Abstract
The present study was designed to explore the role of microRNA-197-3p in regulating the epithelial–mesenchymal cellular transition in ovarian cancer. The results showed that miR-197 to be significantly (P < 0.05) downregulated in human ovarian cancer tissues and cell lines. Overexpression of miR-197 significantly (P < 0.05) reduced the proliferation of OVACAR-3 cancer cells. Additionally, the colony formation of the OVACAR-3 cells was inhibited by 59% relative to control. The migration and invasion of the OVACAR-3 cells was inhibited by 64% and 72%, respectively, upon miR-197 overexpression. Western blot analysis showed miR-197 was found to upregulate the expression of E-cadherin, while the expression of N-cadherin, vimentin, and snail proteins was found to decrease significantly (P < 0.05). TargetScan analysis together with dual luciferase assay revealed that miR-197 exerts its effects by targeting ABCA7 in ovarian cancer. ABCA7 was significantly (P < 0.05) overexpressed in ovarian cancer tissues and cell lines. However, silencing of ABCA7 resulted in significant inhibition of cell proliferation, migration, and invasion. Nonetheless, overexpression of ABCA7 could abolish the tumor-suppressive effects of miR-197 on the OVACAR-3 cells. Taken together, miR-197 acts a tumor-suppressive in ovarian cancer and points towards its therapeutic implications in the treatment of ovarian cancer.
Keywords: microRNA, Ovarian cancer, Epithelial–mesenchymal transition, Metastasis, ATP-binding cassette transporter
Introduction
Ovarian cancer is the seventh most prevalent malignancy in women with more than 238, 000 cases diagnosed and 151, 000 deaths in 2012 across the globe. Among gynecologic cancers, it is ranked third after cervical and uterine cancer (Doherty et al. 2017). Additionally, it is ranked fourth in terms of the mortality caused by this disease (Jayson et al. 2014). Although ovarian cancer has a lower prevalence relative to breast cancer, it is much more lethal, and it is has been reported that the mortality rate of ovarian cancer will increase significantly by the year 2040, the mortality rate of this cancer will rise significantly (Momenimovahed et al. 2019). The high mortality rate of ovarian cancer is ascribed to its late diagnosis where the metastasis to the abdomen region is highly common (Forstner et al. 2016). The advancement in treatment and diagnostic has enabled treatment of early stage ovarian cancer patients with high success rates. However, little clinical success has been achieved for the patients with advanced stage of the disease. Thus, it becomes necessary to understand the progression of ovarian cancer at molecular level to formulate better treatment tactics against this deadly disorder. During the recent times, the studies have emphasized on the exploration of various developmental aspects regulated by microRNAs (miRs). The miRs constitute a group of short non-coding RNAs which act at post-transcriptional level of gene regulation (Bartel 2004). The miRs function in silencing of protein-coding genes at translational level to accomplish their regulatory role (Fabbri et al. 2008). They are seen to regulate the vital features of cell differentiation, maintain cellular identity, and control the apoptosis of eukaryotic cells (Kosik 2010). Besides, the miRs are important to development of various human pathologies (Bandiera et al 2010). The role of miRs in human cancer growth and proliferation is well established (Wang et al. 2016a, b). Many studies have showed that miRs regulate the tumorigenic behavior of cancer cells including the ovarian cancer (Dahiya and Morin 2010). MicroRNA-197-3p (now onwards referred as miR-197) has been shown to be involved the development, tumorigenesis, and progression of different human cancers. It has been shown to control the metastasis of hepatocellular carcinoma cells via modulation of Wnt/β-catenin signaling (Hu et al. 2018). In a similar study, it has also been reported to act as prognostic marker for hepatocellular carcinoma (Ni et al. 2019). Studies have also revealed the role of miR-197 in regulation of drug resistance in gastric cancer cells (Xiong et al. 2015) and colorectal cancer cells (Sun et al. 2015). In yet another study, miR-197 has been shown to suppress the proliferation of glioblastoma cells (Tian et al. 2016). The miR-197 has been shown to function as tumor suppressor in number of human ovarian cancer (Zou et al. 2015). Additionally, it has been reported to regulate the epithelial–mesenchymal transition of cancer cells like those of pancreatic cancer (Hamada et al. 2013). Nonetheless, the role of miR-197 in the regulation of epithelial–mesenchymal transition in ovarian cancer cells is yet to be elucidated. This study was, therefore, designed to investigate role of miR-197 in the regulation of epithelial–mesenchymal transition in ovarian cancer cells via modulation of ATP-binding cassette transporter A7 (ABCA7).
Materials and methods
Clinical tissue specimen and cell line procurement
Fifteen ovarian cancer tissues and as many adjacent normal tissues were obtained from the cancer patients prior to chemotherapy at the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, Hubei, China, 445,000. Informed consent was obtained from the patients and the study was approved by the research ethics committee institute under approval number AHS-111-C52-2019. Standard scientific ethical guidelines were followed for the experimental usage of clinical specimens. The specimens were maintained in the deep freezers at – 80 °C. Four human ovarian cancer cell lines (A2780, SK-OV-3, OVACAR-3, and Caov3) along with the normal ovarian epithelial cell line (HOSE-6–3) were procured from American Type Collection Center (ATCC, USA). The Roswell Park Memorial Institute Medium (RPMI) 1640 medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) was used for culturing of the cell lines. The culturing of cell lines was performed at 37 °C with 5% CO2 using the humidified incubator.
Transfection
The transfection of OVACAR-3 cancer cells was done using the lipofectamine 2000 reagent (Thermo Fisher Scientific) as per the manufacturer’s protocol. The miR-NC (control) and miR-197 mimic (overexpression) constructs along with the silencing construct of ABCA7 (si-ABCA7) and its negative control (si-NC) were purchased from RiboBio company, China. pcDNA3.1 overexpression vector was used for overexpression of ABCA7.
Expression analysis
Total RNA extraction from the clinical tissues and cell lines was performed with the help of RNeasy mini kit (Qiagen) which was then used to synthesize the complementary DNA (cDNA) using miScript Reverse Transcription kit (Qiagen). The cDNA was used as template in the quantitative real-time polymerase chain reaction (qRT-PCR) performed using SYBR Green PCR master mix (Thermo Fisher Scientific). The PCR reactions were performed on the Quant Studio 5.0 Real-Time PCR (Applied Biosystems). The cycling conditions were as follows: 95 °C for 20 s, followed by 40 cycles of 95 °C for 15 s, and 58 °C for 1 min. The relative gene expression levels were determined with 2−∆∆Ct method. Human β-actin was used as internal control in the gene expression studies. The real-time primers of miR-197 were sense, 5′-GTTCACCACCTTCTCCAC-3′ and antisense 5′-GTGCAGGGTCCGAGGT-3′ and the primers for ABCA7 gene were sense, 5′-GTGCTATGTGGACGACGTGTT-3′ and antisense, 5′-TGTCACGGAGTAGATCCAGGC-3′.
Cell viability assay
The proliferation of the stably transfected OVACAR-3 ovarian cancer cells was determined through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. In brief, the transfected cells were cultured in 96-well plates for 0, 12, 24, 48, or 96 h at 37 °C. The wells were inoculated with MTT reagent (Sigma-Aldrich) with 0.5% final concentration. The incubation at 37 °C was prolonged for 4 h again, and then, dimethyl sulfoxide (DMSO) (250 µl) was added for dissolving the formazan crystals. Finally, absorbance at 570 nm was recorded to determine the cell proliferation.
Clonogenic assay
The clonogenic assay was performed to analyze the colony forming potential of the transfected cancer cells. The transfected OVACAR-3 cells were harvested at the exponential phase of growth and were then counted using a hemocytometer. The cells were cultured at low densities (200 cells/well) for 10 days in 6-well plates at 37 °C. The colonies formed were washed with phosphate-buffered saline (PBS), ethanol fixed, and stained with 0.1% crystal violet (Sigma-Aldrich) solution. Finally, the colonies were examined under light microscope and percent colony number was calculated to analyze the cell viability.
Transwell chamber assay
The migration of transfected cancer cells was determined by transwell chamber method. Approximately, 105 transfected cells were cultured in the upper chamber of transwell plate which was separated from the underlying chamber by filter paper of 8 µm pore size. The lower chamber contained RPMI 1640 medium supplemented with 10% FBS. After cell culturing for 48 h at 37 °C, the filter paper was carefully removed, and its upper surface was cleared from the adhering cells. The cells sticking to lower surface were washed with PBS, fixed with 4% paraformaldehyde, and then stained using 0.1% crystal violet. Finally, the cells were examined under high power light microscope to determine the cell migration. Also, the percent cell migration was calculated using random microscopic fields. The invasion of cancer cells was also determined in the same manner except for the transwell chamber plate was fitted with matrigel.
miR target analysis
The potential target of miR-197 was predicted using its online scanning through TargetScan (https://www.targetscan.org/) database. To confirm the target prediction, the specific target region of 3′-UTR of ABCA7 was cloned into the pmirGLO luciferase vector (Promega). Also the mutated 3′-UTR of ABCA7 (MUT) was cloned into the luciferase vector. The OVACAR-3 cancer cells were co-transfected with miR-197-mimics or miR-NC in combination with 100 ng luciferase constructs of ABCA7 3′-UTR WT or MUT. The cells were then cultured for 24 h at 37 °C and luciferase activity was determined using Dual Luciferase Reporter assay kit (Promega) using the manufacturer luciferase assay guidelines.
Western blotting
Using RIPA buffer (Cell Signaling Technology, Massachusetts, United States), the total proteins were isolated from the transfected cancer cells and their quantification was done using Bradford’s method. Following, equal proteins were loaded from each sample and separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The blotting was performed using nitrocellulose membranes. The membranes were exposed to the specific primary antibodies at 4 °C overnight and then exposure of secondary antibodies was given for 2 h at room temperature. Afterwards, the enhanced chemiluminescence reagent was used to visualize the specific protein bands. Human β-actin protein served as the expression control in the immune-blotting studies.
Statistical analysis
The statistical analysis was performed using Graphpad prism 7.0 software and the values were given as mean ± standard deviation. Student’s t test was performed to assess the differences between two groups and the P values < 0.05 were taken as the measure of statistically significant difference.
Results
miR-197 is significantly downregulated in ovarian cancer
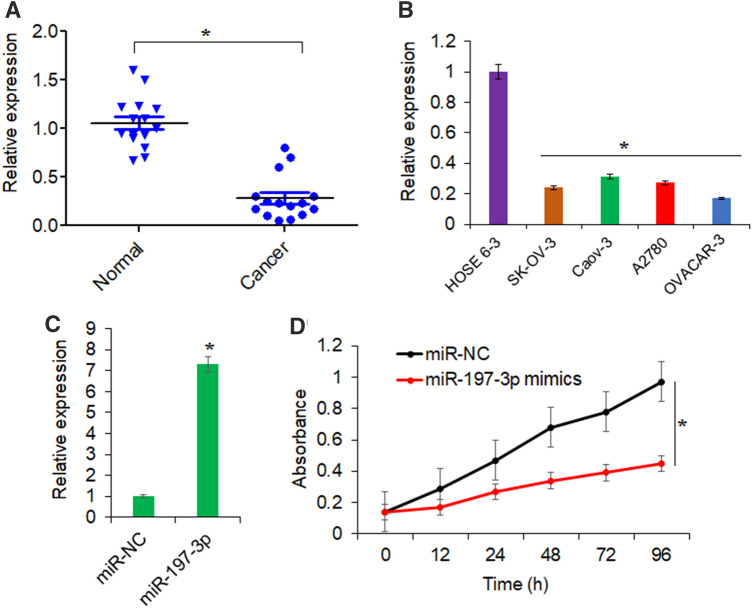
Following the RNA isolation from tissues and cell lines and cDNA synthesis, qRT-PCR was performed. The results showed that ovarian cancer tissues exhibited significantly lower expression of miR-197 when compared with that of the normal tissues (Fig. 1a). Again, it was seen that all the ovarian cancer cell lines (A2780, SK-OV-3, OVACAR-3, and Caov3) possessed markedly lower expression of miR-197 in comparison to the normal ovarian epithelial cell line (HOSE 6–3) (Fig. 1b). The expression of miR-197 was downregulated in ovarian cancer cell lines by up to 5.9-fold relative to normal HOSE-63 cells. Thus, it can be stated that miR-197 is significantly downregulated in human ovarian cancer suggesting its probable cancer regulatory role. Since, lowest expression of miR-197 was observed in case of OVACAR-3 cells, this cell line was used for further experimentation.
Fig. 1.
miR-197 overexpression inhibits the proliferation of ovarian cancer cells. a Relative expression of miR-197 in ovarian cancer tissues and normal adjacent tissues. b Relative expression of miR-197 in ovarian cancer cell lines (A2780, SK-OV-3, OVACAR-3. and Caov3) and normal ovarian epithelial cell line (HOSE 6-3) as determined by the qRT-PCR. c Relative expression of miR-197 in ovarian cancer cell line, OVACAR-3 transfected with miR-197 mimics or miR-NC. d Proliferation of OVACAR-3 ovarian cancer cells transfected with miR-197 mimics or miR-NC as determined by MTT assay. The experiments were performed thrice and presented as mean ± SD (*P < 0.05 for normal vs. cancer tissues, normal vs. cancer cell lines and miR-NC vs. miR-197 mimics)
miR-197 inhibited the growth of OVACAR-3 cells
Approximately about sevenfold increase in the expression of miR-197 was noticed from the OVACAR-3 cancer cells transfected with miR-197 mimics in comparison to normal control cells (Fig. 1c). When the proliferation of miR-197 overexpressing cells was analyzed by the MTT assay, it was found that the cancer cells proliferated at significantly lower rates under miR-197 upregulation (Fig. 1d). Taken together, these findings point towards the tumor-suppressive effects of miR-197 in ovarian cancer.
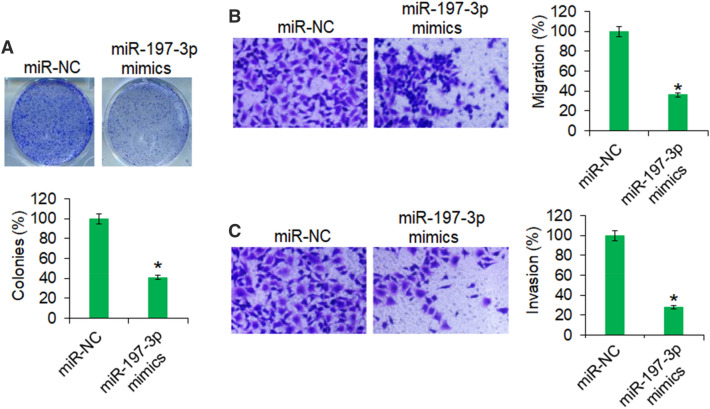
miR-197 inhibited the colony formation, migration, and invasion of OVACAR-3 cells
The colony formation assay revealed that overexpression of miR-197 resulted in significant decrease in the colony formation of the OVACAR-3 cells (Fig. 2a). The colony formation was inhibited by 59% in miR-197 mimic transfected cells relative to the miR-NC transfected cells. Also, the ovarian cancer cells were shown to possess significantly lower migration and invasion capacities under miR-195 overexpression (Fig. 2b, c). The migration and invasion of the OVACAR-3 cells was inhibited by 64% and 72%, respectively, upon miR-197 overexpression.
Fig. 2.
The miR-197 reduces the colony formation and motility of ovarian cancer cells. a Colony formation of OVACAR-3 transfected with miR-197 mimics or miR-NC cells. b Transwell chamber assay showing the migration of OVACAR-3 transfected with miR-197 mimics or miR-NC. c Transwell chamber assay showing the invasion of OVACAR-3 cells transfected with miR-197 mimics or miR-NC. The experiments were performed thrice and presented as mean ± SD (*P < 0.05 for miR-NC vs. miR-197 mimics)
Together, the findings support that miR-197 overexpression in ovarian cancer cells significantly inhibited the motility indicating the therapeutic potential of miR-197 against the human ovarian cancer metastasis.
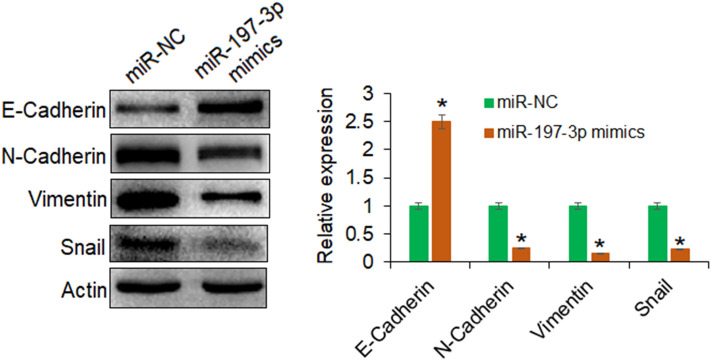
miR-197 inhibits epithelial–mesenchymal transition in OVACAR-3 cells
miR-197 has previously been shown to inhibit the epithelial–mesenchymal transition in pancreatic cancer cells (Hamada et al. 2013). To investigate whether miR-197 excises similar regulatory control in human ovarian cancer, the expression analysis of marker proteins of epithelial–mesenchymal transition was performed. It was shown that the upregulation of miR-197 in ovarian cancer cells enhanced the protein expression of E-cadherin, while the expression of N-cadherin, vimentin, and snail proteins was seen to decrease significantly under miR-197 overexpression (Fig. 3). Thus, it was confirmed that miR-197 negatively regulates the epithelial-to-mesenchymal transition in human ovarian cancer further reflecting its tumor-suppressive role.
Fig. 3.
The miR-197 inhibits the epithelial–mesenchymal transition in ovarian cancer cells. Western blotting analysis showing the expression levels of the epithelial–mesenchymal transition marker proteins in OVACAR-3 transfected with miR-197 mimics or miR-NC. The experiments were performed thrice and presented as mean ± SD (*P < 0.05 for miR-NC vs. miR-197 mimics)
miR-197 exerts tumor-suppressive effects by post-transcriptional suppression of ABCA7
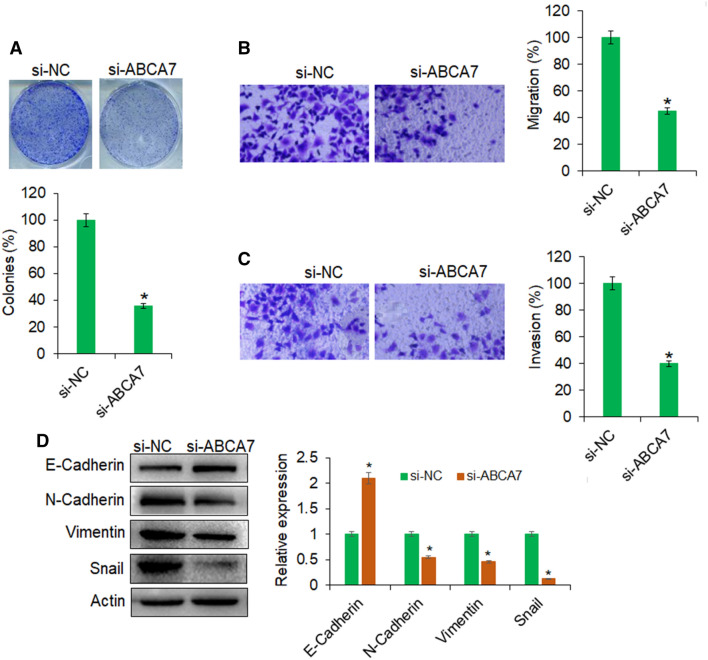
The online bioinformatics showed that ABCA7 is potential target of miR-197 in ovarian cancer cells and specific miR-197 target sequence was predicted in the 3′-UTR of ABCA7 (Fig. 4a). To confirm this, the dual luciferase assay was performed and it was shown that the fall in the luciferase activity of cancer cells, co-transfected with miR-197 mimics and WT 3′-UTR of ABCA7, was because of the interaction of miR-197 with 3′-UTR of ABCA7 (Fig. 4b). Furthermore, support was gained from the gene expression analysis of ABCA7 form tissues and cell lines. The cancer tissues and cell lines exhibited higher transcript abundance of ABCA7 owing to miR-197 repression (Fig. 4c, d). To check whether miR-197 exerted its regulatory control on proliferation and motility of ovarian cancer cells through post-transcriptional silencing of ABCA7, the ABCA7 gene was silencing through RNAi approach and the same was confirmed by qRT-PCR analysis (Fig. 4e). The silencing of ABCA7 in ovarian cancer cells declined their proliferation in the same fashion as under miR-197 overexpression (Fig. 4f). Also, the upregulation of ABCA7 gene in miR-197 overexpressing cancer cells overcame the effects of decline the proliferation and cancer cells proliferated as good as the normal control cells (Fig. 4g). Also, the silencing of ABCA7 gene reduced the clonogenic potential of ovarian cancer cells (Fig. 5a). The migration and invasion of ovarian cancer cells was also significantly restricted under ABCA7 gene silencing (Fig. 5b, c). Again, under the ABCA7 gene silencing, the expression of marker proteins of epithelial–mesenchymal transition was altered in the same manner as under miR-197 overexpression (Fig. 5d). Taken together, the results suggest that miR-197 specifically targeted ABCA7 gene to exert its regulatory effects in ovarian cancer cells. The results further reveal the importance of miR-197/ABCA7 molecular axis to act potential drug-based therapeutic target in the management of human ovarian cancer.
Fig. 4.
The miR-197 exerts its effects by targeting ABCA7 in ovarian cancer. a TargetScan analysis for prediction of miR-197 target. b Dual luciferase reporter assay for showing interaction between miR-197 and 3′-UTR of ABCA7. c Relative gene expression of ABCA7 in ovarian cancer cell lines (A2780, SK-OV-3, OVACAR-3, and Caov3) and normal ovarian epithelial cell line (HOSE 6-3). d Relative gene expression of ABCA7 in ovarian cancer tissues and normal adjacent tissues. e Relative gene expression of ABCA7 in OVACAR-3 transfected with si-NC or si-ABCA7 as determined by qRT-PCR. f MTT assay showing the proliferation of OVACAR-3 ovarian cancer cells transfected with si-NC or si-ABCA7. g MTT assay showing the proliferation of OVACAR-3 ovarian cancer cells transfected with miR-NC, miR-197 mimics, or miR-197 mimics plus pcDNA-ABCA7. The experiments were performed thrice and presented as mean ± SD (*P < 0.05 for normal vs. cancer tissues, normal vs. cancer cell lines; si-NC vs. si-ABCA7 and miR-197 mimics vs. miR-197 mimics + pcDNA-ABCA7)
Fig. 5.
Silencing of ABCA7 inhibits the proliferation, migration, and invasion of ovarian cancer cells: a Clonogenic assay for the assessment of viability of OVACAR-3 transfected with si-NC or si-ABCA7. b Transwell chamber assay for the determination of migration of OVACAR-3 transfected with si-NC or si-ABCA7. c Transwell chamber assay for the determination of invasion of OVACAR-3 transfected with si-NC or si-ABCA7. d Western blotting for the analysis of the expression levels of the epithelial–mesenchymal transition marker proteins in OVACAR-3 transfected with si-NC or si-ABCA7. The experiments were performed thrice and presented as mean ± SD (*P < 0.05 for si-NC vs. si-ABCA7)
Discussion
The aberrant expression of miRs has been shown to have significant bearing on the growth and metastasis of various human cancers (Di Leva et al. 2014). The miRs have thus been proposed to serve as vital prognostic biomarkers in human cancers (Hayes et al. 2014). The miRs have been found to function either as oncogenes or tumor suppressors (Zhang et al. 2007). The therapeutic potential of miRs against human cancers is very well anticipated (Cho 2010). The miR-197 has been reported to be linked with several human cancers (Wang et al. 2016a, b; Ni et al. 2019; Mavridis et al. 2015). Consistent with the previous reports, the miR-197 was found to be significantly downregulated in ovarian cancer. The overexpression of miR-197 was found to inhibit the growth of the ovarian cancer cells. Similar effects of the miR-197 overexpression have been reported from the previous studies also (Chen and Yang 2018). Metastasis of ovarian cancer acts as one of the crucial hindrances in attaining ideal level of clinical success against this malignancy (Forstner et al. 2016). The finding that miR-197 overexpression negatively affected the motility of ovarian cancer cells in vitro thus reveals the therapeutic potential of this biomolecule against the human ovarian cancer. The decline in the cancer cell motility under miR-197 upregulation was also been shown in the previous research investigations (Li et al. 2019). The miRs exert their regulatory effects through selective targeting of protein-coding genes translationally (Fabbri et al. 2008). Similarly, miR-197 has been shown to target several genes in human cells, for instance, mitogen activated protein kinase 1 (Xiong et al. 2015), GAB2 (Tian et al. 2016), and NLK (Zou et al. 2015). Nonetheless, ABCA7 has not been studied as the target of miR-197 in any type of cancer cells. The ABC transporters have been reported to play vital roles in human cancers and contribute to the development of drug resistance in cancer cells through drug effluxing (Lage 2003). The upregulation of ABCA7 has been proposed to act as vital prognostic factor in human cancers like Ewing sarcoma (Pasello et al. 2020). The transition of human cells form epithelial-to-mesenchymal prototype acts as a crucial switch in tumor onset and progression (Yilmaz and Christofori 2009). ABCA7 was found to enhance this transition in ovarian cancer cells through upregulation of TGF-β signaling pathway (Liu et al. 2018). The study further showed that the knockdown of ABCA7 inhibited the migration of ovarian cancer cells as also evident from the results of the present research work. The epithelial–mesenchymal transition triggers the dynamic cellular heterogeneity during the metastasis. E-cadherin, which has higher expression in epithelial cells, acts as cell-to-cell adhesion protein and negatively regulates epithelial–mesenchymal transition and thus the metastasis (Tsuji et al. 2009). Therefore, it acts as the biomarker of epithelial–mesenchymal transition. The results of the present study showed that miR-197 enhanced the protein levels of E-cadherin together with the repression of N-cadherin which is highly expressed in the mesenchymal state (Nakajima et al. 2004). Therefore, miR-197 is suggested to be associated with the epithelial–mesenchymal transition in ovarian cancer cells. Snail is the zinc-finger transcription factor which acts as the transcriptional repressor of E-cadherin (Yuan et al. 2014). The repression of snail protein in ovarian cancer cells via the miR-197/ABCA7 axis might thus have resulted in higher expression of E-cadherin. Vimentin is also a marker of mesenchymal prototype (Lo et al. 2017). The decline of vimentin protein levels under miR-197 upregulation thus suggests that epithelial–mesenchymal transition is negatively regulated by miR-197 via ABCA7 which further highlights the therapeutic potential of miR-197/ABCA7 molecular axis. Taken together, miR-197 acts as tumor-suppressor and inhibits the epithelial-to-mesenchymal transition of ovarian cancer which is essential to their tumorigenicity via post-transcriptional suppression of ABCA7. The results point towards the therapeutic implications of miR-197 in human ovarian cancer cells. Nonetheless, studies on different cell lines and under in vivo conditions are required for further confirmation.
Acknowledgements
The authors acknowledge the experimental assistance from The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, Hubei, China
Author contribution
Conceptualization: WX and XF; methodology: WX. CS, YP, and XF; formal analysis and investigation: WX, CS, and YP; writing—original draft preparation: XF, LQ, and WX; writing—review and editing critically for important intellectual content: XF and LQ; supervision: XF.
Funding
This research did not obtain any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
All the authors declare that he has no conflict of interest. Ethical approval. This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical approval
The study was approved by research ethics committee of The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, Hubei, China under approval number AHS-111-C52-2019.
References
- Bandiera S, Hatem E, Lyonnet S, Henrion-Caude A. MicroRNAs in diseases: from candidate to modifier genes. Clin Genet. 2010;77(4):306–313. doi: 10.1111/j.1399-0004.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang C. miR-197-induced downregulation of lysine 63 deubiquitinase promotes cell proliferation and inhibits cell apoptosis in lung adenocarcinoma cell lines. Mol Med Rep. 2018;17(3):3921–3927. doi: 10.3892/mmr.2017.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WC. MicroRNAs in cancer—from research to therapy. Biochim Biophys Acta. 2010;1805(2):209–217. doi: 10.1016/j.bbcan.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Dahiya N, Morin PJ. MicroRNAs in ovarian carcinomas. Endocr Relat Cancer. 2010;17(1):F77–89. doi: 10.1677/ERC-09-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Ann Rev Pathol. 2014;24(9):287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JA, Jensen A, Kelemen LE, Pearce CL, Poole E, Schildkraut JM, Terry KL, Tworoger SS, Webb PM, Wentzensen N. Current gaps in ovarian cancer epidemiology: the need for new population-based research. J Natl Cancer Inst. 2017;109(10):djx144. doi: 10.1093/jnci/djx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008;14(1):1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- Forstner R, Meissnitzer M, Cunha TM. Update on imaging of ovarian cancer. Curr Radiol Rep. 2016;4(6):31. doi: 10.1007/s40134-016-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Miura S, Hirota M, Kanno A, Masamune A, Kikuta K, Kume K, Unno J, Egawa S, Motoi F. miR-197 induces epithelial–mesenchymal transition in pancreatic cancer cells by targeting p120 catenin. J Cell Physiol. 2013;228(6):1255–1263. doi: 10.1002/jcp.24280. [DOI] [PubMed] [Google Scholar]
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wang P, Lin J, Zheng X, Yang F, Zhang G, Chen D, Xie J, Gao Z, Peng L, Xie C. MicroRNA-197 promotes metastasis of hepatocellular carcinoma by activating Wnt/β-catenin signaling. Cell Physiol Biochem. 2018;51(1):470–486. doi: 10.1159/000495242. [DOI] [PubMed] [Google Scholar]
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- Kosik KS. MicroRNAs and cellular phenotypy. Cell. 2010;143(1):21–26. doi: 10.1016/j.cell.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Lage H. ABC-transporters: implications on drug resistance from microorganisms to human cancers. Int J Antimicrob agents. 2003;22(3):188–199. doi: 10.1016/s0924-8579(03)00203-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu X, Gao F, Wang X. MiR-197 regulates endothelial cell proliferation and migration by targeting IGF1R and BCL2 in Kawasaki disease. Int J Clin Exp Pathol. 2019;12(11):4181. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li Q, Zhou J, Zhang S. ATP-binding cassette transporter A7 accelerates epithelial-to-mesenchymal transition in ovarian cancer cells by upregulating the transforming growth factor-β signaling pathway. Oncol Lett. 2018;16(5):5868–5874. doi: 10.3892/ol.2018.9366. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lo U, Lee CF, Lee MS, Hsieh JT. The role and mechanism of epithelial-to-mesenchymal transition in prostate cancer progression. Int J Mol Sci. 2017;18(10):2079. doi: 10.3390/ijms18102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavridis K, Gueugnon F, Petit-Courty A, Courty Y, Barascu A, Guyetant S, Scorilas A. The oncomiR miR-197 is a novel prognostic indicator for non-small cell lung cancer patients. Br J Cancer. 2015;112(9):1527–1535. doi: 10.1038/bjc.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, Kawaguchi Y. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004;10(12):4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- Ni JS, Zheng H, Huang ZP, Hong YG, Ou YL, Tao YP, Wang MC, Wang ZG, Yang Y, Zhou WP. MicroRNA-197-3p acts as a prognostic marker and inhibits cell invasion in hepatocellular carcinoma. Oncol Lett. 2019;17(2):2317–2327. doi: 10.3892/ol.2018.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasello M, Fanelli M, Mularoni V, Ciotti S, Picci P, Serra M, Scotlandi K. Expression levels of ABCA6 or ABCA7 predict primary Ewing sarcoma progression at diagnosis. Semin Cancer Biol. 2020;60:57–71. [Google Scholar]
- Sun Z, Zhou N, Han Q, Zhao L, Bai C, Chen Y, Zhou J, Zhao RC. MicroRNA-197 influences 5-fluorouracil resistance via thymidylate synthase in colorectal cancer. Clin Transl Oncol. 2015;7(11):876–883. doi: 10.1007/s12094-015-1318-7. [DOI] [PubMed] [Google Scholar]
- Tian LQ, Liu EQ, Zhu XD, Wang XG, Li J, Xu GM. MicroRNA-197 inhibits cell proliferation by targeting GAB2 in glioblastoma. Mol Med Rep. 2016;13(5):4279–4288. doi: 10.3892/mmr.2016.5076. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69(18):7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li H, Cui L, Feng J, Fan Q. MicroRNA-182 suppresses clear cell renal cell carcinoma migration and invasion by targeting IGF1R. Neoplasma. 2016;63:717–725. doi: 10.4149/neo_2016_508. [DOI] [PubMed] [Google Scholar]
- Wang YY, Wu ZY, Wang GC, Liu K, Niu XB, Gu S, Meng JS. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197. Tumor Biol. 2016;37(11):14553–14563. doi: 10.1007/s13277-016-5303-8. [DOI] [PubMed] [Google Scholar]
- Xiong HL, Zhou SW, Sun AH, He Y, Li J, Yuan X. MicroRNA-197 reverses the drug resistance of fluorouracil-induced SGC7901 cells by targeting mitogen-activated protein kinase 1. Mol Med Rep. 2015;12(4):5019–5025. doi: 10.3892/mmr.2015.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, Chen Y, Wu K. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7(1):87. doi: 10.1186/s13045-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–2. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Zou D, Wang D, Li R, Tang Y, Yuan L, Long X, Zhou Q. MiR-197 induces Taxol resistance in human ovarian cancer cells by regulating NLK. Tumor Biol. 2015;36(9):6725–6732. doi: 10.1007/s13277-015-3365-7. [DOI] [PubMed] [Google Scholar]