Abstract
Abortion and miscarriage are common, affecting millions of US women each year. By age 45, one in four women in the USA will have had an abortion, and at least as many will have had a miscarriage. Most individuals seeking abortion services do so before 10 weeks’ gestation when medication abortions are a safe and effective option, using a regimen of oral mifepristone followed by misoprostol tablets. When a pregnancy is non-viable before 13 weeks’ gestation, it is referred to as an early pregnancy loss or miscarriage and can be managed using the same mifepristone and misoprostol regimen. Given their safety and efficacy, mifepristone and misoprostol can be offered in ambulatory settings without special equipment or on-site emergency services. As more patients find it difficult to access clinical care when faced with an undesired pregnancy or a miscarriage, it is important for general internists and primary care providers to become familiar with how to use medications to manage these common conditions. We summarize the most recent evidence regarding the use of mifepristone with misoprostol for early abortion and miscarriage. We discuss clinical considerations and resources for integrating mifepristone and misoprostol into clinical practice. By learning to prescribe mifepristone and misoprostol, clinicians can expand access to time-sensitive health services for vulnerable populations.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-05836-9) contains supplementary material, which is available to authorized users.
KEY WORDS: family planning, medication abortion, miscarriage, women’s health, primary care
INTRODUCTION
Internists provide primary care for many reproductive-age women, and the American College of Physicians has called for enhanced reproductive health education and training.1 Abortion and miscarriage affect one-third of women in the USA who become pregnant annually.2 By age 45, one in four US women will have had an abortion, and at least as many will have had a miscarriage.3, 4 Abortions are most commonly performed in the first trimester, with 91% of US abortions occurring before 13 weeks.5 Until 10 weeks’ gestation, abortion can be induced safely and effectively with medications (mifepristone followed by misoprostol).6 Currently, 80% of abortions occur before 10 weeks’ gestation.5, 7 Thus, the majority of US women seeking abortion care are eligible for a medication abortion.
As pill-based protocols for medication abortion and miscarriage are safe and effective, internists should become familiar with these options for managing such time-sensitive clinical conditions. Historically, obstetrician–gynecologists and family medicine physicians have provided nearly all of the abortion care in the USA. However, a recent survey found that only 7% of obstetrician–gynecologists provided any abortions in the preceding year, and 35% said they would not refer patients for abortion services.8 In fact, there are only slightly more than 1500 abortion providers nationwide.9, 10 Many women seeking clinical services related to abortion and miscarriage must travel considerable distances to obtain care—a particular burden for rural and low-income women.11, 12 Moreover, many patients prefer to receive family planning services from a familiar primary care provider rather than a clinician whom they have never met.13–16 With nearly 100,000 currently practicing in the USA, general internists are in a unique position to close the gap in access to medication management of abortion and miscarriage.10
Since the US Food and Drug Administration (FDA) first approved its use in 2000 for medication abortion, more than 3.7 million US women have used mifepristone in combination with misoprostol for medication abortion.17 Although misoprostol alone can be used to expel pregnancy tissue, combining it with mifepristone increases its efficacy for both abortion and miscarriage.18, 19 Thus, for both medication abortion and medical management of early miscarriage, the standard of care is to provide oral mifepristone followed by misoprostol tablets.4, 18 The use of medication for abortion and miscarriage has evolved since the last review of abortion for generalists in 2004.20
We summarize the most recent evidence supporting mifepristone’s use with misoprostol for abortion and miscarriage. We discuss the drug’s safety profile and common side effects, resources for integration into clinical practice, and legal considerations. By learning how to prescribe mifepristone and misoprostol for abortion and miscarriage, internists can meet the needs of reproductive-age patients and improve access to timely care.
TERMINOLOGY
This review uses the term abortion to indicate an induced abortion, meaning a medication or procedure to end a pregnancy. We use the term miscarriage to refer to early pregnancy loss, defined as a non-viable, intrauterine pregnancy diagnosed by ultrasound before 13 weeks’ gestation.4
SEARCH STRATEGY
We reviewed the medical literature published in the English language using PubMed and detailed search terms for medication abortion and miscarriage. The full list of terms used in the search is included in the supplementary appendix. A landmark National Academies of Science, Engineering, and Medicine (NASEM) report on abortion published in 2018 reviewed more than 9000 peer-reviewed articles published over 30 years.6 Our manuscript includes key articles from that comprehensive report as well as studies published more recently (January 2017–December 2019), which had not been included. Our search yielded 373 studies published during that time period; of these, 59 studies were relevant to US practice of general internal medicine.
MEDICAL MANAGEMENT OF ABORTION
First developed in France in 1982 and known as RU-486, mifepristone has been extensively studied in the USA and worldwide.21 An anti-progestin that competitively binds to progesterone receptors, mifepristone can be used to detach pregnancy tissue from the endometrium. Mifepristone alone has limited effectiveness for medication abortion, but it is highly effective when combined with misoprostol, a prostaglandin analogue, that induces uterine contractions and cervical dilation to aid in the expulsion of pregnancy tissue.
The US FDA first approved a regimen of mifepristone followed by misoprostol in 2000 for medication abortion prior to 49 days’ gestation. The original protocol required three in-clinic visits and used a high dose of mifepristone (600 mg).22, 23 The details of this regimen are outlined in Table 1. Subsequent clinical trials indicated that a lower dose of mifepristone (200 mg) followed by a high dose of misoprostol (800 mcg) was as effective as the original regimen.21, 24, 25 Although mifepristone followed by misoprostol is more effective the earlier it is given in pregnancy and highest at < 42 days (6 weeks), it remains 93% effective for abortions ≤ 70 days (10 weeks) with 2.9% risk of continuing pregnancy.26–28
Table 1.
Comparison of the US Food and Drug Administration (FDA) Labeling for Mifepristone Followed by Misoprostol 2000 vs. 2016
| Original FDA-approved regimen (2000) | Updated FDA-approved regimen (2016) | |
|---|---|---|
| Gestational age limits | 49 days’ gestation | 70 days’ gestation |
| Mifepristone dose and administration | 600 mg on day 1 in clinic | 200 mg on day 1 in clinic |
| Misoprostol dose and administration | 400 mcg orally in clinic on day 3 post-mifepristone | 800 mcg buccally at home 24–48 h post-mifepristone |
| Timing of follow-up assessment | 7–14 days post-mifepristone | 7–14 days post-mifepristone |
| Location of follow-up assessment | In clinic required | In clinic not required |
Consequently, in 2016, the FDA updated the mifepristone label for termination of pregnancies up to 70 days (10 weeks) gestation and reduced the number of mandatory in-person visits to one (Table 1). Direct clinical observation of ingestion of mifepristone and misoprostol is no longer necessary, allowing for successful telemedicine programs.24, 26, 29 Although many patients are eager to complete the medication regimen as soon as possible, taking misoprostol the same day as the mifepristone is less effective than waiting 24–48 h.30, 31
Despite the new clinical protocol in 2016, the FDA made no changes to the Risk Evaluation and Mitigation Strategy (REMS) that has been in place for mifepristone since it was originally approved in 2000.32 The REMS requires that (1) clinicians register in the drug manufacturer’s central database and (2) registered clinicians must order, store, and dispense mifepristone instead of writing a prescription to be filled at a retail pharmacy. These FDA requirements were placed to ensure patient safety and that registered clinicians could only dispense the medication if they could accurately determine gestational age, diagnose ectopic pregnancy, and provide or refer to emergency care if necessary.32 Clinical experts have called for the FDA to remove the REMS from mifepristone to allow pharmacy dispensing, as mifepristone’s safety profile is superior to that of many over-the-counter medications.33, 34
Internationally, studies have shown that multiple doses of misoprostol alone can be used off-label (800 mcg vaginally or sublingually every 3 h for a total of 3 doses) to induce abortions in the first trimester.35 However, with this misoprostol-only regimen, 7% of women had ongoing pregnancies, and 22% required a uterine aspiration procedure to complete the abortion.35, 36 Given the lower efficacy rates and greater need for follow-up procedures, a regimen of mifepristone followed by misoprostol remains the standard of care for providing medication abortion. However, in settings where access to mifepristone has been restricted, a misoprostol-only regimen may be needed or preferred given its relative ease of access (e.g., misoprostol does not require REMS protocol) and lower cost (misoprostol costs $10–$15 whereas mifepristone costs $50–100).37, 38
Overall, the use of mifepristone and misoprostol for medication abortion has been slowly rising in the USA. In 2017, 39% of all abortions before 10 weeks’ gestation were medication abortions.9 However, the vast majority of these occurred in free-standing abortion clinics (95%) rather than in primary care settings.9 General internists who provide mifepristone and misoprostol for their patients reduce practical barriers and burdens for patients seeking time-sensitive care.
MEDICAL MANAGEMENT OF MISCARRIAGE
Up to one-third of all pregnancies end in miscarriage.39–41 When a pregnant patient presents with an early pregnancy loss (often with bleeding and/or cramping), an ultrasound should be used to confirm miscarriage and rule out other pregnancy complications. Once the diagnosis of a miscarriage is confirmed and found to be < 13 weeks’ gestation, patients can be offered expectant management, medical management, or a uterine aspiration procedure.25 All are effective and result in similar long-term outcomes.4 For patients who prefer to expedite the passage of their miscarriage but wish to avoid a uterine aspiration, medical management is a reasonable approach.
Historically, clinicians have treated women experiencing miscarriage with misoprostol 800 mcg (vaginally, orally, or buccally) alone to stimulate uterine contractions and facilitate passage of pregnancy tissue. However, up to 30% of women treated with this misoprostol-only regimen required additional doses of misoprostol or a uterine aspiration procedure, prolonging physical and emotional recovery.42, 43 A 2019 Cochrane Systematic Review found vaginal misoprostol accelerated time to completion of miscarriage compared with placebo or expectant management.43 Notably, it did not lead to increased satisfaction or decreased physical symptoms (e.g., nausea, bleeding).43
Subsequent studies attempted to increase the effectiveness of medical management of miscarriage by adding mifepristone as a “pre-treatment” before misoprostol, in a regimen identical to the combined regimen used for medication abortion.44, 45 A 2018 randomized, controlled trial compared pre-treatment with mifepristone 200 mg followed by misoprostol 800 mcg (intervention group) to misoprostol 800 mcg alone (control group) and found that women pre-treated with mifepristone were less likely to require subsequent uterine aspiration (8.8% vs. 23.5%, RR 0.37, 95% CI 0.21–0.68).18 The intervention group was more likely to complete passage of the gestational sac within 4 days (83.8% vs. 67.1%, RR 1.25, 95% CI 1.09–1.43). The number needed to treat with mifepristone to avoid an intrauterine procedure was 6. Rates of adverse events were low and similar in both groups.
A subsequent systematic review on miscarriage comparing expectant management, medical management with misoprostol alone, medical management with mifepristone and misoprostol, and aspiration procedures found that medical treatments had similar effectiveness to surgical interventions and that mifepristone with misoprostol is more effective than misoprostol alone (RR 1.49, 95% CI 1.09–2.03).46 This finding has also been validated in international trials, and mifepristone pre-treatment has consistently led to improved efficacy compared with misoprostol alone.47, 48 As a result, mifepristone pre-treatment is becoming the new standard of care for medical management of miscarriage.
Recently, ACOG updated their practice bulletin on early pregnancy loss to recommend a 200-mg oral dose of mifepristone be given 24 h before misoprostol administration “when mifepristone is available”—recognizing ongoing obstacles to accessing mifepristone in some communities.4 Clinically, use of mifepristone followed by misoprostol for management of abortion and miscarriage is nearly identical (Table 2). However, the use of mifepristone for miscarriage remains off-label.
Table 2.
Comparison of Mifepristone Followed by Misoprostol for Management of Medication Abortion Versus Early Miscarriage
| Medication abortion | Miscarriage | |
|---|---|---|
| Day 1 (in office) | ||
| History |
• Confirm last menstrual period correlates to GA less than 70 days (ultrasound only needed if concern for ectopic pregnancy or uncertain GA) • Counseling about pregnancy options |
• Ultrasound to confirm diagnosis • Counseling about miscarriage management options |
| • Exclude contraindications (Box 2) | ||
| Exam | • Pelvic examination only considered if concern for ectopic pregnancy or uncertain GA | |
| Lab |
• ± Baseline quantitative serum hCG for comparison at follow-up visit • ± Hemoglobin if concern for anemia • ± STI testing if risk factors identified |
|
| Informed consent |
• Ensure patient understands the process, alternatives, risks, and benefits • Required: sign manufacturer’s Patient Agreement Form (available at earlyoptionpill.com) • Dispense mifepristone 200 mg PO × 1 |
|
| Day 2+ (at home) | ||
| Patient initiates | Take 800 mcg misoprostol buccally or vaginally 24–72 h after taking mifepristone | Take 800 mcg misoprostol vaginally 24–72 h after taking mifepristone |
|
• Ibuprofen 600 mg every 6 h as needed for cramping and pain • Counsel patient on concerning symptoms and return precautions that would require urgent evaluation (Box 2) | ||
| Days 5–14 | ||
| History |
• Assess bleeding and symptoms consistent with passed pregnancy and resolution of pregnancy symptoms • Identify if any concerning symptoms are present (Box 2) |
|
| Lab |
• Quantitative serum hCG should decline 50% by 3 days after medication abortion and 80% by 7 days49, 50 • Ultrasound rarely indicated (e.g., concern for ectopic, ongoing pregnancy) |
|
GA gestational age, hCG human chorionic gonadotropin, STI sexually transmitted infection
INTEGRATION OF MIFEPRISTONE INTO PRIMARY CARE PRACTICE
Ordering and Dispensing Medications
Although mifepristone is available in pharmacies in Canada and many countries worldwide, this is not yet the case in the USA. The US FDA currently requires that mifepristone be ordered, stored, and dispensed by a registered clinician.23 Online resources outlining the steps needed to order mifepristone are readily available.51 Under federal guidelines, no formal medical board certification or privileging is required. Clinics that dispense mifepristone do not need to have an ultrasound on site or the ability to perform a uterine aspiration procedure.
Clinical Protocol and Resources for Implementation
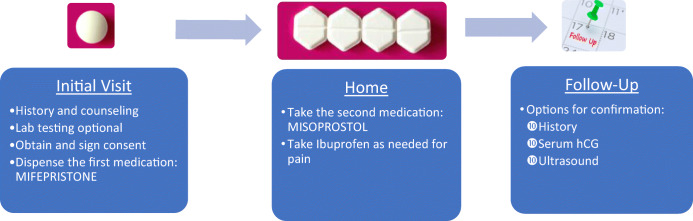
Any licensed physician (and in many states advanced practice clinicians) can provide mifepristone and misoprostol.52 The key steps in providing mifepristone followed by misoprostol for either early abortion or miscarriage are nearly identical (Fig. 1). The first step is to assess gestational age via history of last menstrual period. Ultrasound is used to formally diagnose miscarriage but is not required for abortion unless (1) the gestational age is uncertain or (2) there is concern for ectopic pregnancy.53, 54 Pelvic examination is indicated only for those with an uncertain gestational age (especially if there is concern it is greater than 10 weeks) or concern for ectopic pregnancy.54 There are few contraindications to use of mifepristone and misoprostol (Box 1, 2).22 A history of cesarean section does not preclude use of mifepristone followed by misoprostol for either medication abortion or management of miscarriage.55
Figure 1.
Combining mifepristone and misoprostol to treat undesired pregnancy or miscarriage. hCG, human chorionic gonadotropin
Box 1 Contraindications to Mifepristone56
| • Adrenal insufficiency or chronic adrenal failure | |
| • Concurrent use of long-term corticosteroid therapy | |
| • Ectopic pregnancy | |
| • Bleeding disorder or use of anticoagulant therapy | |
| • Inherited porphyria | |
| • Allergy to mifepristone or misoprostol | |
| • Intrauterine device in place |
Box 2 Concerning Symptoms and Return Precautions22, 50, 54, 57, 58
| • Excessive bleeding: soaking through 2 sanitary napkins per hour for 2 consecutive hours | |
| • Lack of bleeding: no bleeding 24 h after taking misoprostol | |
| • Infectious symptoms: flu-like symptoms that start 24 h after taking misoprostol or fevers, chills, severe abdominal pain, and/or malodorous discharge | |
| • Pain: severe abdominal pain, cramping, and/or bloating | |
| • Ongoing pregnancy symptoms: feeling pregnant (e.g., breast tenderness, nausea) at the follow-up visit |
Although, historically, Rh-negative women who sought abortion in the USA were given Rhogam, current guidelines state that Rh testing and Rhogam are not required if the gestational age is less than 8 weeks.59 Moreover, a recent study suggests Rhogam is likely unnecessary for medication abortion and early miscarriage less than 10 weeks.60 Testing for sexually transmitted infections is not required for low-risk women seeking abortion or miscarriage management but should be offered to those with risk factors. When treatment is needed for a sexually transmitted infection, antibiotics can be provided at the same time as mifepristone.
Patients should be educated about how to use misoprostol at home. A free 1-h video-based online training (https://abortionpillcme.teachtraining.org/) provides examples of primary care counseling about medication abortion and highlights key steps in providing mifepristone and misoprostol. To reduce nausea, the patient medication guide from the manufacturer has a useful illustration to show patients how to use misoprostol buccally by placing two 200-mcg tablets in each cheek pouch (the area between the cheek and gums) for 30 min, after which any pill remnants can be swallowed with water.56 If the tablets are in place for less time (e.g., vomited), patients may need to re-dose the misoprostol.54, 61 Other common off-label but evidence-based regimens for patients prone to nausea include placing misoprostol vaginally; tablets placed in the vagina do not have to be removed after 30 min.62
For managing cramps with medication abortion, ibuprofen is superior to acetaminophen.63 Ibuprofen taken as needed, rather than scheduled, results in equal pain control with less medication use.64 Although non-steroidal anti-inflammatory drugs are first-line therapy and typically sufficient, oral narcotics are occasionally used as adjuvant therapy for pain management.65 However, oral narcotics have not been shown to reduce maximum pain scores or duration of maximum pain, and they have not led to improved satisfaction among women undergoing medical abortion; thus, narcotics should be used with caution.66
After medication abortion or medical management of miscarriage, follow-up should be scheduled for 5–14 days after taking mifepristone and can occur either in person, by telephone, or via electronic messaging (i.e., use of a patient portal).54, 67–70 Follow-up involves assessing symptoms to rule out ongoing pregnancy, such as breast tenderness and nausea, as well as symptoms that could warrant further evaluation to rule out a complication (Box 2). If pregnancy continues after an attempted medication abortion, one must counsel patients about the teratogenicity of misoprostol.71
A number of approaches can be used to confirm a successful medication abortion, including patient self-assessment of symptoms, repeated pregnancy testing, and ultrasound. Measuring serum human chorionic gonadotropin (hCG), a hormone produced in early pregnancy, before and after use of mifepristone and misoprostol is more reliable than ultrasound.54, 72 Compared with baseline, serum hCG should decline 50% by 3 days after medication abortion and 80% by 7 days.49, 72 Although some clinicians opt to use ultrasound to confirm complete passage of tissue, residual echogenic material in the uterus and endometrial thickening may be normal and requires no intervention unless accompanied by cramping, excessive bleeding, or concern for infection.54 Clinicians should be aware that most over-the-counter urine pregnancy tests utilize a cutoff level of hCG for pregnancy detection instead of a range. Thus, standard pregnancy tests may remain positive more than a month after a pregnancy ends and are not helpful in detecting downward trends in hCG levels that would indicate a successful abortion.54
Resources for integrating mifepristone and misoprostol into clinical practice—including electronic health record templates, patient aftercare instructions, and an electronic listserv that offers prompt responses to real-world questions—are available from the Reproductive Health Access Project (RHAP) with additional resources from the Training in Early Abortion for Comprehensive Healthcare (TEACH) program, the National Abortion Federation (NAF), and primary care practice summaries.50, 54, 57 Although primary care is typically team-based, given the rarity of adverse events following use of mifepristone and misoprostol, some general internists (e.g., one author) offer these medications without asking colleagues to take phone calls from their patients who have taken mifepristone. In rare cases when concern for ectopic pregnancy or excessive bleeding arises, referral to a local gynecologist, family physician, or emergency department is warranted.
Safety, Side Effects, and Adverse Events
The 2018 NASEM review concluded that primary care clinicians can safely provide medication abortion in ambulatory settings without on-site ultrasound or emergency services.6 Clinical experience has shown mifepristone to be far safer than antibiotics, antihypertensive agents, insulin, and many other common primary care medications.6, 29, 50, 73 The use of mifepristone followed by misoprostol to end a pregnancy is 14 times safer than continuing a pregnancy to term.74 Medication abortion has no long-term adverse effects on health or fertility.75 In a prospective cohort study of nearly 1000 women comparing those who received an abortion and those who were turned away due to gestational age limits, women who were turned away reported worse anxiety and mental health, as well as worse self-reported physical health outcomes, which persisted 5 years later.75, 76
Patients do not typically experience side effects after taking mifepristone. In contrast, side effects generally occur after taking misoprostol. Common side effects include 4–6 h of severe cramping and vaginal bleeding that is heavier than a typical menstrual period and often includes passage of clots. After the initial 4–6 h, bleeding should gradually decrease and spotting is common for up to 1–2 weeks77 Bleeding that requires urgent evaluation or transfusion is very rare (0.05%).26, 73 Given that bleeding is expected, the risk of anemia should be considered prior to administration of mifepristone and misoprostol, and patients with a hemoglobin < 9.5 g/dl are generally advised to consider a uterine aspiration procedure instead of a medication abortion.50, 78
Flu-like symptoms such as nausea, fever and chills, vomiting, diarrhea, and malaise may occur and should last less than 24 h. Patients should be advised to take non-steroidal anti-inflammatory medication for pain management and call if they experience concerning symptoms that could warrant urgent evaluation (Box 2). Patients who experience no or little bleeding within 24 h of taking misoprostol should be advised to call their clinician, as it could indicate a risk for ongoing or ectopic pregnancy (< 0.6%).79 If a patient requires urgent evaluation (Box 2), the first step is often an ultrasound to identify whether pregnancy tissue remains in the uterus. If a patient has retained pregnancy tissue on ultrasound, they can generally be managed with a repeat dose of misoprostol; however, some individuals may opt for a uterine aspiration procedure.54
Pelvic infections, such as endometritis and sepsis, are exceedingly rare, and prophylactic antibiotics are not routinely recommended.80 Overall, only 0.5–0.9% of patients will need treatment for infection and 0.04–0.9% require hospitalization.73 The need for IV antibiotics is extremely rare (0.006% to 0.093%).73, 81 However, mifepristone has a black box warning reminding providers that if a patient presents with afebrile malaise, Clostridium sordellii should be considered. C. sordellii can lead to toxic shock syndrome, and patients may present with tachycardia, hypotension, and lab abnormalities (e.g., leukocytosis, hemoconcentration).82–84 Case reports of fatal C. sordellii infections have been noted in patients following medication abortion as well as live birth, stillbirth, miscarriage, and procedures for cervical dysplasia. Therefore, it remains unclear whether mifepristone or misoprostol truly plays a causal role in clostridial infections though must be considered given the associated morbidity and mortality.84
Legal Considerations
Although provision of mifepristone and misoprostol is clinically safe, medication abortion is increasingly regulated by laws and policies at the federal and state level.52 In some communities, restrictions may include gestational age limits, physician-only prescribing, requiring the prescriber to be physically on-site (e.g., precluding telemedicine), mandatory waiting periods, mandatory counseling that may be inaccurate and dangerous (e.g., reversal of medication abortions with progesterone), and requirements for the physical characteristics of buildings in which abortions are allowed to occur.85, 86 An up-to-date, state-by-state resource is available online from the Guttmacher Institute.52
Moving Forward
Given that patients in a growing number of communities experience barriers to accessing clinical services, an increasing number of individuals seek to self-manage their abortion.87, 88 Unfortunately, this has resulted in the incarceration of some women for “practicing medicine without a license” and provides a stark reminder of the need to integrate mifepristone into primary care.89 The landscape of abortion is ever-changing, and we are likely to see new models for abortion care emerge, along with further integration into primary care.
SUMMARY
Mifepristone followed by misoprostol can be safely and effectively used to manage early abortion and miscarriage. Recent evidence supports the provision of mifepristone followed by misoprostol in primary care without need for special equipment or emergency services. Primary care providers in many states can easily integrate mifepristone with misoprostol into their practice, thereby dramatically reducing stigma and other barriers their patients may face in accessing time-sensitive reproductive healthcare.
|
Take-Home Points • Mifepristone and misoprostol are highly effective for medical management of early abortion and miscarriage. • Mifepristone and misoprostol can be safely integrated into primary care settings and improve access to time-sensitive clinical services. • Resources are readily available to support the provision of these medications in clinical practice. |
Electronic Supplementary Material
(DOCX 15 kb).
Acknowledgments
We gratefully acknowledge the assistance of Amy Studer, RN, MSN, MSLIS, AHIP, Health Science Librarian, UC Davis Blaisdell Medical Library, in structuring a search of the relevant literature and Erin Hartman, MS, UCSF Department of Medicine, for her review and editorial guidance.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daniel H, Erickson SM, Bornstein SS. Women’s health policy in the United States: an American College of Physicians position paper. Ann Intern Med. 2018;168(12):874–5. doi: 10.7326/M17-3344. [DOI] [PubMed] [Google Scholar]
- 2.Curtin SC, Abma JC, Kost K. Pregnancy rates among U.S. women. 2010. Available at: https://www.cdc.gov/nchs/data/hestat/pregnancy/2010_pregnancy_rates.htm#table 1. Accessed July 3, 2019.
- 3.Jones RK, Jerman J. Population group abortion rates and lifetime incidence of abortion: United States, 2008-2014. Am J Public Health. 2017;107(12):1904–9. doi: 10.2105/AJPH.2017.304042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The American College of Obstetricians and Gynecologists. Early pregnancy loss. Available at: https://www.acog.org/Patients/FAQs/Early-Pregnancy-Loss?IsMobileSet=false. Accessed July 3, 2019.
- 5.Jatlaoui TC, Eckhaus L, Mandel MG, et al. Abortion Surveillance – United States, 2016. MMWR Surveill Summ. 2019;68(11):1–41. doi: 10.15585/mmwr.ss6811a1. [DOI] [PubMed] [Google Scholar]
- 6.National Academies of Sciences, Engineering, and Medicine. The safety and quality of abortion care in the United States. Available at: 10.17226/24950. Accessed July 3, 2019.
- 7.Guttmacher Institute: https://www.guttmacher.org/state-policy/explore/medication-abortion. Accessed on December 17, 2019.
- 8.Desai S, Jones RK, Castle K. Estimating abortion provision and abortion referrals among United States obstetrician-gynecologists in private practice. Contraception. 2018;97(4):297–302. doi: 10.1016/j.contraception.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones R, Witwer E, Jerman J. Abortion incidence and service availability in the United States. 2017. Available at: https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed December 12, 2019.
- 10.Agency for Healthcare Research and Quality. The Number of Practicing Primary Care Physicians in the United States: Primary Care Workforce Facts and Stats No. 1. Available at: https://www.ahrq.gov/research/findings/factsheets/primary/pcwork1/index.html. Accessed December 12, 2019.
- 11.Bearak JM, Burke KL, Jones RK. Disparities and change over time in distance women need to travel to have an abortion in the USA: a spatial analysis. Lancet. 2017;2(11):e492–500. doi: 10.1016/S2468-2667(17)30158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr-Walker J, Jayaweera RT, Ramirez AM, Gerdts C. Experiences of women who travel for abortion: A mixed methods systematic review. PloS One. 2019;14(4):e0209991. doi: 10.1371/journal.pone.0209991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfrey EM, Rubin SE, Khare MM, Gold M. Women’s preference for receiving abortion in primary care settings. J Womens Health. 2010;19(3):547–53. doi: 10.1089/jwh.2009.1454. [DOI] [PubMed] [Google Scholar]
- 14.Page C, Stumbar S, Gold M. Attitudes and preferences toward the provision of medication abortion in an urban academic internal medicine practice. J Gen Intern Med. 2012;27(6):647–52. doi: 10.1007/s11606-011-1956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summit AK, Casey LM, Bennett AH, Karasz A, Gold M. “I don’t want to go anywhere else”: patient experiences of abortion in family medicine. Fam Med. 2015;48(1):30–4. [PubMed] [Google Scholar]
- 16.Wu JP, Godfrey EM, Prine L, Andersen KL, MacNaughton H, Gold M. Women’s satisfaction with abortion care in academic family medicine centers. Fam Med. 2015;47(2):98–106. [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. Mifepristone U.S. post-marketing adverse events summary through 12/31/2018. Available at: https://www.fda.gov/media/112118/download. Accessed July 3, 2019.
- 18.Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161–70. doi: 10.1056/NEJMoa1715726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56(3):385–93. doi: 10.1016/s0015-0282(16)54527-0. [DOI] [PubMed] [Google Scholar]
- 20.Grimes DA, Creinin MD. Induced Abortion: An Overview for Internists. Ann Intern Med. 2004;140(8):620–6. doi: 10.7326/0003-4819-140-8-200404200-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kulier R, Kapp N, Gulmezoglu AM, Hofmeyr GJ, Cheng L, Campana A. Medical methods for first trimester abortion. Cochrane Database Syst Rev. 2011;11:CD002855. doi: 10.1002/14651858.CD002855.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. MIFEPREX (mifepristone) tablets. 200mg for oral administration only. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20687lbl.htm. Accessed July 3, 2019.
- 23.Government Accountability Office. Approval and oversight of the drug mifeprex. 2008 Available at: https://www.gao.gov/new.items/d08751.pdf. Accessed July 3, 2019.
- 24.Schaff EA, Eisinger SH, Stadalius LS, Franks B, Gore BZ, Poppema S. Low-dose mifepristone 200mg and vaginal misoprostol for abortion. Contraception. 1999;59:1–6. doi: 10.1016/s0010-7824(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 25.Neilson JP, Hickey M, Vazquez JC. Medical treatment for early fetal death (less than 24 months) Cochrane Database Syst Rev. 2006;3:CD002253. doi: 10.1002/14651858.CD002253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleland K, Creinin MD, Nucatola D, Nshom M, Trussel J. Significant adverse events and outcomes after medical abortion. Obstet Gynecol. 2013;121(1):166–71. doi: 10.1097/aog.0b013e3182755763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MJ, Creinin MD. Mifepristone with buccal misoprostol for medical abortion: a systematic review. Obstet Gynecol. 2015;126(1):12–21. doi: 10.1097/AOG.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 28.Kapp N, Baldwin MK, Rodriguez MI. Efficacy of medical abortion prior to 6 gestational weeks: a systematic review. Contraception. 2018;97(2):90–9. doi: 10.1016/j.contraception.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Abbas D, Chong E, Raymond EG. Outpatient medical abortion is safe and effective through 70 days gestation. Contraception. 2015;92(3):197–9. doi: 10.1016/j.contraception.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Guest J, Chien PF, Thomson MA, Kosseim ML. Randomized controlled trial comparing the efficacy of same-day administration of mifepristone and misoprostol for termination of pregnancy with the standard 36 to 48-hour protocol. BJOG. 2007;114(2):207–15. doi: 10.1111/j.1471-0528.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Lohr PA, Starling JE, Scott JG, Aiken ARA. Simultaneous Compared With Interval Medical Abortion Regimens Where Home Use Is Restricted. Obstet Gynecol. 2018;131(4):635–41. doi: 10.1097/AOG.0000000000002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. Risk evaluation and mitigation strategies (REMS). Available at: https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems. Accessed July 3, 2019.
- 33.Mifeprex REMS Study Group Sixteen years of overregulation: time to unburden Mifeprex. N Engl J Med. 2017;376:790–4. doi: 10.1056/NEJMsb1612526. [DOI] [PubMed] [Google Scholar]
- 34.Henney JE, Gayle HD. Time to reevaluate U.S. mifepristone restrictions. N Engl J Med. 2019. 10.1056/NEJMp1908305. [DOI] [PubMed]
- 35.Sheldon WR, Durocher J, Dzuba IG, et al. Early abortion with buccal versus sublingual misoprostol alone: a multicenter, randomized trial. Contraception. 2019;99(5):272–7. doi: 10.1016/j.contraception.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Raymond EG, Harrison MS, Weaver MA. Efficacy of misoprostol alone for first-trimester medical abortion: a systematic review. Obstet Gynecol. 2019;133(1):137–47. doi: 10.1097/AOG.0000000000003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NeedyMeds. Mifeprex. [cited 2020 Jan 24];Available from: https://www.needymeds.org/generic-drug/DrugSearch/mifepristone
- 38.GoodRx. Misoprostol. [cited 2020 Jan 24];Available from: https://www.goodrx.com/misoprostol
- 39.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 40.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65(3):503–9. [PubMed] [Google Scholar]
- 41.Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353(8):761–9. doi: 10.1056/NEJMoa044064. [DOI] [PubMed] [Google Scholar]
- 42.Chen BA, Creinin MD. Contemporary management of early pregnancy failure. Clin Obstet Gynecol. 2007;50(1):67–88. doi: 10.1097/GRF.0b013e31802f1233. [DOI] [PubMed] [Google Scholar]
- 43.Lemmers M, Verschoor MA, Kim BV, et al. Medical treatment for early fetal death (less than 24 weeks) Cochrane Database Syst Rev. 2019;6:CD002253. doi: 10.1002/14651858.CD002253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehrnstén L, Altman D, Ljungblad A, Kallner HK. Efficacy of mifepristone and misoprostol for medical treatment for missed miscarriage in clinical practice- a cohort study. Acta Obstet Gynecol Scand. 2019. [DOI] [PubMed]
- 45.Marret H, Simon E, Beucher G, et al. Overview and expert assessment of off-label use of misoprostol in obstetrics and gynaecology: review and report by the Collège national des gynécologues obstétriciens français. Eur K Obstet Gynecol Reprod Biol. 2015;187:80–4. doi: 10.1016/j.ejogrb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Haw B, Murugesu N, Tobias A, Zamora J, Khan KS. Management of first-trimester miscarriage: a systematic review and network meta-analysis. Hum Reprod Update. 2019;25(3):362–74. doi: 10.1093/humupd/dmz002. [DOI] [PubMed] [Google Scholar]
- 47.Dunford A, Fyfe R. Combination therapy with mifepristone and misoprostol for the management of first trimester miscarriage: Improved success. Aust N Z J Obstet Gynaecol. 2018;58(4):438–42. doi: 10.1111/ajo.12747. [DOI] [PubMed] [Google Scholar]
- 48.Sinha P, Suneja A, Guleria K, Aggarwal R, Vaid NB. Comparison of Mifepristone Followed by Misoprostol with Misoprostol Alone for Treatment of Early Pregnancy Failure: A Randomized Double-Blind Placebo-Controlled Trial. J Obstet Gynaecol India. 2018;68(1):39–44. doi: 10.1007/s13224-017-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenquist A, Fortin J, Goldberg AB. Serum human chorionic gonadotropin (hCG) trend within the first few days after medical abortion: a prospective study. Contraception. 2017;95(3):263–8. doi: 10.1016/j.contraception.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Amico JR, Cheng TL, Godfrey EM. Providing Abortion Services in the Primary Care Setting. Prim Care. 2018;45(4):599–613. doi: 10.1016/j.pop.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Reproductive Health Access Project. Mifepristone (Mifeprex) ordering information. Available at: https://www.reproductiveaccess.org/wp-content/uploads/2013/12/mifepristone_ordering.pdf. Accessed July 3, 2019.
- 52.Guttmacher Institute. An overview of abortion laws. Available at: https://www.guttmacher.org/state-policy/explore/overview-abortion-laws. Accessed July 3, 2019.
- 53.Raymond EG, Tan YL, Comendant R, et al. Simplified medical abortion screening: a demonstration project. Contraception. 2018;97(4):292–6. doi: 10.1016/j.contraception.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 54.National Abortion Federation. 2018 clinical policy guidelines. Available at: https://prochoice.org/resources/clinical-policy-guidelines/. Accessed July 3, 2019.
- 55.Mazouni C, Provensal M, Porcu G, et al. Termination of pregnancy in patients with previous cesarean section. Contraception. 2006;73(3):244–8. doi: 10.1016/j.contraception.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Danco Laboratories. Mifeprex medication guide. Available at: http://www.earlyoptionpill.com/wp-content/uploads/2016/01/DAN_MedGuideEng_FINAL.pdf. Accessed July 3, 2019.
- 57.Training in Early Abortion for Comprehensive Healthcare (TEACH). Early abortion training workbook. Available at: https://www.teachtraining.org/training-tools/early-abortion-training-workbook/. Accessed July 3, 2019.
- 58.World Health Organization. Medical Management of Abortion. Available at: https://apps.who.int/iris/bitstream/handle/10665/278968/9789241550406-eng.pdf?ua=1. Accessed January 24,2020.
- 59.Mark A, Foster AM, Grossman D, et al. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99(5):265–6. doi: 10.1016/j.contraception.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Hollenback SJ, Cochran M, Harrington A. “Provoked” feto-maternal hemorrhage may represent insensible cell exchange in pregnancies from 6 to 22 weeks gestational age. Contraception. 2019;S0010-7824(19):30129–5. doi: 10.1016/j.contraception.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 61.Planned Parenthood. Client information: how to take the pills for your abortion and what to expect. Available at: https://www.plannedparenthood.org/files/1314/2308/5667/CI_Buccal_NEW.pdf. Accessed July 3, 2019.
- 62.Hsia JK, Lohr PA, Taylor J, Creinin MD. Medical abortion with mifepristone and vaginal misoprostol between 64 and 70 days’ gestation. Contraception. 2019;S0010-7824(10):30169–6. doi: 10.1016/j.contraception.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Livshits A, Machtinger R, David LB, Spira M, Moshe-Zahav A, Seidman DS. Ibuprofen and paracetamol for pain relief during medical abortion: a double-blind randomized controlled study. Fertil Steril. 2009;91(5):1877–80. doi: 10.1016/j.fertnstert.2008.01.084. [DOI] [PubMed] [Google Scholar]
- 64.Raymond EG, Weaver MA, Louie KS, et al. Prophylactic compared with therapeutic ibuprofen analgesia in first-trimester medical abortion: A randomized controlled trial. Obstet Gynecol. 2013;122(3):558–64. doi: 10.1097/AOG.0b013e31829d5a33. [DOI] [PubMed] [Google Scholar]
- 65.Friedlander EB, Soon R, Salcedo J, et al. Prophylactic pregabalin to decrease pain during medication abortion: A randomized controlled trial. Obstet Gynecol. 2018;132(3):612–8. doi: 10.1097/AOG.0000000000002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colwill AC, Bayer LL, Bednarek P, Garg B, Jensen JT, Edelman AB. Opioid Analgesia for Medical Abortion: A Randomized Controlled Trial. Obstet Gynecol. 2019;134(6):1163–70. doi: 10.1097/AOG.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 67.Clark W, Bracken H, Tanenhaus J, Schweikert S, Lichtenberg ES, Winikoff B. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 Pt 1):264–72. doi: 10.1097/AOG.0b013e3181c996f3. [DOI] [PubMed] [Google Scholar]
- 68.Cameron ST, Glasier A, Dewart H, Johnstone A, Burnside A. Telephone follow-up and self-performed urine pregnancy testing after early medical abortion: A service evaluation. Contraception. 2012;86(1):67–73. doi: 10.1016/j.contraception.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Oppegaard KS, Qvigstad E, Fiala C, Heikinheimo O, Benson L, Gemzell-Danielsson K. Clinical follow-up compared with self-assessment of outcome after medical abortion: a multicentre, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9969):698–704. doi: 10.1016/S0140-6736(14)61054-0. [DOI] [PubMed] [Google Scholar]
- 70.Cameron ST, Glasier A, Johnstone A, Dewart H, Campbell A. Can women determine the success of early medical termination of pregnancy themselves? Contraception. 2015;91(1):6–11. doi: 10.1016/j.contraception.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Coelho KE, Sarmento MF, Veiga CM, et al. Misoprostol embryotoxicity: clinical evaluation of fifteen patients with arthrogryposis. Am J Med Genet. 2000;95(4):297–301. [PubMed] [Google Scholar]
- 72.Fiala C, Safar P, Bygdeman M, Gemzell-Danielsson K. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109(2):190–5. doi: 10.1016/s0301-2115(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 73.Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125(1):175–83. doi: 10.1097/AOG.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 74.Raymond EG, Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstet Gynecol. 2012;119(2 Pt 1):215–9. doi: 10.1097/AOG.0b013e31823fe923. [DOI] [PubMed] [Google Scholar]
- 75.Biggs MA, Upadhyay UD, McCulloch CE, Foster DG. Women’s mental health and well-bring 5 years after receiving or being denied an abortion: a prospective, longitudinal cohort study. JAMA Psychiatry. 2017;74(2):169–78. doi: 10.1001/jamapsychiatry.2016.3478. [DOI] [PubMed] [Google Scholar]
- 76.Ralph LJ, Schwarz EB, Grossman D, Foster DG. Self-reported physical health of women who did and did not terminate pregnancy after seeking abortion services: a cohort study. Ann Intern Med. 2019. 10.7326/M18-1666. [DOI] [PubMed]
- 77.Davis A, Westhoff C, De Nonno L. Bleeding patterns after early abortion with mifepristone and misoprostol or manual vacuum aspiration. J AM Med Womens Assoc. 2000;55(3 Suppl):141–4. [PubMed] [Google Scholar]
- 78.The American College of Obstetricians and Gynecologists Practice Bulletin No. 143: medical management of first-trimester abortion. Obstet Gynecol. 2014;123(3):676–92. doi: 10.1097/01.AOG.0000444454.67279.7d. [DOI] [PubMed] [Google Scholar]
- 79.Raymond EG, Shannon C, Weaver MA, Winikoff B. First-trimester medical abortion with mifepristone 200mg and misoprostol: a systematic review. Contraception. 2013;87(1):26–37. doi: 10.1016/j.contraception.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Achilles SL, Reeves MF. Prevention of infection after induced abortion: SFP guideline 20102. Contraception. 2011;83(4):295–309. doi: 10.1016/j.contraception.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Fjerstad M, Trussel J, Sivin I, Lichtenberg ES, Cullins V. Rates of serious infection after changes in regimens for medical abortion. N Engl J Med. 2009;361(2):145–51. doi: 10.1056/NEJMoa0809146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353(22):2352–60. doi: 10.1056/NEJMoa051620. [DOI] [PubMed] [Google Scholar]
- 83.Winikoff B. Clostridium sordellii infection in medical abortion. Clin Infect Dis. 2006;43(11):1447–8. doi: 10.1086/508895. [DOI] [PubMed] [Google Scholar]
- 84.Ho CS, Bhatnagar J, Cohen AL, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am J Obstet Gynecol. 2009;201:459.e1–7. doi: 10.1016/j.ajog.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 85.Grossman D, White K, Harris L, et al. Continuing pregnancy after mifepristone and “reversal” of first-trimester medical abortion: a systematic review. Contraception. 2015;92(3):206–11. doi: 10.1016/j.contraception.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Creinin MD, Hou MY, Dalton L, Steward R, Chen MJ. Mifepristone antagonization with progesterone to prevent medical abortion: a randomized controlled trial. Obstet Gynecol. 2020;135(1):158–65. doi: 10.1097/AOG.0000000000003620. [DOI] [PubMed] [Google Scholar]
- 87.Aiken ARA, Broussard K, Johnson DM, Padron E. Motivations and Experiences of People Seeking Medication Abortion Online in the United States. Perspect Sex Reprod Health. 2018;50(4):157–63. doi: 10.1363/psrh.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biggs MA, Ralph L, Raifman S, Foster DG, Grossman D. Support for and interest in alternative models of medication abortion provision among a national probability sample of U.S. women. Contraception. 2019;99(2):118–24. doi: 10.1016/j.contraception.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Bazelon E. A Mother in Jail for Helping Her Daughter Have an Abortion. New York Times. Available at: https://www.nytimes.com/2014/09/22/magazine/a-mother-in-jail-for-helping-her-daughter-have-an-abortion.html. Accessed January 24, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb).