Abstract
Abstract
Background
Opioid agonist therapy (OAT) has been shown to reduce mortality in patients with opioid use disorder (OUD), yet mortality in individuals receiving OAT remains higher than in an age- and gender-matched population.
Objective
To identify baseline risk factors in patients who engaged in buprenorphine treatment that are associated with this elevated risk of death.
Design
We performed a retrospective cohort study from January 1, 2007, to December 31, 2018, using a centralized clinical data registry within a multi-hospital health system in Boston, MA, USA.
Participants
All adult patients who had ≥ 2 consecutive encounters with sublingual buprenorphine on the active medication list from January 1, 2007, to December 31, 2018.
Main Measures
We abstracted several sociodemographic, clinical, and healthcare use characteristics from the clinical data registry. The primary outcome was all-cause mortality and the secondary outcome was opioid overdose-related mortality. We performed multivariable cox regression to identify baseline characteristics independently associated with these outcomes.
Key Results
Of 5948 patients in the cohort, the majority were white (80.7%) and male (59.7%), with a mean age of 38.2 years. The all-cause mortality rate was 24.0 deaths per 1000 person-years. Baseline characteristics independently associated with an increased hazard of all-cause mortality included homelessness (adjusted hazard ratio [aHR] = 1.39; 95% confidence interval [CI] = 1.09, 1.78), an opioid on the active medication list (aHR = 1.28; 95% CI = 1.08, 1.52), and entry into the cohort during an inpatient hospitalization (aHR = 1.43; 95% CI = 1.18, 1.73). Homelessness was also associated with an increased hazard of opioid overdose-related mortality (aHR = 1.77; 95% CI = 1.25, 2.50).
Conclusions
We identified several novel and potentially modifiable predictors of mortality among patients engaging in buprenorphine treatment who remain at an increased risk of death compared with the general population. Understanding these risk factors can assist healthcare providers in risk stratification and inform the design of targeted interventions to improve outcomes in a high-risk patient population.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-05779-1) contains supplementary material, which is available to authorized users.
KEY WORDS: buprenorphine, opioid-related disorders, opioid abuse, substance-related disorders, substance abuse
INTRODUCTION
Opioid use disorder (OUD) is responsible for significant morbidity and premature mortality worldwide.1 Opioid overdose mortality rates have risen by more than 200% over the past two decades, with rates climbing from 2.9 per 100,000 persons in 1999 to 14.9 per 100,000 persons in 2017.1–3 In 2017 alone, a total of 47,600 people in the USA died from opioid overdose and over half of these deaths occurred in individuals under 45 years old.2
Opioid agonist therapy (OAT), which includes methadone and buprenorphine, has been shown to be effective in reducing mortality in patients with OUD.1, 4 Methadone, an opioid agonist, has been used for the treatment of OUD since the 1960s and can only be dispensed from federally certified opioid treatment programs. Buprenorphine, a partial opioid agonist, entered the market in the early 2000s and can be prescribed in any office-based setting by a physician who obtains a specialized prescribing waiver.5
Despite these effective treatments, individuals who are initiated on OAT remain at elevated risk of death compared with an age- and gender-matched general population.6 This is partially attributable to the fact that many patients cycle in and out of treatment, with significantly higher mortality rates during out-of-treatment phases.1, 4 However, there may be other factors contributing to this persistently elevated risk of death.
Studies evaluating baseline risk factors associated with mortality in patients treated with methadone have found that medical and psychiatric comorbidities, homelessness, a concurrent benzodiazepine prescription, injection drug use, and alcohol use disorder are associated with an increased risk of mortality.7–12 Though increasing numbers of physicians are prescribing buprenorphine,5, 13 there is limited research evaluating the extent to which baseline characteristics influence the risk of death in patients treated with buprenorphine.
The objective of this study was to identify factors independently associated with all-cause and opioid overdose-related mortality in patients who engage in buprenorphine treatment. We hypothesized that in addition to several characteristics that were previously found to increase the risk of death in patients treated with methadone (e.g., homelessness, medical comorbidities, and anti-psychotic medications), two additional risk factors for death in patients who engage in buprenorphine treatment are serious bacterial infections and a lack of outpatient follow-up when initiated on buprenorphine as an inpatient.
METHODS
Study Design and Population
We conducted a retrospective cohort study utilizing a centralized clinical data registry within a multi-hospital health system in Boston, MA. The Research Patient Data Registry (RPDR) includes comprehensive clinical information for over 4.7 million patients from the Partners Healthcare System, including Massachusetts General Hospital, Brigham and Women’s Hospital, and their associated outpatient systems. The RPDR is populated by data from clinical and billing systems including demographics, inpatient and outpatient encounters, diagnoses, medications, procedures, laboratory values, imaging, progress notes, and discharge summaries.
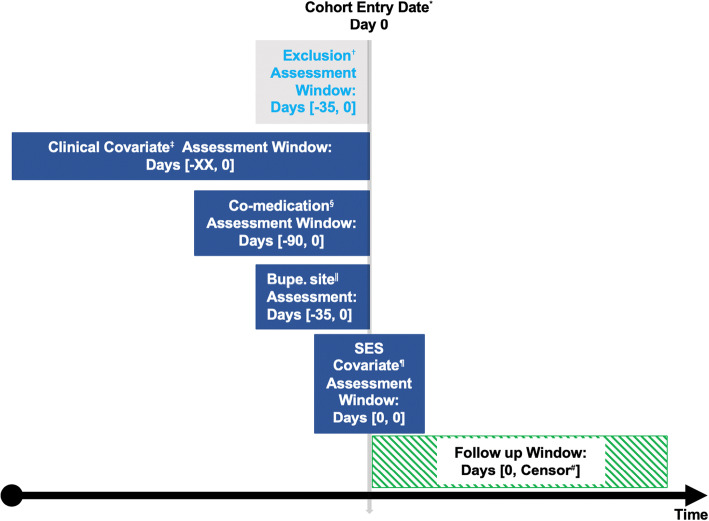
The cohort included all adults (≥ 18 years old) who had ≥ 2 consecutive encounters with sublingual buprenorphine on the active medication list within a source data range from January 1, 2007, to December 31, 2017. Sublingual formulation is approved exclusively for the treatment of OUD (formulations provided in Supplemental Table 2). Buprenorphine treatment was based on the active medication list during each patient encounter rather than actual prescriptions because prescription data is not available in the RPDR. Two consecutive encounters, defined as encounters that occurred within 35 days of each other to allow for a maximum buprenorphine prescription length of 28 days with a 7-day buffer, were required to enter the cohort to exclude those who had buprenorphine entered as an active medication once in error. The cohort entry date was the date of the second encounter. The follow-up window extended from the cohort entry date through December 31, 2018, to allow for at least one full year of follow-up after the last possible cohort entry date. Patients were followed until the primary outcome (death) or end of the study period occurred (Fig. 1, adapted from Schneiweiss et al.14). The Partners Healthcare Institutional Review Board approved this study.]
Figure 1.
Exposure-based cohort entry timeline. The vertical gray arrow depicts the cohort entry date and the horizontal black arrow depicts time over the study period. Each box depicts the time (in days) during which the covariates were assessed in relation to the cohort entry date. *Date of second encounter with buprenorphine on the active medication list; possible cohort entry from January 1, 2007, through December 31, 2017. †Exclusion criteria: < 18 years old and < 2 encounters with buprenorphine on the active medication list. ‡Baseline clinical covariates included coronary artery disease, cerebrovascular disease, congestive heart failure, chronic lung disease, diabetes mellitus, hepatitis C infection, HIV, chronic kidney disease, liver disease, malignancy, homeless status, and alcohol use disorder. §Baseline medications included anti-depressants, anti-psychotics, benzodiazepines, opioids (excluding buprenorphine), and naloxone. ‖Site of the first two encounters with buprenorphine on the active medication list. ¶SES baseline covariates included age, sex, race/ethnicity, preferred language, and study entry year. #Earliest of outcome of interest (death) or end of the study period (December 31, 2018). Adapted from www.repeatinitiative.org/projects.html. Licensed under CC BY.
Outcomes
The primary outcome was all-cause mortality. We identified deaths using the Social Security Death Index (SSDI) and the Massachusetts Registry of Vital Records and Statistics (RVRS). The SSDI is automatically linked to the RPDR; however, a new legislative rule in 2014 prohibits the release of death records for a period of three years. Therefore, we used the SSDI as our primary data source from 2007 through 2015 and the Massachusetts RVRS as our primary data source from 2016 through 2018. Based on the overlapping years (2007–2015), we identified 21 deaths (3%) in the SSDI that were not in the Massachusetts RVRS.
The secondary outcome was opioid overdose-related mortality, defined as having an underlying cause of death related to drug poisoning (International Statistical Classification of Diseases and Related Health Problems, 10th Revision [ICD-10] codes X40-X44, X60–64, X85, and Y10-Y14) and an opioid code (ICD-10 codes T40.0-T40.4, T40.6) listed in the multiple cause of death fields.3, 15, 16 We linked cause of death information from the Massachusetts RVRS to our cohort by using deterministic as well as probabilistic methods (merging on date of birth, gender, first and last names, city, and zip code). For cause of death information not available through the Massachusetts RVRS, we obtained cause of death information from the Centers for Disease Control and Prevention National Death Index.17
Covariates
Baseline covariates for model inclusion were chosen a priori and designed to minimize correlation (e.g., we removed HCV diagnosis codes from the covariate for liver disease). All baseline variables were abstracted from the RPDR using automated methods. Sociodemographic features included age at cohort entry, sex, race/ethnicity, preferred language, and housing status (defined by the presence of housing-related ICD codes prior to cohort study entry).
Baseline clinical characteristics included chronic comorbid conditions, a prior serious bacterial infection, medications, and comorbid substance use disorders (SUDs). Chronic comorbid conditions were defined as having an encounter with an ICD code from a validated ICD code algorithm prior to cohort study entry (Supplemental Table 1).18, 19 A prior serious bacterial infection was defined as having a diagnosis of endocarditis, epidural abscess, septic arthritis, or osteomyelitis prior to study entry using ICD code algorithms from previously published studies of serious bacterial infections and OUD (Supplemental Table 1).20, 21 Medications, including anti-depressants, anti-psychotics, benzodiazepines, opioids (excluding buprenorphine), and naloxone, were defined as having an encounter with a medication listed on the active medication list within 3 months prior to study entry (Supplemental Table 2).22, 23 Comorbid SUDs, including alcohol use disorder (AUD) and other SUDs (excluding OUD), were defined as having an encounter with an ICD code from previously published Health Center Utilization Project algorithms prior to study entry (Supplemental Table 1).24
We also examined the site of the first two encounters that listed buprenorphine as an active medication as a baseline predictor of mortality. Site of encounter was divided into four categories, including outpatient only, outpatient then inpatient (first encounter as an outpatient and second during an inpatient hospitalization), inpatient only (both encounters during an inpatient hospitalization), and inpatient then outpatient (first encounter during an inpatient hospitalization and second as an outpatient). These categories are based on prior studies evaluating inpatient initiation of buprenorphine and linkage to outpatient addiction care.25, 26
An additional baseline characteristic included study entry year. Study entry year was divided into three categories, including 2007 through 2009, 2010 through 2013, and 2014 through 2017, consistent with national trends in opioid use and overdose mortality.2, 27
Statistical Analysis
We used descriptive statistics to summarize the baseline sociodemographic, clinical, and healthcare use characteristics of our cohort. We used the chi-square or Fisher’s exact tests to compare categorical variables and t tests or logrank tests to compare continuous variables.
We performed multivariable cox regression to identify baseline characteristics independently associated with all-cause mortality, reporting hazard ratios (HRs) and 95% confidence intervals (CIs). Using a priori clinical knowledge, we included all possible confounders and significant predictors using the aforementioned variables. We were not at risk of overfitting the model for our primary outcome as we had at least 10 outcomes per variable (in total, we had 664 deaths and 28 variables). We then used a cause-specific hazard model, censoring those who died from other causes, to identify baseline characteristics independently associated with opioid overdose-related mortality.
We performed several diagnostic evaluations of the model, including confirmation that we did not violate the proportional hazards assumption by examining the Schoenfeld and Martingale residuals.
We also performed two pre-specified sensitivity analyses. First, we limited the analysis to individuals with a Massachusetts address to assess for ascertainment bias introduced by using the Massachusetts RVRS as the sole source of identifying deaths from 2016 through 2018. Second, we limited the analysis to individuals with a diagnosis of OUD prior to study entry (in addition to the eligibility criteria of having buprenorphine listed as an active medication) to account for potential misclassification with patients receiving buprenorphine for chronic pain.
A two-sided p value of less than 0.05 was considered statistically significant. All analyses were performed using SAS, version 9.4 (SAS institute). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting an observational cohort study.28
RESULTS
Cohort Characteristics
From January 1, 2007, to December 31, 2017, 5948 adult patients had ≥ 2 consecutive encounters with sublingual buprenorphine on the active medication list with a total of 27,638 person-years of follow-up. The median follow-up time was 4.3 years (IQR = 2.6, 6.0).
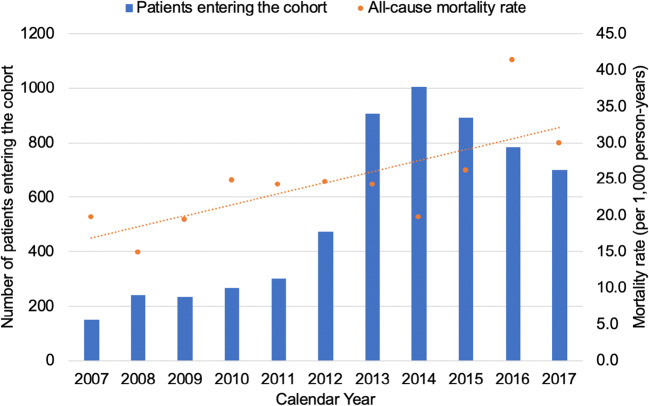
The majority were male (59.7%) and white (80.7%) with a mean of age 38.2 years (SD 13.1 years). Approximately one quarter (25.9%) had chronic lung disease, 26.3% had a hepatitis C viral (HCV) infection, and 50.7% had AUD. The majority of patients had their first two encounters with buprenorphine as an active medication as outpatients only (47.0%) or inpatients only (46.6%) (Table 1). The peak study entry years were from 2013 through 2015 (Fig. 2), while the total number of patients in the RPDR system increased at a relatively stable rate throughout the study period.
Table 1.
Baseline Characteristics of Patients who Engaged in Buprenorphine Treatment from 2007 to 2017
| Vital status | ||||
|---|---|---|---|---|
| Baseline characteristics | All patients (n = 5948) | Alive (n = 5284) | Deceased (n = 664) | p value |
| Sociodemographic features | ||||
| Age, mean (SD) | 38.2 (13.1) | 37.6 (12.9) | 43.5 (14.0) | < 0.0001 |
| Sex, n (%) | ||||
| Male | 3550 (59.7) | 3116 (59.0) | 433 (65.2) | 0.002 |
| Race, n (%) | 0.01 | |||
| White | 4800 (80.7) | 4257 (80.6) | 544 (81.8) | |
| Black | 249 (4.2) | 212 (4.0) | 37 (5.6) | |
| Hispanic | 224 (3.8) | 195 (3.7) | 29 (4.4) | |
| Other | 133 (2.2) | 127 (2.4) | 6 (0.9) | |
| Unknown | 542 (9.1) | 493 (9.3) | 49 (7.4) | |
| Preferred language, n (%) | 0.3 | |||
| English | 5351 (90.0) | 4760 (90.1) | 591 (89.0) | |
| Non-English | 79 (1.3) | 66 (1.2) | 13 (2.0) | |
| Unknown | 518 (8.7) | 458 (8.7) | 60 (9.0) | |
| Housing status, n (%) | ||||
| Homeless | 492 (8.3) | 400 (7.6) | 92 (13.9) | < 0.0001 |
| Clinical characteristics | ||||
| Chronic comorbid conditions, n (%) | ||||
| Coronary artery disease | 246 (4.1) | 186 (3.5) | 60 (9.0) | < 0.0001 |
| Cerebrovascular disease | 147 (2.5) | 122 (2.3) | 25 (3.8) | 0.03 |
| Chronic kidney disease | 247 (4.2) | 185 (3.5) | 62 (9.3) | < 0.0001 |
| Chronic lung disease | 1539 (25.9) | 1282 (24.3) | 257 (38.7) | < 0.0001 |
| Congestive heart failure | 408 (6.9) | 295 (5.6) | 113 (17.0) | < 0.0001 |
| Hepatitis C infection | 1566 (26.3) | 1290 (24.4) | 276 (41.7) | < 0.0001 |
| HIV | 126 (2.1) | 95 (1.8) | 31 (4.7) | < 0.0001 |
| Liver disease | 1171 (19.7) | 952 (18.0) | 219 (33.0) | < 0.0001 |
| Malignancy | 435 (7.3) | 341 (6.5) | 94 (14.2) | < 0.0001 |
| Serious bacterial infection*, n (%) | 468 (7.9) | 371 (7.0) | 97 (14.6) | < 0.0001 |
| Comorbid substance use disorders, n (%) | ||||
| Alcohol use disorder | 3017 (50.7) | 2613 (49.5) | 404 (60.8) | < 0.0001 |
| Other substance use disorder† | 3750 (63.0) | 3304 (62.5) | 446 (67.2) | 0.006 |
| Medications, n (%) | ||||
| Anti-depressant | 2313 (38.9) | 2019 (38.2) | 294 (44.3) | 0.003 |
| Anti-psychotic | 1247 (21.0) | 1054 (19.9) | 193 (29.1) | < 0.0001 |
| Benzodiazepine | 2593 (43.6) | 2257 (42.7) | 336 (50.6) | 0.0001 |
| Opioid‡ | 1752 (29.5) | 1472 (27.9) | 280 (42.2) | < 0.0001 |
| Naloxone | 3976 (66.8) | 3487 (66.0) | 489 (73.6) | < 0.0001 |
| Healthcare use factors | ||||
| Buprenorphine encounter site§, n (%) | 0.15 | |||
| Outpatient only | 2797 (47.0) | 2497 (47.3) | 300 (45.2) | |
| Outpatient then inpatient | 175 (2.9) | 146 (2.8) | 29 (4.4) | |
| Inpatient only | 2774 (46.6) | 2466 (46.7) | 308 (46.4) | |
| Inpatient then outpatient | 161 (2.7) | 140 (2.6) | 21 (3.2) | |
| Unknown | 41 (0.7) | 35 (0.7) | 6 (0.9) | |
| Study entry year, n (%) | < 0.0001 | |||
| 2007–2009 | 620 (10.4) | 516 (9.8) | 104 (15.7) | |
| 2010–2013 | 1950 (32.8) | 1687 (31.9) | 263 (39.6) | |
| 2014–2017 | 3378 (56.8) | 3081 (58.3) | 297 (44.7) | |
*Includes endocarditis, epidural abscess, osteomyelitis, and septic arthritis
†Includes amphetamine use disorder, hallucinogen use disorder, sedative use disorder, and other/polysubstance use disorder
‡Excludes buprenorphine
§Initial buprenorphine encounter site based on the first two encounters with buprenorphine on the active medication list
Figure 2.
Secular trends of entry into the study cohort and all-cause mortality rates. The X-axis depicts calendar year over the study period. The Y-axis on the left depicts the number of patients entering the cohort and is represented by the blue bars. The Y-axis on the right depicts the mortality rate (per 1000 person-years) and is represented by the orange dots. The total Partners Healthcare patient population increased at a relatively stable rate over this time period.
Mortality-Related Statistics
Of the 5948 patients in the cohort, 664 (11.2%) died with a 25th percentile of survival of 11.6 years. The all-cause mortality rate was 24.0 per 1000 person-years, with a rising mortality rate over the study period (Fig. 2). In total, 299 (5.0%) died from an opioid overdose with an opioid overdose-related mortality rate of 10.8 per 1000 person-years.
We identified three sociodemographic characteristics independently associated with an increased hazard of all-cause mortality, including age (adjusted HR [aHR] = 1.24 per 10-year increment; 95% CI = 1.17, 1.33), male sex (aHR = 1.34; 95% CI = 1.13, 1.58), and homelessness (aHR = 1.39; 95% CI = 1.09, 1.78). An increased hazard of opioid overdose-related mortality was associated with male sex (aHR = 1.62; 95% CI = 1.26, 2.10) and homelessness (aHR = 1.77; 95% CI = 1.25, 2.50) (Table 2).
Table 2.
Association Between Baseline Patient Characteristics and Mortality
| All-cause mortality (n = 664) | Opioid overdose mortality (n = 299) | |||
|---|---|---|---|---|
| Baseline characteristics | aHR* (95% CI) | p value | aHR* (95% CI) | p value |
| Sociodemographic features | ||||
| Age (per 10-year increment) | 1.24 (1.17, 1.33) | < 0.001 | 0.96 (0.87, 1.07) | 0.49 |
| Sex | ||||
| Female | Ref | Ref | Ref | Ref |
| Male | 1.34 (1.13, 1.58) | < 0.001 | 1.62 (1.26, 2.10) | < 0.001 |
| Race | ||||
| Non-White | Ref | Ref | Ref | Ref |
| White | 1.24 (1.00, 1.54) | 0.06 | 1.11 (0.80, 1.55) | 0.52 |
| Preferred language | ||||
| English | Ref | Ref | Ref | Ref |
| Non-English | 1.06 (0.60, 1.87) | 0.85 | 0.63 (0.20, 2.04) | 0.44 |
| Housing status | ||||
| Housed | Ref | Ref | Ref | Ref |
| Homeless | 1.39 (1.09, 1.78) | 0.01 | 1.77 (1.25, 2.50) | 0.001 |
| Clinical characteristics | ||||
| Chronic comorbid conditions | ||||
| Coronary artery disease | 1.02 (0.75, 1.38) | 0.92 | 0.68 (0.34, 1.35) | 0.27 |
| Cerebrovascular disease | 0.68 (0.44, 1.03) | 0.07 | 0.68 (0.27, 1.70) | 0.41 |
| Chronic kidney disease | 1.45 (1.09, 1.94) | 0.01 | 1.44 (0.83, 2.52) | 0.20 |
| Chronic lung disease | 1.35 (1.14, 1.61) | < 0.001 | 1.11 (0.85, 1.46) | 0.44 |
| Congestive heart failure | 1.75 (1.38, 2.23) | < 0.001 | 1.15 (0.70, 1.90) | 0.57 |
| Hepatitis C infection | 1.56 (1.29, 1.89) | < 0.001 | 1.83 (1.39, 2.41) | < 0.001 |
| HIV | 1.13 (0.77, 1.67) | 0.53 | 0.89 (0.42, 1.86) | 0.75 |
| Liver disease | 1.06 (0.87, 1.30) | 0.54 | 0.94 (0.69, 1.29) | 0.71 |
| Malignancy | 1.33 (1.04, 1.68) | 0.02 | 0.66 (0.38, 1.16) | 0.15 |
| Serious bacterial infection† | 1.26 (0.99, 1.60) | 0.07 | 1.22 (0.81, 1.83) | 0.34 |
| Comorbid substance use disorders | ||||
| Alcohol use disorder | 1.04 (0.88, 1.25) | 0.64 | 1.05 (0.81, 1.36) | 0.69 |
| Other substance use disorder‡ | 0.91 (0.75, 1.10) | 0.32 | 1.26 (0.95, 1.68) | 0.11 |
| Medications | ||||
| Anti-depressant | 1.05 (0.89, 1.23) | 0.58 | 1.09 (0.86, 1.40) | 0.47 |
| Anti-psychotic | 1.32 (1.10, 1.58) | 0.002 | 1.23 (0.93, 1.62) | 0.16 |
| Benzodiazepine | 1.05 (0.88, 1.24) | 0.61 | 0.99 (0.76, 1.28) | 0.91 |
| Opioid§ | 1.28 (1.08, 1.52) | 0.005 | 1.18 (0.90, 1.55) | 0.23 |
| Naloxone | 0.98 (0.79, 1.21) | 0.84 | 0.78 (0.56, 1.09) | 0.14 |
| Healthcare use factors | ||||
| Buprenorphine encounter site‖ | ||||
| Outpatient only | Ref | Ref | Ref | Ref |
| Outpatient then inpatient | 1.67 (1.14, 2.47) | 0.009 | 1.07 (0.52, 2.21) | 0.86 |
| Inpatient only | 1.43 (1.18, 1.73) | 0.002 | 1.20 (0.88, 1.63) | 0.25 |
| Inpatient then outpatient | 1.07 (0.68, 1.69) | 0.76 | 1.12 (0.56, 2.23) | 0.76 |
| Study entry year | ||||
| 2007–2009 | Ref | Ref | Ref | Ref |
| 2010–2013 | 1.27 (0.97, 1.66) | 0.07 | 1.40 (0.90, 2.19) | 0.13 |
| 2014–2017 | 1.57 (1.18, 2.10) | 0.002 | 2.61 (1.63, 4.19) | < 0.001 |
*Adjusted hazard ratio: results from each column come from one single model adjusting for all covariates in this table
†Includes endocarditis, epidural abscess, osteomyelitis, and septic arthritis
‡Includes amphetamine use disorder, hallucinogen use disorder, sedative use disorder, and other/polysubstance use disorder
§Excludes buprenorphine
‖Initial buprenorphine encounter site based on the first two encounters with buprenorphine on the active medication list
We also identified several clinical characteristics independently associated with an increased hazard of all-cause mortality, including chronic kidney disease (aHR = 1.45; 95% CI = 1.09, 1.94), chronic lung disease (aHR = 1.35; 95% CI = 1.14, 1.61), congestive heart failure (aHR = 1.75; 95% CI = 1.38, 2.23), HCV infection (aHR = 1.56; 95% CI = 1.29, 1.89), and malignancy (aHR = 1.33; 95% CI = 1.04, 1.68), as well as prior receipt of an anti-psychotic medication (aHR = 1.32; 95% CI = 1.10, 1.58) and an opioid medication (aHR = 1.28; 95% CI = 1.08, 1.52). A history of HCV infection was associated with an increased hazard of opioid overdose-related mortality (aHR = 1.83; 95% CI = 1.39, 2.41) (Table 2).
The site of the first two encounters with buprenorphine listed as an active medication was also associated with all-cause mortality (Table 2). When compared with individuals who had their first two encounters as outpatient only, patients who had their first two encounters during an inpatient hospitalization (aHR = 1.43; 95% CI = 1.18, 1.73) and patients who had their first encounter as an outpatient and second encounter as an inpatient (aHR = 1.67; 95% CI = 1.14, 2.47) had an increased hazard of all-cause mortality.
Compared with patients who entered the study from 2007 through 2009, individuals who entered the cohort from 2014 through 2017 had a 1.57-fold (95% CI = 1.18, 2.10) increased hazard of all-cause mortality and a 2.61-fold (95% CI = 1.63, 4.19) increased hazard of opioid overdose-related mortality (Table 2).
In our two sensitivity analyses, limiting the cohort to patients with a Massachusetts address (Supplemental Table 3) and patients who had a documented diagnosis of OUD (Supplemental Table 4), results were similar.
DISCUSSION
In a large cohort of patients who engaged in buprenorphine treatment for OUD, the mortality rate was 12.4-fold higher than that of a similarly aged general population (24.0 deaths per 1000 person-years in our cohort vs. 1.95 deaths per 1000 person-years for those aged 35–44 years in 201729). We identified several baseline sociodemographic, clinical, and healthcare use factors that were associated with mortality in this cohort. While many of the predictors of mortality that we identified are similar to those previously found to be associated with mortality in patients treated with methadone, including chronic lung disease, HCV infection, and an anti-psychotic prescription, several of our findings are novel and potentially modifiable. These findings are particularly important for the development of interventions focused on reducing adverse outcomes in a group of individuals who remain at elevated risk of death despite engagement in effective treatment.
Homeless individuals were at significantly increased risk of both all-cause and opioid overdose-related mortality compared with housed individuals. In addition to having a unique set of risk factors that place homeless individuals at increased risk of death, they also tend to have more severe OUD and higher rates of drug overdose compared with the general population.30–33 Though buprenorphine treatment can be effective in homeless individuals,30 more research is needed to evaluate buprenorphine-related outcomes in this population given their unique set of risk factors for death. This also highlights the important role that social determinants of health may play in addiction care.
An opioid prescription within 3 months prior to cohort entry was also associated with an increased risk of death. Pain control in patients with OUD has historically been difficult to navigate with a concern that under-treated pain can lead to relapse.34, 35 Our findings do raise concern about concurrent opioid prescriptions in those receiving buprenorphine, but further research elucidating this relationship in a more detailed and time-varying manner is needed.
Additionally, we found that patients who had their first two encounters as inpatients were at increased risk of all-cause mortality compared with those who entered the cohort as outpatients. Our findings suggest that this may be related to discontinuation of buprenorphine after discharge, as patients who had their first encounter as an inpatient and second as an outpatient did not have the same increased risk of death. Prior studies evaluating inpatient buprenorphine induction and linkage to outpatient treatment compared with detoxification alone found increased rates of outpatient follow-up and longer times in treatment among individuals who were linked to outpatient care.25, 26, 36 These findings emphasize that linkage to addiction care after hospital discharge is critical for buprenorphine treatment outcomes. We would have expected this association to also be true for opioid overdose-related mortality, as we know that time out of buprenorphine treatment is a significant risk factor for opioid overdose-related mortality1, 37; however, our study may have been underpowered to detect this particular association. We recognize that in this study we cannot fully disentangle whether the increased risk associated with initiating buprenorphine in the hospital is associated with gaps in subsequent OAT or is simply associated with the risks related to being hospitalized (i.e., patients who are hospitalized may be sicker at baseline or the hospitalization itself may increase risk). In future studies, we intend to evaluate this further using time-varying models and extended pharmacy data to explore the association between buprenorphine adherence and mortality risk.
Finally, the risk of all-cause and opioid overdose-related mortality increased in patients who entered the cohort in later years, particularly 2014 through 2017. This likely represents the increased potency of opioids used overtime, such as fentanyl;2, 27 however, this could also reflect a case-mix trend towards providing lower barrier addiction care to patients at the highest risk for adverse addiction-related outcomes.
Limitations
There are several limitations to our study. Data quality due to coding errors is of concern when using a clinical data registry that is reliant on the electronic health record (EHR). Similarly, validation of variable measurement using the EHR is often lacking. However, we did use validated algorithms for the vast majority of the variables, as shown in Supplemental Table 1.
We measured variables only at baseline and did not control for buprenorphine retention, a known risk factor for mortality in patients with OUD. However, we believe this cohort represents a group of patients who had at least some intention of being treated with buprenorphine and understanding baseline risk factors for mortality in this healthcare seeking group is clinically important, especially if additional harm reduction interventions are initiated at the time of buprenorphine initiation. More research is needed to evaluate predictors of buprenorphine retention in a time-varying manner.
In addition, patients in the cohort were not necessarily newly initiated on buprenorphine, as they could have previously been started on buprenorphine in another healthcare system. Similarly, we may have missed patients who received a second subsequent buprenorphine prescription outside of the Partners system. Furthermore, because methadone can only be prescribed at federally certified opioid treatment centers, we do not have information regarding prior methadone use which could alter a patient’s risk of death.
Although our multivariable analysis controlled for a wide range of characteristics potentially associated with OAT and death, the observational nature of the study introduces the possibility of unmeasured confounding. Finally, our findings may not be generalizable to other patient populations, such as rural populations or those located outside of the northeastern United States.
CONCLUSIONS
We identified several sociodemographic, clinical, and healthcare use characteristics independently associated with all-cause mortality in a large cohort of patients who engaged in buprenorphine treatment for OUD. Several of these predictors of mortality have not been previously identified and are potentially modifiable, including homelessness, receipt of an opioid prescription, and initial encounters as an inpatient. Understanding baseline predictors of all-cause mortality can help healthcare providers prognosticate which patients are at highest risk of death and inform the design of interventions aimed towards mitigating this risk in patients with OUD who remain at elevated hazard of death despite effective treatment.
Electronic Supplementary Material
(DOCX 36 kb)
Contributors
No additional contributors.
Funding Information
This study was supported by a Ruth L. Kirschstein Institutional National Research Services Award (T32 2015D006913), the Ryiochi Sasakawa Fellowship Fund, and the Division of General Medicine at Massachusetts General Hospital. These funding sources did not influence the study’s design, conduct, or reporting.
Compliance with Ethical Standards
The Partners Healthcare Institutional Review Board approved this study.
Conflict of Interest
Dr. Baggett receives authorship royalties from UpToDate. The other authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 2.Scholl LSP, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mort Wkly Rep. 2019;67:1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 4.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderks CE. Trends in the Use of Methadone, Buprenorphine, and Extended-release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (Update). The CBHSQ Report: August 22, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed]
- 6.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug Alcohol Depend. 2009;105(1–2):9–15. doi: 10.1016/j.drugalcdep.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Leece P, Cavacuiti C, Macdonald EM, et al. Predictors of Opioid-Related Death During Methadone Therapy. J Subst Abuse Treat. 2015;57:30–35. doi: 10.1016/j.jsat.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 8.McCowan C, Kidd B, Fahey T. Factors associated with mortality in Scottish patients receiving methadone in primary care: retrospective cohort study. BMJ. 2009;338:b2225. doi: 10.1136/bmj.b2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousins G, Teljeur C, Motterlini N, McCowan C, Dimitrov BD, Fahey T. Risk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort study. J Subst Abuse Treat. 2011;41(3):252–260. doi: 10.1016/j.jsat.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Brugal MT, Domingo-Salvany A, Puig R, Barrio G, Garcia de Olalla P, de la Fuente L. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction. 2005;100(7):981–989. doi: 10.1111/j.1360-0443.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- 11.Gossop M, Stewart D, Treacy S, Marsden J. A prospective study of mortality among drug misusers during a 4-year period after seeking treatment. Addiction. 2002;97(1):39–47. doi: 10.1046/j.1360-0443.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 12.Peles E, Schreiber S, Adelson M. 15-Year survival and retention of patients in a general hospital-affiliated methadone maintenance treatment (MMT) center in Israel. Drug Alcohol Depend. 2010;107(2–3):141–148. doi: 10.1016/j.drugalcdep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Wen H, Hockenberry JM, Pollack HA. Association of Buprenorphine-Waivered Physician Supply With Buprenorphine Treatment Use and Prescription Opioid Use in Medicaid Enrollees. JAMA Netw Open. 2018;1(5):e182943. doi: 10.1001/jamanetworkopen.2018.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneeweiss S, Rassen JA, Brown JS, et al. Graphical Depiction of Longitudinal Study Designs in Health Care Databases. Ann Intern Med. 2019. [DOI] [PubMed]
- 15.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAMHSA. Using international classification of diseases (ICD) codes to assess opioid-related overdose deaths. 2018; https://www.samhsa.gov/capt/sites/default/files/capt_resource/using-icd-10-codes-to-assess-opioid-related-overdose-deaths.pdf. Accessed 31 Jan 2019.
- 17.National Center for Health Statistics. National Death Index user’s guide. Hyattsville, MD; 2013. Available from: https://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. Accessed 25 Feb 2019.
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Niu B, Forde KA, Goldberg DS. Coding algorithms for identifying patients with cirrhosis and hepatitis B or C virus using administrative data. Pharmacoepidemiol Drug Saf. 2015;24(1):107–111. doi: 10.1002/pds.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronan MV, Herzig SJ. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832–837. doi: 10.1377/hlthaff.2015.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in Drug Use-Associated Infective Endocarditis and Heart Valve Surgery, 2007 to 2017: A Study of Statewide Discharge Data. Ann Intern Med. 2018. [DOI] [PMC free article] [PubMed]
- 22.The Executive Office of Health and Human Services. Masshealth Drug List Available from: https://masshealthdruglist.ehs.state.ma.us/MHDL/pubthera.do. Accessed 25 Feb 2019.
- 23.Centers for Disease Control. Drug overdose Data Files: Oral morphine milligram equivalents 2017 Available from: https://www.cdc.gov/drugoverdose/datafiles/CDC_Oral_Morphine_Milligram_Equivalents_Sept_2017.xlsx. Accessed 25 Feb 2019.
- 24.Heslin KC, Elixhauser A, Steiner CA. Hospitalizations Involving Mental and Substance Use Disorders Among Adults, 2012. HCUP Statistical Brief #191. Agency Healthc Res Qual. 2015. [PubMed]
- 25.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369–1376. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803–808. doi: 10.1007/s11606-010-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell JK, Gladden RM, Seth P. Trends in Deaths Involving Heroin and Synthetic Opioids Excluding Methadone, and Law Enforcement Drug Product Reports, by Census Region — United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:897–903. [DOI] [PMC free article] [PubMed]
- 28.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 29.Murphy SL, Xu JQ, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief, no 328. Hyattsville: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 30.Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang SW. Mortality among men using homeless shelters in Toronto, Ontario. JAMA. 2000;283(16):2152–2157. doi: 10.1001/jama.283.16.2152. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SW, Orav EJ, O’Connell JJ, Lebow JM, Brennan TA. Causes of death in homeless adults in Boston. Ann Intern Med. 1997;126(8):625–628. doi: 10.7326/0003-4819-126-8-199704150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Baggett TP, Hwang SW, O’Connell JJ, et al. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern Med. 2013;173(3):189–195. doi: 10.1001/jamainternmed.2013.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bounes V, Palmaro A, Lapeyre-Mestre M, Roussin A. Long-term consequences of acute pain for patients under methadone or buprenorphine maintenance treatment. Pain Physician. 2013;16(6):E739–747. [PubMed] [Google Scholar]
- 35.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldiero RM, Parran TV, Jr, Adelman CL, Piche B. Inpatient initiation of buprenorphine maintenance vs. detoxification: can retention of opioid-dependent patients in outpatient counseling be improved? Am J Addict. 2006;15(1):1–7. doi: 10.1080/10550490500418989. [DOI] [PubMed] [Google Scholar]
- 37.Dupouy J, Palmaro A, Fatseas M, et al. Mortality Associated With Time in and Out of Buprenorphine Treatment in French Office-Based General Practice: A 7-Year Cohort Study. Ann Fam Med. 2017;15(4):355–358. doi: 10.1370/afm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)