Abstract
Dengue fever is an extremely common infection in Indonesia, with an estimated 77.96 cases / 100.000 person-years in 2016. However, in 2020 the threat of extremely contagious SARS CoV-2 or COVID-19 in Indonesia emerged, which has infected more than 100.303 persons by July 28, 2020, and expected to grow exponentially except if very strict measures were implemented. There are similar symptoms and laboratory findings with both dengue fever and COVID-19, paving way to dangerous possibilities such as incorrect or delayed initial treatment. This is especially worrisome in the context of the pandemic, where COVID-19 positive patients must be promptly identified, isolated and contact-traced, and eluded diagnosis might possibly endanger communities and healthcare workers.
We present cases of patients who initially presented with symptoms and laboratory findings of dengue fever, including positive NS1 and/or IgM serology results. During the course of illness these patients fail to show characteristic dengue symptoms, and two cases begin to show respiratory symptoms. Upon further investigation with chest X-ray or contact tracing, the patients were indicated for COVID-19 swab test, which yielded positive results. Repeat dengue IgM/IgG returned positive in one case, suggesting dengue coinfection; however in all other cases, the repeat testing returned negative, suggesting that the initial serologies were false positives. These cases highlight the importance of comprehensively studying patients with apparent dengue fever symptoms and serology, and using the appropriate adjuvant test according to the course of the disease, since a serological overlap may exist between the two diseases.
Keywords: COVID-19, Dengue fever, Serology, Case series
Introduction
Dengue, a viral infection caused by the DENV virus, is a major public health concern in Asian countries including Indonesia, with manifestation ranging from mild dengue fever (DF) to severe and life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [1]. In 2017, there were 59,047 and 444 of DHF cases and DHF-associated deaths in Indonesia with 22.55 per 100,000 person-years and 0.75 % of IR and CFR, respectively [2]. Coinciding with the peak of the dengue season- during the rainy seasons of November to April [3] - is the recent, yet significant, outbreak of the novel coronavirus SARS CoV2, or COVID-19. As of July 28, 2020, there are 100.303 confirmed COVID-19 cases in Indonesia, with 170 deaths; numbers are projected to increase [4].
The similarity of symptoms between dengue and COVID-19 often led to confounded diagnosis, with both infections presenting with high fever and flu-like symptoms. Similarly, routine blood test for preliminary screening often show similar patterns, with the characteristic thrombocytopenia in dengue often appearing as well in COVID-19 infection. Physicians often rely on serological tests to confirm dengue and newly disseminated serological test kit to rapidly diagnose COVID-19, but even on this front, there seems to be a serological overlap between the two diseases. This case series aim to show how COVID-19 patients often present with dengue-like symptoms and initially show false positive dengue serology results. A case of dengue and COVID-19 coinfection is also shown, showing the real possibility of coinfection in dengue-endemic countries, and the importance of utilizing the most appropriate serological test according to the course of the disease.
Case Report 1
A 53-year old male patient presented with main complaint of high fever for two days, malaise, and sore throat. There are no complaints of cough, shortness of breath, or nasal congestion. He had a history of travel from Jakarta two days before the onset of symptoms. There are personal history of type II Diabetes under good control. Initial physical exam revealed fever and positive torniquet sign (15 petechiae per square inch). Blood pressure, heart rate and EKG were normal.
In the initial screening the patient presented with thrombocytopenia (Platelet 65.000/μL). leukopenia/lymphocytopenia (WBC 4000/μL, Lymphocyte 121%, Neutrophil 74.6 %; Neutrophil-to-lymphocyte ratio 6.16). HBA1c was 6.9. Serology examination showed positive NS1 dengue results. With these results, the patient chose to undergo outpatient treatment and rest at home with daily home-service routine blood examination, given symptomatic medications including acetaminophen, prophylactic antimicrobials and high-dose vitamin C, and instructed on adequate fluid intake.The patient remained febrile and lethargic despite the normalization of his lab results. On the 7th day after the onset of symptoms, the patient underwent both dengue serology exam and COVID-19 total antibody rapid test. (SARS-CoV-2 Antibody Test, Guangzhou Wondfo Biotech Co Ltd.) Dengue serology was positive for both IgM and IgG. COVID-19 rapid test showed negative result, and at-home treatment was continued.
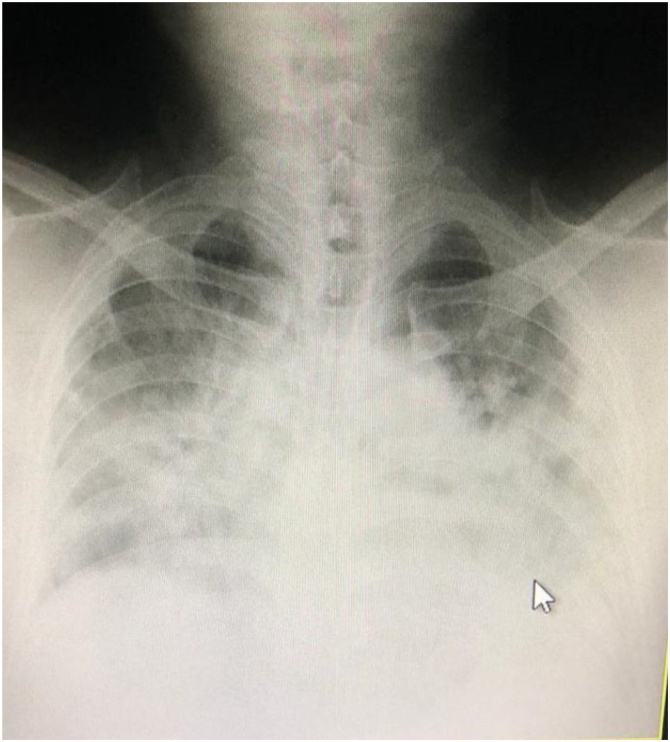
On the 9th day of symptoms, the patient suddenly exhibited shortness of breath and was admitted to the ICU with 92 % oxygen saturation, and given high-flow O2 supplementation with non-rebreathing mask. EKG was normal and routine blood work was normal except for persisting lymphocytopenia. (Neutrophil-to-lymphocyte ratio 4.13) Chest sounds showed rhonchi in all lung fields, but more prominent in the basal area. Chest X-ray showed opacity especially in the basal area of the lung. The patient was referred to a facility with CT scan; upon arrival, the saturation had dropped to 87 %, and chest CT showed ground glass opacity in all lung fields suggestive of SARS-CoV-2 infection. The patient was diagnosed with ARDS and suspected COVID-19 infection, and admitted to the Isolation ICU for intubation and ventilation (Fig. 1).
Fig. 1.
Chest Xray of Case 1.
The patient’s condition deteriorated rapidly. Blood work taken approximately 6 h after mechanical ventilation commenced show leukopenia/lymphocytopenia (WBC 2800/μL, Lymphocyte 13.4 %, Neutrophil 73.4 %; Neutrophil-to-lymphocyte ratio 5.477), hs-CRP 15 mg/L. The patient died a day after admission from severe pneumonia, septic shock, and respiratory failure, and interred according to the COVID-19 protocol. A postmortem swab was taken for confirmation of SARS-CoV-2 infection, and yielded positive results a day later. A RT-PCR exam was later performed on the same sample for dengue, chikungunya, and Zika viruses, and yielded negative results. Repeat blood sample taken from the patient, however, showed positive results for both IgM and IgG. Since 10 days have passed since the onset of the illness until the blood sample used for PCR was taken; the RT-PCR result was deemed inaccurate, and the initial NS1 result combined with positive IgM and IgG suggested that the case involved coinfection with both dengue and COVID-19.
Case Report 2
A 24-year-old male patient, a son of Case #1, presented with no discernable symptoms; however, upon the death of the his father and confirmation of SARS-CoV-2 infection with PCR, the patient was included in a cohort screened with swab test for SARS-CoV-2 PCR. Results came back two days later, showing a positive result. An RT-PCR for dengue, Zika virus and chikungunya was performed on the same sample, all of which yielded negative results.
Blood samples taken for further examination of the patient showed no abnormalities; interestingly, the dengue IgM and IgG yielded positive results. Chest X-ray showed normal findings. The patient was instructed to complete two weeks of self-isolation at a quarantine facility, and treated with vitamin C and prophylactic antimicrobials. The patient remained completely asymptomatic until the end of the two-week period. A swab sample for SARS-CoV-2 PCR was taken at the end of the quarantine period, which returned negative. The patient was declared cured after two consecutive negative PCR results. Another blood sample taken at the end of the quarantine period again returned within normal ranges; IgM and IgG dengue were all negative, suggesting that the initial seroconversion results were a false positive.
Case Report 3
A 26-year old female patient presented to the polyclinic with main complaint of high fever for three days. This was accompanied with malaise and tiredness, and no symptoms of cough or shortness of breath. There are no personal history of other diseases. The patient had taken paracetamol for her fever at home, which had somewhat limited effect. She had a history of travel from Jakarta. Physical examination show fever and positive Torniquet sign (15 petechiae per square inch). In the initial screening she had anemia (Hgb 12.1 g/dL) and thrombocytopenia (Platelet 95.000/μL). Leukocyte count is normal (WBC 7800/μL, Neutrophil 66.0 %, Lymphocyte 23.5 %, Neutrophil-to-lymphocyte ratio 2.8) She has C-reactive protein level of 0.07 mg/dL. Serology examination showed positive IgM dengue results. Upon these results, the patient was initially admitted to the hospital with a diagnosis of DHF grade I, and was given supportive care and fluid resuscitation.
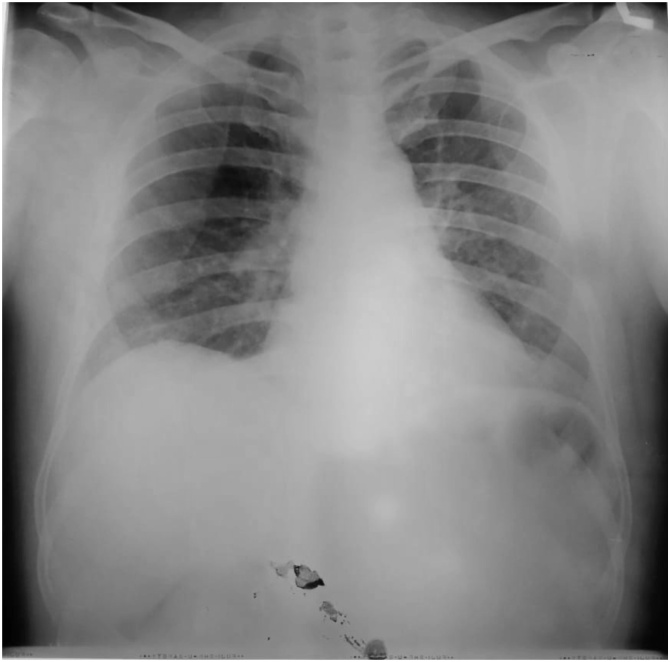
Two days after admittance, the patient began to show respiratory symptoms of cough and shortness of breath. Chest x-ray was indicative of pneumonia, and the patient was indicated for a SARS-CoV-2 swab test and viral RT-PCR. Results two days later was positive for SARS-CoV-2 infection and the patient was readmitted into the isolation ward, with continued supportive care regimen. The patient was discharged on the 12th day of isolation upon two consecutive negative RT-PCR results, with instructions to continue two weeks of self-isolation at home (Fig. 2).
Fig. 2.
Chest Xray of Case 3.
A repeat dengue serology test for both IgM and IgG was performed on the patient after two weeks of home isolation, during which the patient remained symptom-free, and yielded negative results.
Case Report 4
A 42-year old male patient presented to the polyclinic with main complaint of high fever for four days, malaise, palpitations and no cough or shortness of breath. There was no personal history of other diseases. He originally had no history of known contact or travel to endemic areas. Initial physical exam show fever and positive Torniquet sign (15 petechiae per square inch). Blood pressure is normal, heart rate is slightly elevated and the EKG showed sinus tachycardia. Chest sounds were normal.
In the initial screening the patient presented with thrombocytopenia (Platelet 80.000/μL). leukopenia/lymphocytopenia (WBC 3000/μL, Lymphocyte 16.1 %, Neutrophil 75 %; Neutrophil-to-lymphocyte ratio 4.65) Serology examination showed positive IgM and IgG dengue results. With these results, the patient was initially admitted to the hospital with a diagnosis of DHF grade I, and was given supportive care and fluid resuscitation.
Two days into his hospitalization it was discovered an acquaintance he briefly chatted with three days before he began showing symptoms, had suddenly showed characteristic symptoms of COVID-19 with high fever and acute respiratory distress, and was deteriorating rapidly. This acquaintance was later found to belong to a transmission cluster related to a religious meeting in Java.
Chest radiograph was then performed on the patient, showing normal findings. The patient was nevertheless indicated for swab test and viral RT-PCR, with the results issued two days later being positive for SARS-CoV-2 infection.
The patient was admitted into the isolation ward and received continued supportive care regimen. He was discharged in good condition on the 14th day of isolation after two consecutive negative RT-PCR results, and was instructed to continue two weeks of self-isolation at home. Repeat dengue serology tests on the patient a week after discharge yielded normal results, including negative results for both IgM and IgG.
Case Report 5
A 22-year-old male patient presented to the polyclinic with main complaint of a history of high fever for four days and productive coughing for two days. The patient had a history of recent travel from Jakarta, where he had been studying in college. Symptoms began with a general feeling of malaise two days after arrival from Jakarta. High, sudden fever appeared two days later, accompanied with retroorbital pain and two days later, productive coughing that bothered sleep. He also complained of a loss of sense of smell and lack of appetite. The patient didn’t initially undergo any physical examination, since he was assigned to a telemedicine service.
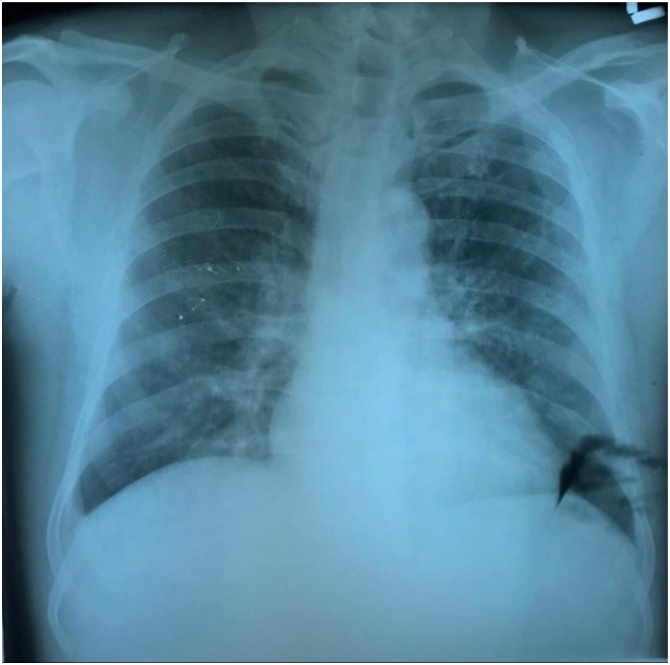
The patient was instructed to undergo mobile laboratory screening and in the initial blood examination the patient presented with thrombocytopenia (Platelet 120.000) and leukopenia/mild lymphocytopenia (WBC 4490/μL, Lymphocyte 19 %, Neutrophil 67 %; Neutrophil-to-lymphocyte ratio 3.52). Serology examination showed positive IgM dengue results. Rapid antibody test for COVID-19 showed negative results. Chest X-ray findings are suggestive of pneumonia, and a nasopharyngeal swab sample are taken. The patient was prescribed vitamin C, antimicrobials, and mucolytic and monitored daily (Fig. 3).
Fig. 3.
Chest Xray of Case 5.
The results from the swab returned positive two days later, and the patient elected to be housed in a quarantine facility. RT-PCR for dengue, zika virus and chikungunya from the sample was negative. By this time the patient was no longer febrile, and his respiratory conditions began to continuously improve. The patient was discharged after 16 days of quarantine after two consecutive negative SARS-CoV-2 PCR results. Follow-up blood test showed normal findings, including negative IgM and IgG results. Therefore, it is concluded that the initial IgM seroconversion was a false positive.
Discussion
The diagnosis of dengue is normally established in practice through characteristic symptoms such as saddleback-pattern fever, and laboratory findings such as thrombocytopenia and signs of plasma leakage. Currently, serological testing is also used to confirm dengue, to some extent being able to determine the onset of dengue infection [5]. The NS1 glycoprotein is produced by all flaviviruses, secreted from mammalian cells, and often used to make early diagnosis of dengue virus infection, as rapid testing kits for it are now available. Acute phase (IgM) dengue antibodies can be detected from serum, blood or saliva from dengue patients after five days since the onset of fever, although the production varies considerably among patients [6]. According to Pan American Health Organization (PAHO) guidelines,by day five of illness, 80 % of cases have detectable IgM antibody, and by day six to ten, 93–99 % of cases have detectable IgM that may persist for over 90 days [7,8].
ELISA for anti-dengue IgG detection is currently widely used for classifying cases based on the kind of infection, primary or secondary. Some protocols use serum dilutions to titer anti-dengue IgG. In others, a ratio of IgM/IgG higher than 1.78 is considered a marker of primary infection, and less is considered a marker of secondary infection [9]. Combining the use of different serological markers, together with dengue PCR if available, greatly enhances the accuracy of dengue diagnosis. For diagnosis using a single serum specimen, a combination of NS1 antigen and IgM ELISA has been proven to be more accurate than one single assay; complimenting the combined test with nucleic-acid based test gives further benefit, especially in dengue-endemic areas where secondary infections are common [10].
Rapid NS1 antigen and NS1 ELISA seems especially promising to specifically confirm the diagnosis of dengue, especially in the early stages. The sensitivity and specificity of rapid NS1 antigen were 55.5 % and 92 %, respectively- however, the disadvantage comes to light in cases where the patient present after more than three days of fever, since the accuracy of NS1 antigen test in the subacute phase decreases considerably. In the study by Solanke et al. [11], the positivity of rapid NS1 antigen on days 4–6 was 39.4 %, decreasing further to 13.1 % on days 7−9. However, in the current resource-limited emergency setting, proper NS1 testing could be the key in differentiating between true dengue infection, false positive infections and possibly coinfections, especially since NS1 seems to have no cross-reaction even with other flaviviruses [12].
Cross-reactivity of both dengue IgM and IgG, however, is known to exist with malaria and leptospirosis; there seems to be cross-reactivity with other flaviviruses as well, such as Zika and Japanese encephalitis [[13], [14], [15]]. The cross-reactivity with flaviviruses is expected as dengue virus and other flaviviruses share a large degree of structural and sequence homology; the same phenomenon occurring with malaria is speculated to result from the elicitation of cross-reactive antibodies or other immune responses that infer cross-protection, or at least partial cross-protection, against symptomatic and severe dengue [15].
Meanwhile, the diagnosis of SARS-CoV-2 infections still rely on viral RNA detection through RT-PCR. Limited availability of reagents and resources in several areas made this method impractical for mass or rapid testing, and therefore serological assays have begun to be used for preliminary diagnosis, although it is not recommended due to lack of commercial reagents that have been vetted by trials and regulatory bodies. The current official Indonesian approach is to use rapid testing as a mass screening tool, although ideally a method with higher sensitivity is used for that purpose [16]. Two cases reported in this case series initially had negative COVID-19 rapid test results, although their PCR results later turned out positive. In one patient, rapid test results were negative on the 7th day of symptom onset. Post-mortem swab from this patient was later found to be positive for SARS-CoV2. With further reports of viral redetection in “cured” patients, this presses for more research on the exact dynamics of immunity towards COVID-19.
This difficulty in distinguishing dengue and COVID-19 is not the first to be reported in literature. Two Singaporean patients were also reported to present with false-positive result from rapid dengue serologic testing, and later confirmed to have SARS-CoV-2 [17]. The same occurred in our case series. Symptomatic patients presented with fever and thrombocytopenia, characteristic of dengue fever, coincidentally at the peak of its season during the COVID-19 outbreak. Classic symptom of cough only exists in one patient upon admission; chest radiograph was normal for all other patients upon admission, possibly calling for a chest CT whenever the facility is available to allow for more sensitive detection of lung abnormalities.
Dengue IgM/IgG test turned out positive in all patients, at which point diagnosis of dengue were often established. The patients only underwent SARS-CoV-2 swab test / RT-PCR upon development of cough during treatment; discovery of known contact with a positive case; or the progression of sudden respiratory distress. Swab tests for all patients later turned out positive. In one patient, initial positive NS1 result and persistent IgM/IgG results suggested a coinfection of dengue and COVID-19. However, in all other patients, IgM/IgG were no longer positive during follow-up testing and dengue PCR results were negative, indicating that the initial IgM/IgG seroconversion were false positives.
These cases add a layer of complexity towards COVID-19 management in dengue endemic areas. Initial missed COVID-19 diagnosis can lead to significant losses, such as exposure of medical workers and other patients to COVID-19 outbreak; failure to impose distancing and isolation measures when patients were “only” diagnosed with dengue fever; delayed contact tracing; or potentially incorrect treatment approach that may lead to harm. It is important for all healthcare professionals to understand that false positive dengue serology results can be a possibility in COVID-19 cases, and approach suspected dengue cases with this in mind.
It is also important to identify the possibility of dengue and COVID-19 coinfection, since it may possibly exert adverse outcome in patients, although any definitive research has yet to be done on this front. The likely key to differ between false-positive seroconversion and co-infection is the NS1 antigen testing, which is highly specific for dengue fever; however, accurate detection of NS1 requires sampling in the very early stages of the disease, which might not be possible for patients who already presented with more than three days of fever. Similarly, dengue PCR decreases in accuracy the further the course of the disease. At this point, the most prudent course might be to screen for COVID-19 infection in all patients suspected of dengue fever; start COVID-19 treatment, monitoring and quarantine sooner than later; and also follow-up daily for symptoms and fluid balance with frequent blood examinations to monitor plasma dynamics and possible plasma leakage in case of a dengue coinfection.
It is very important to correctly diagnose the two infections and understand how they affect each other, both through the clinical care and epidemiological point of view. There also needs to be further study on the cause and significance of COVID-19 and dengue cross-reactivity, and the dissemination of that information to scientists, physicians, and decision makers to refine our current knowledge and guidelines on COVID-19 treatment.
Conclusion
Similar symptoms and laboratory findings between COVID-19 and dengue fever pose a diagnostic challenge, especially in countries such as Indonesia, where dengue infection is extremely common. This necessitates more alertness for COVID-19 infection even when patients present with “characteristic” symptoms and findings of dengue fever. These cases show the possibility of false positive dengue serology in COVID-19 patients, necessitating physicians to rule out dengue IgM/IgG serology as a definitive test for confirmation of dengue fever.
We argue that in the context of the recent outbreak, there is an urgent need for highly accurate yet accessible diagnostic test for SARS-CoV-2, allowing all patients with signs of dengue fever to be accurately screened. In addition, not all patients initially present with the characteristic dry cough or shortness of breath, and chest radiographs of many patients were normal upon admission, possibly encouraging the use of chest CT when the facility is available to diagnose for COVID-19 more accurately.
Authorship contributions
Category 1
Conception and design of study: GJ Kembuan
Acquisition of data: GJ Kembuan
Analysis and/or interpretation of data: GJ Kembuan
Category 2
Drafting the manuscript: GJ Kembuan
Revising the manuscript critically for important intellectual content: GJ Kembuan
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed): GJ Kembuan
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of patients.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship
Declaration of Competing Interest
The following authors have no financial disclosures: GJK
References
- 1.Halstead S.B. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 2.Harapan H., Michie A., Mudatsir M. Epidemiology of dengue hemorrhagic fever in Indonesia: analysis of five decades data from the National Disease Surveillance. BMC Res Notes. 2019;12:350. doi: 10.1186/s13104-019-4379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumarmo Dengue hemorrhagic fever in Indonesia. SE Asian J Trop Med. 1987;18(3):269–274. [PubMed] [Google Scholar]
- 4.Ministry of Health Republic of Indonesia; 2020. COVID-19: situasi kasus Indonesia.https://infeksiemerging.kemkes.go.id/ [Internet] [cited 19 April 2020]. Available from: [Google Scholar]
- 5.Tontulawat P., Pongsiri P., Thongmee C., Theamboonlers A., Kamolvarin N., Poovorawan Y. Evaluation of rapid immunochromatographic NS1 test, anti-dengue IgM test, semi-nested PCR and IgM ELISA for detection of dengue virus. SE Asian J Trop Med. 2011;42(3):570–578. [PubMed] [Google Scholar]
- 6.De Paula S.O., de Fonseca B.A.L. Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz J Infect Dis. 2004;8(6):390–398. doi: 10.1590/s1413-86702004000600002. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization . Scientific Publication No.: 548; 1994. Dengue and dengue hemorrhagic fever in the Americas: guidelines for Prevention and Control. [Google Scholar]
- 8.Lam S.K., Devi S., Pang T. Detection of specific IgM in dengue infections. SE Asian J Trop Med. 1987;18:532–538. [PubMed] [Google Scholar]
- 9.Guzman M.G., Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis. 2004;8(2):69–80. doi: 10.1016/j.ijid.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Teoh B., Sam S., Tan K. The use of NS1 rapid diagnostic test and qRT-PCR to complement IgM ELISA for improved dengue diagnosis from single specimen. Sci Rep. 2016;6:27666. doi: 10.1038/srep27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solanke V.N., Karmarkar M.G., Mehta P.R. Early dengue diagnosis: role of rapid NS1 antigen, NS1 early ELISA, and PCR assay. Trop J Med Res. 2015;18:95–99. [Google Scholar]
- 12.Lapphra K., Sangcharaswichai A., Chokephaibulkit K. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2008;60:387–391. doi: 10.1016/j.diagmicrobio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Priyamvada L., Quicke K.M., Hudson W.H. Dengue antibodies potently cross-react with ZIKV. Proc Natl Acad Sci U S A. 2016;113(28):7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton-Triviño N., Montaña D., Castellanos J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev Saude Publ. 2009;10(2):299–307. doi: 10.1590/s0124-00642008000200010. [DOI] [PubMed] [Google Scholar]
- 15.Bygbjerg I.C., Simonsen L., Schiøler K.L. Elimination of Falciparum Malaria and emergence of severe dengue: an independent or interdependent phenomenon? Front Microbiol. 2018;9:1120. doi: 10.3389/fmicb.2018.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Association of Indonesian Clinical Pathologists; 2020. Alur pemeriksaan rapid test SARS-CoV-2.https://www.pdspatklin.or.id/post/alur-px-rapid-test-covid-19-pds-patklin [cited 4 April 2020]. Available from: [Google Scholar]
- 17.Yan G., Lee C.K., Lam L.T.M. Covert COVID-19 and false positive dengue serology in Singapore. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]