Abstract
Background
The aim of this study was to report the experience of one of the major “hubs” for vascular surgery in Lombardy, Italy, during the first 7 weeks after total lockdown due to COVID-19 pandemic.
Methods
Data from all patients treated at our Department since the decision of the regional healthcare authorities of Lombardy to centralize surgical specialties creating a hub/spoke system (March 9, 2020) were prospectively collected and compared with a retrospectively collected cohort from the same period of year 2019. Primary study end point was defined as primary clinical success. Secondary end points were defined as in-hospital mortality and/or any in hospital major adverse event or lower limb amputation.
Results
One hundred sixteen patients were treated (81 men, 70%; median age: 71 years, IQR 65–81). Thirty-two patients (28%) were addressed from spoke hospitals directly referring to our hub, 19 (16%) from hospitals belonging to other hub/spoke nets, 48 (41%) came directly from our emergency department, and 17 (15%) were already hospitalized for COVID-19 pneumonia. Acute limb ischemia was the most observed disease, occurring in 31 (26.7%), 12 (38.7%) of whom were found positive for COVID-19 pneumonia on admission, whereas 3 (9.7%) became positive during hospitalization. Chronic limb ischemia was the indication to treatment in 24 (20.7%) patients. Six (5.2%) patients underwent primary amputation for irreversible ischemia. Aortic emergencies included 21 cases (18.1%), including 13 (61.9%) symptomatic abdominal aortic or iliac aneurysms, 4 (19.0%) thoracoabdominal aortic aneurysms, 2 (9.5%) cases of acute type B aortic dissection (one post-traumatic). Seventeen (14.7%) patients were admitted for symptomatic carotid stenosis (no COVID-19 patients); all of them underwent carotid endarterectomy. Seventeen (14.7%) cases were treated for other vascular emergencies. Overall, at a median follow-up of 23 ± 13 days, primary clinical success was 87.1% and secondary clinical success was 95.9%. We recorded 3 in-hospital deaths for an overall mortality rate of 2.6%. Compared with the 2019 cohort, “COVID era” patients were older (72 vs. 63 years, P = 0.002), more frequently transferred from other hospitals (44% vs. 21%, P = 0.014) and more frequently with decompensated chronic limb threatening ischemia (21% vs. 3%, P = 0.015); surgical outcomes were similar between the 2 cohorts.
Conclusions
Since its appearance, SARS-CoV-2 has been testing all national healthcare systems which founds themselves facing an unprecedented emergency. Late referral in the pandemic period could seriously worsen limb prognosis; this aspect should be known and addressed by health care providers. Vascular surgical outcomes in pre-COVID and COVID era were comparable in our experience.
Introduction
The first case of COVID-19 infection in Italy was confirmed during the night of February 20th, 2020, in a small town of Lombardy, a district in Northern Italy with 10 million inhabitants. In the week that followed, several nearby towns experienced a very rapid increase in the number of detected cases, which rose to over 530 positive samples by February 28th and 5,830 by March 8th, clearly appointing Lombardy as the epicenter of the Italian epidemic.
Owing to the exponential growth of infected cases and deaths, total lockdown of the entire Italian country was decided by the Prime Minister on March 9th, 2020, imposing national quarantine, restricting the movement of the population except for necessity, work, and health circumstances. The same day, the Regional Authority of Lombardy remodeled the hospitalization system to allocate appropriate resources to treat COVID-19 patients, increase the number of intensive care unit (ICU) beds, and identify “hub” hospitals on which certain highly specialized medical activities (major trauma, neurosurgery, interventional neuroradiology, interventional cardiology, cardiac surgery, and vascular surgery) should have been concentrated to ensure care of urgent cases and diseases whose treatment could not be procrastinated.
For vascular surgery, identified hubs were IRCCS Centro Cardiologico Monzino (Milan), Poliambulanza Foundation Hospital (Brescia), A.O. Ospedale Civile di Legnano (Legnano), and IRCCS San Raffaele Hospital (Milan). The remaining centers of vascular surgery in Lombardy, acted as spokes and, were mainly redirected to the care of COVID-19 patients.
In accordance with this resolution, hub hospitals had to guarantee 24/7 evaluation of all patients presenting with known or suspected vascular disease, taking charge of all cases needing urgent treatment, or deemed unable to wait for more than 30 days. For this purpose, hubs are required to be able to count on several available teams (each composed by at least two surgeons, one anesthesiologist and two nurses) of which at least one on duty. Surgical team composition could be guaranteed also through the collaboration of personnel coming from other accredited public and private hospitals.
As one of the selected vascular hubs, our institution started to serve a population of approximately 3 million inhabitants. During the lockdown period, indication to hospital admission and surgical (open or endovascular) treatment has been restricted only to symptomatic, urgent, or emergent disease. A new emergency room (ER)—dedicated only to cardiac and vascular cases—was created to allow patients accessing by a distinct pathway that should be independent from the contaminated area of the standard ER.
The aim of this study is to report the experience of one of the major “hubs” for vascular surgery in Lombardy during the first 7 weeks after total lockdown due to COVID-19 pandemic in comparison with the 2019 caseload on the same period to highlight the differences attributable to the SARS-CoV-2 infection on vascular surgery unit duty.
Materials and Methods
Data from all patients who were treated at our department during the first 7 weeks of pandemic were prospectively collected. Patients included in the analysis are urgent or emergent cases referred both to our center and by the spokes. Nonurgent admissions/procedures could not be performed in accordance with the national and regional dispositions. In the reported timeframe, routine office visits were not allowed. Already scheduled follow-up visits were postponed. Asymptomatic patients were not evaluated, whereas patient complaining about vascular-related conditions were evaluated by phone calls or—starting from March 26th—through the telemedicine portal made available by our institution, with the possibility of remote counseling and visualization of imaging studies provided by the patient.
Preoperative, intraoperative, postoperative, and predischarge data were then analyzed. The study complies with the Declaration of Helsinki; all patients signed an informed consent to the procedure itself and to allow the use of deidentified collected data for scientific purposes at the moment of the admission. Data were reported in accordance with the Society for Vascular Surgery (SVS) and the Society for Vascular Surgery/American Association for Vascular Surgery (SVS/AAVS) suggested reporting standards.1, 2, 3
All patients accessing the cardiovascular ER were tested for SARS-CoV-2 with nasopharyngeal swab and chest X-ray. Chest computed tomography scan was performed in selected cases to confirm the diagnosis. In case of suspect or confirmed COVID-19 in a patient requiring surgical therapy, full personal protective equipment, such as double sterile gloves, surgical waterproof gowns, goggles for eye protection, and N95 mask, for all surgical team was predisposed outside from the dedicated COVID-19 operating theater. In case of general anesthesia, the surgical team waited outside the room while the patient was intubated. All interventions were performed in negative-pressure operating rooms. All patients were systemically treated with 70 to 100 UI/kg of heparin before artery cross clamping to obtain an activated clotting time longer than 250 seconds.
The primary study end point was defined as a primary clinical success. The secondary end points were defined as one of the following: in-hospital mortality and/or any major adverse event such as any cardiac, pulmonary, intestinal, and neurologic complication occurring in the postoperative period or lower limb amputation. Data from the pandemic period were compared with a control group composed of urgent/emergent patients treated at our division in the same timeframe of 2019 by retrospectively analyzing medical records.
To prepare the database and perform descriptive analysis, the Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA) version 14.0 for Windows was used. The results are presented in tables. Categorical variables are expressed as frequencies and percentages. Continuous variables with normal distribution were expressed as mean and standard deviation; and those with non-normal distribution, as median and interquartile range.
Results
During the study period, 116 consecutive patients (81 men and 35 women; median age: 71 years, IQR 65–81) were treated at our department. The mean preoperative SVS comorbidity score4 was 8 (IQR 4–12), other preoperative and demographical data are summarized in Table I . Details of indications for treatment are reported in Table II . Thirty-two patients (28%) were addressed from spoke hospitals directly referring to our hub, 19 (16%) from hospitals belonging to other hub/spoke nets, 48 (41%) came directly from our ER, and 17 (15%) were already hospitalized for COVID-19 pneumonia.
Table I.
Preoperative risk factors of patients undergoing urgent/emergent surgery in March-April 2020 and 2019
| Variable | 2020: 116 patients | 2019: 34 patients | P |
|---|---|---|---|
| Male | 81 (69.8%) | 19 (65.6%) | 0.65 |
| Median age (years) | 72 (IQR 65–81) | 63 (IQR 54–72) | 0.002 |
| Hypertension (grade ≥ 1) | 99 (85.3) | 31 (91.2%) | 0.379 |
| 1 | 33 (28.4%) | 13 (38.2%) | 0.276 |
| 2 | 42 (36.2%) | 12 (35.3%) | 0.922 |
| 3 | 24 (20.7%) | 6 (17.6%) | 0.697 |
| Smoking (grade ≥ 1) | 78 (67.2%) | 32 (94.1%) | 0.002 |
| 1 | 51 (44.0%) | 21 (61.8%) | 0.068 |
| 2 | 20 (17.2%) | 11 (32.4%) | 0.056 |
| 3 | 7 (6%) | 0 (0%) | 0.176 |
| Diabetes (grade ≥ 1) | 30 (25.9%) | 4 (11.8%) | 0.084 |
| 1 | 7 (6.0%) | 0 (0%) | 0.142 |
| 2 | 16 (13.8%) | 2 (5.9%) | 0.212 |
| 3 | 7 (6.0%) | 2 (5.9%) | 0.871 |
| Renal status (grade ≥ 1) | 46 (39.7%) | 13 (38.2%) | 0.882 |
| 1 | 32 (27.6%) | 10 (29.4%) | 0.835 |
| 2 | 11 (9.5%) | 3 (8.8%) | 0.907 |
| 3 | 3 (2.6%) | 0 (0%) | 0.344 |
| Cardiac status (grade ≥ 1) | 46 (39.7%) | 13 (38.2%) | 0.882 |
| 1 | 23 (19.8%) | 2 (5.9%) | 0.055 |
| 2 | 14 (12.1%) | 3 (8.8%) | 0.600 |
| 3 | 9 (7.8%) | 8 (23.5%) | 0.011 |
| Pulmonary status (grade ≥ 1) | 46 (39.7%) | 13 (38.2%) | 0.853 |
| 1 | 37 (32.2%) | 11 (32.4) | 0.984 |
| 2 | 7 (6.0%) | 1 (2.9%) | 0.141 |
| 3 | 2 (1.7%) | 1 (2.9%) | 0.661 |
| SVS risk score | 8 (IQR 4–12) | 8 (IQR 3–16) | 0.923 |
| ASA score (grade ≥ III) | 92 (79.3%) | 25 (73.5%) | 0.474 |
| III | 56 (48.3%) | 10 (29.4%) | 0.051 |
| IV | 35 (30.2%) | 8 (23.5%) | 0.451 |
| V | 1 (0.9%) | 7 (20.6%) | <0.001 |
| Obesity (BMI > 30) | 10 (8.6%) | 4 (11.7%) | 0.579 |
| Dyslipidemia | 94 (81.0%) | 21 (61.8%) | 0.974 |
| Antiplatelet/anticoagulation therapy | |||
| Single antiplatelet therapy | 54 (46.6%) | 15 (44.1%) | 0.802 |
| Double antiplatelet therapy | 19 (16.4%) | 6 (17.6%) | 0.862 |
| Anticoagulant | 17 (14.7%) | 6 (17.6%) | 0.670 |
| Anticoagulant and any antiplatelet therapy | 9 (7.8%) | 1 (2.9%) | 0.322 |
| Previous PTCA/CABG | 25 (21.6%) | 7 (20.6%) | 0.904 |
| PTCA | 15 (12.9%) | 6 (17.6%) | 0.486 |
| CABG | 7 (6%) | 1 (2.9%) | 0.480 |
| PTCA and CABG | 3 (2.6%) | 0 (0%) | 0.344 |
| Previous stroke or TIA | 13 (11.2%) | 7 (20.6%) | 0.157 |
| History of autoimmune disease | 18 (15.7%) | 4 (11.8%) | 0.575 |
| SARS-CoV-2 positivity on admission | 17 (14.7%) | 0 (0%) | 0.018 |
| Transferred from other hospitals | 51 (44.0%) | 7 (20.6%) | 0.014 |
Hypertension: 1: easily controlled with single drug; 2: controlled with 2 drugs; 3: required more than 2 drugs or uncontrolled.
Smoking: 1: none current, but smoked in last ten years; 2: current, less than 1 pack/day; 3: current, greater than one pack/day.
Diabetes: 1: adult onset; diet controlled; 2: adult onset; oral medication-controlled; 3: adult onset; insulin-controlled.
Renal status: 1: creatinine 1.5–3.0 mg/dL, clearance 30–50 mL/min; 2: creatinine 3.0–6.0 mg/dL, clearance 15–30 mL/min; 3: creatinine > 6.0 mg/dL, clearance < 15 mL/min or on dialysis or with transplants.
Cardiac status: 1: asymptomatic, remote myocardial infarction (MI) by history > 6 months or occult MI by ECG; 2: stable angina, controlled ectopy or symptomatic arrhythmia, drug compensated congestive heart failure (CHF); 3: unstable angina, symptomatic or poorly controlled ectopy or arrhythmia or poorly compensated CHF, MI within 6 months.
Pulmonary status: 1: asymptomatic or mild dyspnea on exertion, mild X-ray parenchymal changes, PFT 65 to 80% of predicted; 2: between 1 and 3; 3: vital capacity less than 1.85 L, FEV less than 35% of predicted, maximal voluntary ventilation less than 28 l/min or less than 50% of predicted, PCO greater than 45 mm Hg, supplemental oxygen use necessary or pulmonary hypertension.
SVS risk score: Society of vascular surgery risk score calculated in accordance with the reporting standards.
ASA score: American Society of Anesthesiologists risk score calculated in accordance with the reporting standards.
BMI: body mass index calculated as weight/height2 and expressed as kg/m2.
PTCA: percutaneous transluminal coronary angioplasty.
CABG: coronary artery bypass graft. Bold indicates the results reaching statistical significance (P < .05).
Table II.
Indication for treatment of patients undergoing urgent/emergent patients in March-April 2020 and 2019
| Variable | 2020: 116 patients | 2019: 34 patients | P |
|---|---|---|---|
| Acute limb ischemia | 31 (26.7%) | 6 (17.6%) | 0.28 |
| Native arterial tree thrombosis | 28 (90.3%) | 5 (83.3%) | |
| Previous femoropopliteal graft thrombosis | 3 (9.7%) | 1 (16.7%) | |
| Chronic limb-threatening limb ischemia | 24 (20.7%) | 1 (2.9%) | 0.015 |
| Irreversible lower limb ischemia, gangrene | 6 (5.2%) | 0 (0%) | 0.176 |
| Aortic pathology | 21 (18.1%) | 6 (17.6%) | 0.951 |
| Stanford B acute AD | 2 (9.5%) | 2 (33.3%) | |
| TAAA | 4 (19.0%) | 2 (33.3%) | |
| TAA | 2 (9.5%) | 0 (0%) | |
| AAA | 13 (61.9%) | 2 (33.3%) | |
| Symptomatic carotid stenosis | 17 (14.7%) | 5 (14.7%) | 0.994 |
| Other | 17 (14.7%) | 16 (47.1%) | <0.001 |
| Cardiogenic shock necessitating ventricular assist devices | 2 (1.7%) | 5 (14.7%) | 0.002 |
AD, aortic dissection; TAAA, thoracoabdominal aortic aneurism; TAA, thoracic aortic aneurysm; AAA, abdominal aortic aneurysm. Bold indicates the results reaching statistical significance (P < .05).
Among patients who were addressed from other hubs/spokes, the median delay between patient arrival at the first emergency unit and patient treatment was 6 hr (IQR 3–12). Overall, at a median follow-up of 23 ± 13 days, primary clinical success was 87.1% and secondary clinical success was 95.9%. We recorded 3 in-hospital deaths for an overall mortality rate of 2.6%. Postoperative data are summarized in Table III .
Table III.
Perioperative outcomes of patients undergoing urgent/emergent patients in March-April 2020 and 2019
| Variable | 2020: 116 patients | 2019: 34 patients | P |
|---|---|---|---|
| Type of intervention | |||
| Open | 77 (66.4%) | 23 (67.6%) | 0.890 |
| Endovascular | 28 (24.1%) | 6 (17.6%) | 0.427 |
| Hybrid | 7 (6.0%) | 5 (14.7%) | 0.101 |
| Angiography and medical therapy | 4 (3.4%) | 0 (0%) | 0.272 |
| Setting | |||
| Urgent (<48h) | 74 (63.8%) | 24 (70.6%) | 0.464 |
| Emergent | 42 (36.2%) | 10 (29.4%) | 0.464 |
| Procedural time (min) | 74 (IQR 49–130) | 75 (IQR 50–150) | 0.642 |
| Intraoperative death | 0 (0%) | 0 (0%) | 0.997 |
| Reintervention | 7 (6.0%) | 5 (14.7%) | 0.128 |
| Clinical success | |||
| Primary | 101 (87.1%) | 29 (85.3%) | 0.789 |
| Secondary | 111 (95.7%) | 31 (91.2%) | 0.196 |
| Adverse eventsa | |||
| In-hospital death | 3 (2.6%) | 4 (11.8%) | 0.026 |
| Major cardiac | 4 (3.4%) | 3 (8.8%) | 0.224 |
| Major pulmonary | 5 (4.3%) | 3 (8.8%) | 0.341 |
| Major renal | 5 (4.3%) | 3 (8.8%) | 0.355 |
| Major cerebrovascular | 2 (1.7%) | 2 (5.9%) | 0.208 |
| Bowel ischemia | 1 (0.8%) | 1 (2.9%) | 0.071 |
| Amputation | 2 (1.7%) | 1 (2.9%) | 0.351 |
| Spinal cord injury | 2 (1.7%) | 1 (2.9%) | 0.380 |
| Perioperative bleeding requiring transfusion | |||
| <3 RBC units | 15 (12.9%) | 10 (37%) | 0.098 |
| ≥3 RBC units | 11 (9.5%) | 2 (7.4%) | 0.302 |
| Length of stay (days) | 4 (IQR 3–6) | 6 (IQR 2–10) | 0.635 |
RBC, red blood cells. Bold indicates the results reaching statistical significance (P < .05).
Major events are described as graded ≥2 according to reporting standards.
Acute Limb Ischemia
Thirty-one patients required treatment for acute limb ischemia. Of them, 12 (38.7%) were found positive for COVID-19 pneumonia on admission, whereas 3 (9.7%) became positive during hospitalization. Twenty-eight patients (90.3%) presented for acute thrombosis of the native arterial tree, 3 (9.7%) for thrombosis of a femoropopliteal bypass graft (1 above the knee, 2 below the knee). Six patients (19.4%) were affected by atrial fibrillation at diagnosis (3 SARS-CoV-2 positive). Thrombectomy of the iliac axis was performed in 9 patients (29.0%) of femoropopliteal arteries in 19 (61.3%), and of below-the-knee and tibial vessels in 10 (32.6%) cases. As adjunctive procedures, 3 femoral endarterectomies, 2 common iliac artery stenting, 1 superficial femoral artery stenting, 2 femorofemoral crossover bypasses, and 2 femoropopliteal bypasses were required to complete the revascularization. We recorded 3 upper limb occlusions. Three patients had more than one limb involved: upper and lower limb in one man affected by COVID-19, and both lower limbs in 2 women affected one by severe mitral valve stenosis and the other by hematological malignancy.
Postoperatively, all patients received systemic anticoagulation with intravenous heparin infusion for the entire hospital stay. One patient treated by means of femorotibioperoneal trunk bypass was reoperated for bleeding after discharge on postoperative day 7. During hospitalization, we recorded 3 reinterventions in 2 patients (6.5%) because of recurrent thrombosis. One patient experienced 2 revascularization failures, and, after a second thrombectomy procedure, eventually underwent major amputation on postoperative day 5; the second patient presented with acute arterial thrombosis on the ipsilateral and contralateral limb on postoperative day 1. All patients with a failed revascularization or requiring a reintervention were tested positive for SARS-CoV-2 either at admission (n = 1) or during the hospitalization (n = 1). Limb salvage was obtained in 29 (93.5%) patients and 2 patients were amputated for recurrent thrombosis with irreversible limb necrosis. Two patients died during hospitalization because of multiorgan failure on postoperative day 4 in both cases; one was SARS-CoV-2 positive and one was tested negative.
Irreversible Subacute Ischemia or Chronic Gangrene
A total of 6 patients (5.2%) presented with the clinical features of irreversible lower limb ischemia and were therefore primarily considered for demolitive surgery. In 3 patients in whom primary amputation was needed for chronic irreversible gangrene, one amputation was performed above the knee, one below the knee, and one involved a single toe. In 3 patients, primary amputation was performed for acute thrombosis with symptoms onset dating several days before hospital access and irreversible limb necrosis already developed (mean delay from symptoms to hospital access 8 ± 3 days). Notably only one of these patients was COVID positive, and the late referral to vascular services was attributable to the cognitive state of the patient, with an advanced dementia, and to patient's living in a facility which did not offer daily medical evaluation of patients. The other 2 patients were both COVID negative and deliberately postponed access to medical services as a consequence of the reported “fear of being infected” by the SARS-CoV-2 if they were hospitalized. Both self-medicated with NSAIDS for some days and eventually came to the emergency department only after complete nerve damage with inability to walk had developed.
One 94-year-old patient (COVID-19 positive) died on postoperative day 4 because of multiorgan failure after major amputation performed for chronic irreversible gangrene.
Chronic Limb Ischemia
Twenty-four patients were treated for chronic limb threatening ischemia (one COVID-19 positive). Of them, 2 (8.3%) received an aortobifemoral bypass, 5 (20.8%) received profundoplasty with common femoral artery endarterectomy, 3 (12.5%) an above-the-knee femoropopliteal bypass, 4 (16.7%) a below-the-knee femoropopliteal bypass, and 6 (25%) percutaneous angioplasty of the iliac, superficial femoral, popliteal, or tibial artery. All the supragenicular grafts were prosthetic, while great saphenous vein was used for infragenicular bypass in 3 cases, and an ePTFE graft was used in one patient with previous bilateral saphenectomy for chronic venous insufficiency. Four patients were treated with vasoactive therapy after angiography showed inadequate runoff to perform revascularization.
Limb salvage was obtained in all patients. One COVID-19 patient needed reintervention during hospitalization for acute thrombosis of a femorotibioperoneal trunk bypass: the patient received emergent thrombectomy of the graft and tibial vessels with successful secondary patency. Postoperatively, all patients received systemic anticoagulation with intravenous heparin infusion for the entire hospital stay and they were discharged with double antiplatelet therapy, or new oral anticoagulants and single antiplatelet therapy in case of below-the-knee bypass grafting. No bleeding complication or mortality was observed in this group.
Aortic Emergencies
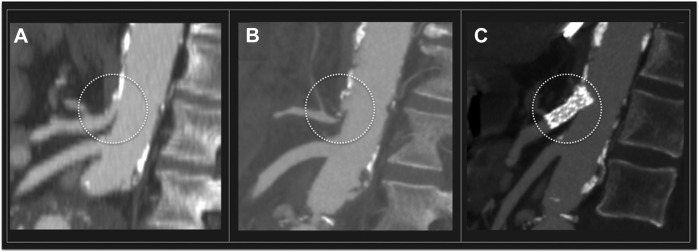
Thirteen patients were treated for abdominal aortic disease (one COVID-19 positive). Indications for treatment included: ruptured abdominal aortic aneurysm (AAA) in 2 cases (15.4%), rapidly growing AAA (more than 1 cm/6 months) in 4 cases (30.8%), symptomatic AAA in 2 cases (15.4%), failed endovascular aneurysm repair (EVAR) requiring open conversion (OC) in 5 cases (23.1%) due to endoleak (two type I, one type II) with rapid sac expansion; aortoenteric fistula (AEF) in 2 cases (15.4%). AEF was secondary to aorto-bifemoral bypass grafting in one case, and to standard EVAR in the other. Graft explantation, duodenal fistula repair, and aortobifemoral bypass grafting using a silver acetate and triclosan impregnated antimicrobial graft (InterGard Synergy, Maquet Getinge Group, Hudson, NH, USA) were performed. No mortality was recorded. One COVID-19-positive patient, submitted to OC underwent reintervention for liver and colonic ischemia secondary to celiac trunk thrombosis (Fig. 1 A, B) on postoperative day 2. First, the patient underwent celiac trunk endovascular recanalization by means of covered stent implantation (Fig. 1C); then, relaparotomy with cholecystectomy and partial colectomy were performed. No other reinterventions or adverse events were recorded in this subgroup of patients.
Fig. 1.
(A) Preoperative CTA showing patency of the celiac trunk (dotted circle) before open surgical conversion. (B) Celiac trunk thrombosis was documented by postoperative CTA. The patient underwent urgent celiac trunk recanalization by means of covered stent. (C) Postprocedural CTA showing patency of the celiac trunk.
Two patients were treated for acute type B dissection. The first patient was referred for post-traumatic type B dissection due to car accident. He underwent zone 2 emergent thoracic endovascular aortic repair closing the primary entry tear, covering the origin left subclavian artery that showed valid retrograde flow through the vertebral artery. No complications were recorded, and the patient was discharged on postoperative day 4. The second patient was treated emergently for thoracoabdominal dissection with malperfusion (lower limb left renal dynamic malperfusion) developing during ascending aorta aneurysm repair. He underwent bare metal stent placement from the distal aortic arch to the infrarenal aorta (PETTICOAT technique) to solve malperfusion, with left renal artery stenting and iliac kissing stenting, but eventually died on postoperative day 3 for multiorgan failure syndrome.
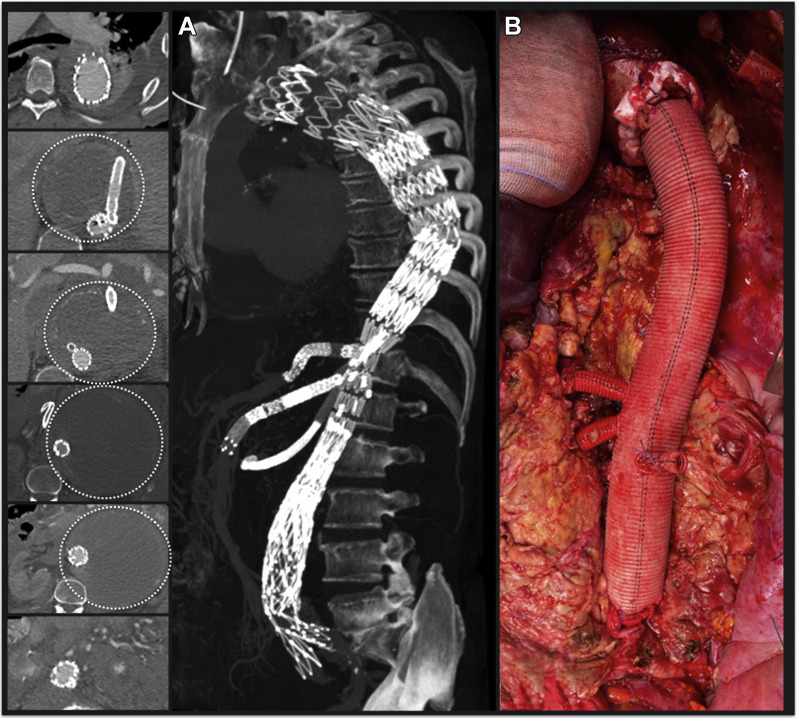
One patient was admitted for failure of a previous branched endovascular aortic repair (BEVAR) leading to thoracoabdominal aneurysm (TAAA) impending rupture (Fig. 2 A). The patient was referred to our institution from another region where he had undergone BEVAR using a T-Branch off-the-shelf stent graft (Cook Medical, Bloomington, IN) for an extent type III TAAA. The first operation was then followed by a number of unsuccessful endovascular secondary procedures, trying to fix multiple endoleaks, but lately resulting in sac growth up to 18 cm. He underwent OC using a multibranched graft. Postoperative course passed uneventfully and the patient was discharged on postoperative day 8 (Fig. 2B).
Fig. 2.
Open conversion after previous endovascular treatment of a thoracoabdominal aneurysm with a branched thoracoabdominal stent graft (BEVAR). (A) Preoperative CTA showing BEVAR with bridging stents for celiac trunk, superior mesenteric artery, and right renal artery. The left renal artery was occluded during the index procedure. Despite the endovascular treatment, a progressive enlargement of the thoracoabdominal aneurysm (16 cm) was reported (dotted circles). (B) Intraoperative photographs showing thoracoabdominal aortic repair with a multibranched surgical prosthesis (see text).
Carotid Artery Stenosis
Seventeen patients were treated for symptomatic carotid artery stenosis (no patient was COVID-19 positive). All patients were treated by means of carotid endarterectomy under local anesthesia and clinical neurological monitoring. Selective shunting was used in 2 patients (11.8%). No neurological events or other major complications were observed. No deaths were recorded in this subgroup.
Other Vascular Emergencies
Three cases of central venous catheters (CVCs) misplacement with inadvertent catheterization of the vertebral (1 case), subclavian (1 case), or superficial femoral (1 case) artery were referred from other hospitals. All procedures were performed in dedicated COVID-19 ICUs. In all cases, patients were treated endovascularly or by manual compression and consequent catheter removal, with no need of open surgical conversion. In one case, a CVC got stuck in the right atrium and was retrieved by means of a goose-neck catheter through percutaneous femoral access. Patients did not experience any other vascular complications and were readmitted to the COVID-19 ICU immediately after the procedure. Two patients were treated for transaxillary Impella (Abiomed Inc., Danvers, MA) implantation, one of whom died for cardiogenic shock in postoperative day 4. The remaining admissions included 6 common femoral artery pseudoaneurysms, 4 femoropopliteal graft infection, and 2 iliocaval venous obstructions.
Comparison with the Pre-COVID Era
The comparison of the pandemic period with a pre-COVID cohort of vascular urgencies/emergencies referred to our center in 2019 is depicted in Table I, Table II, Table III. It is worth noting that the overall preoperative status and postoperative outcomes do not significantly vary between the 2019 and the 2020 cohorts, although a higher proportion of chronic decompensated and acute limb ischemia were operated on during the pandemic (21% vs. 3% of the treated patients, P = 0.015). The main difference in in-hospital death rates (11.8% in 2019 vs. 2.6% in 2020, P = 0.026) must be weighed against the higher proportion of cardiac arrests and cardiogenic shock which needed an expeditious placement of a ventricular assist device in 2019 vs. 2020 (15% vs. 2% of urgent patients, P = 0.002). In fact, of the 4 deaths in the control group, 3 were the consequence of refractory cardiogenic shock in this high-risk category of patients.
Discussion
The impact of vascular emergencies in the clinical daily routine of a tertiary referral center is not negligible, with aortic aneurysms rupture, acute limb ischemia, and symptomatic cerebrovascular disease representing the most frequent conditions requiring immediate management.5, 6, 7
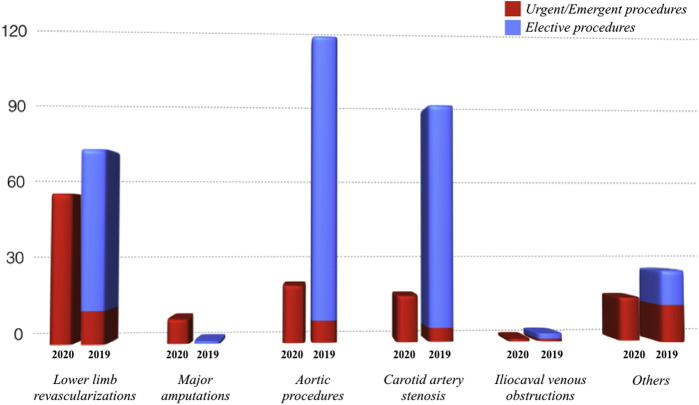
Our institution is a tertiary referral center, performing both elective and emergent procedures. In March and April 2019, we performed 376 interventions of which were 342 (91%) elective cases and 34 (9%) urgencies/emergencies. During the first 7 weeks of lockdown, we performed 116 urgent/emergent interventions with a rise of 340%, and interestingly we found that acute limb ischemia rose 158% (Fig. 3 ), of which 38.7% positive to COVID-19. The reason for the significant increase in the incidence of acute leg ischemia could be related to the pathogenesis of COVID-19 infection. As a matter of fact, several articles reported a link between advanced COVID-19 disease, microvascular inflammation,8 distal vasculitis, and prothrombotic state,9 and this seems to be due to the inflammatory cytokines storm (IL-6 and IL1-beta) that contributes to the procoagulant and proadhesive state of dysfunctional endothelium.10 , 11 Moreover, abnormal coagulation parameters are associated with poor prognosis in COVID-19 patients.12 , 13
Fig. 3.
The graphic showing the comparison between our surgical experience during the COVID period in 2020 and our experience during the same period in 2019, in accordance with the indications to treatment.
In this view, a number of operative internal guidelines reporting modified anticoagulation protocols for arterial and venous thrombosis prophylaxis in COVID-19 patients have been adopted by different teaching hospitals. However, no consensus on a uniform anticoagulation strategy has been reached internationally so far. An adjunctive and somewhat complementary explanation to the rise of acute limb ischemia cases could also be found in the centralization of vascular care, with a larger portion of the regional population being cared by a handful of hospitals.
On the other hand, we did not observe a comparable increase of ruptured and symptomatic aortic aneurysms but contrarily they fell by 50% during the same interval. The reason of this apparent decrease can be postulated to be due to the emergency situation in which most patients, having to face the quarantine, renounced the visits already scheduled, or refused to go to the hospital for fear of SARS-CoV-2 infection. In this scenario, an important aspect to discuss is that we are probably not aware of all patients who were found dead at home, or died during transfer to the hospital, due to vascular emergent disease. In our experience, probably underestimating the real incidence, 2 patients arrived dead at the ER (1 with thoracic aortic dissection and the other with thoracoabdominal aneurysm rupture) having ignored the appearance of symptoms they had initially treated with analgesics throughout the days before (as reported by their relatives on arrival). We will never know how many patients actually died of aneurysm rupture or stroke during pandemic in Lombardy.
A positive perspective derived from the comparative analysis is that we have not documented a worsening in postsurgical outcomes during the pandemic period, possibly meaning that, when the patients are correctly and timely addressed to a high-volume center, the quality of the health care services is guaranteed. This trend is inconsistent with what was published from another Italian group, showing a mortality rate as high as 40% in COVID+ patients with acute limb ischemia,14 whereas the same class of patients in our cohort showed a mortality rate of 12.5%. It cannot be known if SARS-CoV-2-positive patients died of other causes without receiving vascular care or if the combination of high clinical efficacy and services reorganization had played a crucial role to guarantee such outcomes. Generalization to other settings, especially to national health care systems without universal coverage, would require further studies.
Another key point needing to be considered is that, to work properly, the hub/spoke system requires a widespread organization of medical transport to allow patients transfer as soon as possible, to reduce the delay between symptoms, diagnosis, and treatment.
Finally, during pandemic, we observed a significant increase of complications after invasive anesthesiologic procedures, such as CVCs placement. Large numbers of CVCs are placed every year and misplacements are reported15 with an incidence of unintended arterial punctures of 2 to 4.5%, resulting in arterial injury in 0.1 to 0.5% of patients.16 In this scenario, several hypotheses may be postulated for the rising incidence of such a complication in our experience. During COVID-19 pandemic, in a few days, the number of ward beds dedicated to COVID-19 patients increased in Lombardy. In our hospital, ward beds arrived to a total number of 279, and ICU beds also increased from 28 to 72 (54 of them dedicated to patients with COVID-19, and 18 to cardiology and cardiac surgery hub emergencies).17 An extremely high number of complicated patients were treated in emergency conditions and, in this scenario, numerous doctors from other specialties have been enrolled to support the anesthesiologists who have found themselves having to perform a greater number of operations in a much greater number of patients contributing to explain the reason for the growth of this complication.
The monocentric design and the small number of patients may be considered a limitation of this study, preventing us from performing a stratification of results or subgroup analysis. Nevertheless, to the best of our knowledge, it represents the most extensive experience reported in literature regarding the impact that the COVID-19 pandemic has had on the vascular program of a high-volume tertiary center in one of the regions with the highest incidence of infection worldwide.
Conclusion
Since its appearance, SARS-CoV-2 has been testing all national healthcare systems which found themselves facing an unprecedented emergency. Late referral in the pandemic period could seriously worsen limb prognosis; this aspect should be known and addressed by health care providers. Vascular surgical outcomes in pre-COVID and COVID era were comparable in our experience.
Footnotes
No conflicts of interest or funding are to be disclosed.
References
- 1.Chaikof E.L., Blankensteijn J.D., Harris P.L. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 2.Fillinger M.F., Greenberg R.K., McKinsey J.F. Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52:1022–1033.e5. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Timaran C.H., McKinsey J.F., Schneider P.A. Reporting standards for carotid interventions from the Society for Vascular Surgery. J Vasc Surg. 2011;53:1679–1695. doi: 10.1016/j.jvs.2010.11.122. [DOI] [PubMed] [Google Scholar]
- 4.Faizer R., DeRose G., Lawlor D.K. Objective scoring systems of medical risk: a clinical tool for selecting patients for open or endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;45:1102–1108. doi: 10.1016/j.jvs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Patel M.I., Hardman D.T., Fisher C.M. Current views on the pathogenesis of abdominal aortic aneurysms. J Am Coll Surg. 1995;181:371–382. [PubMed] [Google Scholar]
- 6.Wilmink T.B., Quick C.R., Hubbard C.S. The influence of screening on the incidence of ruptured abdominal aortic aneurysms. Vasc Surg. 1999;30:203–208. doi: 10.1016/s0741-5214(99)70129-1. [DOI] [PubMed] [Google Scholar]
- 7.Davies B., Braithwaite B.D., Birch P.A. Acute leg ischaemia in Gloucestershire. Br J Surg. 1997;84:504–508. [PubMed] [Google Scholar]
- 8.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P.P., Blet A., Smyth D. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 10.Nencioni A., Trzeciak S., Shapiro N.I. The microcirculation as a diagnostic and therapeutic target in sepsis. Intern Emerg Med. 2009;4:413–418. doi: 10.1007/s11739-009-0297-5. [DOI] [PubMed] [Google Scholar]
- 11.Boisramé-Helms J., Kremer H., Schini-Kerth V. Endothelial dysfunction in sepsis. Curr Vasc Pharmacol. 2013;11:150–160. [PubMed] [Google Scholar]
- 12.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellosta R., Luzzani L., Natalini G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.04.483. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson F., Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 16.Ezaru C.S., Mangione M.P., Oravitz T.M. Eliminating arterial injury during central venous catheterization using manometry. Anesth Analg. 2009;109:130–134. doi: 10.1213/ane.0b013e31818f87e9. [DOI] [PubMed] [Google Scholar]
- 17.Zangrillo A., Beretta L., Silvani P. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22:91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]