Abstract
Protein kinase Cβ (PKCβ) expressed in mammalian cells as two splice variants, PKCβI and PKCβII, functions in the B cell receptor (BCR) signaling pathway and contributes to B cell development. We investigated the relative role of PKCβII in B cells by generating transgenic mice where expression of the transgene is directed to these cells using the Eµ promoter (Eµ-PKCβIItg). Our findings demonstrate that homozygous Eµ-PKCβIItg mice displayed a shift from IgD+IgMdim toward IgDdimIgM+ B cell populations in spleen, peritoneum and peripheral blood. Closer examination of these tissues revealed respective expansion of marginal zone (MZ)-like B cells (IgD+IgM+CD43negCD21+CD24+), increased populations of B-1 cells (B220+IgDdimIgM+CD43+CD24+CD5+), and higher numbers of immature B cells (IgDdimIgMdimCD21neg) at the expense of mature B cells (IgD+IgM+CD21+). Therefore, the overexpression of PKCβII, which is a phenotypic feature of chronic lymphocytic leukaemia cells, can skew B cell development in mice, most likely as a result of a regulatory influence on BCR signaling.
Subject terms: Chronic lymphocytic leukaemia, B cells, Chronic lymphocytic leukaemia
Introduction
B cell receptor (BCR) signaling plays an essential role at critical stages of B cell development1,2. In the bone marrow spontaneous and environmental ligand-induced tonic signals from this receptor aid the transition of developing B cells from one stage of differentiation to the next until they emerge into peripheral circulation. In the periphery, mature B cells move between secondary lymphoid organs where specific antigen recognition by BCR results in their activation and differentiation to ultimately generate cells that yield specific antibody production and immune memory. Key signaling molecules have been found to be intricately linked to the stage specific responses of BCR signaling. While this is particularly true for the early stages of B cell differentiation where this relationship is well defined, the roles of signaling molecules involved in B cell fate decisions in the periphery are less well characterized and understood2.
One such signaling molecule is Protein kinase Cβ (PKCβ), which plays a central role in the appropriate regulation of B cell development and activation, including BCR signaling3. It has been demonstrated that knock-down of the PKCβ gene, prkcb, in mice results in immunodeficiency that is reminiscent of X-linked immunodeficiency, and corresponds to impaired humoral immune responses and B cell function4. PKCβ plays an important role in B cell development and activation, including BCR signaling3,4.
A key target of PKCβ during BCR engagement is Bruton’s tyrosine kinase (Btk). PKCβ phosphorylates Btk to provide feedback inhibition of BCR signaling by blocking its ability to activate phospholipase Cγ2 (PLCγ2) and stimulate Ca2+ release from intracellular stores5. Genetic studies have confirmed a functional relationship between PKCβ and Btk, showing that there is great similarity of B cell-related phenotypes resulting from targeted disruption of the genes coding for these proteins4,6–8. The gene coding for PKCβ, PRKCB, when transcribed is alternatively spliced to yield PKCβI and PKCβII, which differ from each other within their C-terminal 50 amino acids9,10. However, it is not clear whether these isoforms have redundant or specific functions in B cell development and activation.
Our previous studies of PKCβII in the malignant cells of B lymphoproliferative disorders have shown that overexpression of this PKC isoform is a phenotypic feature of chronic lymphocytic leukaemia (CLL) cells11. In addition, the Tcl1-transgenic mouse model of CLL demonstrates that PKCβ plays a key role in the pathogenesis of CLL, because PKCβ deficient mice do not develop CLL-like disease12. One possible explanation for this is the severe reduction in numbers of marginal zone B cells (MZ B cells) and B-1 B cells in PKCβ knock out mice4, since these are the cells from which CLL is thought to arise13,14. Interestingly, in virtually all mouse models of CLL, malignant cells develop from an expanded B-1 B cell compartment15–19, and this is similar to human disease which is preceded by a condition known as monoclonal B lymphocytosis20. Based on these combined findings, we wanted to investigate the effect of PKCβII overexpression in the B cell compartment. Considering the role of PKCβ in modulating BCR signaling and in B cell development, particularly the development of B-1 and MZ B cells, we hypothesized that transgenic expression of PKCβII within B cells of mice might lead to an expansion in populations of these B cell types.
Materials and methods
Generation of the Eµ-PKCβII tg mice
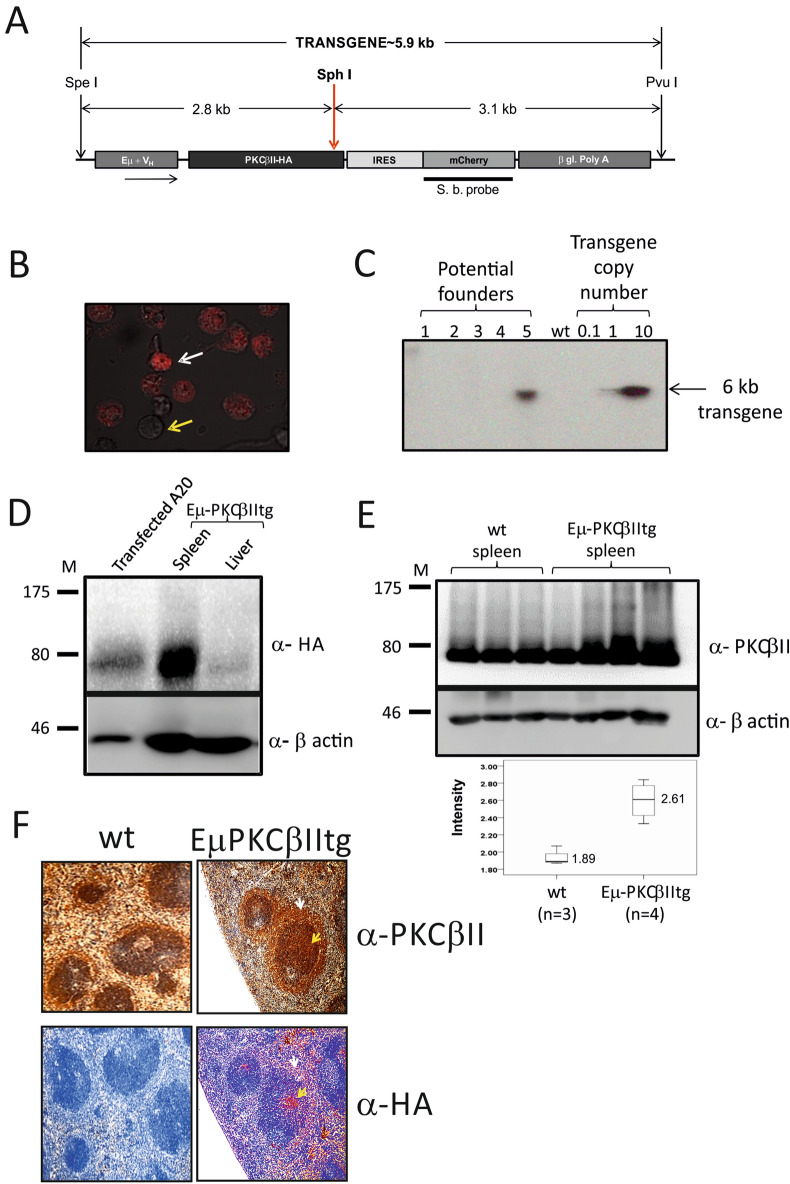
To generate Eµ-PKCβIItg mice, a pBSVE6BK plasmid (kindly provided by Dr. Raif Geha, Harvard Medical School) which exploits the VH promoter and the IgH-enhancer to specifically direct expression of target genes in B cells was used (pEµ vector21). The full length PKCβII coding sequence (2021-bp) was tagged with human influenza HA at its 3′ end by PCR, using the forward 5′-GAGAATCGATCAAGATG-GCTGACCCGGCTGCGGGGCC-3′, and the reverse 5′-GAGAGTCGACTCAAAGAGCGTAATCTGGAACATCGTATGGGTAGCTCTTGACTTCGGGTTTTAAA-3′ primers (Eurofins MWG Operon, London UK) prior to cloning into the ClaI (all restriction enzymes used in this study are from New England Biolabs, Hitchin UK) and SalI sites of the pEµ vector. An intra- ribosomal entry site (IRES) was sub-cloned into the BamHI and SalI sites of the multiple cloning site (MCS) of mCherry, and the IRES-mCherry coding sequence was then sub-cloned into pEµ-PKCβIIHA using Not-I and Apa-I placing it in a 5′→3′ unilateral orientation between the PKCβIIHA and β-poly-globin coding sequences in the Eµ vector (Fig. 1A). This construct was tested in the mouse B lymphoma cell line A2022 where 2 × 106 cells were nucleofected™ with 2 µg pEµ-PKCβIIHA-IRES-mCherry using the solution V transfection kit and programme U-013 according to the manufacturer’s protocol (Lonza, Tewkesbury, UK) and fluorescence associated with the expression of mCherry observed (Fig. 1B). The construct containing pEµ-PKCβIIHA-IRES-mCherry was then digested with Pvu-I and Spe-I, DNA fragment purified and injected into pronuclei of zygotes isolated from C57Bl/CBA F1 hybrid mice. Mice generated from this procedure were screened for the presence of the transgene by Southern blot analysis on genomic DNA isolated from the tail and digested with SphI. Blots were hybridized with the mCherry specific DNA probe labeled with 32P-dCTP (Amersham Megaprime DNA Labelling System), essentially as described previously23. Founders were identified by higher expression, and/or evidence of a single integration site for the transgene (Fig. 1C), and those showing transgene integration in their genome were chosen for further breeding to generate the heterozygous and ultimately homozygous Eµ-PKCβIItg transgenic animals that were analysed in this study. To establish the first-generation of Eμ-PKCβII heterozygous mice, the founder mouse was backcrossed with C57BL/6 wild type mice to generate F1 progeny. The heterozygous mice from this crossing were then selected and inter-crossed to generate F2 progeny. Some of these F2 mice were homozygous, and, at this point, we reconsidered the line established and these mice were used for phenotypical characterization as well as for breeding to expand the line. Eµ-PKCβIItg, littermate wt and non-littermate control mice were all maintained under identical conditions. Mice were generated and bred in-house within the Biological Services Units at the University of Liverpool and the University of Glasgow. Animal experiments were performed with appropriate approval and licenses from the UK Home Office. All experimental procedures involving animals were performed in accordance to guidelines approved by Animal Welfare and Ethical Review Bodies at the University of Liverpool and University of Glasgow and were carried out in accordance with standard animal housing conditions under local and home office regulations.
Figure 1.
Generation of Eµ-PKCβII transgenic mice. (A) Schematic representation of the plasmid construct (pEµ-PKCβII-IRES-mCherry) used to generate transgenic mice. (B) A20 cell line transfected with pEµ-PKCβII-IRES-mCherry plasmid using nucleofection. White arrow indicates fluorescent A20 cells that were succesfully transfected and express mCherry. Yellow arrow indicates those cells which did not take up the plasmid. (C) Southern blot analysis of genomic DNA isolated from tail cuttings of transgenic progeny to identify potential Eµ-PKCβIIItg founder mice. Copy number of the inserted gene was estimated by the accompanying standard curve of plasmid DNA. (D) Immunoblot analysis of protein extracted from splenic and liver tissue of homozygous Eµ-PKCβIItg mice. Western blots were performed using antibodies against HA (upper panel) or β-actin (lower panel). As positive control, protein lysates prepared from A20 cells that had been transiently transfected with pEµ-PKCβII-IRES-mCherry. (E) Upper and midde panel. Respective western blot analysis of PKCβII and β-actin expression in splenic tissue of wt (n = 3) and Eµ-PKCβIItg (n = 4) mice. Lower panel. Comparison of PKCβII expression in wt and Eµ-PKCβIItg mice. Expression of PKCβII was quantitated relative to β-actin. (F) Immunohistochemical staining of spleen sections from Eµ-PKCβIItg and wt mice with anti-HA (stained with Fast Red) and anti-PKCβII antibodies (brown with DAB). With respect to Eµ-PKCβIItg mice, the spleen sections are sequential where upper and lower images can be superimposed using the white arrow which points to the MZ and the yellow arrow which points to the follicle. The images in parts (C–E) have been cropped to efficiently show the relevant details, uncropped images can be viewed in the Supplementary information accompanying this manuscript. All elements of this figure have been published in the PhD thesis of AAA43.
Western blot analysis
Proteins were extracted from tissue samples as described previously23. Briefly, snap frozen tissue samples were homogenised under liquid nitrogen using pestle and mortar. The homogenised tissue was resuspended in buffer containing 20 mM HEPES, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1 mM PMSF and the following protease inhibitors: aprotinin (2 µg/ml), leupeptin (0.5 µg/ml), pepstatin A (1 µg/ml) and soybean trypsin inhibitor (100 µg/ml), subjected to 3 freeze/thaw cycles and then, following centrifugation, the supernatant was collected and protein concentration determined using a modified Bradford (BioRad, Hemel Hampstead UK). Equal amounts of protein were separated by SDS-PAGE, transferred on to Immobilon™ membranes (Millipore, Fisher Scientific UK Ltd, Loughborough, UK), and Ponceau-S staining was used to verify equivalent protein loading. The membranes were probed with the following antibodies according to established protocol11: PKCβII (C-18, Santa Cruz, Insight Biotechnology Ltd, Wembley UK) or HA [clone C29F4, (Cell Signaling Technologies, Hitchin, UK) or clone 16B12 (Covance, Cambridge Bioscience, Cambridge UK)] at a dilution of 1:1,000. Following incubation with secondary HRP-conjugated antibodies, protein bands were visualized using enhanced chemiluminescence (ECL) according to manufacturer’s protocol (GE Healthcare Life Sciences, Little Chalfont UK).
Histopathology and immunohistochemistry
Splenic tissue was fixed in 10% neutral buffered formalin and then embedded in paraffin. To remove paraffin and rehydrate splenic sections a PT Link device (DAKO, Stockport UK) was used according to the manufacturer's instructions. Immunohistochemical staining was performed using a DAKO Auto-stainer automated slide processing system and the EnVision™ FLEX/HRP kit (DAKO, K8012). The primary antibodies used were PKCβII (C-18) and HA (C29F4). Hematoxylin and eosin (H&E) staining of splenic sections was carried out according to standard protocols.
Cell preparation
Peritoneal cells were removed by injecting 5–10 ml ice cold modified PBS (1% BSA + 0.1% Sodium Azide, pH 7.2) into the peritoneal cavity followed by withdrawal of the peritoneal exudates. Spleens were removed and mechanically disrupted to prepare single cell suspensions. Erythrocytes in peripheral blood, peritoneal and splenic samples were lysed by treating with 0.165 M ammonium chloride, prior to washing the cell suspensions.
Flow cytometry
Flow cytometry was employed to characterize mature B cell subsets in spleen, peritoneum and peripheral blood comparing Eµ-PKCβII tg mice with wt mice, as indicated. Cells (2–5 × 106) were suspended in 200 µl modified PBS, then antibody cocktails were added and the cell suspension was incubated on ice for 30 min in the dark. Following this the cells were washed with 300 µl modified PBS, and then centrifuged at 2,000 rpm at 4 °C for 2 min. The supernatant was decanted and the pellet was re-suspended in 300 µl modified PBS. Labeled cells were acquired using a LSR-Fortessa flow cytometer (BD Biosciences) and analyzed using FACSDIVA™ and FlowJo™ (BD Biosciences, UK) software packages. Fluorescence-conjugated antibodies were purchased from BioLegend (London, UK) unless otherwise stated: Living Colors® DsRed mCherry (PT3647-2, Clontech), CD45R/B220-PE (RA3-6B2), CD5-PE/Cy5 (53–7.3), IgD-APC (11-26c.2a), CD43-FITC (S11), IgM-APC/Cy7 (RMM-1), CD21/CD35-PE/Cy7 (7E9), CD24-PerCP/Cy5.5 (M1/69), FITC-Rat IgG2bκ Isotype Ctrl (RTK4530) and CD45R/B220-Alexa Fluor® 488 (RA3-6B2). The percentage of B cell subsets, including FO (IgD+IgMdim), MZ (IgDdimIgM+CD43negCD21+CD24+) and B-1 (B220+IgDdimIgM+CD43+CD24+) B cell populations were largely similar between wt littermate and non-littermate animals (data not shown), therefore non-littermate and littermate wt mice were considered a single group of wt mice.
Calcium flux
Calcium flux analysis in isolated cells was carried out as described previously24. Briefly, splenic cells from Eµ-PKCβII tg hom/het mice were labelled with 1 μM Fura-2 AM (Invitrogen Ltd.) in buffer (145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 10 mM HEPES, 0·18% glucose and 0·2% BSA, adjusted to pH 7.4) at 37 °C for 30 min in the dark. Cells were washed, incubated with 10 μg/ml biotinylated anti-IgM for a further 30 min at 4 °C, washed and re-suspended at 1 × 106/ml in buffer. Cells (1.5 × 106) transferred to the fluorimeter cuvette were allowed to warm to 37 °C for 3 min prior to data acquisition. After recording basal fluorescence, 50 µl avidin was added to crosslink the BCR (final concentration 8.3 µg/ml) and recording continued for a further 3 min. Fluorescence was measured at 340 and 380 nm using a Hitachi F-7000 Fluorescence Spectrophotometer (Hitachi High Technologies America, Inc., Schaumburg, IL, USA).
Ig concentration analysis
Blood was extracted from mice and serum was prepared by pelleting the RBCs at 12,000g for 10 min. The serum was aliquoted and stored at – 20 °C until needed. Assays of IgM concentration in serum were performed using the LEGENDplex™ kit (BioLegend, UK) following the manufacturers’ instructions. IgM concentrations were calculated using the LEGENDplex™ data analysis software dongle.
Statistical analysis
All statistical analyses in this study were performed using GraphPad Prism® 8 software.
Results
Characterization of Eµ-PKCβII transgenic mice
Based on the Southern blotting analysis, the number of pEµ-PKCβIIHA-IRES-mCherry transgene copies integrated in the single site of the founder mouse genome was estimated to be greater than one, but less than 10 copies (Fig. 1C). PKCβIIHA expression was then analysed by Western blot analysis and detected in spleen but not in liver of 6 month-old mice homozygous for the PKCβIIHA transgene (hereafter Eµ-PKCβIItg mice) (Fig. 1D), suggesting that transgene expression is tissue specific. A comparison of total PKCβII expression in protein extracts derived from the splenic tissue showed that PKCβII was expressed at significantly higher levels in Eµ-PKCβIItg mice compared with wt counterparts (Fig. 1E). In addition, analysis of HA expression within the spleen revealed that expression was concentrated within the follicle area of the peri-arteriolar lymphoid sheaths (PALS) and MZ, both of which are B cell rich areas (Fig. 1F). Although total PKCβII expression in the spleen of transgenic and wt mice showed a similar staining pattern, the intensity of staining was always greater in the tissue from transgenic mice where it correlated with that of HA. We were not able to detect the expression of mCherry in Eµ-PKCβIItg mice (data not shown). This may be because expression of a secondary gene from an IRES sequence can be variable and not always efficient in transgenic mice and therefore might have been below detection level25.
Eµ-PKCβIItg mice aged normally and did not show any signs of illness when aged up to 14 months. The WBC count of Eµ-PKCβIItg mice was in a normal range and did not differ from that in wt mice (Table 1). In addition, the spleen weight did not change significantly between Eµ-PKCβIItg mice and wt mice, and although there appeared a small but significant increased ratio of B cells to combined T/B lymphocytes in the spleen of EµPKCβIItg compared to wt mice, this ratio remained similar in the peripheral blood and peritoneum between these animals.
Table 1.
Comparison of spleen weight, WBC count and B/B + T lymphocyte ratio in Eµ-PKCβIItg and wt control mice.
| Wild type | Std | Eµ-PKCβIItg | Std | P value | |
|---|---|---|---|---|---|
| Spleen weight (mg) |
129.4 n = 7 |
9.02 |
120.4 n = 9 |
5.9 | 0.63 |
| WBC count (× 106 per ml) |
3.02 n = 7 |
0.78 |
3.74 n = 11 |
0.62 | 0.41 |
| B cell/B + T cells | |||||
| Spleen |
0.60 n = 10 |
0.046 |
0.73 n = 13 |
0.064 | 0.024 |
| Peritoneum |
0.85 n = 6 |
0.014 |
0.87 n = 10 |
0.024 | 0.25 |
| Peripheral blood |
0.71 n = 4 |
0.063 |
0.65 n = 10 |
0.052 | 0.44 |
B/B + T lymphocyte ratio in spleen, peritoneum and peripheral blood were determined using a FACS-based protocol identifying B cells (B220+CD5− live lymphocytes) and T cells (B220−CD5+ live lymphocytes). Statistical analysis was performed using a Mann–Whitney U-test.
Splenic tissue from Eµ-PKCβIItg mice display an expansion of the MZ B cell population and a concomitant reduction of the FO B cell population
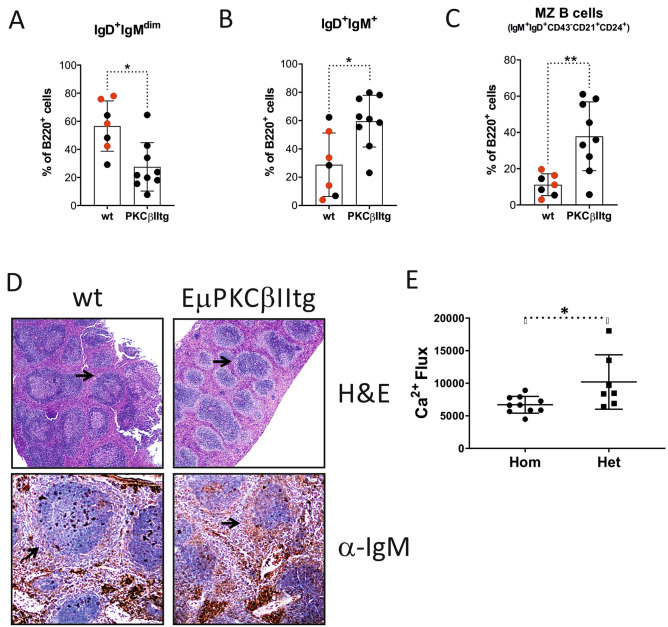
Although total B cell counts were similar between EµPKCβIItg and wt mice, differences were observed between discrete populations. We applied the gating strategy shown in Supplementary Figure 2 for the analysis of B cells within splenic tissue from wt and Eµ-PKCβIItg mice. Thus, B220+ cells were first analysed for surface IgD and IgM expression. Eµ-PKCβIItg mice exhibited an increased percentage of IgD+ IgM+ B cells and decreased percentage of IgD+ IgMdim B cells compared to wt control mice (Fig. 2A,B). Analysis of the IgD+ IgMdim B cells identified them as mainly FO B cells because of their expression of CD21 and CD24, the phenotype of these cells seemed consistent between wt and Eµ-PKCβIItg mice (Supplementary Figure 2), despite lower numbers of these cells in the latter. The IgD+ IgM+ B cells were further gated for CD43 expression, and cells negative for this antigen were further analyzed for CD21 and CD24 expression. The major population identified, IgD+IgM+CD43negCD21+CD24+, are “MZ-like” in phenotype and resemble a described precursor MZ cell population known as marginal zone precursor B cells26,27. This population of “MZ-like” B cells showed significantly greater representation in splenic tissue from Eµ-PKCβIItg compared to wt mice, suggesting a strong bias towards this phenotype in the former (Fig. 2C). The bias towards this MZ-like phenotype was further corroborated by H&E stains showing an extended MZ in Eµ-PKCβIItg compared with wt mice (Fig. 2D, upper panels), identified by anti-IgM staining (Fig. 2D, lower panels). Interestingly, this is the same area that seemed to stain strongly for PKCβII and HA in splenic tissue of transgenic mice (Fig. 1F). Of note, IgD+ IgM+ CD24+ CD43neg cells negative for CD21 were present in splenic tissue from Eµ-PKCβIItg, but not from wt mice. This population may represent immature B cells but was not further analysed in this study. Functional analysis of splenic B cells from transgenic animals showed that BCR-induced Ca2+ flux was significantly suppressed in cells isolated from Eµ-PKCβIItg mice (Fig. 2E), an observation that is in line with the role of PKCβ in regulating the function of Btk and PLCγ2-induced calcium release5. Thus, these data suggest that B cell-targeted over-expression of PKCβII functionally restricts antigen receptor signaling resulting in an expansion of splenic MZ B cells in Eµ-PKCβIItg mice.
Figure 2.
Effect of B cell-targeted expression of PKCβII on B cell populations in splenic tissue from Eµ-PKCβIItg and wt mice. Single cell suspensions prepared from spleens isolated from Eµ-PKCβIItg and wt mice were stained with a cocktail of antibodies containing B220, IgM, IgD, CD43, CD21 and CD24 markers, and then analysed by flow cytometry. Quantitative comparisons were then made of the percentage B220+ cells within the following gates (A) IgD+ IgMdim, (B) IgD+ IgM+, and (C) IgD+ IgM+ CD43− CD21+ CD24+ (MZ-like B cell) for wt and Eµ-PKCβIItg mice as defined by the strategy illustrated in Supplementary Figure 2. Black dots in these graphs refer to wt and homozygous Eµ-PKCβIItg progeny derived from mating heterozygous Eµ-PKCβIItg mice. Red dots refer to wt mice with similar genetic backgrounds (C57BL/6) that are alike in terms of age to the transgenic mice. (D) upper panels H&E staining of splenic tissue from wt and Eµ-PKCβIItg mice. Lower panels anti-IgM staining of spleen sections from wt and Eµ-PKCβIItg mice. These images are representative of n = 2 experiments using splenic tissue from different mice that had been aged in excess of 12 months. Inset arrows indicate MZ. These histogram images have been published in the PhD thesis of AAA43. (E) BCR-induced Ca2+ flux in isolated splenic B cells from heterozygous and homozygous Eµ-PKCβIItg mice. Total flux was calculated as area under the curve is reported in arbitrary units. Statistical analysis for parts (A) (*P = 0.012), (B) (*P = 0.016), (C) (**P = 0.0052) and (E) (*P = 0.024) was performed using a Mann–Whitney U test.
The peritoneum of Eµ-PKCβII transgenic mice contains an elevated B-1 cell population
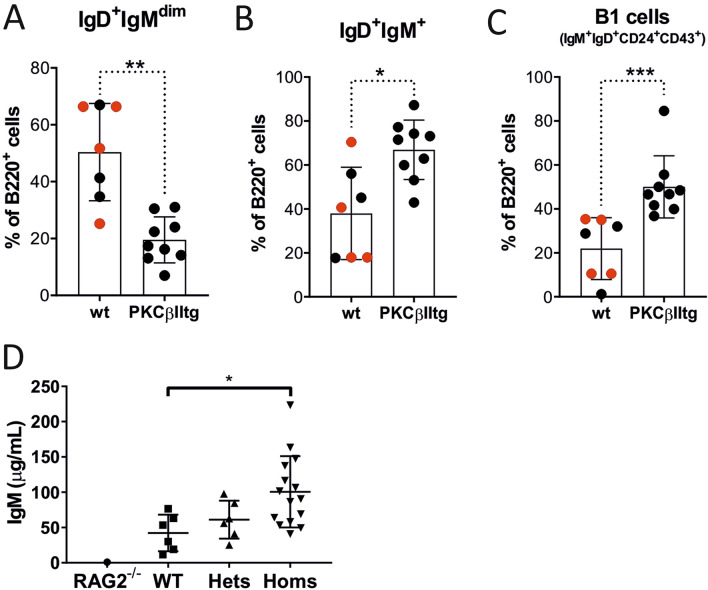
Peritoneal B220+ B cells exhibited a significant decrease in the percentage of IgD+ IgMdim cells, coupled with significant increase in the percentage of IgDdim IgM+ cells in Eµ-PKCβIItg mice when compared to wt mice (Fig. 3A,B, Supplementary Figure 3). Further analyses revealed that the populations of IgD+ IgMdim cells defined by CD24 and CD43 expression were largely similar between wt and Eµ-PKCβIItg mice (Supplementary Figure 3). However, similar analysis of IgDdim IgM+ cells showed that the proportion of CD24+CD43+ cells in Eµ-PKCβIItg mice was significantly increased compared to wt mice (Fig. 3C). These cells carry a B-1 B cell phenotype (B220+ IgM+ IgDdim/− CD43+ CD24hi) and are likely to be B-1a cells because the majority of them are also positive for CD5 (Supplementary Figure 3). Taken together, these results suggest that B cell-targeted over expression of PKCβII results in accumulation of B-1a B cells in the peritoneum of Eµ-PKCβIItg mice.
Figure 3.
Effect of B cell-targeted expression of PKCβII on B cell populations in the peritoneum of Eµ-PKCβIItg and wt mice. Single cell suspensions prepared from peritoneal wash of Eµ-PKCβIItg and wt mice were stained with antibodies to B220, IgM, IgD, CD43, CD24, and CD5 and analyzed by flow cytometry. Quantitative comparisons were then made of the percentage B220+ cells within the following gates (A) IgD+ IgMdim, (B) IgDdim IgM+, and (C) IgDdim IgM+ CD43+ CD24+ (B-1 cells) for wt and Eµ-PKCβIItg mice as defined by the strategy illustrated in Supplementary Figure 3. Black dots in these graphs refer to wt and homozygous Eµ-PKCβIItg progeny derived from mating heterozygous Eµ-PKCβIItg mice. Red dots refer to wt mice with similar genetic backgrounds (C57BL/6) that are alike in terms of age to the transgenic mice. (D) Comparison of IgM levels in serum derived from Rag2 deficient, wt, and heterozygous and homozygous Eµ-PKCβIItg mice. Statistical analysis for parts (A) (**P = 0.0040), (B) (*P = 0.042), and (C) (***P = 0.0010) was performed using a Mann–Whitney U test, for part (D) analysis was done using a one-way ANOVA and Tukey’s multiple comparisons test (P = 0.040).
Serum IgM levels are increased in Eµ-PKCβIItg mice
Consistent with the increased proportion of MZ and B-1a cells observed in spleen and peritoneum, Eµ-PKCβIItg mice also exhibited elevated levels of serum IgM compared to wt animals (Fig. 3D). As a negative control we analysed serum derived from a single Rag1−/− mouse to show the background of detection for the assay used.
Peripheral blood from Eµ-PKCβIItg mice contains an expanded population of immature B cells
When B220+ B cells derived from peripheral blood were analysed for IgD and IgM expression (Supplementary Figure 4), an expansion of IgDdim IgM+/− B cells was evident in Eµ-PKCβIItg mice compared to wt mice as was a reduction of IgD+ IgM+/− B cells (Fig. 4). Analysis of CD21 expression on IgDdim IgM+/− B cells and IgD+ IgM+/− B cells from Eµ-PKCβIItg and wt mice showed that the former were CD21neg while the latter were CD21+ (Supplementary Figure 4). As CD21 is a marker of B cell maturity28, the lack of CD21 expression on IgDdim IgM+/− B cells from Eµ-PKCβIItg mice suggests that these cells are most likely immature B cells. Considering that the WBC and the B: B/T ratio in peripheral blood is not significantly different between Eµ-PKCβIItg and wt mice (Table 1), these results suggest that B cell-directed expression of PKCβII results in a shift towards expansion of the immature B cell population in peripheral blood.
Figure 4.
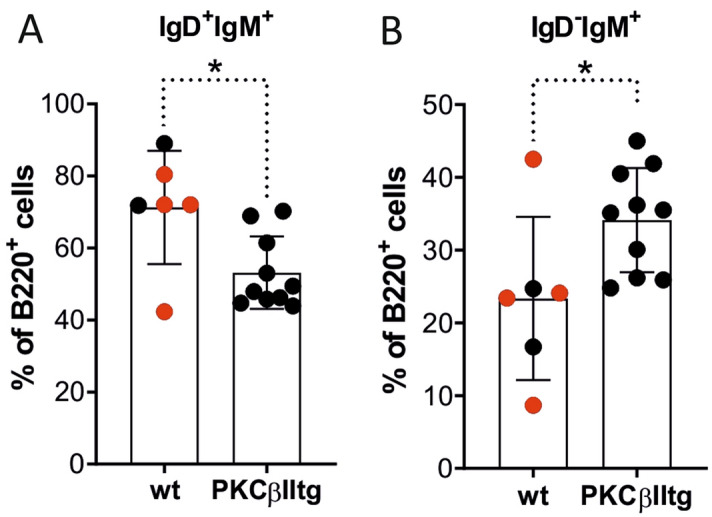
Effect of B cell-targeted expression of PKCβII on B cell populations in peripheral blood of Eµ-PKCβIItg and wt mice. Single cell suspensions prepared from peripheral blood and bone marrow of Eµ-PKCβIItg and wild type mice were stained with antibodies to B220, IgM, IgD, and CD21 and analyzed by flow cytometry. Quantitative comparisons were then made of the percentage B220+ cells within the following gates (A) IgD+ IgM+/−, and (B) IgDdim IgM+/− for wt and Eµ-PKCβIItg mice as defined by the strategy illustrated in Supplementary Figure 4. Black dots in these graphs refer to wt and homozygous Eµ-PKCβIItg progeny derived from mating heterozygous Eµ-PKCβIItg mice. Red dots refer to wt mice with similar genetic backgrounds (C57BL/6) that are alike in terms of age to the transgenic mice. Statistical analysis for parts (A) (*P = 0.030) and (B) (*P = 0.023) was performed using a Mann–Whitney U test.
Discussion
Upregulated expression of PKCβII is frequently observed in the malignant cells of B lymphoproliferative diseases29, and, in particular, CLL where its overexpression is a phenotypic feature11. Because of this, we aimed to analyze the effect of PKCβII overexpression on the B cell compartment by generating a transgenic mouse strain where expression of PKCβII was driven by the Eµ promoter. Consistent with our initial hypothesis, the consequence of B cell-directed PKCβII overexpression is a proportional change in FO, MZ-like, B-1 and immature B cell populations respectively in the spleen, peritoneum and peripheral blood of Eµ-PKCβIItg mice. Further examination of the Eµ-PKCβIItg mice shows suppression of BCR signaling in splenic B cells, and elevated levels of serum IgM. Although expansion of the B-1 cell compartment is a feature of several mouse models of CLL15–19, we did not observe disease in Eµ-PKCβIItg mice and the proportion of B cells to combined B/T cells (taken as total lymphocytes) in these animals remained largely similar to that of wt mice despite aging them for 15 months. We conclude that PKCβII plays an important role in B cell development, with overexpression resulting in biased MZ-like and B-1 B cell development, but not in the pathogenesis of a CLL-like or other B lymphoproliferative disease in Eµ-PKCβIItg mice.
A limitation of this study is that our findings are based on data generated from analysis of animals that were derived from a single founder that was minimally backcrossed into the parental strain. The phenotype we describe could therefore potentially be a result of positional effect or an unidentified factor involved in producing the Eµ-PKCβIItg mice. However, comparison of B cell phenotypes within spleen, peritoneum and peripheral blood from the wt mice we analyzed are similar between littermate and non-littermate (i.e. C57BL/6 background) wt animals. These findings indicate that the genetic background of the strains used to generate Eµ-PKCβIItg mice had little, if any, effect on our observations. Furthermore, the phenomenon we observe is consistent with a mechanism whereby higher expression of PKCβII within B cells of Eµ-PKCβIItg mice could limit BCR signaling by suppressing Btk activation. A well-described role of PKCβ in B cells is the phosphorylation of the S180 residue in Btk leading to downregulation of its function4,5,30. Our own work shows that overexpressed PKCβII in primary CLL cells limits BCR signaling in the presence of pS18011,31. The novel mouse model we describe herein recapitulates these findings and we show that BCR signaling in B cells from Eµ-PKCβIItg mice is suppressed. In contrast, a study describing the phenotype of PKCβ knockout mice showed enhanced BCR-mediated signaling in B cells from these animals4, a phenomenon similar to that observed in a study of PKCβ inhibition on BCR signaling32. This same study also showed that MZ and B1 B cell populations, as well as serum IgM levels were severely reduced in PKCβ knockout mice4. Together, these observations provide support to our interpretation that suppressed BCR signaling resulting from targeted PKCβII overexpresson in Eµ-PKCβIItg mice leads to expansion of MZ-like and B1 B cells within total B cell populations and increased serum IgM levels. Further complementary support can be found in our observation that splenic B cell populations are slightly increased in Eµ-PKCβIItg mice, whilst Leitges et al.4 observed a slight decrease in these populations in their PKCβ knockout mice. Taken together, these observations suggest that the phenotype we observe is most likely due to transgenic overexpression of PKCβII rather than a confounding factor.
In the current study we have focused on PKCβII and have not considered its splice variant PKCβI. Since we have not silenced the expression of the gene that codes for PKCβ in our system, we cannot rule out the contribution of PKCβI to the phenotype we have generated. Future studies crossing the Eµ-PKCβIItg mouse with a PKCβ null mouse would enable us to determine the role of PKCβII in B cells more precisely.
It is intriguing that despite the noted lineage biases observed in B cell populations in the Eµ-PKCβIItg mice, aged animals did not develop a disease endpoint. A possible reason for this could be that overexpression of PKCβII alone is not sufficient to drive the pathogenesis in B lymphoid diseases. However, Eµ-PKCβIItg mice may be more susceptible to the development of these diseases since it has been demonstrated that, for example, in colon cancer, overexpression of PKCβII promotes development of disease by increasing the sensitivity of colonic epithelial cells to carcinogenic stimuli33,34. Indeed, this may be particularly relevant for CLL because this disease appears to develop from a lymphocytosis of B-1 cells in both mice and humans14–17. If this hypothesis is correct, Eµ-PKCβIItg mice crossed with another mouse model of CLL, such as the Tcl-1 model, would exhibit accelerated development of disease because of the expanded population of B-1 cells. An alternative reason for the lack of disease development may be that the expression levels of PKCβII achieved in our mouse model are not high enough to facilitate neoplastic transformation. Our previous work in CLL showed that the malignant cells in this disease express up to sevenfold greater amounts of this PKC isozyme than do normal B cells17. In the current study we were only able to achieve an approximate twofold increase of PKCβII in Eµ-PKCβIItg compared to wt mice.
We propose that the modest increase in protein levels of PKCβII observed in Eµ-PKCβIItg mice alters BCR signaling resulting in skewed representation of B cell population subsets in the periphery with expansion of MZ-like cells, B-1 B cells and immature B cells as we describe in this study. PKCβII in B cells has two key targets; CARMA1 (CARD11) which, when phosphorylated, combines with MALT1 and Bcl10 to form a complex that recruits TAK1 to stimulate activation of the NFκB and JNK signaling pathways and promote cell survival35–37, and Btk where S180 phosphorylation downregulates its function as discussed above5. Hence, overexpression of PKCβII will preserve survival of B cells while limiting their ability to respond to BCR engagement. Such limitation is likely to affect B cell differentiation and our observations can be explained by the "strength of signal" model suggested by Cariappa et al.38,39 where transitional B cells in the periphery respond to strong or "triggered" signals delivered via the BCR with commitment to FO B cell differentiation, while weaker or "tickled" BCR signals lead to differentiation towards MZ and B-1 B cells. The absence of BCR signals results in cell death due to neglect. This is likely the explanation for the phenotype of the MZ-like B cells we identify. These cells possess similarity to MZ precursor B cells26, the presence of which are directly related to BCR signaling strength as is demonstrated in Aiolos- and CD22-deficient mice27,38,40. Weakening of BCR signaling by overexpression of PKCβII is also likely to be responsible for the increased number of immature B cells in peripheral blood we observe in Eµ-PKCβIItg mice. Elimination of self-reactive B cell clones occurs at the pre-B cell stage within bone marrow, and is achieved either by receptor editing whereby B cell development is arrested while further gene rearrangements occur in order to mitigate self-reactivity41, or by the induction of apoptosis by strong BCR signals42. Since both of these processes are driven by Btk8, overexpression of PKCβII within pre-B cells of Eµ-PKCβIItg mice could limit strong self-antigen-driven BCR signaling by suppressing Btk activation thus generating the increased numbers of immature B cells seen here. We propose that the modestly increased levels of PKCβII in Eµ-PKCβIItg mice alters BCR signaling such that B cell subsets become skewed in the periphery as demonstrated; populations of MZ-like cells, B-1 B cells and immature B cells all expand5,8,26,27,35–42.
In conclusion, we have generated a transgenic mouse strain with targeted overexpression of PKCβII within B lineage cells; Eµ-PKCβIItg. We show that the phenotype associated with Eµ-PKCβIItg mice skews development of MZ-like and immature B cells over FO B cells in the spleen and peripheral blood, and of B-1 B cells in the peritoneum, thereby providing in vivo data that support our hypothesis that overexpression of PKCβII might result in an expansion of B1 and MZ B cell populations. Considering the phenotype of the PKCβ knockout mouse3, we have speculated that the increased populations of MZ-like and B-1a cells within the Eµ-PKCβIItg mouse would generate a phenotype where IgM antibodies would be prevalent and this was indeed the case. Thus, we have demonstrated that PKCβII specifically plays an important role in B cell development in mice. This novel mouse strain may be useful in studies of diseases involving BCR signaling, and, in particular, CLL.
Supplementary information
Acknowledgements
The authors would like to acknowledge the assistance provided by the Flow Cytometry and Cell Sorting Facility and Biological Services Units of the University of Liverpool and the University of Glasgow. JRS was in receipt of LLR funding (Ref 13013). AMM was in receipt of LLR funding (Ref. 13012). The experiments reported here also feature in the doctoral thesis of AAA43. This paper is dedicated to Dr Ferahim A. Azar.
Author contributions
A.A.A. designed the study, performed experiments, analyzed data and wrote the paper. A.M.M., A.T., N.M., G.K.M. and K.J.T. performed experiments and analyzed data. J.R.S. and N.V. designed the study, performed experiments, analyzed data and wrote the paper.
Competing interests
JRS has received funding from Verastem Inc., and has a collaborative relationship with the Netherlands Translational Research Center B.V. The other authors of this manuscript have no potential competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nikolina Vlatković and Joseph R. Slupsky.
Contributor Information
Nikolina Vlatković, Email: vlatko@liverpool.ac.uk.
Joseph R. Slupsky, Email: jslupsky@liverpool.ac.uk
Supplementary information
is available for this paper at 10.1038/s41598-020-70191-y.
References
- 1.Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer. 2018;18:148–167. doi: 10.1038/nrc.2017.121. [DOI] [PubMed] [Google Scholar]
- 2.Yam-Puc, J., Zhang, L., Zhang, Y. & Toellner, K. Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Research7, (2018). [DOI] [PMC free article] [PubMed]
- 3.Spitaler M, Cantrell DA. Protein kinase C and beyond. Nat. Immunol. 2004;5:785–790. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- 4.Leitges M, et al. Immunodeficiency in protein kinase cβ-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 5.Kang SW, et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 2001;20:5692–5702. doi: 10.1093/emboj/20.20.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerner JD, et al. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 7.Khan WN, et al. Defective B-cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 8.Maas A, Hendriks RW. Role of Bruton's tyrosine kinase in B cell development. Dev. Immunol. 2001;8:171–181. doi: 10.1155/2001/28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens L, Rhee L, Parker PJ, Ullrich A. Alternative splicing increases the diversity of the human protein kinase C family. DNA. 1987;6:389–394. doi: 10.1089/dna.1987.6.389. [DOI] [PubMed] [Google Scholar]
- 10.Kubo K, Ohno S, Suzuki K. Primary structures of human protein kinase C beta I and beta II differ only in their C-terminal sequences. FEBS Lett. 1987;223:138–142. doi: 10.1016/0014-5793(87)80524-0. [DOI] [PubMed] [Google Scholar]
- 11.Abrams ST, et al. B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase CβII. Blood. 2007;109:1193–1201. doi: 10.1182/blood-2006-03-012021. [DOI] [PubMed] [Google Scholar]
- 12.Holler C, et al. PKCβ is essential for the development of CLL in the TCL1 transgenic mouse model: Validation of PKCβ as a therapeutic target in CLL. Blood. 2009;113:2791–2794. doi: 10.1182/blood-2008-06-160713. [DOI] [PubMed] [Google Scholar]
- 13.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: Cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert M, et al. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J. Exp. Med. 2012;209:2183–2198. doi: 10.1084/jem.20120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bichi R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl. Acad. Sci. USA. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein U, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Planelles L, et al. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 2004;6:399–408. doi: 10.1016/j.ccr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Santanam U, et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc. Natl. Acad. Sci. USA. 2010;107:12210–12215. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michie AM, Nakagawa R, McCaig AM. Murine models for chronic lymphocytic leukaemia. Biochem. Soc. Trans. 2007;035:1009–1012. doi: 10.1042/BST0351009. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): Biology, natural history and clinical management. Leukemia. 2010;24:512–520. doi: 10.1038/leu.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J. Exp. Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 23.Athwal T, et al. Expression of human cationic trypsinogen (PRSS1) in murine acinar cells promotes pancreatitis and apoptotic cell death. Cell Death Dis. 2014;5:e1165. doi: 10.1038/cddis.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCaig AM, Cosimo E, Leach MT, Michie AM. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br. J. Haematol. 2011;153:199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 25.Kozak M. A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res. 2005;33:6593–6602. doi: 10.1093/nar/gki958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava B, Quinn WJ, III, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 28.Allman D, Pillai S. Peripheral B cell subsets. Curr. Opin. Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decouvelaere AV, Morschhauser F, Buob D, Copin MC, Dumontet C. Heterogeneity of protein kinase C β2 expression in lymphoid malignancies. Histopathology. 2007;50:561–566. doi: 10.1111/j.1365-2559.2007.02666.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, et al. A negative-feedback function of PKCβ in the formation and accumulation of signaling-active B cell receptor microclusters within B cell immunological synapse. J. Leuk. Biol. 2015;97:887–900. doi: 10.1189/jlb.2A0714-320R. [DOI] [PubMed] [Google Scholar]
- 31.Abrams ST, Brown BRB, Zuzel M, Slupsky JR. Vascular endothelial growth factor stimulates protein kinase CβII expression in chronic lymphocytic leukaemia cells. Blood. 2010;115:4447–4454. doi: 10.1182/blood-2009-06-229872. [DOI] [PubMed] [Google Scholar]
- 32.Venkataraman C, et al. Selective role of PKCβ enzymatic function in regulating cell survival mediated by B cell antigen receptor cross-linking. Immunol. Lett. 2006;105:83–89. doi: 10.1016/j.imlet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Gökmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase CβII is an early promotive event in colon carcinogenesis. Cancer Res.. 2001;61:1375–1381. [PubMed] [Google Scholar]
- 34.Murray NR, et al. Overexpression of protein kinase CβII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J. Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara H, Maeda S, Watarai H, Kurosaki T. IκB kinase beta-induced phosphorylation of CARMA1 contributes to CARMA1 Bcl10 MALT1 complex formation in B cells. J. Exp. Med. 2007;204:3285–3293. doi: 10.1084/jem.20070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara H, et al. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su TT, et al. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 38.Cariappa A, et al. The Follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 39.Bowers EM, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: Identification of a selective small molecule inhibitor. Chem. Biol. 2010;17:471–482. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samardzic T, et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: An approach by autoreactive B cells to escape tolerance. J. Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 43.Azar, A. A. Generation of PKCβII transgenic mice for the study of chronic lymphocytic leukaemia Ph.D. thesis, University of Liverpool (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.