Abstract
Methylmalonic acidemia (MMA) is a severe, heterogeneous disorder of methylmalonate and cobalamin (cbl; vitamin B12) metabolism with a poor prognosis that can cause brain damage. Identifying the magnetic resonance imaging (MRI) findings of MMA might help to make accurate diagnoses earlier in the disease course and exploring the relationship between neuropsychological scores and MRI findings, when therapy is more effective and to improve therapeutic efficacy. Cerebral MRI studies from 37 children with MMA were evaluated by a neuroradiologist. Clinical and imaging data were collected from each patient. All tests were performed during routine investigations and in accordance with the ethical principles of the Declaration of Helsinki. Informed consent was obtained from the guardians of all patients for inclusion in the study. The most common and significant findings were periventricular white matter changes (78.4%), ventricular dilation (29.7%) and cerebral atrophy (40.5%). According to the developmental quotient, the 37 patients were divided into the normal intelligence subgroup (NI, developmental quotient ≥ 85) and the low intelligence subgroup (LI, developmental quotient < 85). The incidence of corpus callosal thinning, cortical atrophy, subcortical white matter changes, and ventricular dilation (grades 0–3) was significantly higher in the LI subgroup than in the NI subgroup (P < 0.05). The incidence of no-mild and moderate-severe ventricular dilation was significantly higher in the LI subgroup than in the NI subgroup (P < 0.05). Ventricular dilatation, cerebral atrophy, white matter changes, and corpus callosal thinning are the main MRI abnormalities in MMA patients, and these manifestations are significantly correlated with delayed development in children.
Subject terms: Paediatric research, Metabolic disorders, Neurological disorders, Neuroscience
Introduction
Methylmalonic acidemia (MMA) is a lethal, severe, heterogeneous disorder of methylmalonate and cobalamin (cbl; vitamin B12) metabolism with a poor prognosis, as noted in 1967 in the first report of this disease1. Defects in methylmalonyl-CoA mutase (MCM) or its coenzyme, cobalamin, lead to the accumulation of methylmalonic acid, which is characteristic of methylmalonic acidemia (MMA)2–4.
According to the type of enzyme deficiency, there are two categories of MMA: MCM deficiency (also referred to as the mutant type (mut type)) and coenzyme cobalamin metabolism disorder (or the cobalamin type). A mutation in the gene encoding MCM causes the mut type; this mutation alters the activity of the allosteric enzyme. The cobalamin type is caused by congenital defects in the synthesis, activation or transport of cobalamin (the allosteric coenzyme), which affect the function of MCM by disrupting the synthesis of its coenzyme deoxyadenosylcobalamin. Accumulation of homocysteine and occurs in isolation or combined with MMA mutase deficiency results from deficiency of this enzyme defects of intracellular cobalamin metabolism as well as in nutritional deficiency or disturbed uptake or transport of vitamin B12. The cobalamin type can be subdivided into six subtypes (cblA, cblB, cblC, cblD, cblF, and cblH). The mut type and the cblA, cblB, and cblH deficiency types present as MMA alone and are classified as isolated MMA; the cblC, cblD, and cblF deficiency types are classified as MMA combined with homocysteinemia5,6, MMA combined with homocysteinemia is the main biochemical type of MMA in China, accounting for 60–80% of cases7, and cblC is the most common subtype8,9.
MMA often causes damage to multiple body systems, especially the central nervous system10. Although the incidence is low, the mortality and disability rates are very high11. This disease has diverse and nonspecific clinical manifestations,the main manifestations are feeding difficulties, intellectual disabilities, ataxia, abnormal muscle tone, convulsions, epilepsy, and lethargy12,13. As a result, patients often need to undergo many tests before a correct diagnosis can be made. Symptoms of neurological damage are common in patients with MMA, and brain imaging findings are often used to rule out congenital or acquired cerebral abnormalities. Magnetic resonance imaging (MRI) causes no radiation damage and is suitable for brain examinations in children. This modality is increasingly used in neuroimaging studies of genetic metabolic diseases. Identifying the magnetic resonance imaging (MRI) findings of MMA might help to make accurate diagnoses earlier in the disease course and exploring the relationship between neuropsychological scores and MRI findings, when therapy is more effective and to improve therapeutic efficacy14.
Materials and methods
Patients and diagnosis
This study was approved by the Institutional Review Board (IRB) of Jinan Maternal and Child Care Hospital, Shandong. Informed consent was obtained from the guardians of all patients for inclusion in the study. Thirty-seven patients were diagnosed with MMA according to the MMA diagnostic criteria and were included in the study. The standard protocol for the diagnosis of MMA was increased plasma methylmalonyl carnitine or increased urine methylmalonic acid, as detected by gas chromatography-mass spectrometry (GC/MS) and the propionate incorporation test. The levels of propionyl carnitine (C3), C3/acetylcarnitine (C2) were measured by tandem mass spectrometry methionine in dried blood spots. To further confirm the diagnosis, the levels of organic acids in urine were measured with GC/MS in patients with suspected MMA. In addition, the concentration of total plasma homocysteine (tHcy) and hyperammonemia was measured15, detailed genetic data and experimental data in Supplementary materials. Nineteen patients underwent genetic newborn screening for neonatal genetic diseases after birth, and 18 patients underwent blood and genetic screening after exhibiting suspicious symptoms (SS). MRI examination and neuropsychological tests were performed on these patients before treatment. The neuropsychological test used a pediatric examination table of neuropsychological development for 0- to 6-year-old patients (CNBSR 2016). All tests were performed as a part of routine clinical and biochemical investigations and were performed in accordance with the ethical principles of the Declaration of Helsinki.
Demographic and clinical data acquisition
We gathered clinical data, laboratory test results, gene type, including age at the time of MRI and symptoms for each patient. These data were obtained from the genetic metabolic disease clinic, and medical history was obtained from the guardian if needed. Cerebral MRI scans of the patients were retrieved, and those who had no previous cerebral MRI were excluded. We identified 37 studies from 70 patients who had been diagnosed with genetic metabolic diseases. The MRI findings of these 37 patients were reviewed, including the findings of 27 (73.0%) males and 10 (27%) females (see Table 1). Thirty-seven patients received standardized treatment. After vitamin B12 stress test, vitamin B12 is effective in patients with cblC, the patients with mut did not respond. The patients with cblC were treated with hydroxocobalamin (OHCbl) (1 mg/day, intramuscular injection), betaine (100–500 mg/kg/day, oral administration), folic acid(5–0 mg/day, oral administration),vitamin B6 (10–30 mg/day, oral administration), sodium benzoate(150–250 mg/kg/day, improve hyperammonemia), levocarnitine (50 mg/kg/day, oral administration), low protein and high-energy diet to reduce the accumulation of toxic metabolites. The patients with mut were treated with Vitamin B6 (10–30 mg/day, oral administration),Sodium benzoate(150-250 mg/kg/day, improve hyperammonemia), L-carnitine (50–100 mg/kg/d, orally or intramuscular injection) and were restricted natural protein. Some patients with motor system damage need to carry out sensory, motor function rehabilitation training and language cognitive ability training in order to facilitate the growth and development of patients.
Table 1.
Demographic data and clinical findings in 37 children with MMA.
| Patient | Gender | AP (m) | AI (m) | Gene type # | The group of by clinical screening* | First presenting symptom | 0–6-year-old pediatric examination table of neuropsychological development | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Big activity sport | Fine sport | Adaptive capacity | Language capacity | Social behavior | Development quotient | Developmental quotient evaluation | |||||||
| 1 | M | 18 | 19 | cbIC (MMA-h) | SS | Developmental regression; hypotonia; respiratory distress | 33.1 | 24.8 | 0 | 33.1 | 0 | 18.2 | Low |
| 2 | F | 23 | 23 | cbIC (MMA-h) | SS | Psychomotor delay | 81 | 71 | 65 | 55 | 71 | 68.6 | Low |
| 3 | F | 6 | 7 | cbIC (MMA-h) | SS | Growth retardation | 58.8 | 49 | 39.2 | 49 | 53.9 | 50 | Low |
| 4 | M | 6 | 6 | cbIC (MMA-h) | SS | Growth retardation; seizure | 83.3 | 90.9 | 75.8 | 90.9 | 90.9 | 86.4 | Normal |
| 5 | M | 1 | 1 | cbIC (MMA-h) | SS | Respiratory distress | 86 | 89 | 88 | 90 | 91 | 88.8 | Normal |
| 6 | M | 30 | 32 | cbIC (MMA-h) | SS | Coma; recurrent vomiting | 85.5 | 87 | 90 | 93 | 89.5 | 89 | Normal |
| 7 | M | 21 | 24 | cbIC (MMA-h) | SS | Lethargy | 90 | 94 | 86 | 91.5 | 94 | 91.1 | Normal |
| 8 | F | 47 | 48 | mut(i-MMA) | SS | Developmental regression | 87 | 90 | 91 | 93 | 88 | 89.8 | Normal |
| 9 | M | 60 | 60 | mut(i-MMA) | NS | Screening | 93.2 | 93.2 | 93.2 | 103 | 93.2 | 95 | Normal |
| 10 | F | 41 | 41 | cbIC (MMA-h) | SS | Skeletal abnormality; lethargy | 42.4 | 72.7 | 69.6 | 72.7 | 90.9 | 69 | Low |
| 11 | M | 19 | 19 | cbIC (MMA-h) | SS | Recurrent vomiting | 70 | 69.5 | 72 | 73 | 71 | 71.1 | Lower |
| 12 | M | 35 | 35 | cbIC (MMA-h) | NS | Screening; lethargy | 109.2 | 70.2 | 98.8 | 88.4 | 88.4 | 91 | Normal |
| 13 | M | 10 | 10 | cbIC (MMA-h) | SS | Developmental regression | 97.8 | 73.4 | 73.4 | 73.4 | 59.8 | 75.5 | Lower |
| 14 | M | 10 | 11 | cbIC (MMA-h) | NS | Screening | 69 | 103.4 | 107.8 | 94.8 | 94.8 | 94 | Normal |
| 15 | M | 0.5 | 0.5 | cbIC (MMA-h) | NS |
Screening; emesis psychomotor delay |
89 | 80 | 81 | 82 | 79 | 82.2 | Lower |
| 16 | M | 5 | 6 | cbIC (MMA-h) | NS | Screening | 70.3 | 85.9 | 93.8 | 93.8 | 85.9 | 85.9 | Normal |
| 17 | M | 3 | 4 | cbIC (MMA-h) | NS | Screening; poor feeding | 81.4 | 69.8 | 87.2 | 69.8 | 69.8 | 75.6 | Lower |
| 18 | F | 1.1 | 1.5 | cbIC (MMA-h) | NS | Screening | 82 | 65.6 | 69.7 | 57.4 | 73.8 | 69.7 | Low |
| 19 | M | 1.0 | 1.5 | cbIC (MMA-h) | NS | Screening | 75.9 | 75.9 | 71.4 | 53.6 | 71.4 | 69.6 | Low |
| 20 | M | 1 | 1 | cbIC (MMA-h) | NS | Screening | 108 | 85.2 | 108 | 68.2 | 90.9 | 92 | Normal |
| 21 | M | 0.4 | 0.5 | cbIC (MMA-h) | NS | Screening | 80 | 78 | 81 | 76 | 83 | 79.6 | Lower |
| 22 | M | 4 | 5 | cbIC (MMA-h) | NS | Screening | 119.2 | 72.8 | 79.5 | 79.5 | 69.5 | 84.0 | Lower |
| 23 | F | 5 | 5 | cbIC (MMA-h) | NS | Screening | 85.9 | 82 | 82 | 85.9 | 78.1 | 82.8 | Lower |
| 24 | M | 3 | 3 | cbIC (MMA-h) | SS | Poor feeding; convulsion | 62.5 | 80.4 | 80.4 | 71.4 | 89.3 | 76.8 | Lower |
| 25 | M | 4.5 | 5 | mut(i-MMA) | NS | Screening | 63.4 | 77.5 | 77.5 | 84.5 | 77.5 | 76.1 | Lower |
| 26 | F | 5 | 6 | cbIC (MMA-h) | NS | Screening | 67 | 93 | 56.8 | 56.8 | 82 | 70 | Lower |
| 27 | M | 7 | 9 | cbIC (MMA-h) | SS | Seizure | 84.4 | 61.7 | 80.2 | 74.1 | 61.7 | 72.8 | Lower |
| 28 | M | 2.6 | 2.9 | cbIC (MMA-h) | SS | Recurrent vomiting | 36 | 38 | 33 | 40 | 37 | 36,8 | Low |
| 29 | M | 3 | 4 | cbIC (MMA-h) | NS | Screening; Seizure | 64.9 | 32.5 | 45.5 | 64.9 | 51.9 | 51.9 | Low |
| 30 | M | 24 | 26 | cbIC (MMA-h) | SS | Developmental regression | 29 | 30 | 33 | 32 | 29 | 30.6 | Low |
| 31 | M | 4 | 5 | cbIC (MMA-h) | NS | Screening | 83.3 | 76.4 | 90.3 | 83.3 | 83.3 | 83.3 | Lower |
| 32 | M | 58 | 60 | cbIC (MMA-h) | SS | Agitation | 89 | 91 | 93 | 85 | 82 | 88 | Normal |
| 33 | F | 54 | 55 | cbIC (MMA-h) | SS | Psychomotor delay | 46.6 | 32.9 | 43.9 | 38.4 | 41.1 | 40.6 | Low |
| 34 | M | 11 | 13 | cbIC (MMA-h) | NS | Screening | 77.5 | 77.5 | 81.4 | 73.6 | 77.5 | 77.5 | Lower |
| 35 | M | 7.1 | 7.7 | cbIC (MMA-h) | NS | Screening | 40 | 39 | 38 | 37 | 41 | 39 | Low |
| 36 | F | 11 | 12 | cbIC (MMA-h) | NS | Screening; agitation | 88.4 | 73.2 | 79.3 | 61 | 64 | 73.2 | Lower |
| 37 | F | 70 | 72 | cbIC (MMA-h) | SS | Microcephalus | 24.3 | 28.4 | 18.2 | 20.3 | 20.3 | 22.3 | Low |
AP age at presentation, AI age at the time of imaging, m months, ,i-MMA isolated MMA, MMA-h MMA combined with homocysteinemia.
*NS stands for the patients were genetically newborn screening for neonatal genetic diseases after birth, SS stands for the patients received blood and genetic screening after suspected symptoms. Developmental quotient evaluation: normal, 85–++4; lower, 70–84; low,≤69.
MRI parameters and image interpretation
Patient imaging data were obtained using a 1.5-T MR scanner (Achieva, Philips Medical Systems) and a 16-channel phased-array head coil with T2-weighted imaging (T2WI) (repetition time (TR): 2,100 ms; echo time (TE): 100 ms), T1-weighted imaging (T1WI) (TR: 568 ms; TE: 15 ms), T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) (TR: 700 ms; TE: 115 ms), and diffusion-weighted imaging (DWI) (TR: 3,090 ms; TE: 99 ms; b = 0 to 1,000) sequences with a field of view (FOV) of 230 × 190, slice thickness of 5 mm, gap of 0.5 mm, axial scanning, and sagittal T2WI (TR: 2,100 ms; TE: 90 ms) sections. The scan time was approximately 10 min.
Each image was evaluated by a senior pediatric radiologist who was unaware of the diagnosis. There are several reported scoring schemes for the severity of cortical atrophy and ventricular dilation, including the semiquantitative scale devised by Yue et al.16 and the age-related white matter changes (ARWMC) rating scale established by Wahlund LO17. After referencing the evaluation criteria proposed in the above two documents, the findings were scored as mild, moderate or severe based on a gross visual assessment and simplified scheme by the pediatric radiologist. We studied the findings in two different subgroups of MMA patients identified by newborn screening (NS) and MMA patients identified by suspected symptoms (SS): the normal intelligence (NI) subgroup and the low intelligence (LI) subgroup.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (Version 21.0 for Windows; SPSS, Chicago, Ill)18 .P value of < 0.05 was considered to be statistically significant. Before performing individual analyses, the distributions of data sets were checked for normality. The measurement data were expressed as mean (standard) deviation, and the counting data as n (%). Chi-square test was used to compare the differences of image features between different groups. Considering that all variables in correlation analysis are classified variables or grade variables, Kendall coefficient is used for correlation analysis.
Results
Among 37 patients (27 males and 10 females), SS of MMA were found in 18 patients, and 19 patients underwent genetic screening after birth for genetic metabolic diseases (mean age, 16.51 ± 19.52 months; age range, 0.5 to 54 months; age at the time of MRI, 17.35 ± 19.72 months; age range, 0.5 to 60 months). Patients with SS were diagnosed by MRI or were screened within no more than one month. These patients’ symptoms included seizure, developmental regression, hypotonia, psychomotor delay, recurrent vomiting, respiratory distress, agitation, coma, lethargy, growth retardation, and poor feeding. According to the table of neuropsychological development used in the examination of 0- to 6-year-old patients (CNBSR 2016), children who underwent the neuropsychological test were assigned a developmental quotient and divided into the normal developmental quotient and low developmental quotient subgroups. The classification standards were as follows: ≥ 130, excellent; 115–129, intelligent; 84–114, normal; 70–84, lower; ≤ 69, low. Scores ≥ 84 were considered normal, and scores < 84 were considered low. Among the 37 patients, the mean developmental quotient was 71.38 ± 20.83 (range, 18.2–94); 11 patients (29.7%) had a normal developmental quotient, and 26 patients (61.3%) had a low developmental quotient, as shown in Table 1.
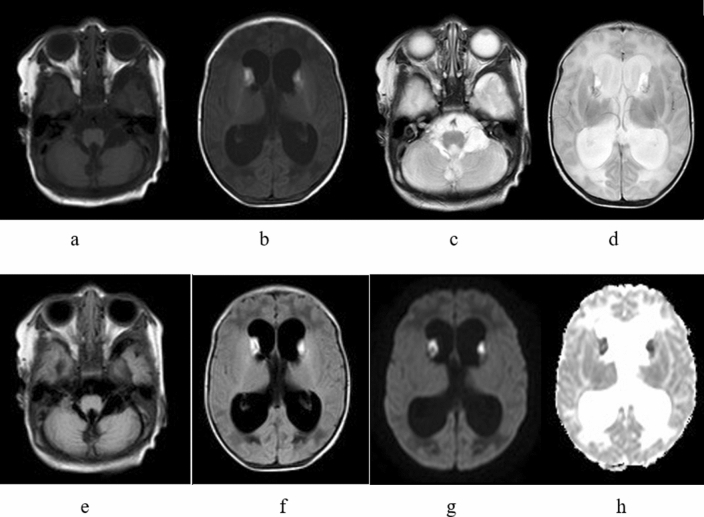
The MRI findings were ventricular dilation, cortical atrophy (sulcal widening), corpus callosal thinning, brainstem thinning, subcortical white matter changes, periventricular white matter changes and signal changes consistent with focal infarct. The most common findings were periventricular white matter changes (78.4%), ventricular dilation (29.7%) and cerebral atrophy (40.5%), which were the most significant and severe imaging manifestations (see Table 2 for details). Symmetrical signal abnormalities were found in the bilateral caudate nuclei of one patient, as shown in Fig. 1.
Table 2.
The MRI findings in 37 children with MMA.
| Patient | The group of by clinical screening | Cerebral sulcus widened | Cerebellar atrophy | Brainstem thinning | Corpus callosum thinning | Cortical atrophy (sulcal widening) | Ventricular dilation (0–3) | Subcortical white matter change | Periventricular white matter change | Internal capsule change | Signal change consistent with focal infarct | Abnormal signal in basal ganglia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SS | − | − | − | + | − | Severe | Frontal | − | − | − | − |
| 2 | SS | − | − | − | − | − | − | − | Mild | − | − | − |
| 3 | SS | + | + | − | + | + | Severe | − | Mild | − | − | − |
| 4 | SS | + | + | − | − | + | − | − | − | − | − | − |
| 5 | SS | + | − | − | − | − | − | − | Mild | − | − | − |
| 6 | SS | − | − | − | − | − | − | − | Mild | − | − | − |
| 7 | SS | + | − | − | − | + | − | − | Mild | − | − | − |
| 8 | SS | − | − | + | − | − | − | − | Mild | − | − | − |
| 9 | NS | − | − | − | − | − | − | − | Mild | − | − | − |
| 10 | SS | + | + | − | + | + | Moderate | Frontal | Mild | + | − | − |
| 11 | SS | + | − | + | + | + | − | Frontal and parietal | Mild | − | − | − |
| 12 | NS | − | − | − | − | − | − | − | Mild | − | − | − |
| 13 | SS | − | − | − | + | − | − | Parietal | Mild | − | − | − |
| 14 | NS | − | − | − | − | − | − | Parietal | Mild | − | − | − |
| 15 | NS | − | − | − | − | − | − | Diffuse | Mild | + | − | − |
| 16 | NS | + | − | − | − | − | − | − | − | − | − | − |
| 17 | NS | + | + | − | + | + | Mild | − | − | − | − | − |
| 18 | NS | − | − | − | − | − | − | − | Mild | − | − | − |
| 19 | NS | + | − | − | + | − | Severe | Parietal | Moderate | − | + | + |
| 20 | NS | − | − | − | − | − | − | − | Mild | − | + | − |
| 21 | NS | − | − | − | − | − | − | − | Mild | − | − | − |
| 22 | NS | − | − | − | − | − | − | − | Mild | − | − | − |
| 23 | NS | − | − | − | + | − | − | − | Mild | + | − | − |
| 24 | SS | − | − | − | − | − | − | − | Mild | − | − | − |
| 25 | NS | − | − | − | − | − | − | Parietal | Mild | − | − | − |
| 26 | NS | + | − | − | + | + | Mild | − | − | − | − | − |
| 27 | SS | + | − | − | − | + | − | Parietal | − | − | − | − |
| 28 | SS | + | + | + | + | + | Mild | Frontal and parietal | Moderate | − | − | − |
| 29 | NS | + | − | − | − | + | − | − | − | − | − | − |
| 30 | SS | + | + | + | + | + | Severe | Diffuse | Severe | + | − | − |
| 31 | NS | + | − | − | + | + | Moderate | Frontal and parietal | Mild | − | − | − |
| 32 | SS | − | − | − | − | − | − | − | Mild | − | − | − |
| 33 | SS | − | − | − | − | − | − | − | Mild | − | − | − |
| 34 | NS | + | − | − | − | − | − | Frontal and parietal | Mild | − | − | − |
| 35 | NS | + | + | + | + | + | Severe | Diffuse | Moderate | − | + | − |
| 36 | NS | − | − | − | + | + | − | − | Mild | − | − | − |
| 37 | SS | − | − | − | + | + | Severe | − | − | − | − | − |
Figure 1.
(a-h) (b, d, f, e, h); hydrocephalus with small subcerebellar vermis, consistent with the Dandy–Walker variant (patient no. 19. MRI showing T1WI, T2WI, T2WI-FLAIR and DWI hyperintensity in the bilateral caudate nuclei and hypointensity in the corresponding ADC images, consistent with hemorrhage a, c, e).
In this study, 19 patients were identified by newborn genetic screening for neonatal-based diseases after birth, and 18 patients were identified by blood and genetic screening after exhibiting suspected symptoms; these patients were divided into the NS group and the SS group, respectively. The main imaging findings showed no significant difference between the two subgroups, but the signal change consistent with focal infarct was nearly statistically significant (P = 0.051), as shown in Table 3.
Table 3.
Differences in MR imaging findings between MMA patients with different clinical subgroups.
| MR imaging finding | The group by clinical screening (N, %) | χ2 | P | |
|---|---|---|---|---|
| SS (18) | NS(19) | |||
| Cerebral sulcus widened | 9 (50.0) | 8 (42.1) | 0.232 | 0.746 |
| Cerebellar atrophy | 5 (27.8) | 2 (10.5) | 1.793 | 0.232 |
| Brainstem thinning | 4 (22.2) | 1 (5.3) | 2.275 | 0.180 |
| Corpus callosum thinning | 8 (44.4) | 7 (36.8) | 0.222 | 0.743 |
| Cortical atrophy (sulcal widening) | 9 (50.0) | 6 (31.6) | 1.301 | 0.325 |
| Ventricular dilation (0–3 grade) | ||||
| 0 | 12 (66.7) | 14 (73.7) | 1.128 | 0.770 |
| Mild | 1 (5.6) | 2 (10.5) | ||
| Moderate | 1 (5.6) | 1 (5.3) | ||
| Severe | 4 (22.2) | 2 (10.5) | ||
| Subcortical white matter change | 7 (38.9) | 7 (36.8) | 0.016 | 0.898 |
| Periventricular white matter change | ||||
| 0 | 4 (22.2) | 4 (21.1) | 1.347 | 0.718 |
| Mild | 12 (66.7) | 13 (68.4) | ||
| Moderate | 1 (5.6) | 2 (10.5) | ||
| Severe | 1 (5.6) | 0 | ||
| Internal capsule change | 2 (11.1) | 2 (10.5) | 0.003 | 0.954 |
| Signal change consistent with focal infarct | 0 | 3 (15.8) | 3.093 | 0.051 |
| Abnormal signal in basal ganglia | 1 | 0 | − | − |
In this study, 19 patients were identified by newborn genetic screening for neonatal-based diseases after birth, and 18 patients were identified by blood and genetic screening after exhibiting SS; these patients were divided into the NS group and the SS group, respectively. The main imaging findings were not significantly different between the two subgroups, but the signal change consistent with focal infarct was nearly statistically significant (P = 0.051), as shown in Table 3. Regarding sex, significantly more male patients than female patients had subcortical white matter changes (see Table 4, Fig. 2).
Table 4.
Differences in imaging findings between MMA patients with different gender subgroups.
| MR imaging finding | The group by gender (N, %) | χ2 | P | |
|---|---|---|---|---|
| Male (27) | Female (10) | |||
| cerebral sulcus widened | 14 (51.9) | 3 (30.0) | 1.403 | 0.288 |
| Cerebellar atrophy | 5 (18.5) | 2 (20.0) | 0.010 | 0.999 |
| Brainstem thinning | 4 (14.8) | 1 (10.0) | 0.145 | 0.704 |
| Corpus callosum thinning | 9 (33.3) | 6 (60.0) | 2.153 | 0.142 |
| Cortical atrophy (sulcal widening) | 10 (37.0) | 5 (50.0) | 0.509 | 0.476 |
| Ventricular dilation (0–3 grade) | ||||
| 0 | 20 (74.1) | 6 (60.0) | 0.922 | 0.820 |
| Mild | 2 (7.4) | 1 (10.0) | ||
| Moderate | 1 (3.7) | 1 (10.0) | ||
| Severe | 4 (14.8) | 2 (20.0) | ||
| Subcortical white matter change | 13 (48.1) | 1 (10.0) | 4.515 | 0.034 |
| Periventricular white matter change | ||||
| 0 | 6 (22.2) | 2 (20.0) | 1.456a | 0.885 |
| Mild | 17 (63.0) | 8 (80.0) | 1.345 | 0.764 |
| Moderate | 3 (11.1) | 0 | – | – |
| Severe | 1 (3.7) | 0 | – | – |
| Internal capsule change | 2 (7.4) | 2 (20.0) | 1.200 | 0.291 |
| Signal change consistent with focal infarct | 3 (11.1) | 0 | 1.209 | 0.272 |
| Abnormal signal in basal ganglia | 1 | 0 | – | – |
Figure 2.
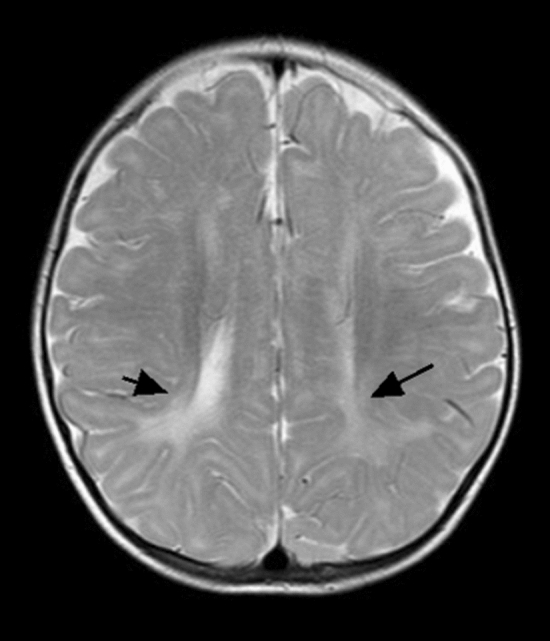
Patient no. 19. axial T2WI showing symmetrical hyperintensity in the matter of the posterior horn of bilateral ventricles.
According to developmental quotient, 37 patients were divided into the NI subgroup (developmental quotient ≥ 85) and the LI subgroup (developmental quotient less than 85). The incidence of corpus callosal thinning, cortical atrophy (sulcal widening), subcortical white matter changes, and ventricular dilation (0 to 3 grade) was significantly higher in the LI subgroup than in the NI subgroup (P < 0.05). The incidence of 0-mild and moderate-severe ventricular dilation was significantly higher in the LI subgroup than in the NI subgroup (P < 0.05), as shown in Table 5, Figs. 3 and 4.
Table 5.
Differences in imaging findings between MMA patients with different developmental quotient subgroups.
| MR imaging finding | The group by developmental quotient (N, %) | χ2 | P | |
|---|---|---|---|---|
| Normal (11) | Lower (26) | |||
| Cerebral sulcus widened | 4 (36.4) | 13 (50.0) | 0.579 | 0.447 |
| Cerebellar atrophy | 1 (9.1) | 6 (23.1) | 0.986 | 0.321 |
| Brainstem thinning | 1 (9.1) | 4 (15.4) | 0.262 | 0.609 |
| Corpus callosum thinning | 0 | 15 (57.7) | 10.673 | 0.001 |
| Cortical atrophy (sulcal widening) | 2 (18.2) | 13 (50.0) | 3.246 | 0.052 |
| Ventricular dilation (0–3 grade) | ||||
| 0–mild | 11 (100) | 18 (69.2) | 5.366 | 0.049a |
| Moderate–severe | 0 | 8 (30.8) | 4.318 | 0.038a |
| Subcortical white matter change | 1 (9.1) | 13 (50.0) | 5.500 | 0.019 |
| Periventricular white matter change | ||||
| 0 | 3 (27.3) | 5 (19.2) | 1.807 | 0.730a |
| Mild | 9 (81.8) | 16 (61.5) | ||
| Moderate | 0 | 3 (11.5) | ||
| Severe | 0 | 1 (3.8) | ||
| Internal capsule change | 0 | 4 (15.4) | 1.897 | 0.168 |
| Signal change consistent with focal infarct | 1 (9.1) | 2 (7.7) | 0.020 | 0.887 |
| Abnormal signal in basal ganglia | 0 | 1 (3.8) | – | |
aFisher's exact probability method is used.
Figure 3.
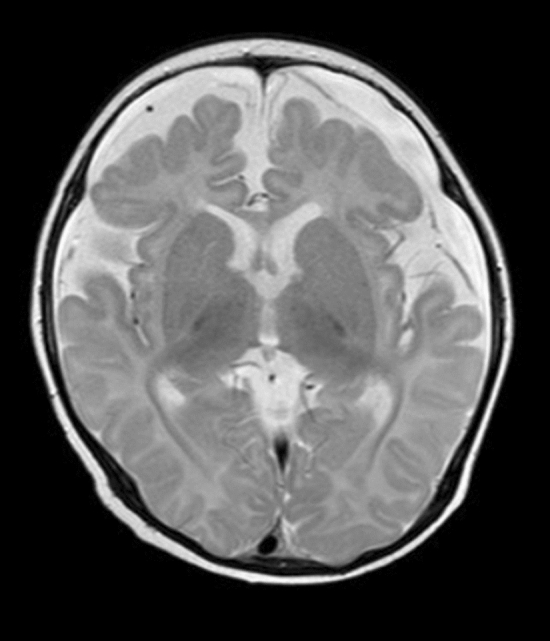
Patient no. 17. axial T2WI showing decreased bilateral frontal lobe volume and widened subarachnoid space.
Figure 4.
(a–d) Patient no. 30. MRI showing severe ventricular dilation and corpus callosal thinning (a, sagittal T2WI).
Finally, we analyzed the correlation between developmental quotient and ventricular dilation/periventricular white matter changes (grade 0–3) and found that the developmental quotient was significantly negatively correlated with the ventricular dilation grade, as shown in Table 6.
Table 6.
Correlation analysis between developmental quotient grouping and ventricular dilation/ periventricular white matter change (0–3 grade).
| MR imaging finding | Correlation coefficient | P |
|---|---|---|
| Ventricular dilation | − 0.546 | <0.001 |
| Periventricular white matter change | − 0.096 | 0.566 |
Discussion
MRI has become the preferred imaging examination method for newborns or infants because it uses no radiation and has features including high tissue contrast and high spatial resolution. Thirty-seven children with MMA underwent routine MRI examinations, which can be used to observe delayed myelin development or dysplasia and to evaluate brain development. The most common imaging findings in the MMA patients in this study were subcortical white matter changes (37.8%), periventricular white matter changes (78.4%), ventricular dilation (29.7%) and cerebral atrophy (40.5%). The autopsy report confirmed that neuropathological damage in MMA included encephalatrophy, reactive gliosis, hypomyelination, multifocal cerebellar hemorrhage, and depletion or hypodevelopment of the cerebellar external granule cells in children older than 10 months of age2,19. Compared with previous autopsy studies, the MRI findings in this study were similar to those in a previous study.
In 2008, 52 patients were reviewed and reported in a research paper20. The MRI findings included ventricular dilation, cortical atrophy, and periventricular white matter abnormalities. Similar to the imaging abnormalities in this study, the incidence rate of these features varies among studies. This may be due to the long time span of previous studies and the use of slightly inconsistent imaging methods. In this study, MRI manifestations were observed in patients at the same medical institution. According to the literature from the past 30 years, the main central nervous system manifestations of MMA are as follows: prominence of the ventricles21, myelination abnormalities22, cortical atrophy and sulcal widening23,24, periventricular white matter abnormalities25, internal capsule changes26, basal ganglia changes 27, cerebellar atrophy, subcortical white matter changes, and thinning of the corpus callosum28–30.
Due to the large difference in the number of patients in the isolated MMA (i-MMA) and MMA combined with homocysteinemia (MMA-h) group, no comparisons were made regarding the imaging manifestations. In mainland China, combined MMA and homocysteinemia (cblC type) is the main type of MMA. MMA needs to be diagnosed by laboratory methods, and relatively high concentrations of methylmalonic acid and methyl citrate in patient urine, as determined by GC/MS, can lead to a definitive diagnosis of the disorder. Plasma homocysteine can be measured to identify the gene types involved in MMA13,31. Nineteen patients underwent newborn genetic screening for NS group, and 18 patients underwent blood and genetic screening after exhibiting SS group. We found that MMA, as a congenital metabolic disease, caused no significant differences in terms of imaging manifestations between the two groups of patients, whether MMA was found by early neonatal gene screening or MRI examinations after SS were observed. This finding indicates that MRI examination is an essential means of identifying central nervous system damage in MMA patients5,13. In this study, there were 27 male and 10 female patients, suggesting that the incidence of the disease may be different between the sexes. Because of the low incidence of the disease and the lack of imaging findings in both sexes for comparison, there were greater white matter changes in male patients with MMA than in female patients with MMA. The sex differences in the clinical manifestations and prognosis of patients should be fully considered.
No standardized assessments of the symptoms or neuropsychological status of patients have been conducted. One difference between this study and previous studies is that the table of neuropsychological development used in the examination of 0- to 6-year-old patients (CNBSR 2016) was applied in all 37 pediatric patients when MRI examinations were performed. The development quotient was calculated to evaluate the neuropsychological development status of the MMA patients (Table 1). The incidence of corpus callosal thinning, cortical atrophy, and ventricular dilation on imaging findings was higher in the LI subgroup than in the NI subgroup. Abnormal MRI findings provide a visual basis for understanding morphological changes in the impaired neuropsychological development of MMA patients. It is suggested that the correlation between the damage to the central nervous system and the neuropsychological developmental state of children with MMA can be evaluated based on MRI findings to objectively evaluate the curative effect of standard treatments.
There were 11 patients with ventricular dilation, of whom 8 children showed moderate to severe mental retardation, suggesting that ventricular dilation is an important imaging feature of neuropsychological developmental disorders in children with MMA. Previous studies in the literature have reported that ventricular dilatation is also common in MMA patients, and some scholars have suggested that its formation mechanism might be related to intracerebral vascular stiffness, causing the pliancy of arteries to decrease, arterial pressure to increase, arterial delivery pressure to remain the same, and intracerebral pressure to increase and leading to hydrocephalus32. The toxicity of high concentrations of cysteine metabolites to the vascular wall is the main cause of vascular endothelial damage33. In our study, follow-up imaging findings of MMA patients after treatment were lacking; these images could help to explain the correlation between changes in the degree of ventricular dilatation and neuropsychological scores after treatment. Longitudinal research with more than one MRI scan from each patient will be indispensable to verify this hypothesis.
We observed cerebral sulcal widening and cerebellar atrophy and found that the LI group had a markedly higher incidence of cortical atrophy (sulcal widening) than the NS group. According to previous reports, the most common imaging manifestation of MMA is cerebral atrophy20, which is characterized by different degrees of cerebral and cerebellar sulcal deepening, cortical atrophy, and widening of the extracerebral space. The cause of brain shrinkage is actually a reduction in the volume of the white matter, which is known as stunted growth. Cerebral atrophy can directly affect the development of the cerebral nervous system in newborns or infants. Diffuse supratentorial white matter edema, which was reported in an Italian study34, was not observed in our patients. In spite of reports of cerebellar hemorrhage in metabolic disorders35,36, there were no MRI findings of this complication in these patients. In this study, conventional MRI sequences were performed,however, susceptibility-weighted sequences that can more sensitively detect hemorrhage were not performed. Moreover, there were seven patients with cerebellar atrophy that could have conceivably resulted from cerebellar lesions. However, such findings were not significantly different among the different groups. There have been recent reports of hemorrhagic and necrotic foci in the brainstem as well as hypomyelination and spongy changes in the brainstem nuclei19. Five children showed brainstem atrophy, and four children showed internal capsular changes in this study; these incidence rates are higher than those observed in previous studies20.
The abnormal white matter changes in MMA patients are usually located in the anterior and posterior corners of both lateral ventricles and in the centrum semiovale with a symmetrical distribution. Mild delays in myelination can be difficult to identify on cerebral MRI; T2WI hyperintensity around the ventricles and subcortical white matter T2WI hyperintensity were observed. The incidence of subcortical white matter hyperintensity was significantly higher in the SS group (50%) than in the NS group (9%). The mechanism driving this result may be related to the brain S-adenosine methionine deficiency caused by metabolic abnormalities37. No children older than 1 year showed this manifestation, which is consistent with the results of a study by Brismar and Ozand showing that MMA leads to some delays in myelination rather than an “absent development”21. If myelin sheath abnormalities cannot be diagnosed and effectively treated at an early stage, the volume of white matter will decrease and lead to a poor prognosis. Corpus callosal thinning, which was reported by Enns et al.24, is common in patients with white matter changes, either acquired or hereditary. Abnormal MRI signals and corpus callosum thinning in bilateral ventricles and the periventricular white matter in the centrum semiovale may be related to myelin dysplasia or developmental delays38. Hypomyelination and delayed myelination have been found in patients with MMA. It is tempting to assume that callosal atrophy is a reflection of prevalent abnormal white matter changes in MMA patients.
The basal ganglia (especially the pallidum) were the most frequently affected areas in MMA patients. The formation mechanism may be related to the decreased activity of cytochrome C oxidase and succinic acid dehydrogenase, resulting in the accumulation of toxic organic acid metabolites and leading to damage to the basal ganglia with high energy requirements, especially the globus pallidus39–41. According to previous research, the basal ganglia were affected in MMA patients21,22,25,26. Both necrosis and hemorrhage have been reported. In the medical imaging literature, calcification of the basal ganglia has been repeatedly mentioned. Calcification could be the end result of either hemorrhage or necrosis27,28. In the acute attack stage of MMA, the globus pallidus showed low density on computed tomography (CT) images. MRI showed hypointensity on T1WI, hyperintensity on T2WI, hyperintensity on DWI, hypointensity on apparent diffusion coefficient (ADC) imaging and a symmetrical distribution. However, in our study, no abnormal signals were found in the globus pallidus, and a large sample of cases should be further examined. In our study, one patient (no. 19) presented symmetrical T1WI hyperintensity in the caudate nucleus, which showed DWI hyperintensity and corresponding ADC hypointensity (Fig. 1). The patient was diagnosed with bleeding by MRI findings, accompanied by severe ventricular dilatation, corpus callosal thinning, and white matter changes, with a developmental quotient of only 69.6. This patient should also be studied longitudinally to understand the relationship between changes in imaging characteristics and changes in the neuropsychological development scale through follow-up observation.
One strength of this study is that all patients underwent MRI examinations using the same MRI equipment at the same medical institution with the exact same scanning parameters. In future studies, we may consider incorporating quantitative susceptibility maps (QSM) into the diagnosis of basal ganglia lesions. It is possible that changes in the globus pallidus do not appear until advanced stages of the disease42. All patients underwent MRI examinations after neonatal genetic screening or after the appearance of SS of MMA. The examination period was early, which may be why no calcification was observed in the basal ganglia. Furthermore, considering the image thickness and the slice gap used in our MRI protocol, it is possible that tiny abnormalities in the basal ganglia were omitted.
Some limitations of our study also have to be mentioned. We did not explored the relationship between MRI abnormalities and mutation data or clinical metabolic that will be further elucidated in subsequent studies, and not fully considered consequences of an inborn error of metabolism (IEM) or effects due to delayed diagnosis and possible medical mismanagement. This study was a preliminary cross-sectional design study of brain manifestations MRI findings in patients with MMA, which is part of our overall IEM study. Furthermore, we continue to expand the sample size and the observations of the relationships between i-MMA and MMA-h patient imaging characteristics and neuropsychological scores. We will also apply multi-modal MRI [the brain structure of 3D T1WI, QSM, blood oxygen level-dependent (BOLD), magnetic resonance spectroscopy (MRS) etc.] in these studies to obtain additional morphological and functional data, and the relevant results will be published in subsequent studies. No follow-up data is a limitation of this study. The clinicians look forward to understanding the improvement of neuropsychological scores of patients with MMA. Parents are very eager to know the rehabilitation status of the patients to understand the changing trends of their conditions, which is important for the patients and their parents to establish confidence in the next treatment. In future studies, we should continue to follow up with the patients included in this study for many years to obtain neuropsychological scores and MRI data at multiple time points.
Conclusion
Ventricular dilatation, cerebral atrophy, white matter changes and corpus callosal thinning are the main MRI abnormalities in MMA patients, and these manifestations are significantly correlated with delayed development in children. The presence of relevant clinical and MRI findings should raise a high degree of suspicion of MMA in pediatric radiologists and pediatricians. MRI findings can be considered an important basis for determining the severity of MMA in individual patients and assessing prognosis.
Supplementary information
Acknowledgements
This work was supported by grants from the Technology Development Plan of Jinan (201301049, 201602206, 201907052) and the Medical and Health Science and Technology Development Project of Shandong Province (2016WS0529).
Author contributions
L.Y., B.G. and L.G. wrote the main manuscript text. X.L. prepared Figures 1-4. X.L. prepared the clinical data and imaging data. X.W. and L.G. revised the main manuscript text. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Linfeng Yang and Bin Guo.
Supplementary information
is available for this paper at 10.1038/s41598-020-70113-y.
References
- 1.Lindblad B, Lindblad BS, Olin P, Svanberg B, Zetterstrom R. Methylmalonic acidemia—A disorder associated with acidosis hyperglycinemia and hyperlactatemia. Acta Paediatr. Scand. 1968;57:417. doi: 10.1111/j.1651-2227.1968.tb07314.x. [DOI] [PubMed] [Google Scholar]
- 2.Cui YZ, Han JX, Zhou X, Cui Y, Han J. Methylmalonic acidemia: Current status and research priorities. Intractable Rare Dis. Res. 2018;7:73–78. doi: 10.5582/irdr.2018.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui SM, Mahoney MJ, Rosenberg LE. The natural-history of the inherited methylmalonic acidemias. Am. J. Hum. Genet. 1980;32:A119–A119. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 4.Matsui SM, Mahoney MJ, Rosenberg LE. The natural-history of the inherited methylmalonic acidemias. N. Engl. J. Med. 1983;308:857–861. doi: 10.1056/nejm198304143081501. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, et al. Clinical, biochemical, and molecular analysis of combined methylmalonic acidemia and hyperhomocysteinemia (cblC type) in China. J. Inherit. Metab. Dis. 2010;33:S435–S442. doi: 10.1007/s10545-010-9217-0. [DOI] [PubMed] [Google Scholar]
- 6.Fowler B, Leonard JV, Baumgartner MR. Causes of and diagnostic approach to methylmalonic acidurias. J. Inherit. Metab. Dis. 2008;31:350–360. doi: 10.1007/s10545-008-0839-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40:52–57. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrillocarrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J. Inherited Metab. Dis. 2012;35:91–102. doi: 10.1007/s10545-011-9364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J. Inherited Metab. Dis. 2012;35:91–102. doi: 10.1007/s10545-011-9364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QL, et al. Predictors of survival in children with methymalonic acidemia with homocystinuria in Beijing, China: A prospective cohort study. Indian Pediatr. 2015;52:119–124. doi: 10.1007/s13312-015-0584-3. [DOI] [PubMed] [Google Scholar]
- 11.Thompson GN, Christodoulou J, Danks DM. Metabolic stroke in methylmalonic acidemia. J. Pediatr. 1989;115:499–500. doi: 10.1016/S0022-3476(89)80867-4. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JL, Venditti CP. Methylmalonic and propionic acidemias: Clinical management update. Curr. Opin. Pediatr. 2016;28:682–693. doi: 10.1097/mop.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han BJ, et al. Clinical presentation, gene analysis and outcomes in young patients with early-treated combined methylmalonic acidemia and homocysteinemia (cb1C type) in Shandong province, China. Brain Dev. 2016;38:491–497. doi: 10.1016/j.braindev.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Naidu S, Moser HW. Value of neuroimaging in metabolic diseases affecting the CNS. Am. J. Neuroradiol. 1991;12:413–416. [PMC free article] [PubMed] [Google Scholar]
- 15.Weisfeld-Adams JD, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol. Genet. Metab. 2010;99:116–123. doi: 10.1016/j.ymgme.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue NC, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: Data from the Cardiovascular Health Study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 17.Wahlund LO, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 18.18Altman, D. G. Practical Statistics for Medical Research. 1st edn (Chapman and Hall, London, 1991).
- 19.Kanaumi T, Takashima S, Hirose S, Kodama T, Iwasaki H. Neuropathology of methylmalonic acidemia in a child. Pediatr. Neurol. 2006;34:156–159. doi: 10.1016/j.pediatrneurol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Radmanesh A, et al. Methylmalonic acidemia: brain imaging findings in 52 children and a review of the literature. Pediatr. Radiol. 2008;38:1054–1061. doi: 10.1007/s00247-008-0940-8. [DOI] [PubMed] [Google Scholar]
- 21.Brismar J, Ozand PT. CT and NR of the brain in disorders of the propionate and methylmalonate metabolism. Am. J. Neuroradiol. 1994;15:1459–1473. [PMC free article] [PubMed] [Google Scholar]
- 22.Biancheri R, et al. Cobalamin (Cbl) C/D deficiency: Clinical, neurophysiological and neuroradiologic findings in 14 cases. Neuropediatrics. 2001;32:14–22. doi: 10.1055/s-2001-12217. [DOI] [PubMed] [Google Scholar]
- 23.Gebarski SS, Gabrielsen TO, Knake JE, Latack JT. Cerebral CT findings in methylmalonic and propionic acidemias. Am. J. Neuroradiol. 1983;4:955–957. [PMC free article] [PubMed] [Google Scholar]
- 24.Enns GM, et al. Progressive neurological deterioration and MRI changes in cblC methylmalonic acidaemia treated with hydroxocobalamin. J. Inherit. Metab. Dis. 1999;22:599–607. doi: 10.1023/a:1005517727451. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich R, et al. Acute extrapyramidal syndrome in methylmalonic acidemia - Metabolic stroke involving the globus pallidus. J. Pediatr. 1988;113:1022–1027. doi: 10.1016/s0022-3476(88)80574-2. [DOI] [PubMed] [Google Scholar]
- 26.Desousa C, Piesowicz AT, Brett EM, Leonard JV. Focal changes in the globi-pallidi associated with neurological dysfunction in methylmalonic acidemia. Neuropediatrics. 1989;20:199–201. doi: 10.1055/s-2008-1071292. [DOI] [PubMed] [Google Scholar]
- 27.Korf B, Wallman JK, Levy HL. Bilateral lucency of the globus-pallidus complicating methylmalonic acidemia. Ann. Neurol. 1986;20:364–366. doi: 10.1002/ana.410200317. [DOI] [PubMed] [Google Scholar]
- 28.Yesildag A, et al. Magnetic resonance imaging and diffusion-weighted imaging in methylmalonic acidemia. Acta Radiol. 2005;46:101–103. doi: 10.1080/02841850510020888. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi M, et al. Magnetic resonance imaging and spectroscopy in a patient with treated methylmalonic acidemia. J. Comput. Assist. Tomogr. 2003;27:547–551. doi: 10.1097/00004728-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Pui M, Ahmad MJN. Magnetization transfer imaging diagnosis of intracranial tuberculomas. Neuroradiology. 2002;44:210–215. doi: 10.1007/s002340100693. [DOI] [PubMed] [Google Scholar]
- 31.Han LS, et al. Clinical features and MUT gene mutation spectrum in Chinese patients with isolated methylmalonic acidemia: Identification of ten novel allelic variants. World J. Pediatr. 2015;11:358–365. doi: 10.1007/s12519-015-0043-1. [DOI] [PubMed] [Google Scholar]
- 32.Greitz D, Greitz T, Hindmarsh T. A new view on the CSF-circulation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr. 2010;86:125–132. doi: 10.1111/j.1651-2227.1997.tb08850.x. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann W, Knapp JP. Hyperhomocysteinemia: A new risk factor for degenerative diseases. Clin. Lab. 2002;48:471. [PubMed] [Google Scholar]
- 34.Rossi A, et al. Early-onset combined methylmalonic aciduria and homocystinuria: Neuroradiologic findings. Am. J. Neuroradiol. 2001;22:554–563. [PMC free article] [PubMed] [Google Scholar]
- 35.Prafull D, Curless RG, Steinman L. Cerebellar hemorrhage complicating methylmalonic and propionic acidemia. Neurology. 1982;32:A217–A217. doi: 10.1212/WNL.32.2.217. [DOI] [PubMed] [Google Scholar]
- 36.Dave P, Curless RG, Steinman L. Cerebellar hemorrhage complicating methylmalonic and propionic acidemia. Arch. Neurol. 1984;41:1293–1296. doi: 10.1001/archneur.1984.04050230079025. [DOI] [PubMed] [Google Scholar]
- 37.Weisfeld-Adams JD, et al. Neurologic and neurodevelopmental phenotypes in young children with early-treated combined methylmalonic acidemia and homocystinuria, cobalamin C type. Mol. Genet. Metab. 2013;110:241–247. doi: 10.1016/j.ymgme.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 38.39Yeilda, A., et al. Magnetic resonance imaging and diffusion-weighted imaging in methylmalonic acidemia. Acta Radiol.46, 101–103 (2005). [DOI] [PubMed]
- 39.Federica D, Sara B, Santorelli FM, Carlo DV. Methylmalonic and propionic aciduria. Am. J. Med. Genet. Part C Semin. Med. Genet. 2010;142C:104–112. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- 40.Michel SJ, Given CA, Robertson WC. Imaging of the brain, including diffusion-weighted imaging in methylmalonic acidemia. Pediatr. Radiol. 2004;34:580–582. doi: 10.1007/s00247-004-1155-2. [DOI] [PubMed] [Google Scholar]
- 41.Baker EH, et al. MRI characteristics of globus pallidus infarcts in isolated methylmalonic acidemia. AJNR Am. J. Neuroradiol. 2015;36:194–201. doi: 10.3174/ajnr.A4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinh BC, Melhem ER, Barker PB. Multi-slice proton MR spectroscopy and diffusion-weighted imaging in methylmalonic acidemia: Report of two cases and review of the literature. Am. J. Neuroradiol. 2001;22:831–833. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.