Abstract
Background
Hospitalization of patients with opioid use disorder (OUD) is increasing, yet little is known about opioid agonist therapy (OAT: methadone and buprenorphine) administration during admission.
Objective
Describe and examine patient- and hospital-level characteristics associated with OAT receipt during hospitalization in the Veterans Health Administration (VHA).
Participants
A total of 12,407 unique patients, ≥ 18 years old, with an OUD-related ICD-10 diagnosis within 12 months prior to or during index hospitalization in fiscal year 2017 from 109 VHA hospitals in the continental U.S.
Main Measure
OAT received during hospitalization.
Key Results
Few admissions received OAT (n = 1914; 15%) and when provided it was most often for withdrawal management (n = 834; 7%). Among patients not on OAT prior to admission who survived hospitalization (n = 10,969), 2.0% (n = 203) were newly initiated on OAT with linkage to care after hospital discharge. Hospitals varied in the frequency of OAT delivery (range, 0 to 43% of qualified admissions). Patients with pre-admission OAT (adjusted odds ratio [AOR] = 15.30; 95% CI [13.2, 17.7]), acute OUD diagnosis (AOR = 2.3; 95% CI [1.99, 2.66]), and male gender (AOR 1.52; 95% CI [1.16, 2.01]) had increased odds of OAT receipt. Patients who received non-OAT opioids (AOR 0.53; 95% CI [0.46, 0.61]) or surgical procedures (AOR 0.75; 95% CI [0.57, 0.99]) had decreased odds of OAT receipt. Large-sized (AOR = 2.0; 95% CI [1.39, 3.00]) and medium-sized (AOR = 1.9; 95% CI [1.33, 2.70]) hospitals were more likely to provide OAT.
Conclusions
In a sample of VHA inpatient medical admissions, OAT delivery was infrequent, varied across the health system, and was associated with specific patient and hospital characteristics. Policy and educational interventions should promote hospital-based OAT delivery.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-05815-0) contains supplementary material, which is available to authorized users.
KEY WORDS: opioid agonist therapy, methadone, buprenorphine, hospital medicine, opioid use disorder
INTRODUCTION
Surging opioid-related hospitalizations challenge the acute care delivery system in the United States (U.S.).1 Opioid-related hospitalizations are associated with increased readmissions2 and 12% of patients admitted with an OUD-related condition leave the hospital against medical advice.3 OUD-related admissions disproportionately burden public payers3,4 and cost more than non-opioid-related admissions.3,5 Opioid agonist therapy (OAT)—buprenorphine or methadone6—is provided infrequently and variably during hospitalization7 or upon discharge.8 Underutilization occurs although OAT delivery during hospitalization is feasible9,10 and OAT receipt is associated with decreased illicit opioid use upon discharge,9 reduced 30- and 90-day readmissions,11 and increased post-hospital substance use disorder (SUD) treatment engagement.11,12
Veterans are particularly vulnerable as they are twice as likely to die from accidental opioid overdose than non-veterans.13 OUD diagnoses within the Veterans Health Administration (VHA) have increased nearly twofold between 2004 (n = 30,093)14 and 2017 (n = 54,078).15 The VHA’s initiatives to increase OAT access16 include a system-wide requirement that all VHA facilities provide access to OAT16 and follow national OUD treatment guidelines.17 In fiscal year 2017, 41% (n = 22,179) of VHA patients with OUD received an OUD-related pharmacotherapy.15 At the facility-level, OUD-related pharmacotherapy delivery ranged from 2 to 76% across the VHA system.15 To date, little is known about inpatient practice for this patient population. This multi-hospital retrospective study examines variation in OAT delivery and receipt for hospitalized VHA patients with OUD.
METHODS
Study Design and Cohort
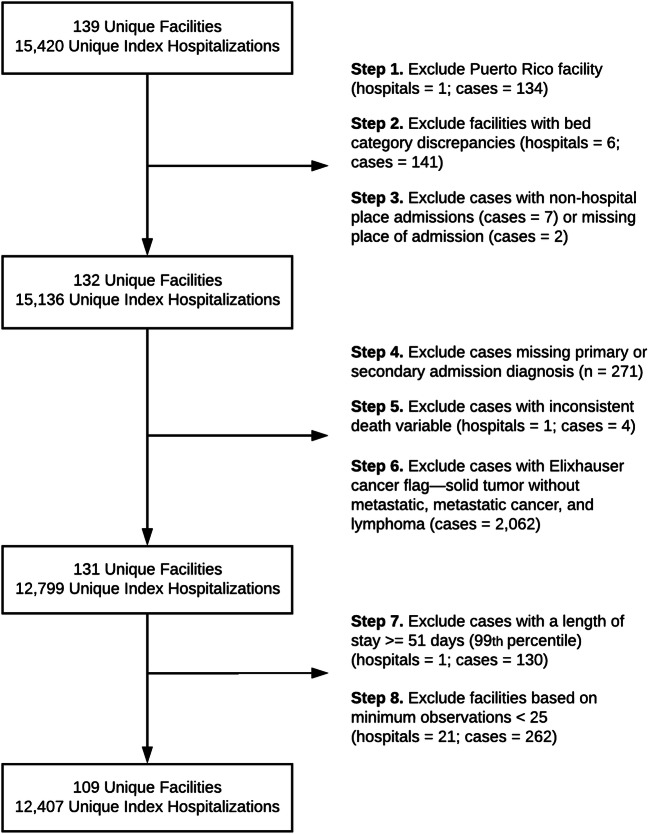
A retrospective sample of unique acute medical and surgical inpatient admissions from fiscal year 2017 was extracted from the VHA Corporate Data Warehouse, a database containing national VHA patient electronic health record data. Eligible individuals were aged 18 or older with a primary or secondary OUD ICD-10 diagnosis from any source (inpatient, outpatient, and community care paid for by VHA) in the year preceding index hospitalization or during index hospitalization in fiscal year 2017. Facilities were restricted to “acute care hospitals” with at least 500 acute bed days of care delivered during the study period and at least 25 index admissions. Patients were excluded if they did not have a primary or secondary admission code, if they had an Elixhauser cancer flag,18 and if they had a hospital length of stay (LOS) greater than or equal to the 99th percentile (median 82 days, range 51 to 1652 days). The 99th percentile LOS cutoff was chosen to capture those admitted with OUD-related infections warranting 4 to 6 weeks of inpatient antibiotics and to exclude chronic hospitalization (see Fig. 1). The Veterans Affairs Portland Health Care System Institutional Review Board approved this study (no. 4045).
Figure 1.
Study sample selection.
Study Variables
Variable selection and construction were informed by the existing literature, data availability, and prior qualitative research.19,20 Study variables included patient demographics (age, gender, race, ethnicity), patient diagnoses present on admission (co-occurring mental health and SUD diagnoses), admission characteristics (intensive care unit [ICU] or surgical services received), and admission-related diagnoses (OUD-related infection, OUD-related diagnoses). Hospital characteristics included admission volume, acute OUD diagnosis volume (the percentage of admissions with an acute OUD diagnosis during hospitalization), hospital geographic location, and hospital size.
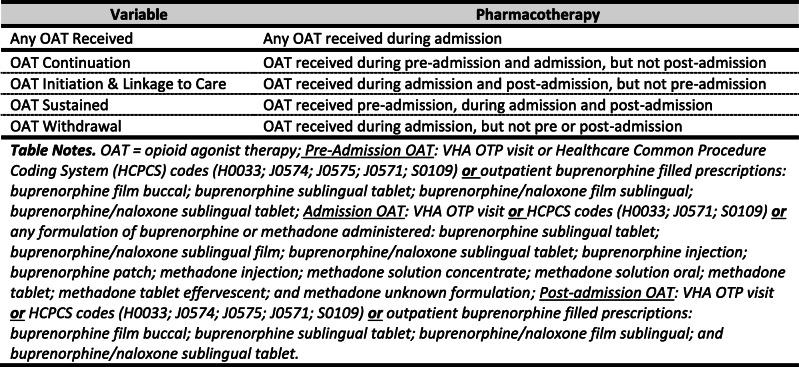
Pharmacotherapy variables were coded for three time periods: (1) 30 days pre-admission; (2) during admission; and (3) 30 days post-admission. Non-OAT pharmacotherapy included benzodiazepines, non-OAT opioids (e.g., oxycodone), naltrexone, naloxone, inpatient use of first-line opioid withdrawal adjuvant (clonidine), and second-line withdrawal adjuvants (baclofen, gabapentin/pregabalin, tizanidine) (see Appendix Table 1 for additional details). Admission OAT was categorized by four OAT delivery scenarios (OAT continued, OAT initiated with linkage to care, OAT for withdrawal, and OAT sustained) (see Table 1). Categories and calculations involving post-admission care excluded patients who died during admission.
Table 1.
Hospital OAT Delivery by Scenario
Statistical Analysis
RStudio21–27 was used for data management and descriptive statistics. Multilevel logistic regression modeling used Stata28 with an alpha value of 0.05. The dependent variable (level 1) was any OAT received (yes/no) during hospitalization, and covariates were level 1 (patient) and level 2 (hospital) continuous, binary, or categorical variables (see Appendix Table 2 for model covariates). Covariate inclusion was based on literature review, study aims, and model fit. Comparative model fit for nested models used the log-likelihood ratio test, the Akaike information criterion, and the Bayesian information criterion. Regression coefficients, standard errors, adjusted odds ratios (AOR) with 95% confidence intervals, and the intraclass correlation coefficient (ICC) were reported. A sensitivity analysis examined whether a narrower OAT administration definition during hospitalization changed study findings.
RESULTS
Patient Characteristics
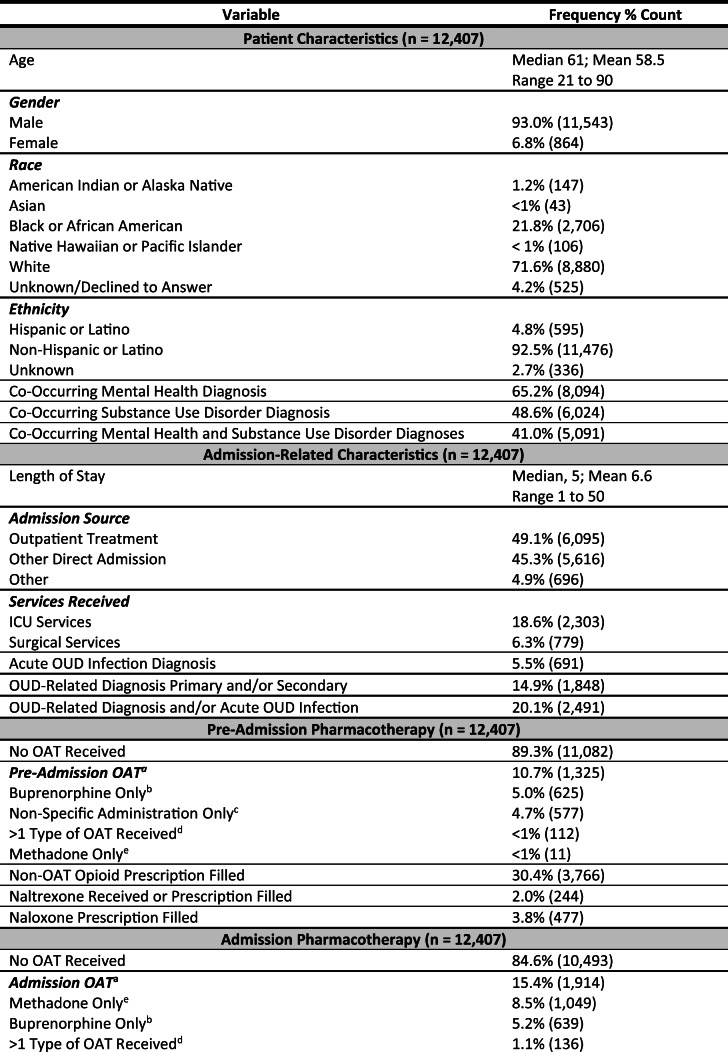
The study cohort included 12,407 unique patients with index hospitalizations from 109 VHA acute care hospitals in the continental U.S. Most patients were male (n = 11,543; 93%), white (n = 8880; 72%) or Black (n = 2706; 22%), and non-Hispanic or Latino (n = 11,476; 93%) with a median age of 61 years (range 21 to 90). Over half of the patients (n = 8094; 65%) had at least one co-occurring mental health diagnosis and nearly half had at least one co-occurring SUD diagnosis (n = 6024; 49%).
Admission-Related Characteristics
The median length of hospital stay was 5 days (range 1 to 50 days). Nearly 20% of the patients (n = 2303) received ICU services and 6% (n = 779) received surgical services. OUD-related infection or primary or secondary OUD-related diagnoses occurred in 20% of admissions (n = 2491) and 1% of the patients died during admission (n = 119).
Pre- and Post-pharmacotherapy
Approximately one in ten patients received OAT in the 30 days prior to admission (n = 1325; 11.5%) and in the 30 days post-hospital discharge (n = 1420; 11.6%). Thirty percent of patients (n = 3766) had an opioid prescription filled in the 30 days before admission and 35% (n = 4250) filled an opioid prescription in the 30 days after discharge.
Admission Pharmacotherapy
The majority of patients did not receive OAT during admission (n = 10,493; 85%). In the 15% of admissions with OAT (n = 1914), methadone was more common (n = 1049; 55%) than buprenorphine (n = 639; 33%). A small number of patients (n = 136; 7.1%) received more than one type of OAT and 4.7% (n = 90) had non-specific administration. For patients on OAT prior to admission (n = 1325), 65% (n = 867) had their OAT continued during admission and 35% (n = 458) had their OAT discontinued during admission—regardless of receipt post-admission.
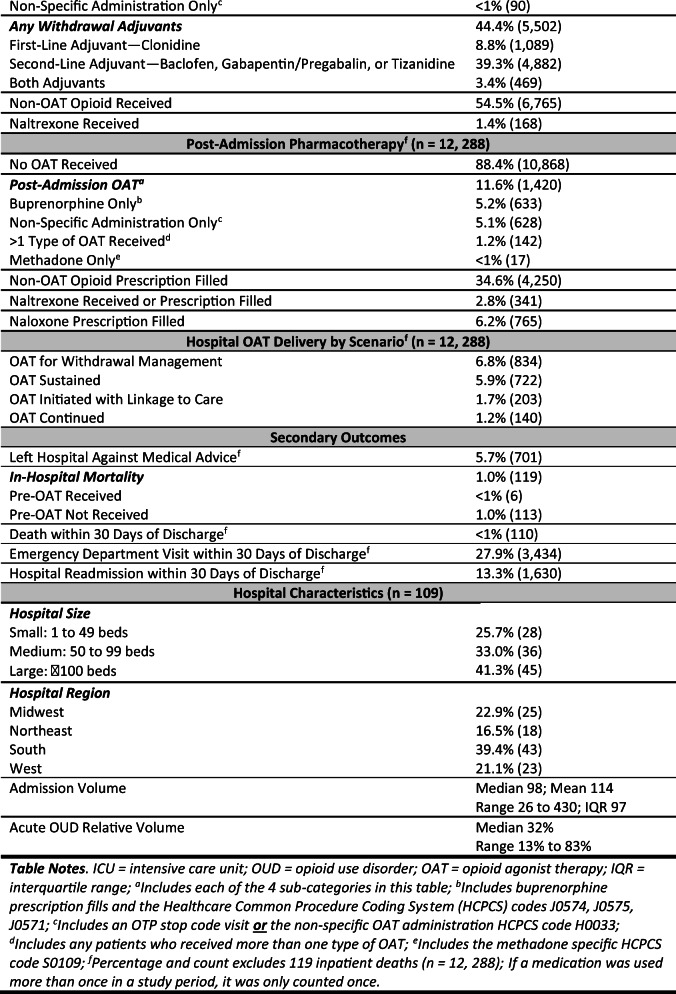
Related to withdrawal management, 9% of patients received the VHA’s recommended first-line adjuvant for opioid withdrawal—clonidine—and 39% (n = 1089) received a second-line adjuvant (baclofen, gabapentin/pregabalin, or tizanidine).29 Over half of the patients (55%; n = 6765) received at least one non-OAT opioid (e.g., oxycodone) during admission.
Hospital OAT by Delivery Scenario
When hospital OAT was delivered for patients not on pre-admission OAT, it was most often provided as withdrawal management (n = 834; 44%) and infrequently initiated during hospitalization with linkage to care upon discharge (n = 203; 11%). For patients on pre-admission OAT, it was most often delivered as a sustained medication (n = 722; 38%)—OAT received before, during, and after admission—and less often continued during admission, but with subsequent discontinuation after discharge (n = 140; 8%) (see Table 2 for additional details on patient and admission-related characteristics and pharmacotherapy).
Table 2.
Patient, Admission, and Hospital Characteristics
System-Wide OAT Delivery
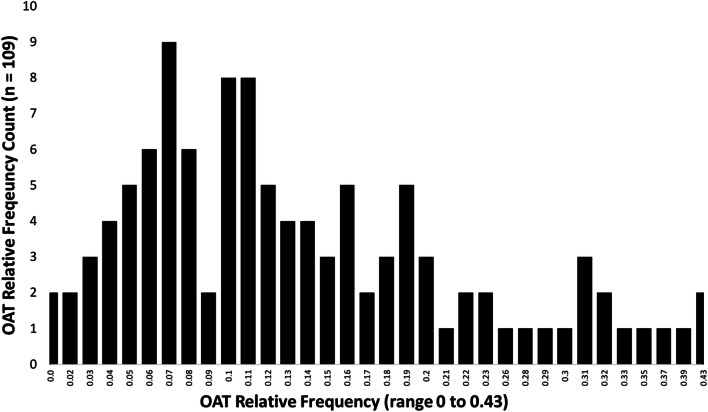
Across the 109 VHA hospitals, the median OAT delivery frequency during admission was 11% (SD 0.10; range 0 to 43%). The data had a non-normal distribution skewed towards less OAT delivery (see Fig. 2). The frequency of OAT delivery scenario in each hospital varied. For example, two hospitals did not provide any OAT, and nearly half of the hospitals (48%; n = 52) did not have a single admission in which OAT was initiated with linkage to care (range 0 to 16 admissions). Measures of variation (ICC) are reported in Appendix Table 3.
Figure 2.
Hospital OAT delivery relative frequency histogram.
Hospital- and Patient-Level Associations with OAT Receipt
Patient-Level Covariates
In the fully specified model, 13 covariates were associated with hospital OAT receipt. Six covariates increased the odds of receiving OAT during hospitalization: pre-admission OAT receipt (AOR 15.3; 95% CI [13.2, 17.7]); an OUD diagnosis or OUD-related infection during admission (AOR 2.30; 95% CI [1.99, 2.66]); male gender (AOR 1.52; 95% CI [1.16, 2.01]); receipt of adjuvant medication for opioid withdrawal during admission (AOR 1.52; 95% CI [1.32, 1.75]); an opioid withdrawal diagnosis (AOR 1.47; 95% CI [1.12, 1.92]); and an increased length of hospital stay (AOR 1.04; 95% CI [1.03, 1.05]). Seven covariates were associated with decreased odds of OAT receipt: the receipt of pre-admission naltrexone (AOR 0.26; 95% CI [0.12, 0.56]); an unintentional overdose diagnosis (AOR 0.29; 95% CI [0.16, 0.52]); the receipt of admission naltrexone (AOR 0.31; 95% CI [0.14, 0.66]); pre-admission non-OAT opioid receipt (AOR 0.49; 95% CI [0.41, 0.58]); non-OAT opioid receipt during admission (AOR 0.53; 95% CI [0.46, 0.61]); surgical service receipt during admission (AOR 0.75; 95% CI [0.57, 0.99]); and having a co-occurring SUD diagnosis (AOR 0.77; 95% CI [0.67, 0.88]) (see Appendix Table 4).
Hospital-Level Covariates
Four hospital-level covariates were associated with hospital OAT receipt. Patients admitted to large-sized (AOR 2.04; 95% CI [1.39, 3.00]) and medium-sized (AOR 1.90; 95% CI [1.33, 2.70]) hospitals had increased odds of OAT receipt compared with small hospitals. Patients admitted to hospitals located in the northeast (AOR 1.80; 95% CI [1.30, 2.49]) and west (AOR 1.62; 95% CI [1.19, 2.22]) had increased odds of OAT receipt compared with those in the south (see Table 3 for the fully specified model output).
Table 3.
Multilevel Logistic Regression Model: Hospital OAT Delivery
| Parameters | B | SE | AOR | 95% CI | |
|---|---|---|---|---|---|
| Intercept | − 2.78*** | 0.00 | 0.04 | 0.03 | 0.13 |
| Patient-level covariates | |||||
| Age | 0.00 | 0.08 | 1.00 | 1.00 | 1.01 |
| Gender: male (ref. female) | 0.42** | 0.18 | 1.52 | 1.16 | 2.01 |
| Race: non-white (ref. white) | − 0.05 | 0.14 | 0.95 | 0.82 | 1.11 |
| Race: unknown (ref. white) | − 0.18 | 0.21 | 0.84 | 0.59 | 1.19 |
| Ethnicity: Hispanic (ref. non-Hispanic) | 0.02 | 0.07 | 1.02 | 0.77 | 1.34 |
| Ethnicity: unknown (ref. non-Hispanic) | 0.17 | 0.07 | 1.2 | 0.80 | 1.81 |
| Acute OUD diagnosis/infection | 0.83*** | 0.07 | 2.30 | 1.99 | 2.66 |
| Co-occurring substance use disorder diagnosis | − 0.26*** | 0.30 | 0.77 | 0.67 | 0.88 |
| Co-occurring mental health diagnosis | − 0.04 | 0.14 | 0.97 | 0.84 | 1.11 |
| Unintentional overdose diagnosis during admission | − 1.24*** | 0.00 | 0.29 | 0.16 | 0.52 |
| Opioid withdrawal diagnosis during admission | 0.38** | 0.08 | 1.47 | 1.12 | 1.92 |
| Hospital length of stay | 0.04*** | 0.14 | 1.04 | 1.03 | 1.05 |
| ICU services received during admission | − 0.13 | 0.14 | 0.88 | 0.74 | 1.03 |
| Surgical services received during admission | − 0.29* | 0.08 | 0.75 | 0.57 | 0.99 |
| Pre-admission OAT received | 2.73*** | 0.12 | 15.3 | 13.1 | 17.7 |
| Pre-admission non-OAT opioid prescription filled | − 0.72*** | 0.38 | 0.49 | 0.41 | 0.58 |
| Pre-admission benzodiazepine prescription filled | 0.03 | 0.08 | 1.03 | 0.81 | 1.32 |
| Pre-admission naltrexone received or prescription filled | − 1.33*** | 0.00 | 0.26 | 0.12 | 0.56 |
| Pre-admission gabapentin/pregabalin prescription filled | − 0.12 | 0.14 | 0.89 | 0.77 | 1.04 |
| Admission source: other (ref. outpatient) | − 0.13 | 0.07 | 0.88 | 0.66 | 1.16 |
| Admission source: direct (ref. outpatient) | − 0.01 | 0.07 | 0.99 | 0.85 | 1.15 |
| During admission non-OAT opioid received | − 0.64*** | 0.07 | 0.53 | 0.46 | 0.61 |
| During admission adjuvant received | 0.42*** | 0.39 | 1.52 | 1.32 | 1.75 |
| During admission benzodiazepine received | − 0.08 | 0.08 | 0.92 | 0.80 | 1.06 |
| During admission naltrexone received | − 1.17** | 0.09 | 0.31 | 0.14 | 0.66 |
| Hospital-level covariates | |||||
| Acute OUD diagnoses volumea | − 0.02** | 0.01 | 0.98 | 0.97 | 0.99 |
| Hospital size: medium (ref. small) | 0.64*** | 0.18 | 1.90 | 1.33 | 2.70 |
| Hospital size: large (ref. small) | 0.71*** | 0.20 | 2.04 | 1.39 | 3.00 |
| Census region: midwest (ref. south) | 0.24 | 0.16 | 1.27 | 0.93 | 1.72 |
| Census region: northeast (ref. south) | 0.59*** | 0.17 | 1.80 | 1.30 | 2.49 |
| Census region: west (ref. south) | 0.48** | 0.16 | 1.62 | 1.19 | 2.22 |
| Admission volume | 0.00 | 0.00 | 1.00 | 1.00 | 1.00 |
Bold indicates statistical significance; p < 0.05*; p < 0.01**; p < 0.001***
aThis relatively small effect size likely has little practical relevance, thus, was omitted from the manuscript text
Sensitivity Analysis
A pre-specified sensitivity analysis examined if a narrower OAT definition would change study findings, specifically, removing non-FDA-approved formulations of OAT for the treatment of OUD (e.g., injectable methadone) from the admission OAT variable. In this sensitivity analysis, surgical service received during admission was no longer statistically significant. No other differences were observed with statistical significance or the directionality of the relationships between covariates and the primary outcome (see Appendix Table 5).
DISCUSSION
This retrospective cohort analysis of 12,407 VHA hospitalized individuals with a past year or admission-related ICD-10 OUD diagnosis suggests that hospital OAT receipt was rare. This study is the first to examine hospital OAT delivery across multiple hospitals. A limited research base has examined hospital OAT delivery. A 10-year (2004 to 2014) retrospective chart review of patients with intravenous drug use–associated infective endocarditis—from a single Massachusetts hospital—observed that only 11% of OUD admissions had plans for OAT or naltrexone at discharge.7 Our national study confirms and expands upon these findings. Only 15% of patients in our study received any OAT during admission (n = 1914) and the vast majority (91%) of patients not on OAT prior to admission did not receive any OAT during hospitalization (n = 10,035).
Further, only 203 patients in our cohort were initiated on OAT and subsequently linked to care—a nationally recommend best-practice.6,30 Instead, patients naïve to OAT in the 30 days prior to admission were more likely to receive OAT for withdrawal management (n = 834; 7%), representing a missed opportunity to continue OUD treatment beyond hospitalization. Moreover, hospital admission interrupted ongoing outpatient OUD treatment. Over a third of patients had their outpatient OAT discontinued during admission. Contemporary guidance strongly recommends continuing outpatient OAT during admission, including for patients who are undergoing surgical procedures.6,31 It is possible that evolving practice recommendations were not reflected in our sample. Anesthesiologists may recommend buprenorphine discontinuation prior to surgery. However, only 6% of our cohort received surgery and only 12% of those patients were on OAT prior to admission; thus, it is unlikely that this is driving observed findings. Moreover, OAT discontinuation during admission may reflect challenges related to care transitions.20
Previous research in the VHA describes system-wide variation in OUD-related pharmacotherapy delivery, which ranged from 2 to 76% of qualified patients per facility.15 That analysis, however, did not examine variation specifically for hospital admissions. Our study aligns with and builds upon these prior findings. We observed OAT delivery frequency per hospital ranging from 0 to 43% of qualified admissions.
Prior VHA research suggests that specific patient characteristics are positively associated with OAT receipt including male gender, age 56 years or older, and those without a co-occurring mental health diagnosis.32 Gender disparities in SUD treatment and engagement have been described in other care delivery settings.33 Our study builds upon these findings, suggesting that women-identified patients may be less likely to receive OAT during hospitalization. Associations from this study also suggest that outpatient OAT preceding hospitalization influences subsequent hospital OAT delivery, highlighting the importance of OAT engagement prior to admission. Further, specific care received during admission decreased OAT receipt; for example, patients who received non-OAT opioids or surgical procedures were less likely to receive OAT during hospitalization. These two clinical scenarios should not influence hospital OAT administration. Finally, immutable hospital characteristics (e.g., size and location) influenced OAT delivery and may reflect unmeasurable internal hospital attributes (e.g., resources or culture).20 These findings could also reflect the contribution of elements outside the hospital, for example, local beliefs about addiction and availability of community-based treatment resources.20
Study Limitations
This is an observational, unmatched, retrospective cohort study; thus, causal relationships cannot be established. There are limitations to the generalizability of study findings because of the cohort (Veterans, older, white, mostly men) and the health system setting (VHA); however, given that the VHA is an integrated health system that has prioritized OAT delivery, it is possible that VHA OAT delivery outperforms non-VHA hospitals. We elected to include patients with an OUD diagnosis in the prior year, not just patients with an admission diagnosis; thus, for 80% of patients, OUD was not the primary reason for hospitalization. The pragmatic study sample selection may be seen as a limitation, but we believe it reflects the realities of acute care delivery for patients hospitalized with complications related to OUD and other chronic illnesses. Patients in our cohort may have been misclassified with an OUD diagnosis and thus were not valid OAT candidates. The challenges of using diagnosis-based denominators for cross-facility comparisons are discussed elsewhere.34 Conversely, OUD is also underdiagnosed and eligible patients may have been inadvertently excluded. Further, our study only includes VHA pharmacotherapy data; thus, it is possible that patients received OAT after discharge at a non-VHA facility. Nonetheless, this is unlikely to significantly influence our results because there were only six cases with post-admission non-VHA OAT receipt in the original data extraction. Another study limitation was our inability to discern why OAT was not delivered (e.g., patients may have declined OAT). Finally, the study data are from 2017, a specific moment in time that does not capture potential changes in practice over time.
Implications for Practice, Research, and Policy
The findings from this study may motivate practice improvement, future research, and inform policy to increase hospital-based OAT delivery in the midst of the opioid-related overdose crisis.
Practice Improvement
National authorities recommend OAT continuation or initiation in the hospital6 and a National Academies of Science, Engineering, & Medicine consensus report concluded that: “Withholding or failing to have available all classes of FDA-approved medication for the treatment of opioid use disorder in any care or criminal justice setting is denying appropriate medical treatment”(p. 3).30 Further, hospitalization is a reachable moment for treatment initiation and engagement.35,36 Unfortunately, our study suggests that hospital OAT delivery frequency may be far from optimal. The current practice is not only a missed opportunity for treatment engagement, but may also cause harm by disrupting life-saving care.
A recent systematic review suggests that the provision of addiction-related services for hospitalized patients with OUD improves patient, provider, and health care outcomes.37 Interventions to improve OAT delivery may include an organizational intervention—the addiction consult service (ACS), which provides clinical, educational, and policy-based addiction services and programming in the hospital.38 ACSs, however, are unlikely to be available or feasible across all hospitals. Further, it is likely that many hospital providers have limited addiction training39 and are less confident in providing OAT and delivering other OUD-related services. To address this issue, the VHA and other national hospital decisionmakers could publish specific guidance promoting evidence-based addictions hospital care40 or create educational campaigns encouraging hospital-based OAT delivery. These initiatives would likely need to address provider knowledge gaps and addiction-related stigma,20,41 describe pathways to OAT after discharge, and identify policies impeding care inside and outside the hospital setting.20
Future Research
Research should explore barriers to OAT initiation during hospitalization at the VHA and reasons for practice variation at patient, hospital, and system levels. Given the VHA’s prioritization of OUD treatment, it is possible that the VHA may be outperforming non-VHA hospitals. Future research may confirm this impression. Policymakers and researchers need to consider data access issues. One of the primary challenges to studying hospital OAT delivery is the widespread use of diagnosis-related groupings (DRG) in hospital billing. DRG billing allows hospitals to bill payers through a bundled payment algorithm to account for illness acuity.42 Study replication using Medicaid claims data, for example, is not feasible because most admission-related medications are not captured in the bundled claims data.
Policy Interventions
The VHA has already mandated national standards to enhance services for patients with OUD. Additional policy interventions outside the VHA may be warranted. Policies requiring all hospitals to offer OAT could be leveraged through hospital-related accrediting bodies (e.g., the Joint Commission). At present, there are no accreditation requirements related to hospital care for persons with OUD and SUDs. It is within the authority of the Joint Commission to require reporting and performance measurement for OAT and to mandate addiction-related technologies for hospital accreditation (e.g., presence of addiction physicians or ACS). Another approach is local legislation. In August 2018, the Massachusetts legislature passed House Bill 4866, Prevention and Access to Appropriate Care and Treatment of Addiction,43which requires Massachusetts’ emergency departments to offer OAT for patients with an opioid overdose and to link them to outpatient services.43 Similar policies could be created for inpatient service delivery. Finally, there is interest in reforming restrictive federal OAT policies, specifically, to abolish buprenorphine X-waiver requirements.44
CONCLUSIONS
In a retrospective, unmatched pragmatic VHA patient cohort, hospital OAT delivery varied widely, was infrequently delivered, and was most commonly administered as a continued outpatient medication or for withdrawal management. This study is the first multisite description of hospital OAT delivery and reveals characteristics that require further exploration to understand how to increase OAT access to patients hospitalized with OUD.
Electronic Supplementary Material
(DOCX 66.1 kb)
(DOCX 36.1 kb)
Acknowledgments
Dr. Priest’s dissertation committee.
Funding Information
This study was funded by the National Institute on Drug Abuse (F30 DA044700, R33 DA035640, UG1 DA015815), the Greenlick Family Scholarship Fund, and the United States Department of Veterans Affairs Health Services Research & Development (IK2HX001516). The funding organizations were not involved in the design of the study, data collection, data analysis, the interpretation of data, or writing of the manuscript.
Compliance with Ethical Standards
The Veterans Affairs Portland Health Care System Institutional Review Board approved this study (no. 4045).
Conflict of Interest
Dr. Lovejoy reports grants from VA Health Services Research & Development during the conduct of the study and grants from National Institutes of Health outside the submitted work. Dr. Priest reports grants from National Institutes of Health and the Greenlick Family Scholarship Fund during the conduct of the study. Drs. Englander, McCarty, and Shull have nothing to disclose.
Disclaimer
The contents of the manuscript are those of the authors and do not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
Prior Presentations
Dr. Priest’s dissertation defense (February 2019); AMERSA’s Annual Conference (November 2019); and OHSU Family Medicine Grand Rounds (November 2019).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weiss AJ, Elixhauser A, Barret ML, Steiner CA, Bailey MK, O’Malley L. HCUP statistical brief #219: Opioid-related inpatient stays and emergency department visits by state, 2009–2014. Agency for Healthcare Research and Quality;2016. [PubMed]
- 2.Peterson C, Liu Y, Xu L, Nataraj N, Zhang K, Mikosz CA. US National 90-Day Readmissions After Opioid Overdose Discharge. Am J Prev Med. 2019. doi: 10.1016/j.amepre.2018.12.003. [DOI] [PMC free article] [PubMed]
- 3.Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832–837. doi: 10.1377/hlthaff.2015.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss AJ, Heslin KC. HCUP statistical brief #239: Payers of opioid-related inpatient stays and emergency department visits nationally and by state, 2010 and 2015. Agency for Healthcare Research and Quality;2018. [PubMed]
- 5.Weiss AJ, Elixhauser A. HCUP statistical brief #180: Overview of hospital stays in the United States, 2012. Agency for Healthcare Research and Quality;2014. [PubMed]
- 6.Substance Abuse and Mental Health Services Administration. Treatment Improvement Protocol 63: Medications for opioid use disorder. 2018.
- 7.Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2015;129(5):481–485. doi: 10.1016/j.amjmed.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Naeger S, Ali MM, Mutter R, Mark T, Hughey L. Prescriptions filled following an opioid-related hospitalization. Psychiatr Serv. 2016;67(11):1262–1264. doi: 10.1176/appi.ps.201500538. [DOI] [PubMed] [Google Scholar]
- 9.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Internal Medicine. 2014;174(8):1369–1376. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services – Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1–5. doi: 10.1016/j.jsat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019. doi: 10.1097/ADM.0000000000000499. [DOI] [PubMed]
- 12.Englander H, Dobbertin K, Lind BK, et al. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: A propensity matched analysis. J Gen Intern Med. 2019;34(12). doi: 10.1007/s11606-019-05251-9. [DOI] [PMC free article] [PubMed]
- 13.Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393–396. doi: 10.1097/MLR.0b013e318202aa27. [DOI] [PubMed] [Google Scholar]
- 14.Oliva EM, Trafton JA, Harris AH, Gordon AJ. Trends in opioid agonist therapy in the Veterans Health Administration: Is supply keeping up with demand? Am J Drug Alcohol Abuse. 2013;39(2):103–107. doi: 10.3109/00952990.2012.741167. [DOI] [PubMed] [Google Scholar]
- 15.Finlay AK, Binswanger IA, Timko C, et al. Facility-level changes in receipt of pharmacotherapy for opioid use disorder: Implications for implementation science. J Subst Abuse Treat. 2018;95:43–47. doi: 10.1016/j.jsat.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Substance abuse. 2018;39(2):139–144. doi: 10.1080/08897077.2018.1452327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Veterans Affairs. VA/DoD clinical practice guidelines: Management of substance use disorder (SUD). 2017; https://www.healthquality.va.gov/guidelines/mh/sud/. Accessed 2/9/2020.
- 18.Agency for Healthcare Research and Quality. Beta Elixhauser Comorbidity Software for ICD-10-CM. 2018; https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp#description. Accessed 2/9/2020.
- 19.Priest KC. Hospital-based services for opioid use disorder: A study of supply-side attributes. Dissertations and Theses. 2019;Paper 4829. doi: https://pdxscholar.library.pdx.edu/open_access_etds/4829
- 20.Priest KC, Englander H, McCarty D. “Now hospital leaders are paying attention”: A qualitative study of internal and external factors influencing addiction consult services. J Subst Abuse Treat. 2020;110:59–65. doi: 10.1016/j.jsat.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RStudio: Integrated development for R. [computer program]. Boston, MA RStudio, Inc.; 2015.
- 22.Car [computer program]. Thousand Oaks, CA: SAGE Publications; 2011.
- 23.Dunn.test: Dunn’s Test of multiple comparisons using rank sums [computer program]. 2017.
- 24.icd: Comorbidity calculations and tools for ICD-9 and ICD-10 codes [computer program]. 2018.
- 25.Wickham H. plyr: The split-apply-combine strategy for data analysis. Journal of Statistical Software. 2011;40(1). doi: 10.18637/jss.v040.i01
- 26.Psych: Procedures for personality and psychological research [computer program]. Evanston, IL: Northwestern University; 2018.
- 27.tidyverse: Easily install and load the ‘Tidyverse’. [computer program]. 2017.
- 28.Stata Statistical Software: Release 15 [computer program]. College Station, TX: StataCorp LLC; 2017.
- 29.U.S. Department of Veterans Affairs. VHA opioid taper decision tool. 2016.
- 30.National Academies of Sciences, Engineering, and Medicine. Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press; 2019. [PubMed]
- 31.Haber L, D’eFries T, Martin M. Things We Do for No Reason™: Discontinuing Buprenorphine When Treating Acute Pain. J Hosp Med. 2019;14(10):633. doi: 10.12788/jhm.3265. [DOI] [PubMed]
- 32.Oliva EM, Harris AHS, Trafton JA, Gordon AJ. Receipt of opioid agonist treatment in the Veterans Health Administration: Facility and patient factors. Drug Alcohol Depend. 2012;122(3):241–246. doi: 10.1016/j.drugalcdep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Lind BK, McCarty D, Gu Y, Baker R, McConnell JK. Predictors of substance use treatment initiation and engagement among adult and adolescent Medicaid recipients. Substance abuse. 2019:1–7. doi: 10.1080/08897077.2018.1550467. [DOI] [PMC free article] [PubMed]
- 34.Harris AHS, Rubinsky AD, Hoggatt KJ. Possible alternatives to diagnosis-based denominators for addiction treatment quality measures. J Subst Abuse Treat. 2015;58:62–66. doi: 10.1016/j.jsat.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339–342. doi: 10.12788/jhm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: A qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296–303. doi: 10.1007/s11606-016-3919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weimer M, Morford K, Donroe J. Treatment of opioid use disorder in the acute hospital setting: A critical review of the literature. Current Addiction Reports. 2014;2019:1–16. doi: 10.1007/s40429-019-00267-x. [DOI] [Google Scholar]
- 38.Priest KC, McCarty D. Role of the hospital in the 21st Century opioid overdose epidemic: The addiction medicine consult service. J Addict Med. 2019;Mar/Apr 13(2):104–112. doi: 10.1097/ADM.0000000000000496. [DOI] [PMC free article] [PubMed]
- 39.Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752–758. 10.12788/jhm.2993. [DOI] [PubMed]
- 40.Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: Hospitalists’ role in addressing substance use disorder. J Hosp Med. 2019;Online October 2019. doi: 10.12788/jhm.3311. [DOI] [PMC free article] [PubMed]
- 41.Ashford RD, Brown AM, McDaniel J, Curtis B. Biased labels: An experimental study of language and stigma among individuals in recovery and health professionals. Substance use & misuse. 2019:1–9. doi: 10.1080/10826084.2019.1581221. [DOI] [PMC free article] [PubMed]
- 42.Quinn K. New directions in Medicaid payment for hospital care. Health Aff (Millwood). 2008;27(1):269–280. doi: 10.1377/hlthaff.27.1.269. [DOI] [PubMed] [Google Scholar]
- 43.WBUR News & Wire Services. Lawmakers send opioid bill to Baker’s desk. 2018; https://www.wbur.org/commonhealth/2018/08/01/opioid-legislation-to-governor. Accessed 2/9/2020.
- 44.Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: X the X waiver. JAMA Psychiatry. 2019;76(3):229–230. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 66.1 kb)
(DOCX 36.1 kb)