Abstract
The current research reports the antibacterial effects of silver (Ag) and citric acid coated iron oxide (Fe3O4) NPs on Escherichia coli wild type and kanamycin-resistant strains, as well as on Salmonella typhimurium MDC1759. NPs demonstrated significant antibacterial activity against these bacteria, but antibacterial effect of Ag NPs is more pronounced at low concentrations. Ag NPs inhibited 60–90% of S. typhimurium and drug-resistant E. coli. The latter is more sensitive to Fe3O4 NPs than wild type strain: the number of bacterial colonies is decreased ~ 4-fold. To explain possible mechanisms of NPs action, H+-fluxes through the bacterial membrane and the H+-translocating FOF1-ATPase activity of bacterial membrane vesicles were studied. N,N′-Dicyclohexylcarbodiimide (DCCD)-sensitive ATPase activity was increased up to ~ 1.5-fold in the presence of Fe3O4 NPs. ATPase activity was not detected by Ag NPs even in the presence of DCCD, which confirms the bactericidal effect of these NPs. The H+-fluxes were changed by NPs and by addition of DCCD. H2 yield was inhibited by NPs; the inhibition by Ag NPs is stronger than by Fe3O4 NPs. NPs showed antibacterial effect in bacteria studied in concentration-dependent manner by changing in membrane permeability and membrane-bound enzyme activity. The FOF1-ATPase is suggested might be a target for NPs.
Subject terms: Biochemistry, Biophysics, Microbiology
Introduction
In recent years, the increase of the bacterial infections and the development of antimicrobial resistance have been avowed as one of the key problems of the biomedicine of twenty-first century, which require a finding of novel antibacterial agents. Nanoparticles (NPs) are considered a great alternative to antibiotics, since they show a high potential to solve the problem of antibiotic resistance. Various NPs are used in biomedicine, pharmaceutics, cosmetics, food industry, textile coating, etc.1–5.
In particular, silver (Ag) NPs and iron oxide (Fe3O4) have attracted much attention in biomedical field4–9. Ag NPs show a pronounced antibacterial activity not only against various pathogens, but also against bacterial biofilms10–12. Effect of NPs depends on the particle size, shape, charge, surface characteristics and magnetic properties1,13,14. For example, at the same concentration 10 nm Ag NPs are more effective than 100 nm Ag NPs against methicillin-resistant Staphylococcus aureus15. Moreover, Ag NPs demonstrate the stronger inhibitory effect than other NPs11,16. Ag NPs can physically interact with the cell surface of bacteria due to their large surface area, providing better interaction with bacteria13. Franchi et al.10 reported that Ag NPs can damage bacterial cell membranes, which provide to increasing of permeability of bacterial membrane. The Ag NPs can preferably affect the respiratory chain in bacterial cells16.
Iron oxide NPs such as Fe3O4 and γ-Fe2O3 NPs due to their super paramagnetic, high magnetic susceptibility and other properties are promising agents for biomedical applications. Nowadays, they have been used in drug delivery systems to deliver various compounds such as peptides, DNA molecules, chemotherapeutic, and hyper-thermic drugs1,17,18. These systems have a potential to minimize the side-effects and the required concentration of drugs, as well as decrease the damage of normal tissues. In these systems, magnetic targeting of drug delivery is considered as the most efficient way17.
The antimicrobial action of NPs is probably a result of their interaction with bacterial membrane, which can lead to alterations of membrane-bound mechanisms, membrane damage and the bacterial death9,19–21. However, the antibacterial mechanisms of NPs action are not fully explored and in this case, the study of the effects of NPs on various bacteria is important for finding out of the NPs' possible action mechanisms.
It is known, that susceptibility of microorganisms to NPs depends not only on NPs’ characteristics, but also on stabilizer type. Due to the tendency of NPs to aggregation, their use requires the stabilization. Stabilizer, which used for the magnetic NPs, can affect the properties of NPs22–27. Oleic acid was suggested as a promising stabilizer for magnetic NPs22–24. Oleic acid can form a protective monolayer around the NPs, which is necessary for monodispersed magnetic NPs formation22–24. However, stabilization of magnetic NPs with oleic acid makes the NPs soluble only in organic solvents and therefore limits their use in biomedicine22,23. By the way, for biomedical applications in aqueous media, the hydrophobic stabilizer needs to be replaced by a hydrophilic one23.
In our previous studies oleic acid coated Fe3O4 NPs’ effects on Gram-negative Escherichia coli BW25113, ampicillin-resistant E. coli DH5α-pUC18, kanamycin-resistant E. coli pARG-25 stains and Gram-positive Enterococcus hirae ATCC9790 growth and membrane-associated mechanisms have been investigated20,21. Our results showed that the Fe3O4 NPs demonstrate different effects on Gram-negative and Gram-positive bacteria. Gram-positive E. hirae displayed higher susceptibility to NPs than Gram-negative E. coli, because the components of cell wall of Gram-positive and Gram-negative bacteria have different pathways for NPs adsorption2,7. Stabilizer oleic acid had no any effect on growth properties of investigated bacteria.
Citric acid is another stabilizer, which able to stabilize magnetic NPs, but there are few data in literature about citric acid coated NPs antibacterial properties. It has been shown that treating NPs by citric acid in aqueous solution stabilized the NPs surfaces25–27. The citric acid coated NPs remained stable in aqueous solution even in a week, and this stability is sustained under an electric field in aqueous media27. Study of citric acid stabilized NPs antibacterial properties is very important for the regulation of various bacteria growth in biomedicine and biotechnology.
In the present work, the antibacterial effects and possible mechanisms of Ag NPs and citric acid coated Fe3O4 NPs on E. coli wild type and drug-resistant strains as well as in comparison with Salmonella typhimurium MDC1759, have been studied for the first time. Additional characteristics of NPs were determined. In order to analyze the role of different membrane-bound systems and to find out probable targets H+-translocating FOF1-ATPase activity, H+-fluxes through the bacterial membrane and H2 yield were also investigated.
Results
Growth characteristics of E. coli wild type and drug-resistant strains, and S. typhimurium in the presence of Ag NPs and citric acid coated Fe3O4 NPs
Escherichia and Salmonella species are the most common foodborne human pathogens causing to various types of illnesses9,28–30. In this case, it is important to study the effect of Ag and Fe3O4 NPs (coated by citric acid) on E. coli wild type and antibiotic-resistant strains, as well as S. typhimurium MDC1759 strain, for revealing the action mechanisms. The growth parameters of E. coli wild type K-12, kanamycin-resistant pARG-25, and S. typhimurium MDC1759 strains in the presence of Ag and Fe3O4 NPs have been investigated. Bacteria grown in the presence of kanamycin (50 μg mL−1) were used as positive control. The negative controls are the strains, cultivated without antibiotic.
The results obtained show antibacterial effects of Ag and Fe3O4 NPs. The intensity of NPs effects depends on the type of NPs, their concentrations and bacterial strains. Both NPs showed inhibitory effect on E. coli growth (Fig. 1). Moreover, the effect of Fe3O4 NPs also depends on the type of stabilizer. Stabilization of NPs by various compounds enhances an antimicrobial activity of NPs8,26,31.
Figure 1.
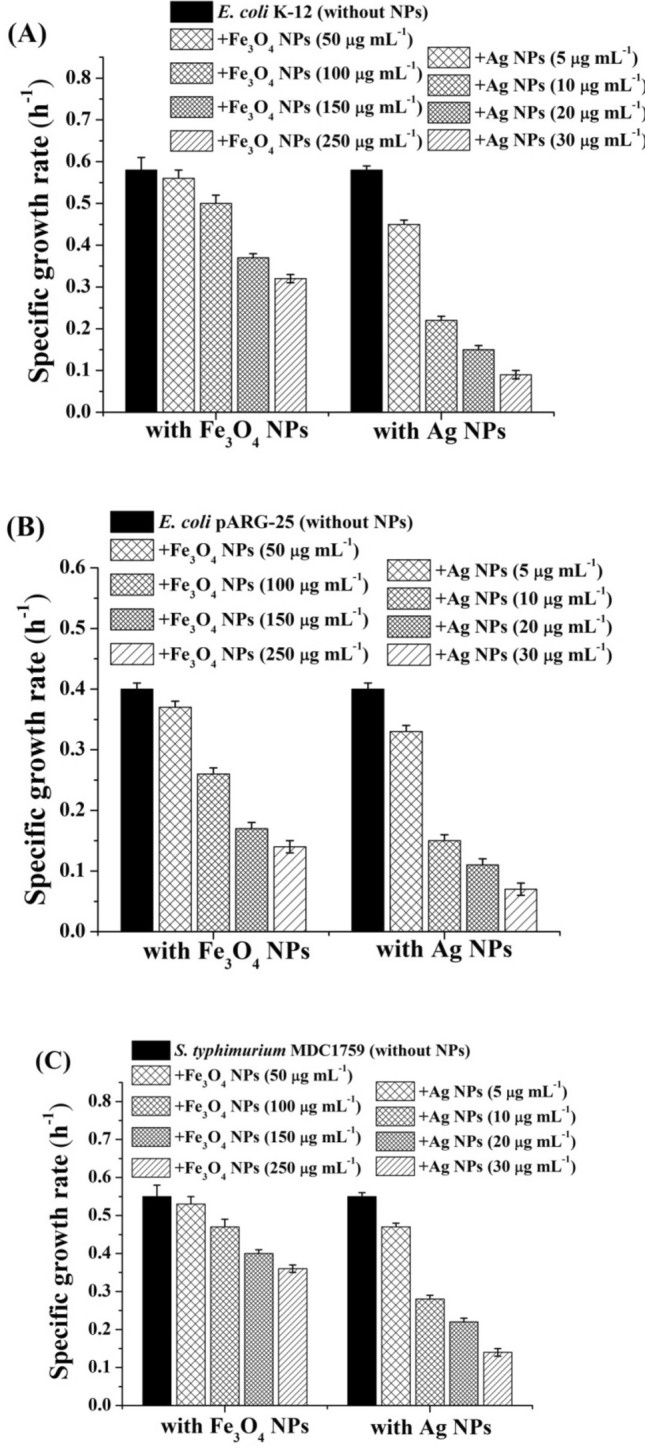
Specific growth rates of E. coli K-12 (A) kanamycin-resistant E. coli pARG-25 (B), and S. typhimurium MDC1759 (C) strains in the presence of citric acid coated Fe3O4 and Ag NPs various concentrations. Control was without nanoparticles addition.
In the presence of citric acid coated Fe3O4 NPs (100–250 μg mL−1) inhibition of E. coli K-12 growth was observed (Fig. 1A). NPs demonstrated concentration dependent effect on E. coli growth (Fig. 3). The specific growth rate of E. coli K-12 at NPs' concentration 50 μg mL−1 was similar to the control, and decreased ~ 1.2-fold at 100 μg mL−1 NPs (Fig. 3). The maximal inhibitory effect has been obtained at the concentration 250 μg mL−1, which led to the decrease in bacterial specific growth rate by ~ 2.0-fold, indicating the bacteriostatic effect of the Fe3O4 NPs. The antibacterial effect of Fe3O4 NPs may be due to several mechanisms. ROS together with superoxide radicals (O2−), hydroxide radical (OH−) and singlet oxygen formed by Fe3O4 NPs could be the reason of inhibition. Similar results were obtained in other studies showing the antibacterial activity of Fe3O4 NPs against E. coli19. In our previous study oleic acid coated Fe3O4 NPs show antibacterial activity against E. coli BW25113 strain21. However, the citric acid coated Fe3O4 NPs demonstrate more pronounced antibacterial activity than Fe3O4 NPs coated by oleic acid at the same concentration. The maximal inhibitory effect of oleic acid coated Fe3O4 NPs has been observed at the 500 μg mL−1 concentration21. Moreover, citric acid does not show any effect on growth rates of investigated bacteria (not shown).
Figure 3.
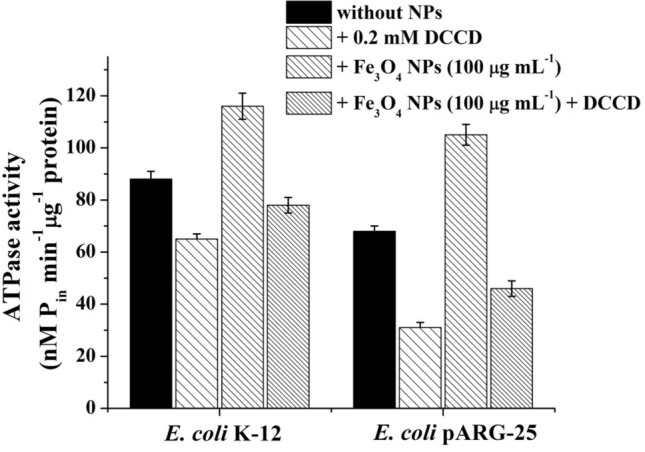
ATPase activity of E. coli K-12 and kanamycin-resistant E. coli pARG-25 membrane vesicles in the presence of citric acid coated Fe3O4 NPs (100 μg mL−1) and DCCD (0.2 mM). Control was without NPs addition.
Ag NPs show more pronounced bactericidal effect than Fe3O4 NPs (Fig. 1). Moreover, Ag NPs display more expressed antibacterial effect at low concentrations. In the presence of 10 μg mL−1 Ag NPs a 2.6-fold suppression of growth of E. coli K-12 was observed (see Fig. 1A). The 20–30 μg mL−1 Ag NPs led to 4.0–6.5-fold decrease in bacterial growth rate, indicating the bactericidal effect of these concentrations.
Moreover, kanamycin-resistant E. coli pARG-25 strain had more susceptibility to Fe3O4 NPs than E. coli wild type strain (Fig. 1B). In the presence of 100–250 μg mL−1 Fe3O4 NPs a 1.5–3-fold inhibition of bacterial growth was observed (see Fig. 1B). Similar results were obtained with ampicillin-resistant E. coli DH5α-pUC18 strain (not shown). Citric acid coated Fe3O4 NPs demonstrated more noticeable antibacterial effect than Fe3O4 NPs stabilized by oleic acid20. In the case of Ag NPs, as seen in Fig. 1B, there were no obvious differences between antibacterial effect of these NPs on wild type and drug-resistant bacteria.
The effect of Fe3O4 NPs on S. typhimurium MDC1759 growth rate is the same with the action of iron oxide NPs on E. coli K-12 strain (Fig. 1C). Antibacterial effect of Ag NPs on S. typhimurium MDC1759 was less pronounced than on E. coli both strains (Fig. 1C). At the same time, S. typhimurium demonstrated susceptibility to the high concentrations of Ag NPs: 20 μg mL−1 Ag NPs led to ~ 2.5-fold decrease in growth specific rate (Fig. 1C).
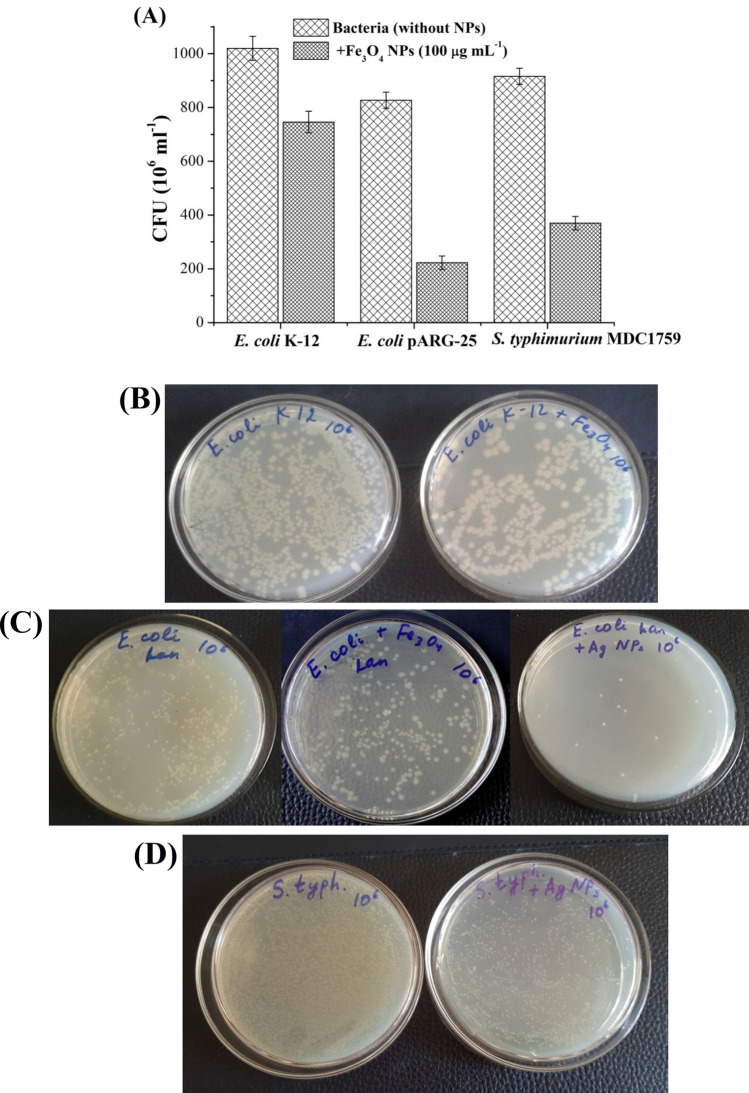
Figures 2 and 3 display the number of viable colonies of E. coli K-12 and pARG-25, and S. typhimurium MDC1759, grown in the absence and presence of 100 μg mL−1 Fe3O4 NPs. CFU of E. coli K-12 was decreased 1.3-fold in the presence of Fe3O4 NPs (Fig. 2A,B). At the same time, S. typhimurium MDC1759 and drug-resistant E. coli strain has more susceptibility to Fe3O4 NPs (stabilized by citric acid) than wild type strain: CFU was decreased 2.5–4-fold, respectively (see Fig. 2A). In the case of kanamycin-resistant E. coli strains Fe3O4 NPs coated by citric acid showed noticeable antibacterial effect in comparison with oleic acid stabilized Fe3O4 NPs20. Ag NPs inhibited ~ 60 to 90% of S. typhimurium MDC1759 and drug-resistant E. coli viable colonies (Fig. 2C,D).
Figure 2.
The number of viable colonies of E. coli K-12, kanamycin-resistant E. coli pARG-25, and S. typhimurium MDC1759 strains grown in the presence of citric acid coated Fe3O4 NPs (100 μg mL−1) (A). The colonies of E. coli K-12 grown in the absence and presence of Fe3O4 NPs (100 μg mL−1) (B). The colonies of E. coli pARG-25 grown in the absence and presence of Fe3O4 NPs (100 μg mL−1) and Ag NPs (10 μg mL−1) (C). The colonies of S. typhimurium MDC1759 cultivated in the absence and presence of Ag NPs (10 μg mL−1) (D).
Thus, NPs show significant antibacterial activity against used bacteria. Different effects of Fe3O4 and Ag NPs on bacterial growth may be due to the difference in the interaction between the bacterial cells and NPs2,11. Small size of NPs can contribute to their antibacterial activity7,11. NPs can interact closely with bacterial membranes and the inactivation of bacteria could be due to their penetration into the bacterial cell1,9,11.
H+-fluxes through the bacterial membrane and H+-translocating ATPase activity in E. coli wild type and drug-resistant strains in the presence of Ag NPs and citric acid coated Fe3O4 NPs
In order to explain the possible mechanisms and find out probable targets of the effect of NPs changes in H+-fluxes through the bacterial membrane and H+-translocating FOF1-ATPase activity in E. coli wild type and drug-resistant strains were investigated.
H+-coupled membrane transport has been determined in E. coli wild type, drug-resistant strains, and S. typhimurium in the absence and presence of citric acid stabilized Fe3O4 and Ag NPs (Table 1). Fe3O4 NPs suppressed energy-dependent H+-efflux in E. coli K-12 and pARG-25 strains ~ 3.0 to and ~ 1.3-folds, respectively (see Table 1). H+-fluxes also decreased in the presence of DCCD, an inhibitor of the H+-translocating ATPases (see Table 1). The presence of Ag NPs in the assay medium led to the increase in H+ fluxes, in comparison with Fe3O4 NPs. A more noticeable effect was observed in E. coli K-12: H+-fluxes were increased ~ 1.2-fold. The effect of Ag NPs on E. coli pARG-25 was weaker in comparison with E. coli wild type strain (see Table 1). At the same time, in the presence of Ag NPs the H+-fluxes were increased even in the presence of DCCD, indicating that Ag NPs affected the bacterial membrane leading to changes in membrane structure and permeability. The same results were obtained with E. coli BW 251131. In case of S. typhimurium both NPs suppressed energy-dependent H+-efflux even by addition of DCCD (Table 1).
Table 1.
The changes of H+-fluxes across the bacterial membranes of E. coli K-12, drug-resistant E. coli pARG-25 and S. typhimurium MDC1759 strains in the presence of citric acid coated Fe3O4 (100 μg mL−1) stabilized by citric acid, and Ag NPs (10 μg mL−1).
| Bacteria and conditions* | H+-fluxes (mmol H+ min−1 (1010 cells)−1 | H+-fluxes + DCCD** (mmol H+ min−1 (1010 cells)−1 |
|---|---|---|
| E. coli K-12 (without NPs) | 2.50 ± 0.02 | 1.10 ± 0.02 |
| E. coli K-12 + Fe3O4 NPs |
0.75 ± 0.01 P*** < 0.01 |
0.68 ± 0.01 P < 0.01 |
| E. coli K-12 + Ag NPs |
2.87 ± 0.02 P < 0.05 |
1.25 ± 0.02 P < 0.05 |
| E. coli pARG-25 (without NPs) | 2.32 ± 0.02 | 1.05 ± 0.02 |
| E. coli pARG-25 + Fe3O4 NPs |
1.80 ± 0.02 P < 0.01 |
0.98 ± 0.01 P < 0.01 |
| E. coli pARG-25 + Ag NPs |
2.55 ± 0.02 P < 0.05 |
1.15 ± 0.01 P < 0.01 |
| S. typhimurium MDC1759 (without NPs) | 1.50 ± 0.02 | 0.83 ± 0.01 |
| S. typhimurium MDC1759 + Fe3O4 NPs |
1.16 ± 0.02 P < 0.01 |
0.27 ± 0.02 P < 0.01 |
| S. typhimurium MDC1759 + Ag NPs |
1.32 ± 0.02 P < 0.01 |
0.34 ± 0.01 P < 0.01 |
*The bacteria were washed and transferred into Tris–phosphate buffer; bacterial cells were treated with NPs for 10 min.
**The bacterial cells were treated with 0.2 mM DCCD for 10 min.
***P is difference between the values of experimental simples and appropriate control.
The membrane-bound H+-translocating FOF1-ATPase activity of E. coli membrane vesicles was analyzed in the presence of citric acid coated Fe3O4 and Ag NPs to reveal their effects on the ATPase. In facultative anaerobic bacteria, such as E. coli, the H+-translocating ATPase is reversible depending on bacterial growth conditions32. During the preparation of membrane vesicles, spheroplasts from Gram-negative E. coli were obtained.
Fe3O4 NPs led to a ~ 1.3 to 1.5-fold increase in total FOF1-ATPase activity in membrane vesicles of E. coli K-12 and pARG-25, respectively (Fig. 3). DCCD-sensitive ATPase activity was increased ~ 1.2- and 1.5-folds in E. coli K-12 and pARG-25, respectively (see Fig. 3). In the case of Ag NPs, ATPase activity was not detected even in the presence of DCCD (no shown), which confirms the bactericidal effect of these NPs. Therefore, the effect of NPs on the ATPase can be responsible for the antibacterial effect.
NPs can directly affect the FOF1-ATPase, because ATPase activity was changed even in the absence of DCCD, an inhibitor of H+-translocating systems; or this effect can be intermediated by membrane-associated formate hydrogen lyase (FHL) complexes, which are responsible for H2 production in E. coli. The bacterial membrane permeability can be changed during the bacterial growth in the presence of NPs.
The redox potential changes and H2 production ability in E. coli wild type and drug-resistant strains in the presence of Ag NPs and citric acid coated Fe3O4 NPs
The redox potential (Eh) is an important factor, which characterizes the metabolic activity of bacteria under various growth conditions1. To reveal the action mechanisms of Ag and Fe3O4 NPs on both E. coli strains, the kinetics of Eh during the bacterial growth has been studied. The anaerobic growth (6 h) of bacteria was accompanied by a drop in the value of Eh from a positive value (+ 120 ± 10 mV) at the beginning of the growth lag phase to a negative value (− 580 ± 15 mV) for E. coli K-12 and (− 550 ± 10 mV) for E. coli pARG-25 (Fig. 4A). This decrease indicates an enhancement of reduction processes that characterizes bacterial metabolism under anaerobic conditions and the generation of H21,20. The addition of NPs resulted in a delayed redox potential drop (Fig. 4A). In the presence of Fe3O4 NPs value of the Eh decreased up to (− 540 ± 10 mV) for E. coli K-12 and (− 500 ± 10 mV) for E. coli pARG-25 (see Fig. 4A). Ag NPs exhibited more pronounced effect: in the presence of 10 μg mL−1 Ag NPs, the Eh values of E. coli K-12 and pARG-25 cells were decreased up to (− 410 ± 5 mV) and (− 400 ± 5 mV), respectively (see Fig. 4A). The inhibition of bacterial growth in the presence of NPs can be coupled with Eh or with a direct effect of NPs on the bacterial membrane.
Figure 4.
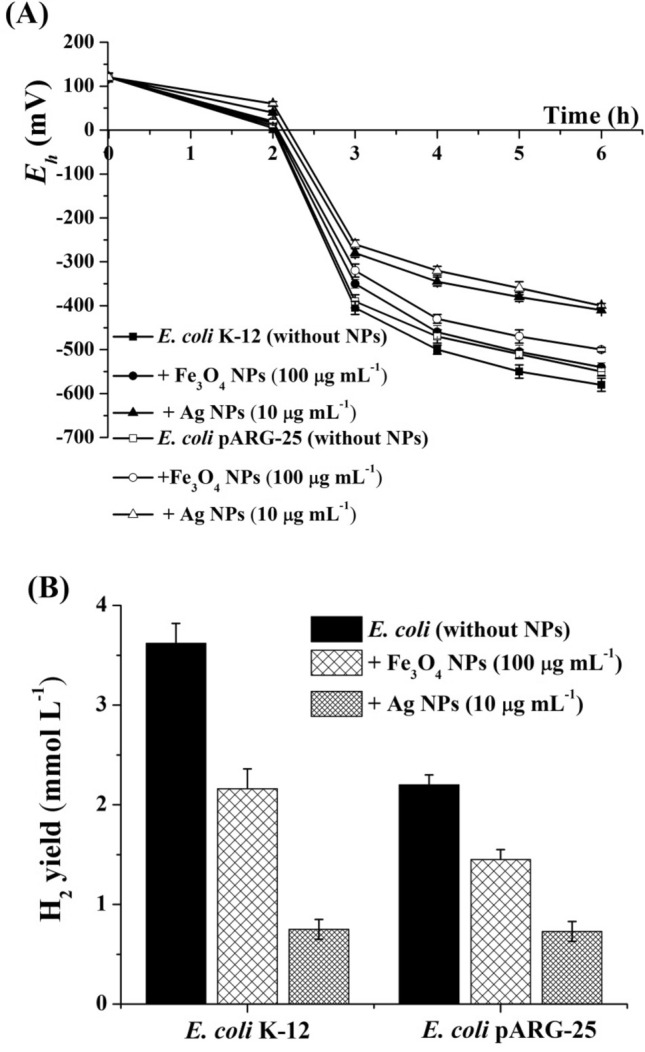
The changes of redox potential (A) and the H2 yield (B) in E. coli K-12 and kanamycin-resistant E. coli pARG-25 strains during anaerobic growth in the presence of citric acid coated Fe3O4 (100 μg mL−1) and Ag NPs (10 μg mL−1). Control was without NPs addition.
A correlation between the decrease of Eh and production of H2 is shown for E. coli, which can generate H2 under the action of FHL complexes32,33. During glucose fermentation, H2 production was determined during 6 h anaerobic growth in both strains: 3.62 and 2.20 mmol H2 L−1 in E. coli K-12 and pARG-25 cells, respectively (Fig. 4B). The H2 production by E. coli coupled with the activity of membrane-associated H2-producing enzymes—hydrogenases, which are involved in H2 metabolism in E. coli21,32,33. Ag and Fe3O4 NPs inhibited H2 yield in both E. coli strains (see Fig. 4B). H2 production by both strains in the presence of 100 μg mL−1 citric acid coated Fe3O4 NPs was ~ 1.5 to 1.7-fold lower in comparison with control cells (see Fig. 4B). Citric acid coated Fe3O4 NPs demonstrated more noticeable effect on H2 yield than NPs stabilized by oleic acid20,21. In the presence of 100 μg mL−1 oleic acid coated NPs H2 yield in E. coli pARG-25 was decreased 1.2-fold in comparison with control, and was not changed significantly in E. coli BW2511320,21. In the presence of Ag NPs H2 yield in E. coli K-12 was ~ 5.0-fold lower than H2 yield in control cells (see Fig. 4B).
The results point out that NPs show antibacterial effect in both bacteria in concentration-dependent manner by changing membrane-bound enzyme activity.
Discussion
Nowadays, due to the development of drug-resistant bacteria, the search of new more effective antibacterial agents required. Various NPs are suggested as novel antimicrobial agents against different pathogens owing to their unique physicochemical properties1,4–7. As it was shown in our previous work, oleic acid coated Fe3O4 NPs display different effect on the growth properties and membrane activity of Gram-negative Escherichia coli BW 25113 and Gram-positive Enterococcus hirae ATCC 979021. Iron oxide NPs demonstrate better antibacterial effect on Gram-negative, than on Gram-positive bacteria21. This effect can be coupled with the components of cell wall of Gram-positive and Gram-negative bacteria. Gram-positive bacteria have a thick peptidoglycan layer, teichoic acids and pores, which allow penetration of external molecules, including NPs2. In contrast, the cell wall of Gram-negative bacteria has a thin peptidoglycan layer between the cytoplasmic membrane and the outer membrane, which forms a penetration barrier for external molecules.
Stabilizer such as oleic acid can be used not only to prevent aggregation of magnetic NPs, but also for protection of NPs22–24. Citric acid also can be used to stabilize the magnetic NPs for biomedical application25–27.
In this study, antimicrobial effects and possible mechanisms of Fe3O4 NPs (coated by citric acid) and Ag NPs on E. coli K-12 wild type and pARG-25 kanamycin-resistant strains have been investigated. Citric acid coated Fe3O4 NPs exhibit significant antibacterial activity against E. coli both strains, but kanamycin-resistant E. coli pARG-25 strain is more susceptible to Fe3O4 NPs than wild type strain. Moreover, the effect of Fe3O4 NPs depended of stabilizer type. Citric acid coated Fe3O4 NPs demonstrated more noticeable action against drug-resistant bacteria than oleic acid stabilized NPs20. In the presence of 250 μg mL−1 citric acid coated Fe3O4 NPs a ~ threefold inhibition of bacterial growth was observed, whereas the same concentration of oleic acid coated NPs suppressed growth rate ~ 2-fold20.
Ag NPs exhibit a more pronounced bactericidal effect in comparison with Fe3O4 NPs. Moreover, Ag NPs have a more expressed antibacterial effect at low concentrations. The low concentration effect of Ag NPs has been reported by various researchers10–12,34. There were no obvious differences in bactericidal activity between E. coli wild type and drug-resistant strains, confirming that Ag NPs have a broad spectrum of action. Ag NPs are assumed to affect not only the growth of both E. coli strains, but also the energy-dependent H+-coupled membrane transport and ATPase activity in bacteria. The increase of H+-fluxes in the presence of DCCD indicated the significant effect of Ag NPs on the bacterial membrane structure and permeability. Probably Ag NPs change the permeability of the bacterial membrane and inhibit the cell respiration by penetrating via cell wall9,10,14. These studies confirm that the FOF1-ATPase, which plays a crucial role in bacterial metabolism, can be a sensitive target for metals NPs action. The effect of NPs used on the FOF1-ATPase can be responsible for the antibacterial effect, and this ATPase can be a target for NPs. Similar data were obtained in mammalian cells, where Ag NPs inhibited mitochondrial ATPase activity of rat liver cells35.
Different effects of Fe3O4 and Ag NPs on E. coli ATPase activity may be coupled to the differences in the interaction of NPs with bacterial cell wall. The antimicrobial activity of metal NPs is a result of NPs interaction with bacterial membranes and their penetration into the bacterial cell, causing membrane damage and bacterial death2,7–9.
Thus, metal NPs studied exhibited antibacterial activity against bacteria, including drug-resistant strains, and they can be applied in biomedicine for the treatment of various infections and in biotechnology and the food industry for controlling bacterial growth.
Materials and methods
Bacterial strains, cultivation conditions and growth determination
This study was performed with S. typhimurium MDC1759, E. coli K-12 wild type and kanamycin-resistant pARG-25 strains (Microbial Depository Center, National Academy of Science, Yerevan, Armenia). Bacteria grown in the presence of kanamycin (50 μg mL−1) were used as positive control. The negative controls are the strains, cultivated without antibiotic. These strains were cultivated in peptone medium at 37 °C and pH 7.520,21. Anaerobic conditions, favorable for intestine microorganisms, including pathogenic ones, were maintained20. For creation of anaerobic conditions O2 was bubbled out from media by autoclaving at 120 °C for 20 min, and then bottles were closed by press caps. To reach anaerobic conditions the bottles were kept sealed to maintain anoxic conditions and all experiments were performed under strict absence of O220,33.
The growth of bacteria was determined by measuring the optical density at 600 nm using Spectro UV–Vis Auto spectrophotometer (Labomed, Los Angeles, USA). The concentration of initial inoculum was 108 of number of colony forming units (CFU) mL−1. Specific growth rate was determined as the quotient of ln2 division on doubling time of absorbance over the interval, when the logarithm of absorbance of the culture at 600 nm increased with time linearly (logarithmic growth phase), and it was expressed as h−1 as decribed20,21. Colloidal Ag NPs ("Silverton", "Tonus-Less", Armenia) in the concentration range from 5 to 30 μg mL−1 and Fe3O4 NPs (stabilized by citric acid) from 50 to 250 μg mL−1 were added to growth medium with inoculum.
Characterization of Ag and Fe3O4 nanoparticles
The structure, form and size of Ag NPs (synthesized by electrochemical method36) were investigated using TEM image and atomic force microscopy method by appropriate microscope (NT-MDT Nanoeducator 2, Russia). The data showed that Ag NPs have spherical form with average size of ~ 30 nm (Fig. 5).
Figure 5.
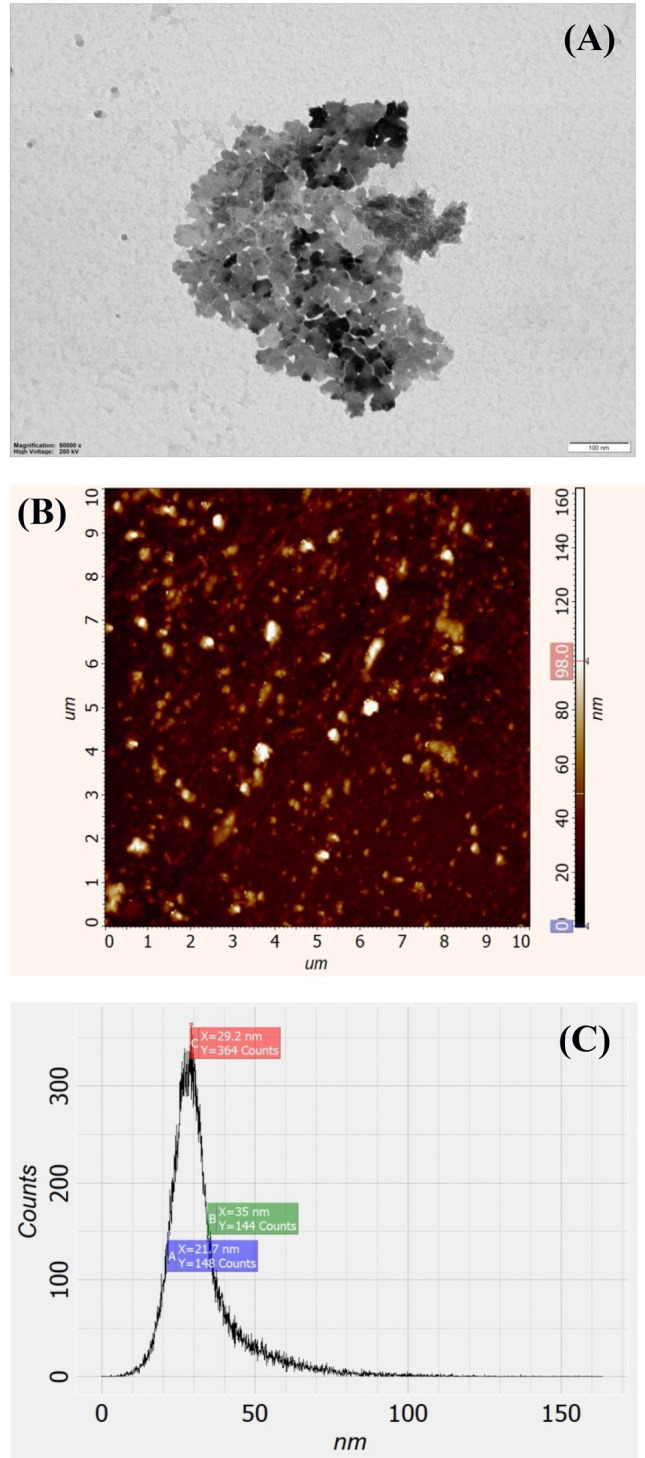
TEM image (A) and atomic force microscopy of Ag NPs using appropriate microscope (B). Ag NPs distribution in the colloid solution depending of their size (C). For details, see “Materials and methods”.
Fe3O4 NPs coated with citric were synthesized by co-precipitation method20,37. Fe3O4 NPs have round form and average size of 10 nm (Fig. 6). The synthesized Fe3O4 NPs were characterized also by X-ray powder diffraction (XRD) to determine the sample phases and average particle size of the dried powder. The XRD pattern of the sample was recorded on diffractometer system EMPYREAN using CuKα (λ = 1.5406 Å) radiation at room temperature in the range of 10° to 120° in the 2θ scale, Generator Settings 40 mA, 45 kV. The XRD patterns of the dried sample of Fe3O4 NPs and diffraction peaks parameters are shown in Fig. 6 and Table 2. XRD data can be used to distinguish the crystallinity and the average size of nanoparticles. The strongest reflection from the (311) diffraction peak indicate of a cubic spinel structure. The reflection from other planes (022), (040), (151) and (044) also correspond with a cubic unit cell38.
Figure 6.
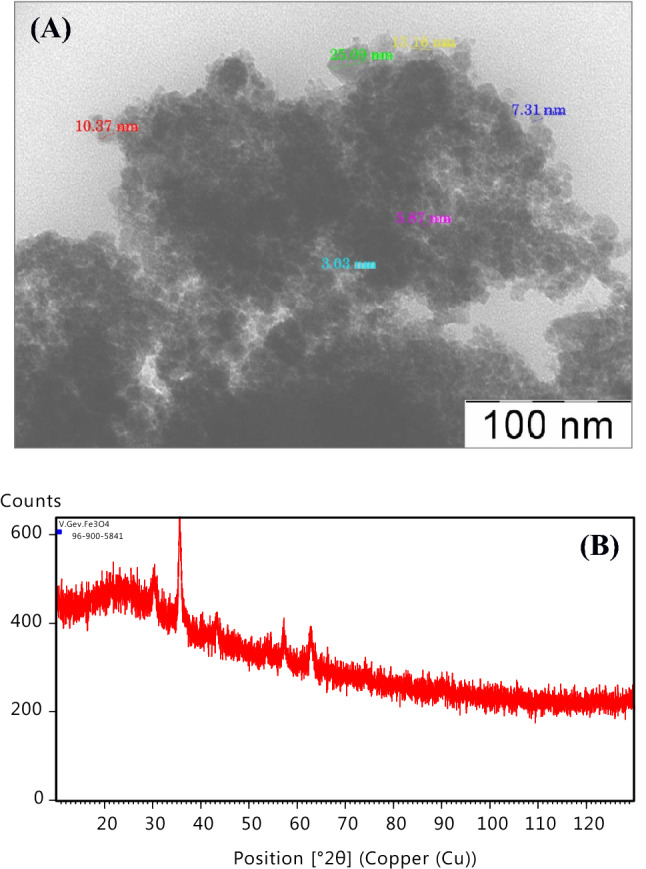
TEM image of synthesized Fe3O4 NP (A). The sizes of some nanoparticles indicated immediately in the figure. XRD pattern of the Fe3O4 NPs (B).
Table 2.
Peak list of Fe3O4 NPs. All the peaks of XRD patterns were analyzed and indexed using ICDD data base.
| Pos (°2θ) | Height (cts) | FWHM left (°2θ) | d-spacing (Å) | Rel. Int (%) | Tip width | Matched by |
|---|---|---|---|---|---|---|
| 30.2360 | 123.24 | 0.5117 | 2.95596 | 47.15 | 0.6140 | Fe24O32 |
| 35.5989 | 261.39 | 0.3582 | 2.52199 | 100.00 | 0.4298 | Fe24O32 |
| 43.2865 | 68.51 | 0.4093 | 2.09024 | 26.21 | 0.4912 | Fe24O32 |
| 57.2379 | 63.41 | 0.7164 | 1.60953 | 24.26 | 0.8596 | Fe24O32 |
| 62.8740 | 79.50 | 0.5117 | 1.47813 | 30.41 | 0.6140 | Fe24O32 |
It can be noticed from XRD pattern and Table 2 that the 2θ position, d-spacing and intensity of the diffraction peaks are in good agreement with the standard pattern for Ref. Code 96-900-5841.
Testing of bacterial sensitivity to NPs
To determine the sensitivity of E. coli strains to used NPs, bacteria were grown in the presence of Fe3O4 NPs (100 μg mL−1) and Ag NPs (10 μg mL−1), after which various dilutions (106–109 folds) of bacterial suspension were applied20. Then 100 μL of each sample was transferred on nutrient agar plates, which were incubated at 37 °C. Nutrient agar plates contained peptone media with 1.5% bacteriological agar. The numbers of bacterial viable colonies were counted after 24 h of incubation for determination of CFUs presented in each sample by: T = 10 × n × 10m, where n is the number of bacterial viable colonies, m is the number of dilution. NPs free plates incubated under the same conditions were used as control20.
Determination of H+-fluxes through bacterial membrane
The H+-flux through the bacterial membrane in whole bacterial cells was determined using appropriate selective electrode (HJ1131B, HANNA Instruments, Portugal), as described11,20,21. Bacteria were cultivated in the presence of Fe3O4 NPs (100 μg mL−1) or Ag NPs (10 μg mL−1). Bacterial cells were transferred into the 150 mM Tris–phosphate buffer (pH 7.5), and then energy source—glucose (11 mM) was added. The H+-flux was expressed as a change in the ion external activity in mmol H+ per min per 1010 cells11,20. The bacterial cultures were incubated with 0.2 mM N,N′-dicyclohexylcarbodiimide (DCCD), an inhibitor of the FOF1-ATPase, for 10 min11.
ATPase activity assay
Bacterial membrane vesicles were obtained by the Kaback method, as described21,32. ATPase activity was determined by amount of inorganic phosphate (Pi), liberated after adding 3 mM ATP to membrane vesicles11,21,32. Pi was measured by the Tausski and Shorr method, as described11,32. ATPase activity was expressed in nmol Pi per µg protein per min. Bacteria were cultivated in the presence of Fe3O4 NPs (100 μg mL−1) orAg NPs (10 μg mL−1). For DCCD studies, membrane vesicles were incubated with 0.2 mM DCCD for 10 min.
The medium pH, redox potential and H2 yield determinations
The pH of the medium was measured during bacterial growth at certain time intervals (from 0 to 6 h) by a pH-meter (HANNA Instruments, Portugal) with pH-selective electrode (HJ1131B), as described20,33. The initial pH was adjusted at 7.5 ± 0.1 by 0.1 M NaOH or 0.1 M HCl. The medium redox potential (Eh) was measured during bacterial growth using a pair of redox [platinum (Pt) and titanium–silicate (Ti–Si)] electrodes, as described20,33. Eh kinetics determined using the pair of redox electrodes during culture growth gives information about main redox processes and also H2 evaluation20,33. The H2 yield in E. coli, cultivated in the presence of Fe3O4 NPs (100 μg mL−1) or Ag NPs (10 μg mL−1), was calculated by the decrease of Eh to low negative values during bacterial growth, as described early21,33, and expressed in mmol H2 per L.
Reagents, data processing and others
Yeast extract, peptone, Tris (aminomethane), Agar–Agar Kobe from Carl Roth GmbH (Germany); glucose, DCCD from Sigma Aldrich (USA), and other reagents of analytical grade were used. The average data are presented from 3 independent experiments; error bars are presented on figures. The validity of the differences between different series of experiments was evaluated by Student criteria (P)20.
Acknowledgements
The authors thank Narine Mnatsakanyan (“Tonus-Les”, Armenia) for supplying Ag NPs. This work was supported by Basic support from Russian-Armenian University and Committee of Science, Ministry of Education, Science, Culture and Sport of Armenia.
Author contributions
L.G. performed the main part of experimental work and data processing; H.B. provided atomic force microscopy of NPs; V.G. supplied Fe3O4 NPs and provided XRD characterization; L.G. and A.T. wrote the manuscript; A.T. supervised and coordinated the research, designed the study, edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Figures 5 and 6, where the incorrect TEM images of nanoparticles were used for panels 5A and 6A.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/30/2021
Editor’s Note: The Editors are currently investigating concerns that have been raised relating to the reliability of data presented in this article. Appropriate editorial action will be taken once the investigation is complete and all parties have been given the opportunity to respond in full.
Change history
6/1/2021
A Correction to this paper has been published: 10.1038/s41598-021-90986-x
References
- 1.Trchounian A, Gabrielyan L, Mnatsakanyan N. Nanoparticles of various transition metals and their applications as antimicrobial agents. In: Saylor Y, Irby V, editors. Metal Nanoparticles: Properties, Synthesis and Applications. New York: Nova Science Publishers; 2018. pp. 161–211. [Google Scholar]
- 2.Wang L, Hu Ch, Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghunath A, Perumal E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents. 2017;49:137–152. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Arias LS, Pessan JP, Vieira APM, de Lima TMT, Delbem AKB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7:46. doi: 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SH, Jun B-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019;20:865. doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdușel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials. 2018;8:681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrielyan L, Trchounian A. Antibacterial activities of transient metals nanoparticles and membranous mechanisms of action. World J. Microbiol. Biotechnol. 2019;35:162. doi: 10.1007/s11274-019-2742-6. [DOI] [PubMed] [Google Scholar]
- 8.Khatoon N, Alam H, Khan A, et al. Ampicillin silver nanoformulations against multidrug resistant bacteria. Sci. Rep. 2019;9:6848. doi: 10.1038/s41598-019-43309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SB, White SB, Steadman CS, et al. Silver-coated magnetic nanocomposites induce growth inhibition and protein changes in foodborne bacteria. Sci. Rep. 2019;9:17499. doi: 10.1038/s41598-019-53080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franci G, Falanga A, Galdiero S, Palomba L, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vardanyan Z, Gevorkyan V, Ananyan M, Vardapetyan H, Trchounian A. Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport. J. Nanobiotechnol. 2015;13:69. doi: 10.1186/s12951-015-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanosc. Res. Lett. 2014;9:373. doi: 10.1186/1556-276X-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong X, Wen J, Xiong X, Hu Y. Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ. Sci. Pollut. Res. Int. 2016;23:4489–4497. doi: 10.1007/s11356-015-5668-z. [DOI] [PubMed] [Google Scholar]
- 14.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayala-Núñez NV, Lara Villegas HH, del Carmen Ixtepan Turrent L, Rodríguez Padilla C. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: Nanoscale does matter. NanoBiotechnology. 2009;5:2. doi: 10.1007/s12030-009-9029-1. [DOI] [Google Scholar]
- 16.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Chen BA. Application and development of magnetic iron oxide nanoparticles in tumor targeted therapy. Chin. J. Cancer. 2010;29:118–122. doi: 10.5732/cjc.009.10153. [DOI] [PubMed] [Google Scholar]
- 18.Mody VV, Cox A, Shah S, Singh A, Bevins W, Parihar H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014;4:385–392. doi: 10.1007/s13204-013-0216-y. [DOI] [Google Scholar]
- 19.Chatterjee S, Bandyopadhyay A, Sarkar K. Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J. Nanobiotechnol. 2011;9:34. doi: 10.1186/1477-3155-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabrielyan L, Hakobyan L, Hovhannisyan A, Trchounian A. Effects of iron oxide (Fe3O4) nanoparticles on Escherichia coli antibiotic-resistant strains. J. Appl. Microbiol. 2019;126:1108–1116. doi: 10.1111/jam.14214. [DOI] [PubMed] [Google Scholar]
- 21.Gabrielyan L, Hovhannisyan A, Gevorgyan V, Ananyan M, Trchounian A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019;103:2773–2782. doi: 10.1007/s00253-019-09653-x. [DOI] [PubMed] [Google Scholar]
- 22.Lai CW, Low FW, Ta MF, Hamid ShDA. Iron oxide nanoparticles decorated oleic acid for high colloidal stability. Adv. Polym. Technol. 2018;37:1712–1721. doi: 10.1002/adv.21829. [DOI] [Google Scholar]
- 23.Patil RM, Shete PB, Thorat ND, et al. Non-aqueous to aqueous phase transfer of oleic acid coated iron oxide nanoparticles for hyperthermia application. RSC Adv. 2014;4:4515–4522. doi: 10.1039/C3RA44644A. [DOI] [Google Scholar]
- 24.Gelbrich T, Feyen M, Schmidt AM. Magnetic thermoresponsive core-shell nanoparticles. Macromolecules. 2006;39:3469–3472. doi: 10.1021/ma060006u. [DOI] [Google Scholar]
- 25.Răcuciu M, Creangă DE, Airinei A. Citric-acid–coated magnetite nanoparticles for biological applications. Eur. Phys. J. 2006 doi: 10.1140/epje/i2006-10051-y. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Mak KY, Leung CW, et al. Effect of synthesis conditions on the properties of citric-acid coated iron oxide nanoparticles. Microelectr. Eng. 2013;110:329–334. doi: 10.1016/j.mee.2013.02.045. [DOI] [Google Scholar]
- 27.Yokoyama S, Suzuki I, Motomiya K, Takahashi H, Tohji K. Aqueous electrophoretic deposition of citric-acid-stabilized copper nanoparticles. Coll. Surf. A Physicochem. Eng. Aspects. 2018;545:93–100. doi: 10.1016/j.colsurfa.2018.02.056. [DOI] [Google Scholar]
- 28.Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. 2012;3:71–87. doi: 10.4161/gmic.19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foulquié Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006;106:1–24. doi: 10.1016/j.ijfoodmicro.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Akbar A, Sadiq M, Ali I, Muhammad N, et al. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 2019;17:36–42. doi: 10.1016/j.bcab.2018.11.005. [DOI] [Google Scholar]
- 31.Espinosa-Cristobal LF, et al. Bovine serum albumin and chitosan coated silver nanoparticles and its antimicrobial activity against oral and nonoral bacteria. J. Nanomater. 2015 doi: 10.1155/2015/420853. [DOI] [Google Scholar]
- 32.Blbulyan S, Trchounian A. Impact of membrane-associated hydrogenases on the FoF1-ATPase in Escherichia coli during glycerol and mixed carbon fermentation: ATPase activity and its inhibition by N, N’-dicyclohexylcarbodiimide in the mutants lacking hydrogenases. Arch. Biochem. Biophys. 2015;579:67–72. doi: 10.1016/j.abb.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Trchounian K, Poladyan A, Trchounian A. Enhancement of Escherichia coli bacterial biomass and hydrogen production by some heavy metal ions and their mixtures during glycerol vs glucose fermentation at a relatively wide range of pH. Int. J. Hydrogen Energy. 2017;42:6590–6597. doi: 10.1016/j.ijhydene.2017.02.003. [DOI] [Google Scholar]
- 34.Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Chichova M, Shkodrova M, Vasileva P, et al. Influence of silver nanoparticles on the activity of rat liver mitochondrial ATPase. J. Nanopart. Res. 2014;16:2243. doi: 10.1007/s11051-014-2243-3. [DOI] [Google Scholar]
- 36.Khaydarov RA, Khaydarov RR, Gapurova O, Estrin Y, Scheper Th. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2009;11:1193–1200. doi: 10.1007/s11051-008-9513-x. [DOI] [Google Scholar]
- 37.Kekutia Sh, Saneblidze L, Mikelashvili V, et al. A new method for the synthesis of nanoparticles for biomedical applications. Eur. Chem. Bull. 2015;4:33–36. [Google Scholar]
- 38.Vaidyanathan G, Sendhilnathan S, Arulmurugan R. Structural and magnetic properties of Co1− xZnxFe2O4 nanoparticles by co-precipitation method. J. Magnet. Magnet. Mat. 2007;313:293–299. doi: 10.1016/j.jmmm.2007.01.010. [DOI] [Google Scholar]