Abstract
Introduction
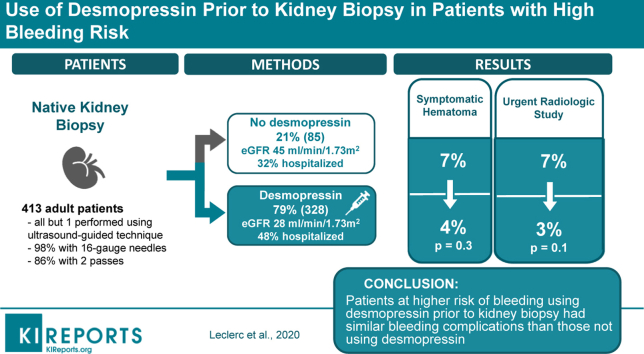
To prevent bleeding after native kidney biopsy (NKB), nephrologists often prescribe desmopressin, especially for patients with reduced estimated glomerular filtration rate (eGFR) at risk of uremia-related platelet dysfunction. However, only 1 randomized study has suggested a beneficial effect for desmopressin in patients with eGFR ≥60 ml/min per 1.73 m2. This retrospective cohort study aimed to evaluate desmopressin effect on postbiopsy bleeding in all patients, regardless of eGFR and other comorbidities.
Methods
In this retrospective cohort study, all adult patients who underwent an NKB from April 1, 2013, to April 30, 2018, in a tertiary hospital were identified. The association between desmopressin use and bleeding complications, including hemoglobin fall, transfusion, hematoma, symptomatic hematoma, urgent radiologic study, and hypotension, was analyzed using multivariable logistic regression models.
Results
A total of 413 native kidney biopsies were studied, 79% of which were performed after receiving desmopressin. Patients receiving desmopressin had worse chronic kidney disease (eGFR 28 vs. 45 ml/min per 1.73 m2; P < 0.001) and were more often hospitalized (48% vs. 32%; P = 0.009). Despite higher bleeding risk, patients using desmopressin had a similar likelihood of symptomatic hematomas (odds ratio [OR], 0.39; 95% confidence interval [CI], 0.13–1.14) and a lower need for urgent radiologic studies (OR, 0.33; 95% CI, 0.11–0.98).
Conclusion
Patients at higher risk of bleeding using desmopressin before kidney biopsy had bleeding complications similar to those not using desmopressin. These results highlight potential important clinical and financial benefits of desmopressin use before kidney biopsy.
Keywords: bleeding, complication, desmopressin, kidney biopsy, radiologic studies
Graphical abstract
NKB is essential to determine diagnosis1 and prognosis in many renal diseases. Since its first description in 1951,2 technical changes have lowered the bleeding risks of NKB. However, a recent prospective study3 showed that 8% of hospitalized patients with acute kidney injury (AKI) received transfusions and 2% needed angiographic intervention, outlining a still significant bleeding risk.
To prevent bleeding, nephrologists often prescribe desmopressin before the NKB. Also known as 1-deamino-8-D-arginine vasopressin (DDAVP), this synthetic derivative of the antidiuretic hormone vasopressin improves uremia-related platelet dysfunction by increasing levels of von Willebrand factor and factor VIII.4 Thus, some postulated that it lowers the bleeding risk in patients with reduced eGFR; however, only 1 randomized study5 has suggested a beneficial effect for desmopressin before NKB in patients with eGFR ≥60 ml/min per 1.73 m2, whereas current evidence in patients with eGFR <60 ml/min per 1.73 m2 is conflicting.6, 7, 8
The present study evaluates the use of desmopressin before NKB and its impact on bleeding and other complications, after controlling for recognized factors affecting bleeding risks, including kidney function at biopsy time.
Methods
Study Design
Patients with NKB performed from April 1, 2013, to April 30, 2018, at Maisonneuve-Rosemont Hospital, a single tertiary care teaching hospital in Montreal, were considered for this retrospective cohort study. Patients aged ≥18 years were included. All biopsies but allograft biopsies and those performed in another hospital were analyzed. No other exclusion criteria were used. The study was approved by our institution’s Research Ethics Committee, in agreement with the Declaration of Helsinki.
Biopsy Procedure
All NKBs but 1 were performed using an ultrasound-guided technique. Computed tomography scan was used in 1 case. A standard protocol was used for >95% of patients to prescribe hydration and i.v. desmopressin (0.3 μg/kg) if deemed necessary. Decision to use desmopressin or not was made by the attending nephrologist. Only 3 interventional radiologists did the procedures. Spring-loaded automated biopsy devices (Bio-Pince; Argon Medical Device, Frisco, TX) were used in 72% of NKBs and manual biopsy needles (Tru-Cut; Merit Medical, South Jordan, UT) were used in the remainder. The proportion of biopsies with manual needle was similar in study groups (25.3 vs. 24.7% for no desmopressin group; P = 0.76) and were all performed by the same radiologist. Needles were 16-gauge in 98% of NKBs, the remaining being 18-gauge. Most patients (86%) had 2 passes collected. When necessary, all patients stopped antiplatelet therapy and anticoagulation before the kidney biopsy, according to relevant guidelines.9 Outpatients were ambulatory patients admitted the morning of their NKB. Among outpatients, 95% were kept under observation until the medical visit the next day, and 5% were released on the same day. Inpatients had their NKB while already hospitalized for another reason. All patients had bed rest of ≥6 hours after the NKB, during which vital signs, diuresis, and urine color were monitored closely. No radiologic examination was performed routinely after the biopsy.
Data Collection
Data were collected by reviewing medical records, and included demographics, renal presentation, comorbidities, and medications. The morning before and after each NKB, vital signs, complete blood count, coagulation studies, and serum sodium and creatinine were noted. Data on the use of desmopressin and the NKB procedure itself were also extracted. Finally, any adverse events, including hemoglobin fall, transfusion, hematoma, symptomatic hematoma, hypotension, AKI, additional ultrasonography or radiologic study, and angio-embolization were collected. Data for all variables were missing in <5% of cases, except for post-NKB serum sodium, serum creatinine, and coagulation studies, which were missing in 9%, 9%, and 86% of cases, respectively.
Outcomes and Covariates
Chronic kidney disease was classified for eGFR level, estimated with the Chronic Kidney Disease–Epidemiology Collaboration equation,10 according to the Kidney Disease: Improving Global Outcomes Chronic Kidney Disease guidelines.11 Elevated blood pressure at the time of the NKB was diagnosed if systolic blood pressure was ≥140 mm Hg and diastolic blood pressure was ≥90 mm Hg.12 AKI was diagnosed and classified according to the Kidney Disease: Improving Global Outcomes AKI guidelines.13 Hypotension was diagnosed if 1 of the following was present <24 hours after NKB: (i) a systolic blood pressure <90 mm Hg, (ii) a mean arterial pressure <65 mm Hg, (iii) a fall in systolic blood pressure ≥40 mm Hg, (iv) orthostatic hypotension (a fall of systolic blood pressure ≥20 mm Hg and diastolic blood pressure ≥10 mm Hg while standing), or (v) the need for i.v. fluids or vasopressors.14 Hematomas were deemed symptomatic if they caused pain, hypotension, fall in hemoglobin, or need for transfusions. Postbiopsy ultrasonography was defined as an abdominal ultrasound performed <72 hours after the biopsy, prescribed by the interventional radiologist or the attending nephrologist. Some were performed by attending nephrologists in response to an urgent clinical situation, and others were prescribed for asymptomatic drop in hemoglobin levels. Urgent postbiopsy radiologic studies were defined as abdominal scans, angioscans, or angiographies that were obtained <72 hours after the biopsy, in all cases due to a clinical deterioration that needed urgent intervention. Such a clinical deterioration was defined as pain, hypotension, fall in hemoglobin, or need for transfusions. The median time between the biopsy and these studies was 23 hours with an interquartile range (IQR) of 9 to 31 hours. All patients undergoing angio-embolization needed an urgent postbiopsy radiologic procedure before their intervention.
Statistical Analysis
Baseline characteristics, clinical parameters, and complication frequencies were compared between patients who received and did not receive desmopressin using Wilcoxon test or t-tests for continuous variables and χ2 or Fischer tests for dichotomous variables. Subgroup analyses were performed, stratifying for inpatients versus outpatients. Multivariable logistic regressions were conducted to evaluate associations between desmopressin and different outcomes of interest (i.e., hemoglobin fall, transfusion, hematoma, symptomatic hematoma, hypotension, AKI, postbiopsy ultrasonography, or urgent radiologic study). We reported ORs with 95% CIs. All baseline characteristics, comorbidities, and clinical parameters were considered in the variable selection process if they had a significance of P ≥ 0.2 or appeared to be clinically significant. A sensitivity analysis was performed to examine the effect of using a propensity score of receiving desmopressin as covariate on logistic regression model OR estimates. All P values were 2 sided and considered statistically significant if P < 0.05. Statistical analysis was performed by N.E. using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
In total, 413 NKBs (187 female) were analyzed (Figure 1), with a median age at biopsy of 61 years (IQR, 45–70 years) (Table 1). Patients with all stages of chronic kidney disease were represented, including 11% on dialysis. Median eGFR at biopsy was 30 ml/min per 1.73 m2 (IQR, 16–62 ml/min per 1.73 m2). At time of NKB, 26% of patients had AKI (with 61% of Kidney Disease: Improving Global Outcomes stage 3). Patients who were biopsied while on dialysis had a suspicion of rapidly progressive glomerulonephritis. The median proteinuria was 3.0 g/d (IQR, 1–6 g/d) and 157 (38%) patients presented with a nephrotic syndrome. Hematuria was present in 198 (48%) patients. Important comorbidities included hypertension (73% of patients), monoclonal gammopathy (10%), and cirrhosis (2%). Only 1 patient had a bleeding disorder (hemophilia B). Kidney malformations were rare, with only 3 patients with a solitary kidney and 2 with horseshoe kidneys (Supplementary Table S1). The median platelet count was 225 ×109/l (IQR: 179–283 ×109/l) and only 4% of patients had a platelet count <100 ×109/l. Only 3 patients (0.7%) had an international normalized ratio >1.5 and the median international normalized ratio was 1.01 (IQR, 0.95–1.08). Only 10 patients (2%) had an activated partial thromboplastin time >40 seconds and the median activated partial thromboplastin time was 29.8 seconds (IQR, 27.4–32.3 seconds).
Figure 1.
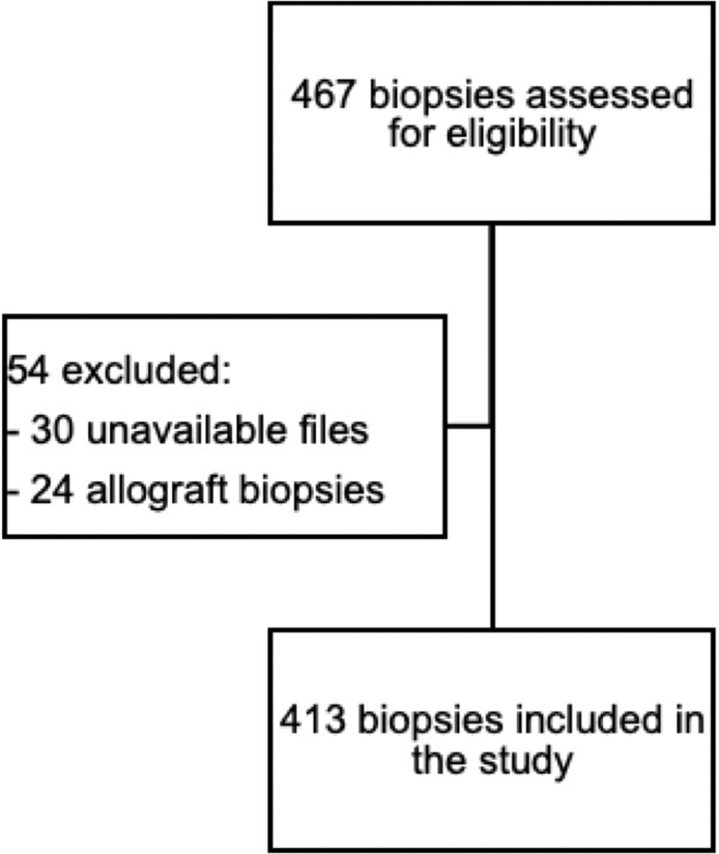
Study flow diagram.
Table 1.
Patient characteristics
| Variables | All patients (n = 413) | Without desmopressin (n = 85) | With desmopressin (n = 328) | P value |
|---|---|---|---|---|
| Age, yr | 61 (45–70) | 64 (51–69) | 60 (44–70) | 0.12 |
| Female | 187 (45) | 43 (51) | 144 (44) | 0.27 |
| Serum creatinine, mg/dl | 2.0 (1.2–3.4) | 1.4 (0.9–2.1) | 2.2 (1.3–3.6) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 30 (16–62) | 45 (28–82) | 28 (15–51) | <0.001 |
| CKD stage | 0.002 | |||
| 1 (≥90) | 57 (14) | 17 (20) | 40 (12) | |
| 2 (60–89) | 52 (13) | 16 (19) | 36 (11) | |
| 3a (45–59) | 47 (11) | 10 (12) | 37 (11) | |
| 3b (30–44) | 68 (16) | 18 (21) | 50 (15) | |
| 4 (15–29) | 93 (23) | 15 (18) | 78 (24) | |
| 5 (<15) | 94 (23) | 8 (9) | 86 (26) | |
| AKI | 106 (26) | 16 (19) | 90 (27) | 0.13 |
| Proteinuria, g/d | 3.0 (1.0–6.0) | 3.0 (2.0–7.0) | 3.0 (1.0–6.0) | 0.11 |
| Hematuria | 198 (48) | 37 (44) | 161 (49) | 0.32 |
| Hypertension | 302 (73) | 63 (74) | 239 (73) | 0.81 |
| Elevated blood pressure before biopsy | 166 (40) | 37 (44) | 129 (39) | 0.46 |
| Hemoglobin, g/dl | 11.2 (9.7–12.9) | 11.7 (10.3–13.5) | 11.0 (9.6–12.7) | 0.004 |
| Platelet, ×109/l | 225 (179–283) | 224 (193–281) | 226 (171–285) | 0.40 |
| INR | 1.01 (0.95–1.08) | 1.01 (0.95–1.07) | 1.01 (0.95–1.08) | 0.68 |
| aPTT, s | 29.8 (27.4–32.3) | 29.6 (27.3–31.9) | 29.8 (27.3–32.3) | 0.57 |
AKI, acute kidney injury defined according to Kidney Disease: Improving Global Outcomes guidelines; aPTT, activated partial thromboplastin time; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate using Chronic Kidney Disease–Epidemiology Collaboration equation; INR, international normalized ratio.
Data are expressed as a median (interquartile range) or n (percent).
Characteristics of Patients Receiving Desmopressin
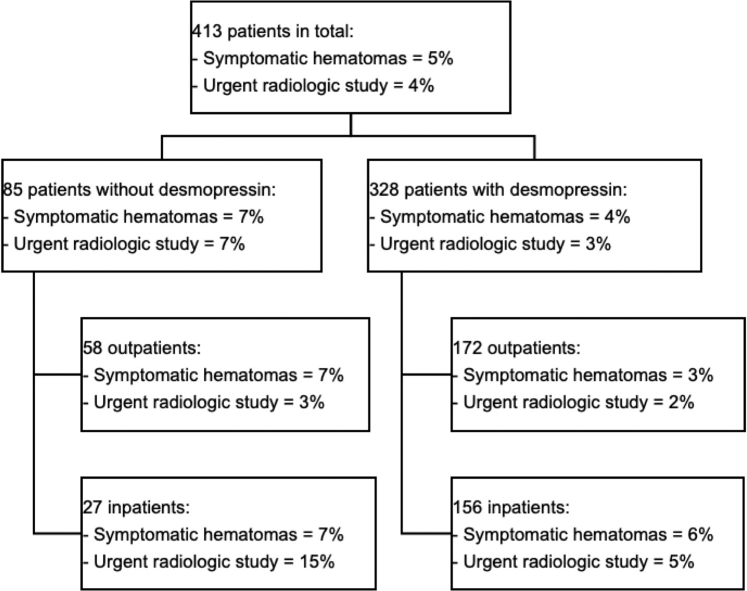
Of all NKBs, 328 (79%) were done after receiving desmopressin (Figure 2). Desmopressin was prescribed more often to hospitalized patients (48 vs. 32%; P = 0.009) and to those with lower eGFR (28 vs. 45 ml/min per 1.73 m2; P < 0.001) and lower hemoglobin level (11.0 vs. 11.7 g/dl; P = 0.004). It was prescribed less frequently to patients with suspected primary glomerulonephritis (56 vs. 50%; P = 0.01) (Supplementary Table S2). Kidney size was similar between the 2 groups (11.4 vs. 11.1 cm for the desmopressin group; P = 0.1).
Figure 2.
Bleeding complications.
Bleeding Complications
At 24 hours, a fall in hemoglobin ≥1.0 g/dl was experienced in 165 (40%) patients and a fall ≥2.0 g/dl in 31 (8%) patients (Table 2). The median fall in hemoglobin after 24 hours was 0.7 g/dl (IQR, 0.3–1.3 g/dl). A transfusion was needed in 21 (5%) patients. Forty-six patients (11%) developed hematoma, 20 (5%) symptomatic hematoma, 7 (2%) macroscopic hematuria, and 2 (<1%) urinary obstruction. Ultrasonography was needed after NKB in 23 (6%) patients and an urgent radiologic study in 17 (4%). Four (1%) patients underwent angio-embolization. After NKB, 39 (9%) patients were hypotensive. Hypotension lasted on average 3 hours. AKI presenting or worsening after the NKB was present in 2% of cases, with only 1 of those being of Kidney Disease: Improving Global Outcomes stage 3.
Table 2.
Complications postbiopsy
| Complications | All patients (n = 413) | Without desmopressin (n = 85) | With desmopressin (n = 328) | P value |
|---|---|---|---|---|
| Hemoglobin fall >1.0 g/dl | 165 (40) | 23 (27) | 142 (43) | 0.006 |
| Hemoglobin fall >2.0 g/dl | 31 (8) | 4 (5) | 27 (8) | 0.36 |
| Transfusion | 21 (5) | 2 (2) | 19 (6) | 0.81 |
| Hematoma | 46 (11) | 9 (11) | 37 (11) | 0.86 |
| Symptomatic hematoma | 20 (5) | 6 (7) | 14 (4) | 0.27 |
| Hypotension | 39 (9) | 8 (9) | 31 (9) | 0.87 |
| Acute kidney injury | 10 (2) | 1 (1) | 9 (3) | 0.69 |
| Ultrasonography postbiopsy | 23 (6) | 3 (4) | 20 (6) | 0.43 |
| Urgent postbiopsy radiologic study | 17 (4) | 6 (7) | 11 (3) | 0.13 |
Data are expressed as an absolute frequency (percent).
Desmopressin Use and Bleeding Complications
Unadjusted data showed a higher proportion of hemoglobin fall ≥1.0 g/dl in patients receiving desmopressin (43 vs. 27%; P = 0.006), but no difference in hemoglobin fall ≥1.5 g/dl, ≥2.0 g/dl, and ≥2.5 g/dl. Furthermore, there were no differences in hematoma, symptomatic hematoma, transfusion, and additional ultrasonography or radiologic study. When using multivariable logistic regressions adjusting for eGFR at biopsy time, desmopressin was associated with a hemoglobin fall ≥1.0 g/dl (OR, 2.34; 95% CI, 1.35–4.08), but once again not with a hemoglobin fall ≥1.5 g/dl, ≥2.0 g/dl, and ≥2.5 g/dl. Desmopressin use was associated with a lower likelihood of urgent radiologic studies (OR, 0.33; 95% CI, 0.11–0.98) and with a trend toward fewer symptomatic hematomas (OR, 0.39; 95% CI, 0.13–1.14) (Tables 3 and 4). Similarly, desmopressin use was associated with a hemoglobin fall ≥1.0 g/dl (OR, 2.65; 95% CI, 1.50–4.70), less urgent radiologic studies (OR, 0.27; 95% CI, 0.09–0.81), and a trend toward fewer symptomatic hematomas (OR, 0.41; 95% CI, 0.14–1.20) in our sensitivity analysis with propensity score adjustment. Other aforementioned complications, including hyponatremia and hypotension, were not statistically significantly associated with desmopressin use.
Table 3.
Multivariable correlates of symptomatic hematomas after biopsy in all patients
| Variable | Unadjusted OR |
Adjusted OR |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Female sex | 0.99 | 0.40–2.45 | 1.02 | 0.40–2.58 |
| Age | 0.97 | 0.94–0.99 | 0.96 | 0.93–0.98 |
| eGFR | 1.00 | 0.99–1.01 | 0.99 | 0.98–1.01 |
| Desmopressin | 0.59 | 0.22–1.58 | 0.39 | 0.13–1.14 |
CI, confidence interval; eGFR, estimated glomerular filtration; OR, odds ratio.
The model is adjusted for eGFR, age, and sex.
Table 4.
Multivariable correlates of urgent radiologic studies after biopsy in all patients
| Variable | Unadjusted OR |
Adjusted OR |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Female sex | 1.38 | 0.52–3.65 | 1.49 | 0.55–4.00 |
| Age | 1.00 | 0.97–1.03 | 0.98 | 0.96–1.01 |
| eGFR | 0.99 | 0.97–1.01 | 0.98 | 0.96–1.00 |
| Desmopressin | 0.46 | 0.16–1.27 | 0.33 | 0.11–0.98 |
CI, confidence interval; eGFR, estimated glomerular filtration; OR, odds ratio.
The model is adjusted for eGFR, age, and sex.
Inpatients Versus Outpatients
In total, 44% of all NKBs were performed during acute hospitalizations. Inpatients received desmopressin before 85% of NKBs, whereas outpatients received desmopressin 75% of the time (Figure 2). Inpatients receiving desmopressin had significantly lower eGFR (46% with eGFR of <15 ml/min per 1.73 m2) compared with inpatients not receiving desmopressin (27% with eGFR of <15 ml/min per 1.73 m2, P = 0.02). When adjusted for eGFR at the time of the biopsy, desmopressin was associated with a lower likelihood of urgent radiologic studies in hospitalized patients (OR, 0.21; 95% CI, 0.05–0.92). There was also a trend toward reduction in symptomatic hematomas in ambulatory patients (OR, 0.30; 95% CI, 0.07–1.20).
Other Risk Factors for Bleeding Complications
Female sex (OR, 2.27; 95% CI, 1.41–3.68) and higher serum creatinine (OR, 1.14; 95% CI, 1.01–1.29) were associated with hemoglobin fall >1.0 g/dl (Supplementary Table S3). A low platelet count or elevated international normalized ratio and activated partial thromboplastin time were not associated with hemoglobin fall. However, low platelets were associated with a higher risk of hematomas (OR, 1.61; 95% CI, 1.06–2.05). Transfusions were more frequent in patients with lower hemoglobin (OR, 2.87; 95% CI, 1.82–5.00) and higher serum creatinine on the day of NKB (OR, 1.64; 95% CI, 1.20–2.32). Higher erythrocyte mean corpuscular volume was associated with higher frequencies of hemoglobin fall >1.0 g/dl (OR, 1.42; 95% CI, 1.02–2.02), hemoglobin fall >2.0 g/dl (OR, 3.32; 95% CI, 1.78–6.42), symptomatic hematoma (OR, 3.39; 95% CI, 1.43–8.47), hypotension (OR, 2.79; 95% CI, 1.51–5.29), and AKI (OR, 3.03; 95% CI, 1.12–8.32). Being on dialysis and having AKI at the time of the NKB were the main variables associated with a longer hospital stay.
Discussion
This study showed that there are no differences in bleeding risk between patients receiving desmopressin pre-NKB and those who did not, despite a worse kidney function and a higher risk of bleeding in the desmopressin group. This study also showed a reduction in urgent postbiopsy radiologic studies prescribed in the first 72 hours for an acute clinical deterioration. This association was more pronounced in hospitalized patients.
The only randomized trial5 on the use of desmopressin before NKB was a single-center trial that included 162 low-risk patients with eGFR ≥60 mL/min/1.732. It showed a significant reduction in minor complications only (defined as hematoma <2 cm or macroscopic hematuria), but not in major complications (defined as hematoma ≥2 cm, transfusion, or angio-embolization). The clinical meaningfulness of <2 cm hematomas detected post-NKB is doubtful. Previous studies have shown that they are almost always present post-NKB if an ultrasonography or scan is performed routinely.1 Other authors studying NKB complications did not consider asymptomatic hematomas at all15 or only if they were ≥5 cm in size,3 because they were not associated with adverse outcomes. This explains why, in this study, postbiopsy ultrasounds were analyzed separately from other urgent radiologic studies. Postbiopsy ultrasounds tend to be prescribed by the radiologist who found the intervention more difficult than usual or by the nephrologist who had a small suspicion of bleeding. Some physicians in other centers even prescribe them routinely. Thus, those ultrasounds have a higher chance of finding minor asymptomatic hematomas with an unknown (and probably negligible) significance. Other radiologic procedures (e.g., scans and angioscans) are used when there is a severe postbiopsy clinical deterioration, as defined earlier in this article. Scans and angioscans are rarely, if ever, prescribed for asymptomatic patients or to follow asymptomatic hematomas. Furthermore, scans and angioscans were always prescribed before an angio-embolization when patients needed one. Thus, choosing to analyze these other radiologic studies separately allowed this study to highlight the effect of desmopressin on bleeding complications more severe than those evaluated by Manno et al.5
In addition, the fact that ultrasound and radiologic studies were compiled up to 72 hours after the biopsy allowed the identification of most significant complications. Indeed, in a study from Marwah and Korbet,16 98% of complications were observed in the first 24 hours, the only one being noted after that was 1 case of gross hematuria. Another study, from Simard-Meilleur et al.,15 showed that 100% of complications were seen in less than 8 hours in outpatients and 90% of complications were observed in less than 24 hours in inpatients. Those 2 studies show that using imagery studies at 72 hours was a reasonable outcome that allowed measurement of almost all significant complications in our population.
By going back to the aforementioned randomized trial by Manno and colleagues,5 it is obvious that it included only patients with eGFR ≥ 60 ml/min per 1.73 m2, who have a lower risk of bleeding and being affected by uremia-related platelet dysfunction. By opposition, our study examined any patient who received desmopressin, including most high-risk patients with eGFR <60 ml/min per 1.73 m2. Even in this high-risk population, it was able to show a reduction in a more significant clinical outcome (i.e., urgent radiologic studies prescribed in the context of clinical deterioration). The observed trend for a reduction in symptomatic hematomas consistent across all groups is also suggesting a small but beneficial effect associated with desmopressin use. Another study by Athavale et al.17 demonstrated a reduced likelihood of bleeding in high-risk patients, but a higher likelihood of bleeding in low-risk patients. However, by opposition to our study, they excluded patients with bleeding time >10 seconds, platelet count <50 × 109/l, international normalized ratio >1.5, or aPTT >40 seconds. One can hypothesize that the inclusion of sicker patients and a smaller number of events probably explain why our study did not show the same “positive” effect of desmopressin. In patients at lower risk of bleeding (with lower serum creatinine), we also observed in our cohort a greater proportion of hemoglobin fall >1.0 g/dl in the desmopressin group (which was the main driver of the composite outcome used in the study by Athavale and colleagues17), but our results did not suggest higher risks of other “hard” outcomes (e.g., symptomatic hematomas, hypotension, AKI), which is more in line with other studies like the randomized trial from Manno et al.5
An unexpected finding was the association between desmopressin and hemoglobin fall >1.0 g/dl that was in opposition with lack of association with hemoglobin fall >1.5 g/dl, >2.0 g/dl, and >2.5 g/dl. It is possible that this finding is due to hemodilution. As patients were fasting since midnight when they had their prebiopsy blood tests, they might have been mildly dehydrated and hemoconcentrated. During the procedure, most received i.v. fluids. Combining this with the antidiuretic effect of desmopressin probably explains why 40% of patients receiving desmopressin before NKB had a hemoglobin fall >1.0 g/dl due to hemodilution, far fewer had a more significant fall, most likely to be caused by real bleeding. This hypothesis is supported by the observation that patients receiving desmopressin had on average a greater post-NKB fall in natremia of −0.6 mmol/l (P = 0.03) and in creatinine of −0.2 mg/dl (P = 0.03) compared with patients not receiving desmopressin.
Desmopressin has previously been associated with cases of severe hyponatremia,6 often when patients were encouraged to drink large amounts of water post-NKB. In our study, a non–statistically significant association was found between desmopressin and hyponatremia (10% vs. 4%; P = 0.08); however, no patients had severe symptomatic hyponatremia and the average additional fall in natremia compared with patients not receiving desmopressin was −0.6 mmol/l. The clinical significance of this desmopressin-induced fall in natremia remains unclear.
In addition, our study allowed us to delineate different post-NKB bleeding risk factors. As previously reported,3,15,18,19 female sex, hypertension, and a higher creatinine were associated with hemoglobin falls. We also identified a risk factor for post-NKB complications that, to our knowledge, was not described in the literature. Higher erythrocyte mean corpuscular volume was strongly associated with hemoglobin fall, symptomatic hematoma, hypotension, and AKI. We hypothesized that elevated erythrocyte mean corpuscular volume might indicates reticulocytosis, and thus an ongoing cause for anemia that should be corrected, if possible, before undergoing NKB. This is an interesting finding, as a recent study has shown an association between all-cause mortality and high erythrocyte mean corpuscular volume in patients with chronic kidney disease.20 However, the exact related physiopathology remains unclear and speculative.
The biopsy technique was done in accordance with contemporary-era evidence-based practice.1 In our cohort, samples were sufficient for pathology diagnosis in 96.1% of cases and had 21 glomeruli on average, more than aimed for in guidelines.1 The post-NKB complication rate in our center was comparable to the average rates described in the literature.1,18
Our study has several strengths. First, our cohort is one of the largest in the literature to analyze the association between desmopressin use and post-NKB bleeding in the contemporary era. Second, all patients were included in our cohort, regardless of eGFR, comorbidities, or bleeding risk, which is more consistent with the current pattern of desmopressin use in clinical practice. Finally, the NKB procedure was standard for all patients and was executed by the same 3 interventional radiologists with contemporary techniques.
Unfortunately, our study had some limitations, mostly related to its retrospective design with no comparative cohort from another center. Most importantly, an indication bias played an important role because nephrologists prescribed desmopressin more often to patients at higher risk of bleeding. This probably reduced the magnitude of desmopressin effect on bleeding post-NKB. Furthermore, hemodilution was an important factor, as described earlier. In addition, despite the large number of patients undertaking NKB in our cohort, few experienced biopsy complications. Hence, it limited the power of our study to prove whether desmopressin has an impact on adverse events. Finally, hospitalized patients were monitored more closely and had more radiologic studies, sometimes performed for unrelated reasons. This obviously increases the chance of identifying non–clinically significant hematomas.
In conclusion, our study identified that patients receiving desmopressin before an NKB did not have a higher likelihood of bleeding despite being significantly more at risk. Furthermore, desmopressin significantly lowered the need for urgent radiologic studies for all patients and had a tendency to reduce symptomatic hematomas. These findings show that desmopressin might have important clinical and financial benefits when prescribed before NKB. However, a prospective study seems essential to clearly evaluate which patients benefit the most from desmopressin use with respect to bleeding complications.
Disclosure
All the authors declared no competing interests.
Acknowledgments
All expenses related to this study were paid by Division of Nephrology, Maisonneuve-Rosemont Hospital. LPL is a Fonds de recherche du Québec-Santé Junior 1 Scholar. A-CN-F is a Fonds de recherche du Québec-Santé Junior 1 Scholar. J-PL is a Fonds de recherche du Québec-Santé Junior 2 Scholar.
Footnotes
Table S1. Patient comorbidities and medication. Data are expressed as n (%). Patients receiving antiplatelet agents or anticoagulation stopped therapy before biopsy according to guidelines.
Table S2. Histologic diagnoses. Data are expressed as n (%).
Table S3. Risk factors for bleeding. OR, odds ratio; CI, confidence interval. This multivariate logistic regression model is adjusted for age, sex, desmopressin use, hypertension, creatinine, hemoglobin and mean corpuscular volume.
STROBE Checklist.
Supplementary Material
References
- 1.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iversen P., Brun C. Aspiration biopsy of the kidney. Am J Med. 1951;11:324–330. doi: 10.1016/0002-9343(51)90169-6. [DOI] [PubMed] [Google Scholar]
- 3.Moledina D.G., Luciano R.L., Kukova L. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13:1633–1640. doi: 10.2215/CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannucci P.M., Remuzzi G., Pusineri F. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8–12. doi: 10.1056/NEJM198301063080102. [DOI] [PubMed] [Google Scholar]
- 5.Manno C., Bonifati C., Torres D.D. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: a randomized controlled trial. Am J Kidney Dis. 2011;57:850–855. doi: 10.1053/j.ajkd.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Lim C.C., Siow B., Choo J.C.J. Desmopressin for the prevention of bleeding in percutaneous kidney biopsy: efficacy and hyponatremia. Int Urol Nephrol. 2019;51:995–1004. doi: 10.1007/s11255-019-02155-9. [DOI] [PubMed] [Google Scholar]
- 7.Peters B., Hadimeri H., Molne J. Desmopressin (Octostim(R)) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: a pilot study. Nephrology (Carlton) 2018;23:366–370. doi: 10.1111/nep.13004. [DOI] [PubMed] [Google Scholar]
- 8.Stratta P., Canavese C., Marengo M. Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest. 2007;37:954–963. doi: 10.1111/j.1365-2362.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 9.Douketis J.D., Spyropoulos A.C., Spencer F.A. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S–e350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summary of recommendation statements. Kidney Int Suppl. 2013;3:5–14. doi: 10.1038/kisup.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nerenberg K.A., Zarnke K.B., Leung A.A. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Summary of Recommendation Statements Kidney Int Suppl. 2012;2:8–12. doi: 10.1038/kisup.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaieski D.F., Mikkelsen M.E., Parsons P.E. Evaluation of and initial approach to the adult patient with undifferentiated hypotension and shock. In: Kluwer W., editor. UpToDate. UpToDate; Waltham, MA: 2019. [Google Scholar]
- 15.Simard-Meilleur M.C., Troyanov S., Roy L. Risk factors and timing of native kidney biopsy complications. Nephron Extra. 2014;4:42–49. doi: 10.1159/000360087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwah D.S., Korbet S.M. Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? Am J Kidney Dis. 1996;28:47–52. doi: 10.1016/s0272-6386(96)90129-8. [DOI] [PubMed] [Google Scholar]
- 17.Athavale A., Kulkarni H., Arslan C.D., Hart P. Desmopressin and bleeding risk after percutaneous kidney biopsy. BMC Nephrol. 2019;20:413. doi: 10.1186/s12882-019-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corapi K.M., Chen J.L., Balk E.M., Gordon C.E. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73. doi: 10.1053/j.ajkd.2012.02.330. [DOI] [PubMed] [Google Scholar]
- 19.Charu V., O’Shaughnessy M.M., Chertow G.M., Kambham N. Percutaneous kidney biopsy and the utilization of blood transfusion and renal angiography among hospitalized adults. Kidney Int Rep. 2019;4:1435–1445. doi: 10.1016/j.ekir.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh Y.-P., Chang C.-C., Kor C.-T. Mean corpuscular volume and mortality in patients with CKD. Clin J Am Soc Nephrol. 2017;12:237–244. doi: 10.2215/CJN.00970116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.