Abstract
Background
The relationship between risk factor or biomarker trajectories and contemporaneous short-term clinical outcomes is poorly understood. In diabetes patients, it is unknown whether hemoglobin A1c (HbA1c) trajectories are associated with clinical outcomes and can inform care in scenarios in which a single HbA1c is uninformative, for example, after a diagnosis of coronary artery disease (CAD).
Objective
To compare associations of HbA1c trajectories and single HbA1c values with short-term mortality in diabetes patients evaluated for CAD
Design
Retrospective observational cohort study
Participants
Diabetes patients (n = 7780) with and without angiographically defined CAD
Main Measures
We used joint latent class mixed models to simultaneously fit HbA1c trajectories and estimate association with 2-year mortality after cardiac catheterization, adjusting for clinical and demographic covariates.
Key Results
Three HBA1c trajectory classes were identified: individuals with stable glycemia (class A; n = 6934 [89%]; mean baseline HbA1c 6.9%), with declining HbA1c (class B; n = 364 [4.7%]; mean baseline HbA1c 11.6%), and with increasing HbA1c (class C; n = 482 [6.2%]; mean baseline HbA1c 8.5%). HbA1c trajectory class was associated with adjusted 2-year mortality (3.0% [95% CI 2.8, 3.2] for class A, 3.1% [2.1, 4.2] for class B, and 4.2% [3.4, 4.9] for class C; global P = 0.047, P = 0.03 comparing classes A and C, P > 0.05 for other pairwise comparisons). Baseline HbA1c was not associated with 2-year mortality (P = 0.85; hazard ratios 1.01 [0.96, 1.06] and 1.02 [0.95, 1.10] for HbA1c 7–9% and ≥ 9%, respectively, relative to HbA1c < 7%). The association between HbA1c trajectories and mortality did not differ between those with and without CAD (interaction P = 0.1).
Conclusions
In clinical settings where single HbA1c measurements provide limited information, HbA1c trajectories may help stratify risk of complications in diabetes patients. Joint latent class modeling provides a generalizable approach to examining relationships between biomarker trajectories and clinical outcomes in the era of near-universal adoption of electronic health records.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-05848-5) contains supplementary material, which is available to authorized users.
KEY WORDS: diabetes, hemoglobin A1c trajectory, cardiovascular disease, mortality
INTRODUCTION
In the era of electronic health records (EHR), repeated measurements of biomarkers and disease risk factors are readily available, but most clinical guidelines recommend treatment decision-making based on solitary measurements. While guidelines for common chronic diseases such as diabetes and hypertension base recommendations on the last available value,1, 2 there may be clinical scenarios in which the last available measurement is a poor predictor of clinical outcomes. In such scenarios, the integration of serial biomarker measurements into trajectories may be clinically informative. That is, the biomarker trajectory may reflect risk of an important clinical outcome, irrespective of a causal mechanism. However, the relationship between risk factor or biomarker trajectories and clinical outcomes remains poorly understood.
Diabetes mellitus, a disease in which a biomarker—hemoglobin A1c (HbA1c)—is measured serially to monitor glycemic control, provides a model clinical context in which to evaluate differential associations of a single HbA1c value and longitudinal trajectories with clinical outcomes. HbA1c is a predictor of diabetes complications and mortality,1, 3–10 and professional society guidelines recommend serial HbA1c monitoring. However, the guidelines do not offer diabetes management recommendations based on HbA1c trajectory.1, 11 Thus, clinical providers typically adjust diabetes treatment based on the last available HbA1c measurement, even though that single value does not predict meaningful clinical outcomes in certain clinical settings.
One clinical scenario in which the last available HbA1c measurement is not associated with short-term clinical outcomes occurs after myocardial infarction (MI) or percutaneous coronary intervention (PCI) for coronary artery disease (CAD).12–15 In this study, we used a real-world cohort of diabetes patients undergoing diagnostic evaluation for CAD to identify glycemic control trajectories, describe the characteristics of patients with distinct HbA1c trajectories, and compare the associations of HbA1c trajectories and single HbA1c measurements with short-term mortality.
METHODS
Study Cohort
We included all US veterans diagnosed with diabetes prior to October 2015 who underwent coronary angiography for indications related to CAD in the US Veterans Affairs (VA) healthcare system between October 2005 and September 2016. Data were recorded in a standardized fashion through the VA Clinical Assessment, Reporting, and Tracking Program (CART).16 We identified diabetes patients as those with at least one ICD-9 diabetes diagnosis code for inpatient encounters or at least two ICD-9 diabetes codes for outpatient visits on separate days, excluding codes for secondary diagnosis of diabetes.17 To avoid confounding by diabetes duration, we included individuals who met the diabetes definition within 24 months of their index cardiac catheterization and excluded those who had a qualifying diabetes diagnosis code more than 2 years prior to catheterization or were newly diagnosed with diabetes at the time of catheterization. To be included, participants had to have at least 6 months of records in the VA preceding index catheterization, at least one HbA1c measurement in the 24 months preceding angiography, and at least one HbA1c measurement during follow-up. We included individuals whose indication for angiography was related to CAD (chest pain, stable angina, positive functional study, or ischemic heart disease). For patients with multiple angiographies, we used HbA1c values after their first cardiac catheterization for this study and excluded patients with a prior MI or coronary revascularization. Loss to follow-up was defined as those with fewer than 3 months of follow-up time (without an outcome event) after catheterization. The Colorado Multiple Institution Review Board provided human subjects oversight and approval.
Exposures, Outcomes, and Covariates
The primary exposure was HbA1c trajectory during follow-up after cardiac catheterization, using the last HbA1c measurement before catheterization and all HbA1c measurements up to 2 years after catheterization. The primary outcome was 2-year all-cause mortality measured using VA vital status data. All multivariable models were adjusted for demographics (age, sex, race), cardiovascular risk factors (hypertension, hyperlipidemia, Framingham risk score, smoking status, body mass index), comorbidities (heart failure [HF], chronic obstructive pulmonary disease, post-traumatic stress disorder, peripheral artery disease, chronic kidney disease, dialysis, depression), post-angiography revascularization (none, PCI, coronary artery bypass graft), diabetes duration, and medication adherence at baseline for cardioprotective medications (HMG-CoA reductase inhibitors [statins], angiotensin-converting enzyme inhibitors [ACEi] or angiotensin receptor blockers [ARB], and beta-blockers) based on proportion of days covered (PDC) dichotomized at a threshold of ≥ 0.8.18 In secondary analyses, we included CAD burden as a covariate and in an interaction term with HbA1c trajectory using standard definitions of flow-limiting stenosis19 (Online Supplement, Table S7). All variables included in multivariable models are described in the Online Supplement (Table S7).
Statistical Analyses
We opted to model HbA1c trajectories and associations with mortality jointly using joint latent class mixed models,20, 21 overcoming limitations of the two-stage approach to trajectory analysis used in prior studies of diabetes patients,22–24 particularly that the two-stage study design precludes estimation of contemporaneous associations between trajectories and outcomes.25, 26 Joint latent class modeling provides an alternative approach to trajectory analysis in which the risk factor trajectories and their associations with an outcome can be estimated contemporaneously.20, 21 The rationale for the study design is described in detail in the Online Supplement.
We performed the analyses in three steps. First, we fit a series of latent class trajectory models to determine the functional form of time and number of latent classes that best fit the data.25, 26 We used Bayes information criteria (BIC) as the primary measure of model fit, and secondarily assessed the mean posterior probability of class membership across classes. Models with a third-degree fixed effect for time, a second-degree random time effect, and three latent HbA1c trajectory classes with an autoregressive covariance structure to account for correlation between repeated HbA1c measurements best fit the data. Details of model selection are available in the Online Supplement. As a sensitivity analysis of the trajectory models, we verified that a three-trajectory model best fit the data after limiting the sample to individuals using at least one diabetes medication at baseline (Fig. S1).
The second step in the analysis was to compare patient-level demographics, cardiovascular risk factors, diabetes-related variables, and other covariates across HbA1c trajectory classes. We used chi-square tests for categorical data and Kruskal-Wallis tests for continuous or ordinal data to compare variables across HbA1c trajectory classes. The third step in the analysis was to test associations between HbA1c trajectory classes and mortality using joint latent class mixed models (R package lcmm). This approach jointly identifies subgroups of patients with different HbA1c trajectories after index catheterization and estimates associations with mortality contemporaneously.20, 21 For the joint latent class survival model, class-specific baseline mortality risk was modeled with a Weibull distribution, and the effects of covariates were assumed constant over time. Finally, we used an omnibus interaction test to assess whether HbA1c trajectory classes and their associations with mortality varied across levels of CAD burden. As a sensitivity analysis, we repeated the primary analyses in participants without HF at baseline.
We also performed an exploratory analysis comparing diabetes medication prescriptions and adherence across individuals in different HbA1c trajectory classes. Based on prescription drug fills in the VA at baseline and 0–6, 6–12, and 12–24 months after catheterization, individuals were classified as being prescribed metformin, sulfonylureas, insulin, or other diabetes medication classes. We compared the classes of diabetes medications prescribed and the number of distinct medication classes used for each individual during each of the time intervals. For adherence, we compared PDC (dichotomized at 0.8) for ACEi/ARB, beta-blockers, statins, sulfonylureas, and metformin at 12 months after cardiac catheterization among individuals who had survived for a year.
All analyses were performed in R (version 3.1, R Foundation for Statistical Computing, Vienna, Austria), and statistical code is available upon request.
RESULTS
We studied 7780 diabetes patients with a mean diabetes duration of 1.1 years at the time of cardiac catheterization. Of these, 7492 (96%) were men, 6141 (79%) were white, and their average age was 62 years. The majority of participants had obstructive CAD on the index catheterization (62%), while 20% had non-obstructive CAD, and 18% had no CAD. Demographic, comorbidity, and treatment-related variables for the full study cohort are summarized in Table 1. Participants had a median of 2 HbA1c measurements in the first 6 months of follow-up and 5 over the 2-year follow-up period (Table S8).
Table 1.
Characteristics of All Study Participants and Stratified by Hemoglobin A1c (HbA1c) Trajectory Class After Cardiac Catheterization
| All participants | Hemoglobin A1c trajectory class | P value | |||
|---|---|---|---|---|---|
| Class A | Class B | Class C | |||
| n = 7780 | n = 6934 | n = 364 | n = 482 | ||
| Age (years), mean (SD) | 61.9 (8.3) | 62.3 (8.2) | 58.4 (8) | 58.1 (8) | < 0.0001 |
| Male, n (%) | 7492 (96.3) | 6683 (96.4) | 347 (95.3) | 462 (95.9) | 0.51 |
| Race, n (%) | < 0.0001 | ||||
| White | 6141 (78.9) | 5545 (80) | 255 (70.1) | 341 (70.7) | |
| Black | 1426 (18.3) | 1200 (17.3) | 99 (27.2) | 127 (26.3) | |
| Other | 213 (2.7) | 189 (2.7) | 10 (2.7) | 14 (2.9) | |
| Diabetes duration (years), mean (SD) | 1.1 (0.6) | 1.1 (0.6) | 1 (0.6) | 1.2 (0.5) | < 0.0001 |
| Baseline HbA1c (%), mean (SD) | 7.2 (1.5) | 6.9 (1.0) | 11.6 (1.6) | 8.5 (1.6) | < 0.0001 |
| Family History of CAD, n (%) | 1398 (18) | 1235 (17.8) | 58 (15.9) | 105 (21.8) | 0.05 |
| Tobacco, n (%) | 4367 (56.1) | 3889 (56.1) | 198 (54.4) | 280 (58.1) | 0.55 |
| Hypertension, n (%) | 7060 (90.7) | 6296 (90.8) | 322 (88.5) | 442 (91.7) | 0.25 |
| Hyperlipidemia, n (%) | 6819 (87.6) | 6093 (87.9) | 312 (85.7) | 414 (85.9) | 0.23 |
| BMI (kg/m2), mean (SD) | 33.1 (6.2) | 33 (6.2) | 32.9 (6.6) | 33.7 (6.6) | 0.13 |
| Framingham risk score, mean (SD) | 19.7 (10.9) | 19.8 (10.9) | 19.4 (11.3) | 18.7 (10.7) | 0.03 |
| PAD, n (%) | 1013 (13) | 912 (13.2) | 49 (13.5) | 52 (10.8) | 0.32 |
| HF, n (%) | 1086 (14) | 925 (13.3) | 72 (19.8) | 89 (18.5) | < 0.0001 |
| COPD, n (%) | 1264 (16.2) | 1149 (16.6) | 52 (14.3) | 63 (13.1) | 0.08 |
| CKD, n (%) | 1053 (13.5) | 927 (13.4) | 57 (15.7) | 69 (14.3) | 0.40 |
| Dialysis, n (%) | 112 (1.4) | 105 (1.5) | 4 (1.1) | 3 (0.6) | 0.24 |
| Depression, n (%) | 2414 (31) | 2130 (30.7) | 115 (31.6) | 169 (35.1) | 0.13 |
| PTSD, n (%) | 1396 (17.9) | 1257 (18.1) | 56 (15.4) | 83 (17.2) | 0.38 |
| Presentation, n (%) | 0.26 | ||||
| Stable angina | 216 (2.8) | 198 (2.9) | 9 (2.5) | 9 (1.9) | |
| Positive functional study | 1825 (23.5) | 1630 (23.5) | 79 (21.7) | 116 (24.1) | |
| Ischemic heart disease | 522 (6.7) | 465 (6.7) | 30 (8.2) | 27 (5.6) | |
| Chest pain | 5217 (67.1) | 4641 (66.9) | 246 (67.6) | 330 (68.5) | |
| Acute coronary syndrome | 504 (6.5) | 433 (6.2) | 33 (9.1) | 38 (7.9) | |
| Post-angiography revascularization, n (%) | 0.08 | ||||
| None | 4878 (62.7) | 4335 (62.5) | 225 (61.8) | 318 (66) | |
| PCI | 1831 (23.5) | 1634 (23.6) | 100 (27.5) | 97 (20.1) | |
| CABG | 1071 (13.8) | 965 (13.9) | 39 (10.7) | 67 (13.9) | |
| Coronary artery disease (CAD) status | 0.09 | ||||
| No CAD | 1377 (17.7) | 1242 (17.9) | 55 (15.1) | 80 (16.6) | |
| Non-obstructive CAD | 1566 (20.1) | 1392 (20.1) | 62 (17) | 112 (23.2) | |
| Obstructive CAD | 4837 (62.2) | 4300 (62) | 247 (67.9) | 290 (60.2) | |
| ACE inhibitor/ARB | < 0.0001 | ||||
| Not prescribed | 2339 (30.1) | 2127 (30.7) | 103 (28.3) | 109 (22.6) | |
| Prescribed | 5441 (69.9) | 4807 (69.3) | 261 (71.7) | 373 (77.4) | |
| PDC < 0.8 | 1164 (21.4) | 985 (20.5) | 65 (24.9) | 114 (30.6) | |
| PDC ≥ 0.8 | 4277 (78.6) | 3822 (79.5) | 196 (75.1) | 259 (69.4) | |
| Beta-blocker | 0.02 | ||||
| Not prescribed | 1569 (20.2) | 1423 (20.5) | 69 (19.0) | 77 (16.0) | |
| Prescribed | 6211 (79.8) | 5511 (79.5) | 295 (81.0) | 405 (84.0) | |
| PDC < 0.8 | 1090 (17.5) | 945 (17.1) | 61 (20.7) | 84 (20.7) | |
| PDC ≥ 0.8 | 5121 (82.5) | 4566 (82.9) | 234 (79.3) | 321 (79.3) | |
| Statins | 0.0001 | ||||
| Not prescribed | 967 (12.4) | 877 (12.6) | 43 (11.8) | 47 (9.8) | |
| Prescribed | 6813 (87.6) | 6057 (87.4) | 321 (88.2) | 435 (90.2) | |
| PDC < 0.8 | 1567 (23.0) | 1345 (22.2) | 94 (29.3) | 128 (29.4) | |
| PDC ≥ 0.8 | 5246 (77.0) | 4712 (77.8) | 227 (70.7) | 307 (70.6) | |
Abbreviations: CAD coronary artery disease, BMI body mass index, PAD peripheral artery disease, HF heart failure, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, PTSD post-traumatic stress disorder, ACE inhibitor angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, Statin HMG-CoA-reductase inhibitor, HbA1c hemoglobin A1c, PDC proportion of days covered
Joint latent class mixed models with three HbA1c trajectory classes best fit the data: individuals with stable glycemia (class A, n = 6934, 89%), individuals with a decline in HbA1c after catheterization (class B, n = 364, 4.7%), and individuals with an increase in HbA1c after catheterization (class C, n = 482, 6.2%) (Fig. 1). Mean HbA1c at the time of catheterization was highest in class B (11.6%), lowest in class A (6.9%), and intermediate in class C (8.5%) (Table 1). By 6 months after cardiac catheterization, class C individuals had the highest predicted HbA1c in the trajectory model (9.6% [95% CI 9.3, 9.9] for class C, 8.8% [8.5, 9.1] for class B, and 7.0% [6.9, 7.1] for class A; Fig. 1). Compared to class B and class C individuals, those in class A were older on average, more likely to be white, less likely to have HF, had the highest Framingham cardiovascular risk score on average, and were more likely to be adherent to cardioprotective medications at baseline (Table 1).
Fig. 1.
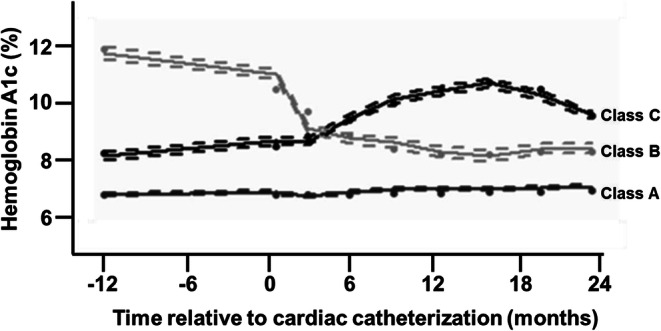
Hemoglobin A1c trajectory classes in individuals with diabetes who undergo cardiac catheterization. Predicted and observed longitudinal hemoglobin A1c (HbA1c) trajectories plotted over time from a three-class joint latent class mixed model that includes the last HbA1c value preceding cardiac catheterization (day 0) and all measurements during up to two 2 years of follow-up. Predicted values for class members indicated with solid dots, observed values indicated with solid lines, and 95% confidence intervals indicated with dashed lines.
Crude mortality rates across HbA1c trajectory classes were 4.3% in class A, 4.7% in class B, and 5.0% in class C. Crude 2-year mortality based on HbA1c values, as opposed to trajectory classes, was higher in individuals with HbA1c ≥ 9% than in those with HbA1c < 7% or 7–9%, based on a single baseline measurement or based on the mean of all HbA1c measurements over 12 or 24 months of follow-up after cardiac catheterization (Table S9). In multivariable joint latent class mixed models, adjusted 2-year mortality differed across trajectory classes (adjusted mortality rate of 3.0% [95% CI 2.8, 3.2] in class A, 3.1% [95% CI 2.1, 4.2] in class B, and 4.2% [95% CI 3.4, 4.9] in class C; P = 0.047 for difference between classes; Fig. 2a). Adjusted mortality differed significantly between classes A and C (P = 0.03) but not between classes A and B (P = 0.9) or between classes B and C (P = 0.5) (Fig. 2a). In identical multivariable Cox proportional hazard models, baseline HbA1c was not associated with 2-year mortality (Table 2). The HbA1c trajectory classes observed were similar among participants without HF at baseline compared to the full study sample (Fig. S2). Among those without HF, the predicted survival probability curves diverged initially between classes A and C (adjusted 1-year mortality of 1.47% in class A vs 2.37% in class C) but came together at two years resulting in a non-significant association between trajectory class and 2-year mortality (Fig. S2, P = 0.95).
Fig. 2.
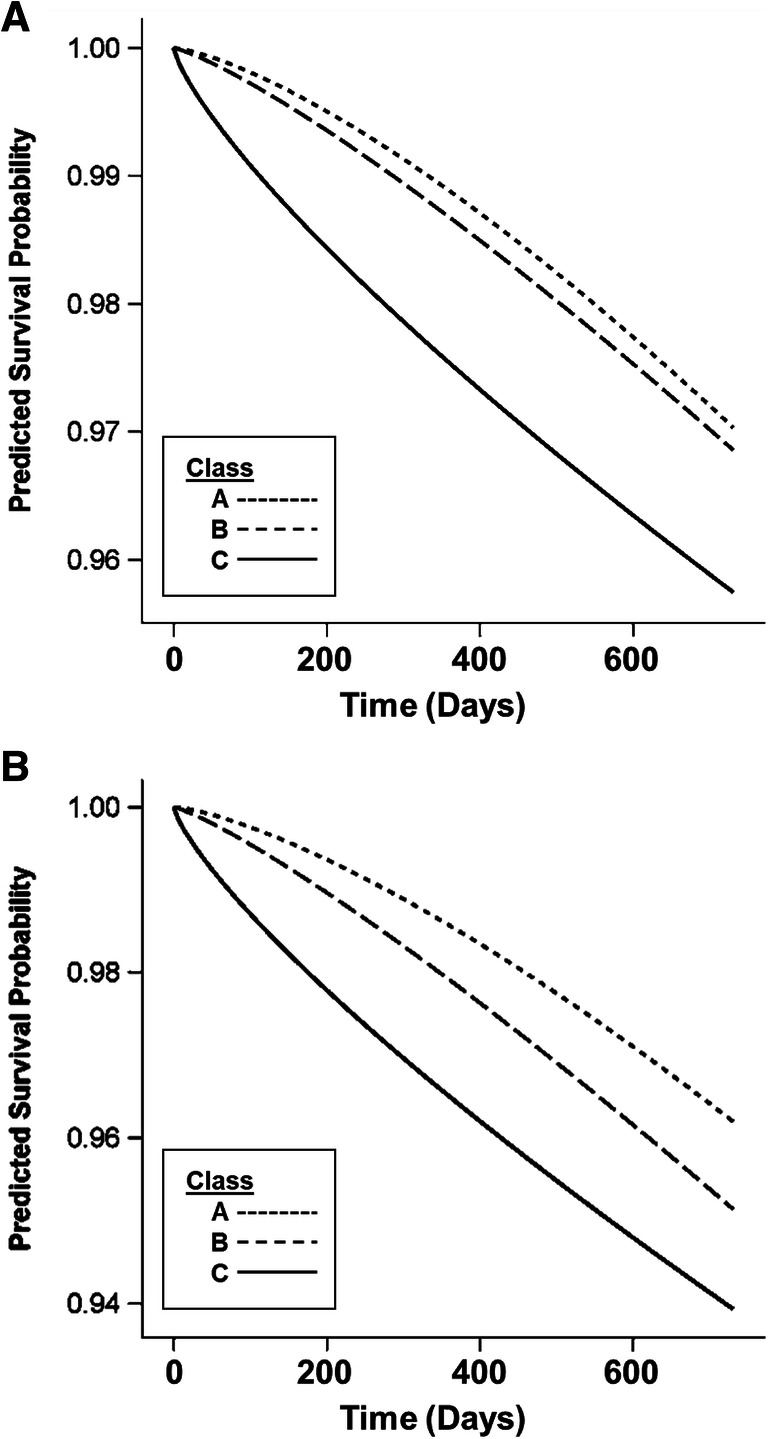
Predicted survival probability over 2 years after cardiac catheterization for individuals in three hemoglobin A1c trajectory classes. Predicted survival probability based on multivariable joint latent class mixed models, adjusted for demographic and clinical covariates, in the full study cohort (a) and in those diagnosed with obstructive coronary artery disease at the time of cardiac catheterization (b).
Table 2.
Association Between Baseline HbA1c and 2-Year Mortality
| Hazard ratio (95% CI) |
P value* | |
|---|---|---|
| HbA1c < 7% (53 mmol/mol) | Ref | – |
| 7% ≤ HbA1c < 9% (53–74 mmol/mol) | 1.01 (0.96, 1.06) | 0.81 |
| HbA1c ≥ 9% (≥ 75 mmol/mol) | 1.02 (0.95, 1.10) | 0.58 |
*Type 3 Wald test P = 0.85 for difference across 3 baseline HbA1c groups
The association between HbA1c trajectory class and mortality did not differ between those with no CAD, non-obstructive CAD, and obstructive CAD (interaction P = 0.1). Mortality events were less common among those with no CAD or non-obstructive CAD than in those with obstructive CAD such that the joint model results were driven by those with obstructive CAD. Among individuals with obstructive CAD, variation in baseline clinical characteristics across trajectory classes was similar to that observed for the full study population (Table S10), and mortality differed across trajectory classes in multivariable joint latent class mixed models (adjusted mortality rate of 3.8% [95% CI 3.5, 4.1] in class A, 4.9% [95% CI 3.6, 6.2] in class B, and 6.1% [95% CI 4.9, 7.2] in class C; P = 0.039 for difference between classes; Fig. 2b). Mortality differed between classes A and C (P = 0.04) but not between classes A and B (P = 1.0) or between classes B and C (P = 1.0) (Fig. 2b).
In an exploratory analysis of diabetes medication prescriptions, individuals in class A were more likely to be on no medications (Fig. 3a, Table S11) and were less likely to be prescribed insulin (Fig. 3b, Table S12) across all time points examined. Individuals in HbA1c trajectory classes B and C were similar with regard to the number of diabetes medications and medication classes prescribed at baseline and across all timepoints during follow-up (Fig. 3a, b, Tables S11 and S12). Adherence to cardioprotective medications was lower in all three-trajectory classes at 12 months after cardiac catheterization than at baseline, but the relative proportions of adherence between classes A, B, and C were similar at baseline (Table 1) and 12 months (Table S13). Individuals in class A had higher adherence to sulfonylureas and metformin than those in classes B and C at 12 months after catheterization (Table S13). Sulfonylurea adherence was higher in class C individuals than in class B, while metformin adherence was higher in class B than in class C (Table S13). Crude 2-year mortality was numerically higher in individuals with lower adherence to ACEi/ARB, beta-blocker, statin, and sulfonylurea, but did not differ across levels of metformin adherence (Table S13). There were too few mortality events to compare the associations of adherence with mortality between HbA1c trajectory classes.
Fig. 3.
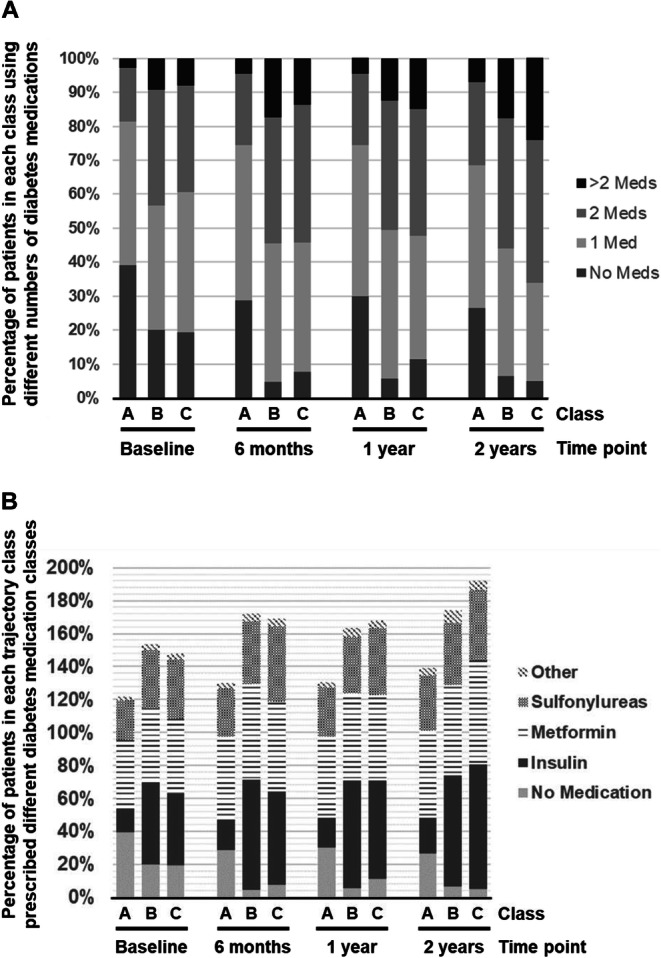
Diabetes medication distribution over two years of follow-up across HbA1c trajectory classes. Proportion of participants in HbA1c trajectory Class A, B, and C on 0, 1, 2, or > 2 diabetes medications at baseline, 6, 12, and 24 months after cardiac catheterization (A). Proportion of participants in each HbA1c trajectory class prescribed no diabetes medications, insulin, metformin, sulfonylurea, or other diabetes medication at baseline, 6, 12, and 24 months after cardiac catheterization (B).
DISCUSSION
In this study, we found three glycemic control trajectories in diabetes patients without and with objectively determined CAD receiving real-world clinical care. Two of the trajectory classes exhibited a qualitative inflection point at or near the time of cardiac catheterization, suggesting the clinical event may have impacted diabetes self-management or treatment in a subset of patients in this cohort. Relative to the most common trajectory—maintaining a stable, low HbA1c after catheterization—the trajectory with intermediate baseline HbA1c and gradually increasing HbA1c after catheterization was associated with higher short-term mortality. In contrast, a trajectory characterized by high baseline HbA1c but declining after catheterization did not have higher mortality than the low, stable HbA1c trajectory. Notably, HbA1c at the time of cardiac catheterization was not associated with mortality.
Our analysis suggests that glycemic control trajectories may be more informative for predicting clinical outcomes than a single HbA1c measurement at the time of cardiac catheterization and CAD diagnosis. We have previously shown that HbA1c examined as a time-varying predictor after cardiac catheterization was associated with 2-year all-cause mortality only for HbA1c values < 6% relative to a reference of 6.5 < HbA1c ≤ 7%.27 The joint latent class modeling approach taken here complements the prior work by examining associations of time-varying patterns in HbA1c, rather than individual values, with mortality. In other words, two individuals with the same HbA1c value measured several months after cardiac catheterization may have distinguishable risk of mortality when that single value is placed in the context of an HbA1c trajectory based on serial measurements.
We found relatively minor differences in descriptive characteristics, if any, between the patients in each HbA1c trajectory class, suggesting that HbA1c trajectory may not be easy to predict at the time of coronary angiography based on the variables we examined. While the mortality differences between trajectory classes were small, the association between trajectories and a clinically important outcome lends credence to this approach. In fact, most EHR systems are already able to present longitudinal HbA1c measurements to clinical providers, so adding HbA1c trajectories to clinical dashboards used to inform diabetes care may be an easily adopted change.
Prior trajectory analyses applied to HbA1c in diabetes patients focused on long-term glycemic control trajectories, their predictors, and their associations with subsequent rather than contemporaneous clinical outcomes.22–24 The prior work, therefore, describes how glycemic control over the natural history of diabetes predicts future clinical outcomes (“legacy effect” of glycemia28, 29) and identifies predictors of specific glycemic control trajectories. To further understand associations of the natural history of glycemic control in diabetes patients with clinical outcomes, joint latent class mixed models could be applied to individuals with similar baseline HbA1c values that diverge over time. However, our goal in this study was to describe the glycemic control trajectories observed after an important clinical event, the diagnosis of CAD, and to evaluate whether serial HbA1c measurements from real-world clinical care could inform diabetes care contemporaneously. Indeed, our data suggest that serial HbA1c measurements occurring at or near the frequency recommended by the ADA (every 6 months)1, 11 could be used to determine whether individuals with recently diagnosed CAD exhibit high-risk glycemic control trajectories, complementing prior work evaluating long-term HbA1c trajectories and individual time-varying HbA1c measurements.
The exploratory analysis of diabetes treatments prescribed across HbA1c trajectory classes suggests that differences in medication prescribing alone are unlikely to explain the different glycemic control trajectories. Class A individuals were more likely to be on no diabetes medications and less likely to be on insulin at baseline, suggesting that differences in diabetes severity at baseline could partly explain better outcomes in this group. Despite divergent HbA1c trajectories, patients in classes B and C were similar in treatment at baseline and during follow-up, consistent with providers basing treatment decisions on single HbA1c values rather than HbA1c trajectories. Prior studies have described higher rates of diabetes treatment intensification after acute myocardial infarction in individuals with higher HbA1c,30, 31 and we observed a similar increase in treatment in trajectory class B and C individuals compared to those in class A.
It is important to point out that our results suggest that HbA1c trajectories may help classify diabetes patients into different short-term mortality risk categories, but do not imply a causal relationship between the trajectories and mortality. That is, the HbA1c trajectory itself may not be a useful target of a clinical intervention yet may be an indicator that other intervenable factors—e.g., medication adherence or other diabetes-related health behaviors—may need attention. Moreover, our analysis did not comprehensively evaluate variables that may be associated with both HbA1c trajectory and mortality, for example, socioeconomic factors such as housing and income stability. Future work is needed to better define the causes and consequences of the different trajectories observed in this study. The exploratory evaluation of medication adherence performed in this study confirms crude associations of adherence with 2-year mortality and suggests variation in adherence across trajectory classes. Recent work in a similar patient population found that HbA1c at the time of acute myocardial infarction was correlated with adherence to cardioprotective medications in the following year,32 providing a plausible link between the rising HbA1c in class C individuals and higher short-term mortality. Evaluating time-varying medication adherence as a predictor of trajectory classes and as a mediator of their associations with mortality would be computationally challenging in the framework of the joint modeling approach used in this study and will be evaluated in future research.
Our study has several limitations. First, we cannot conclude causality between glycemic control trajectories and short-term mortality, nor can we infer that any patient characteristics causally determine trajectory class membership. Second, the trajectory models are inherently subjective as they depend on model fit statistics and investigator-determined criteria for model selection. Third, the use of a clinical registry limited to individuals who had undergone cardiac catheterization makes our study susceptible to selection bias. That is, individuals who have undergone coronary angiography may not represent the general population of diabetes patients without and with CAD. Finally, our study population is predominantly male, limiting generalizability of the results.
Our study also has several clinically relevant implications. By focusing on short-term mortality, our study evaluated an important clinical outcome in a high-risk population at a vulnerable time—patients with diabetes in the first 2 years after CAD diagnosis. We provide observational evidence that HbA1c trajectories may be informative for classifying risk in diabetes patients, possibly more so than the last available HbA1c measurement upon which most guidelines currently base diabetes management recommendations. In the current era of near-universal adoption of electronic health records and interest in the “learning healthcare system”,33 computational tools or dashboards that report biomarker or risk factor trajectories in addition to the last available measurement warrant further study to determine if they can help improve outcomes.
Electronic Supplementary Material
(DOCX 360 kb)
Acknowledgments
SR is supported by American Heart Association Award 17MCPRP33670728 and VA Career Development Award IK2-CX001907-01. SAB is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK109200. LC is supported by VA HSR&D IIR 14-048-3. JEBR, AEB, and PMH are supported by grants from the US Department of Veterans Affairs. PMH is supported by grants from National Institutes of Health, National Heart, Lung, and Blood Institute. TMM is supported by grant funding from the NIH NCATS (1U24TR002306-01: A National Center for Digital Health Informatics Innovation). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest
PMH served on a Steering Committee for a clinical trial on medication adherence for Janssen, Inc., and is the Deputy Editor for Circulation: Cardiovascular Quality and Outcomes, but reports no conflicts of interest with this study. TMM discloses consulting for Creative Educational Concepts, Inc., employment at BJC HealthCare/Washington University School of Medicine, and honoraria payments from Brown University, Washington State Clinical Outcomes Assessment Program (COAP), Virginia Mason, University of Utah, New York Presbyterian, Westchester Medical Center, and Sentara Heart Hospital, but none represent a conflict of interest with this study. The remaining authors have no conflicts of interest relevant to the work in this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S103–S23. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 4.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes care. 2015;38(9):1777–803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes care. 2011;34(6):1329–36. doi: 10.2337/dc10-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of internal medicine. 2004;141(6):413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 8.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England journal of medicine. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Annals of internal medicine. 2004;141(6):421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 10.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S34–S45. doi: 10.2337/dc19-S004. [DOI] [PubMed] [Google Scholar]
- 12.Chan CY, Li R, Chan JY, Zhang Q, Chan CP, Dong M, et al. The value of admission HbA(1c) level in diabetic patients with acute coronary syndrome. Clin Cardiol. 2011;34(8):507–12. doi: 10.1002/clc.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller SR, Bergenstal RM, White WB, Kupfer S, Bakris GL, Cushman WC, et al. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: the EXAMINE trial. Diabetes Obes Metab. 2017;19(5):664–71. doi: 10.1111/dom.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemesle G, Bonello L, de Labriolle A, Maluenda G, Syed AI, Collins SD, et al. Prognostic value of hemoglobin A1C levels in patients with diabetes mellitus undergoing percutaneous coronary intervention with stent implantation. The American journal of cardiology. 2009;104(1):41–5. doi: 10.1016/j.amjcard.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 15.Zheng J, Cheng J, Zhang Q, Qi C, Wang T, Xiao X. Association between glycosylated hemoglobin level and cardiovascular outcomes in diabetic patients after percutaneous coronary intervention. Medicine (Baltimore). 2016;95(19):e3696. doi: 10.1097/MD.0000000000003696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program) The American journal of cardiology. 2014;114(11):1750–7. doi: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 18.Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA internal medicine. 2014;174(2):186–93. doi: 10.1001/jamainternmed.2013.12944. [DOI] [PubMed] [Google Scholar]
- 19.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proust-Lima C, Sene M, Taylor JM, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: a review. Statistical methods in medical research. 2014;23(1):74–90. doi: 10.1177/0962280212445839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Herazo-Maya JD, Molyneaux PL, Maher TM, Kaminski N, Zhao H. Regularized latent class model for joint analysis of high-dimensional longitudinal biomarkers and a time-to-event outcome. Biometrics. 2019;75(1):69–77. doi: 10.1111/biom.12964. [DOI] [PubMed] [Google Scholar]
- 22.Laiteerapong N, Karter AJ, Moffet HH, Cooper JM, Gibbons RD, Liu JY, et al. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: The Diabetes & Aging Study. J Diabetes Complications. 2017;31(1):94–100. doi: 10.1016/j.jdiacomp.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo M, Lim WY, Tan CS, Ning Y, Chia KS, van Dam RM, et al. Longitudinal trends in HbA1c and associations with comorbidity and all-cause mortality in Asian patients with type 2 diabetes: a cohort study. Diabetes Res Clin Pract. 2017;133:69–77. doi: 10.1016/j.diabres.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Walraven I, Mast MR, Hoekstra T, Jansen AP, van der Heijden AA, Rauh SP, et al. Distinct HbA1c trajectories in a type 2 diabetes cohort. Acta diabetologica. 2015;52(2):267–75. doi: 10.1007/s00592-014-0633-8. [DOI] [PubMed] [Google Scholar]
- 25.Muthen B, Brown CH, Masyn K, Jo B, Khoo ST, Yang CC, et al. General growth mixture modeling for randomized preventive interventions. Biostatistics. 2002;3(4):459–75. doi: 10.1093/biostatistics/3.4.459. [DOI] [PubMed] [Google Scholar]
- 26.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–91. [PubMed] [Google Scholar]
- 27.Raghavan S, Liu WG, Michael Ho P, Plomondon ME, Baron AE, Caplan L, et al. Coronary artery disease severity modifies associations between glycemic control and both mortality and myocardial infarction. J Diabetes Complications. 2018. [DOI] [PMC free article] [PubMed]
- 28.Chalmers J, Cooper ME. UKPDS and the legacy effect. The New England journal of medicine. 2008;359(15):1618–20. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 29.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study) Diabetes care. 2019;42(3):416–26. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolker JM, Spertus JA, McGuire DK, Lind M, Tang F, Jones PG, et al. Relationship between glycosylated hemoglobin assessment and glucose therapy intensification in patients with diabetes hospitalized for acute myocardial infarction. Diabetes care. 2012;35(5):991–3. doi: 10.2337/dc11-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolker JM, Sun D, Conaway DG, Jones PG, Masoudi FA, Peterson PN, et al. Importance of measuring glycosylated hemoglobin in patients with myocardial infarction and known diabetes mellitus. The American journal of cardiology. 2010;105(8):1090–4. doi: 10.1016/j.amjcard.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamek KE, Ramadurai D, Gunzburger E, Plomondon ME, Ho PM, Raghavan S. Association of diabetes mellitus status and glycemic control with secondary prevention medication adherence after acute myocardial infarction. J Am Heart Assoc. 2019;8(3):e011448. doi: 10.1161/JAHA.118.011448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality, Rockville, MD. Learning health systems. Content last reviewed November 2017. http://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. 2017; Accessed May 28, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 360 kb)


