimentation-disinfection) and 255 copies/L of SARS-CoV-2 viral RNA was detected only in adjusting tank. The wastewater coming from the second hospital was treated by a series of treatment steps in the following order of adjusting tank-septic tank-adjusting tank-moving bed biofilm reactor (MBBR)-sedimentation-disinfection, in which SARS-CoV-2 viral RNA was detected in three units, first adjusting tank (633 copies/L), MBBR (not detected-505 copies/L), and sedimentation tank (not detected-2208 copies/L). In the third hospital treatment unit, which consisted of two units (preliminary disinfection tank followed by septic tank), SARS-CoV-2 viral RNA was detected in the range of 557–18744 copies/L only in septic tank when 800 mg/L sodium hypochlorite was used. However, SARS-CoV-2 was not detected when 6700 mg/L of sodium hypochlorite was applied [55].
Although investigations conducted by Wang et al. [49] and Ciejka et al. [53] depicting disinfection and removal of coronavirus are included, two later studies [54,55] are of particular interest as they dealt with hospital sewage disinfection to inactivate SARS-CoV-2. Considering the current scenario of worldwide infection, the evaluation of the commonly adopted municipal wastewater treatment technologies in removing SARS-CoV-2 is urgently needed to prevent transmission. For non-centralized hospital wastewater treatment facilities containing high amount of suspended solids, higher dosage of disinfectant may be required as suspended solids may shield and protect the virus. However, the application of high dosage may also form disinfection byproducts which will eventually create ecological risk [54].
5. Issues of serious concern, wastewater surveillance, and urgent research needs
Presence of SARS-CoV-2 in patient’s feces has been reported recently [[56], [57], [58]] which raised concern about possible transmission via fecal-oral [59] and aerosols-borne route [60]. Besides, during the wastewater treatment process, the infected stool particles present in wastewater may form aerosols and act as a transmission route [61]. The first study which put some light on the risk of sanitation workers due to the presence of novel coronavirus in wastewater is conducted by Zaneti et al. [62]. The quantitative microbial risk assessment study considering three stages of COVID-19 pandemic (moderate, aggressive and extreme) suggested that only in case of moderate scenario, the risk is below the WHO benchmark. Therefore, it is of utmost importance to ensure usage of personal protective equipment for handling untreated wastewater. Adaptation of decentralized wastewater treatment facilities, monitoring SARS-CoV-2 in wastewater, policy intervention, improved sanitation, and usage of point-of-use device may significantly help fighting battle against COVID-19 for low-income countries [63]. Increased monitoring on WWTPs and nearby areas is also needed to prevent the transmission from humans to wildlife [64]. To test the hypothesis of possible fecal-oral transmission route, a framework is suggested by Heller et al. [65] considering the persistence of virus and environmental dynamics. On the contrary, the task force of Diamond Princess, a commercial cruise ship (712 COVID-19 affected persons), reported that the transmission through wastewater is less probable [66].
The urgency of developing WBE as an indicator of the scale of infection and to control the spread of COVID-19 has been expressed by Christian Daughton, former scientist of the United States Environmental Protection Agency and pioneer of the WBE concept [67,68]. Other researchers ([17,61,[69], [70], [71], [72]]; Hata and Honda, 2020) also supported the emerging need for developing wastewater-based epidemiology to assess and manage the COVID-19 pandemic. The computational modeling study by Hart and Halden [16] identified WBE as a rapid, cost-effective, and robust tool for tracing COVID-19. Although some recent studies [18,19,[25], [26], [27], [28]] made serious efforts to correlate viral RNA concentration in wastewater with clinically confirmed COVID-19 cases, the application of WBE in combating COVID-19 pandemic is still in a preliminary stage. Currently, RNA based RT-PCR testing is the most commonly used method for SARS-CoV-2 detection in wastewater, which requires time and skilled personnel. The use of WBE can be accelerated by developing easy, paper-based devices to detect SARS-CoV-2 [73]. Electrospun nanofiber membranes which has the property of attracting virus genetic material can be used as a monitoring tool for WBE [74]. Above all, standardization of detection method, limit of detection, sampling point, sampling techniques are required for the success of WBE in forestalling COVID-19 and rapid progress of the field by compiling all the available findings.
Evaluation of the conventional wastewater treatment processes as well as advanced treatment technologies in removing SARS-CoV-2 require special attention [75]. Membrane bioreactors are suggested to work efficiently in this regard by filtering the coronaviruses attached to suspended solids [76]. The use of ultraviolet based advanced oxidation processes for the disinfection of SARS-CoV-2 containing wastewater should also be evaluated [76]. The inactivation rate of SARS-CoV using chlorine and chlorine dioxide has been reported by Wang et al. [44]. The performances of several other disinfection technologies commonly used by WWTPs (sodium hypochlorite, ultraviolet radiation, and ozone) need to be examined. Study considering disinfectant dose and contact time requirement with varying virus concentration in wastewater is needed. The measurement of residual chlorine after the disinfection process is of equal importance as the treated wastewater will eventually end up in rivers and lakes and may cause risk to aquatic ecosystems [77].
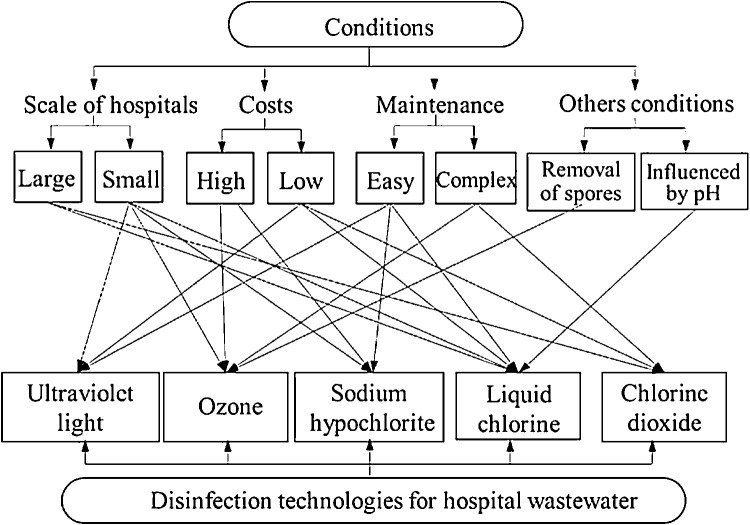
The general disinfection system of hospital wastewater consists of units in the following order of primary disinfection-sedimentation-dechlorination-moving bed biofilm reactor-disinfection [78] and the treatment series can be adopted to reduce the risk associated with hospital wastewater. Among the common disinfectants used for hospital wastewater (e.g., liquid chlorine, sodium hypochlorite, chlorine dioxide, ultraviolet radiation, and ozone), selection of suitable technology depends on various factors, i.e., investment and cost of operation, safety, the quantity of wastewater, the supply of disinfectants, level of operation management, etc. For the ease of selection of disinfection technology to treat hospital wastewater during COVID-19 pandemic, visual representation is shown in Fig. 1 [78].
Fig. 1.
Schematic for the ease of selection of disinfection technology for hospital wastewater depending on various factors. Reprinted with permission from {Wang et al. [78]}. Copyright {2020} Elsevier B.V.
6. Conclusions
The paper describes occurrence, survival, and disinfection/removal methods of coronavirus in wastewater with special emphasis on application of WBE to detect and forestall spread of COVID-19. Numerous investigations reported presence of SARS-CoV-2 RNA in municipal wastewater, sewage sludge, and hospital wastewater. Although fecal-oral transmission route is a point of concern, it is important to evaluate the stability of SARS-CoV-2 in infectious stage in different kinds of wastewater before drawing any conclusion. The knowledge of the scientific community regarding infectivity of SARS-CoV-2 in wastewater needs to be strengthened. The factors influencing persistence of SARS-CoV-2 in wastewater (for example, temperature, presence of organic matter and suspended solids) needs to be thoroughly studied for the implementation of WBE. The evaluation of the generally adopted municipal and hospital WWTP technologies in removing SARS-CoV-2 will surely help in answering questions related to transmission risk associated with wastewater. The investigations dealing with coronavirus in wastewater are heterogeneous, considering aim, methodology, and findings. As discrepancies in results were found among published articles, research on coronavirus related to wastewater needs to progress further for drawing concrete conclusions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Zhang Xiwang
References
- 1.WHO . 2003. SARS (Severe Acute Respiratory Syndrome)https://www.who.int/ith/diseases/sars/en/ (last accessed on 30 April 2020) [Google Scholar]
- 2.Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- 4.WHO . 2020. Pneumonia of Unknown Cause–China.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 5.WHO . Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV); 2020. Statement on the Second Meeting of the International Health Regulations.https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 6.Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh Mdon, Sall A.A., Schuchat A., Ungchusak K., Wieler L.H. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. 2015;1(6):735–746. doi: 10.1039/c5ew00125k. [DOI] [Google Scholar]
- 8.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-A scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wartecki A., Rzymski P. On the coronaviruses and their associations with the aquatic environment and wastewater. Water. 2020;12:1598. doi: 10.3390/w12061598. [DOI] [Google Scholar]
- 11.WHO . 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19; p. 2020. Interim guidance, 23 April. [Google Scholar]
- 12.Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8(5):e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green H., Wilder M., Collins M., Fenty A., Gentile K., Brittany L., Zeng T., Middleton F.A., Larsen D.A. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. MedRxiv. 2020 doi: 10.1101/2020.05.21.20109181. [DOI] [Google Scholar]
- 19.Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Edward H., Casanovas-massana A., Ko A.I., Malik A.A., Wang D., Wang M., Weinberger D.M., Omer S.B. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. MedRxiv. 2020 doi: 10.1101/2020.05.19.20105999. [DOI] [Google Scholar]
- 20.Ampuero M., Valenzuela S., Valiente-Echeverría F., Soto-Rifo R., Barriga G.P., Chnaiderman J., Rojas C., Guajardo-Leiva S., Díez B., Gaggero A. SARS-CoV-2 detection in sewage in Santiago, chile - preliminary results. MedRxiv. 2020 doi: 10.1101/2020.07.02.20145177. [DOI] [Google Scholar]
- 21.Wu F., Xiao A., Zhang J., Moniz K., Endo A., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., Matus M., McElroy K.A., Nagler J., Rhode S.F., Santillana M., Tucker J.A., Wuertz S., Zhao S., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. MedRxiv. 2020 doi: 10.1101/2020.06.15.20117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan E.H., Wang D., Wang M., Malik A.A., Zulli A.A., Peccia J. Aligning SARS-CoV-2 indicators via an epidemic model: application to hospital admissions and RNA detection in sewage sludge. MedRxiv. 2020 doi: 10.1101/2020.06.27.20141739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallejo J.A., Rumbo-Feal S., Conde-Pérez K., López-Oriona Á., Tarrío J., Reif R., Ladra S., Rodiño-Janeiro B.K., Nasser M., Cid Á., Veiga M.C., Acevedo A., Lamora C., Bou G., Cao R., Poza M. Highly predictive regression model of active cases of COVID-19 in a population by screening wastewater viral load. MedRxiv. 2020 doi: 10.1101/2020.07.02.20144865. [DOI] [Google Scholar]
- 24.Trottier J., Darques R., Mouheb N.A., Partiot E., Bakhache W., Deffieu M.S., Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of montpellier. France. MedRxiv. 2020 doi: 10.1101/2020.07.08.20148882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randazzo W., Cuevas-Ferrando E., Sanjuan R., Domingo-Calap P., Sanchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. MedRxiv. 2020 doi: 10.1101/2020.04.23.20076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu F., Xiao A., Zhang J.B., Gu X.Q., Lee W.L., Kauffman K., Hanage W.P., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T.B., Chai P.R. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. MedRxiv. 2020 doi: 10.1101/2020.04.05.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. MedRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema G., Heijnen L., Elsinga G., Italiaander R. Presence of SARS-Coronavirus-2 in sewage. MedRxiv. 2020 doi: 10.1101/2020.03.29.20045880. [DOI] [PubMed] [Google Scholar]
- 29.Kocamemi B.A., Kurt H., Hacıoglu S., Yaralı C., Saatci A.M., Pakdemirli B. First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. MedRxiv. 2020 doi: 10.1101/2020.05.03.20089417. [DOI] [Google Scholar]
- 30.Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. MedRxiv. 2020 doi: 10.1101/2020.05.12.20099358. [DOI] [Google Scholar]
- 31.Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. MedRxiv. 2020 doi: 10.1101/2020.04.15.20066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Moja L., Gismondo M.R., Salerno F. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. MedRxiv. 2020 doi: 10.1101/2020.05.01.20086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E.F., Paitan Y., Bitkover E., Berchenko Y., Kushmaro A. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. MedRxiv. 2020 doi: 10.1101/2020.04.26.20073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. MedRxiv. 2020 doi: 10.1101/2020.06.18.20135277. [DOI] [PubMed] [Google Scholar]
- 35.Kumar M., Patel A.K., Shah A.V., Rava J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. MedRxiv. 2020 doi: 10.1101/2020.06.16.20133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hata A., Honda R., Hara-Yamamura H., Meuchi Y. Detection of SARS-CoV-2 in wastewater in 1 Japan by multiple molecular assays implication for wastewater-based epidemiology (WBE) MedRxiv. 2020 doi: 10.1101/2020.06.09.20126417. [DOI] [Google Scholar]
- 37.Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for monitoring. MedRxiv. 2020 doi: 10.1101/2020.05.25.20112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis K., Keeling D., Yetka K., Larson A., Gonzalez R. Wastewater SARS-CoV-2 concentration and loading variability from grab and 24- hour composite samples. MedRxiv. 2020 doi: 10.1101/2020.07.10.20150607. [DOI] [Google Scholar]
- 39.Chavarria-miró G., Anfruns-estrada E., Guix S., Paraira M., Galofré B., Sánchez G., Pintó R.M., Bosch A. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. MedRxiv. 2020 doi: 10.1101/2020.06.13.20129627. [DOI] [Google Scholar]
- 40.Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., Schörner M.A., Barazzetti F.H., Christoff A.P., Oliveira L.F.V.D., Bazzo M.L., Wagner G., Hernández M., Rodriguez-Lázaro D. SARS-CoV-2 in human sewage in Santa Catalina, Brazil, November 2019. MedRxiv. 2020 doi: 10.1101/2020.06.26.20140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Rosa G., Mancini P., Ferraro G.B., Veneri C., Iaconelli M., Bonadonna L., Luca Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. MedRxiv. 2020 doi: 10.1101/2020.06.25.20140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Döhla M., Wilbring G., Schulte B., Kümmerer B.M., Diegmann C., Sib E., Richter E., Haag A., Engelhart S., Eis-Hübinger A.M., Exner M., Streeck H., Schmithausen R.M. SARS-CoV-2 in environmental samples of quarantined households. MedRxiv. 2020 doi: 10.1101/2020.05.28.20114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., Ahmad J., Safdar R.M., Angez M., Alam M.M., Rehman L., Mujtaba G., Hussain J., Ali J., Akthar R., Malik M.W., Baig Z.I., Rana M.S., Usman M., Ali M.Q., Ahad A., Badar N., Umair M., Tamin S., Ashraf A., Tahir F., Ali N. Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network : an epidemiological gateway to an early warning for COVID-19 in communities. MedRxiv. 2020 doi: 10.1101/2020.06.03.20121426. [DOI] [Google Scholar]
- 44.Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.U., Wang G.J., Qiu Y.H., Wu H.H., Li J.W. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods. 2005;128(1–2):156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bibby K., Viau E., Peccia J. Viral metagenome analysis to guide human pathogen monitoring in environmental samples. Lett. Appl. Microbiol. 2011;52(4):386–392. doi: 10.1111/j.1472-765X.2011.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredin E. First detection of Sars-Cov-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;37 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 51.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10–14. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- 53.Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D., Yang Y., Huang X., Jiang J., Li M., Zhang X., Ling H., Li J., Liu Y., Li G., Li W., Yi C., Zhang T., Jiang Y., Xiong Y., Hu Z., Wang X., Deng S., Zhao P., Qu J. SARS-CoV-2 spillover into hospital outdoor environments. MedRxiv. 2020 doi: 10.1101/2020.05.12.20097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., et al. Washington state 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Hu Y., Yu Y., Zhang X., Li B., Wu J., Li J., Wu Y., Xia X., Tang H., Xu J. Positive result of Sars-Cov-2 in faeces and sputum from discharged patient with COVID-19 in Yiwu. China. J. Med. Virol. 2020:1–10. doi: 10.1002/jmv.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T., Cui X., Zhao X., Wang J., Zheng J., Zheng G., Guo W., Cai C., He S., Xu Y. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 2020;92:909–914. doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhowmick G.D., Dhar D., Nath D., Ghangrekar M.M., Banerjee R., Das S., Chatterjee J. Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. Npj Clean Water. 2020;3:32. doi: 10.1038/s41545-020-0079-1. [DOI] [Google Scholar]
- 60.Arslan M., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8(4):104006. doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaneti R.N., Girardi V., Spilki F.R., Mena K., Westphalen A.P.C., Colares E.R.D.C., Pozzebon A.G., Etchepare R.G. QMRA of SARS-CoV-2 for workers in wastewater treatment plants. MedRxiv. 2020 doi: 10.1101/2020.05.28.20116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: Any sustainable preventive measures to curtail the scourge in low-income countries? MedRxiv. 2020 doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franklin A.B., Bevins S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taskforce for the COVID-19 Cruise Ship Outbreak Environmental sampling for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during a coronavirus disease (COVID-19) outbreak aboard a commercial cruise ship. MedRxiv. 2020 doi: 10.1101/2020.05.02.20088567. [DOI] [Google Scholar]
- 67.Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murakami M., Hata A., Honda R., Watanabe T. Letter to the editor: wastewater-based epidemiology can overcome representativeness and stigma issues related to COVID-19. Environ. Sci. Technol. 2020;54:5311. doi: 10.1021/acs.est.0c02172. [DOI] [PubMed] [Google Scholar]
- 70.Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lodder W., Husman A.M.D.R. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farkas K., Hillary L.S., Malham S.K., McDonald J.E., Jones D.L. Wastewater and public health: the potential of wastewater surveillance for monitoring COVID-19. Curr. Opin. Environ. Sci. Health. 2020;17:14–20. doi: 10.1016/j.coesh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- 74.Venugopal A., Ganesan H., Raja S.S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K.S.M., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr. Opin. Environ. Sci. Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. 2020;6:1213–1216. doi: 10.1039/d0ew90015j. [DOI] [Google Scholar]
- 77.Zhang H., Tang W., Chen Y., Yin W. Disinfection threatens aquatic ecosystems. Science. 2020;368(6487):146–147. doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]