Abstract
Introduction
Thrombocytopenia, anasarca, myelofibrosis, renal dysfunction, and organomegaly (TAFRO) syndrome is a severe subtype of idiopathic multicentric Castleman’s disease, characterized by thrombocytopenia, anasarca, fever, reticulin myelofibrosis, and organomegaly. Renal complication of this disease can be life-threatening and sometimes requires hemodialysis, but it has not been elucidated in detail.
Methods
Case-series was designed to evaluate the renal histology of patients with TAFRO syndrome treated at our hospital.
Results
Seven patients were eligible to the criteria. All of them had severe diuretic-resistant anasarca and 6 of 7 had mild proteinuria (<1 g daily). On light microscopy, all patients showed glomerular endotheliopathy characterized by endothelial cell swelling and a double contour of the glomerular basement membrane with mesangiolysis or mesangial loosening. Immunofluorescent staining and electron microscopy did not detect immune deposits in any patient. Electron microscopy revealed endothelial cell swelling with diffuse expansion of the subendothelial space, loss of mesangial architecture, and loss of endothelial cell fenestrations. Treatment with glucocorticoids and molecular-targeting agents, including tocilizumab and rituximab, improved renal dysfunction and anasarca. In 4 of 7 patients with persistent thrombocytopenia, hemorrhagic events occurred despite platelet transfusion or thrombopoietin receptor antagonist therapy.
Conclusion
Severe diuretic-resistant anasarca with mild proteinuria and severe glomerular endotheliopathy were common characteristics of renal dysfunction due to TAFRO syndrome. In addition, endothelial changes mediated via interleukin (IL)-6 and vascular endothelial growth factor (VEGF) that lead to vascular hyperpermeability and water leakage might contribute to anasarca, because molecular-targeting therapy directed against IL-6 or VEGF improved renal dysfunction and severe endothelial damage.
Keywords: endotheliopathy, TAFRO syndrome, VEGF
Graphical abstract
TAFRO syndrome is a systemic inflammatory disorder that is characterized by thrombocytopenia, anasarca (including pleural effusion and ascites), fever, renal insufficiency, and organomegaly (including hepatosplenomegaly and lymphadenopathy).1 TAFRO syndrome is classified as a subtype of idiopathic multicentric Castleman’s disease (iMCD), with manifestations that include a poor performance status, severe renal dysfunction (estimated glomerular filtration rate <30 or creatinine >3.0 mg/dl), anasarca, anemia, and pulmonary involvement (iMCD-TAFRO), unlike iMCD without TAFRO syndrome (iMCD–not otherwise specified).2
Severe renal dysfunction is recognized as a life-threatening complication of iMCD-TAFRO, but little is known about it. Iwaki et al.3 reported that the median serum creatinine level at the time of lymph node biopsy was 0.96 mg/dl (range, 0.52–6.08 mg/dl) in 25 patients with TAFRO syndrome and 5 patients required hemodialysis due to progressive renal dysfunction. However, the findings on urinalysis, the prognosis, and the renal histopathology were not elucidated by their study.
We previously reported on the renal histology of an 80-year-old woman with TAFRO syndrome, which was characterized by diffuse glomerular endotheliopathy with endothelial cell swelling and edematous expansion of the subendothelial space under the glomerular basement membrane (GBM) without immunoglobulin deposits.4 We also reported an 84-year-old man with TAFRO syndrome whose renal histology was similar to the first case.5 Subsequently, we have experienced another 5 cases of this syndrome. In this report, we analyze the clinical and histopathological features of all 7 patients with TAFRO syndrome.
Methods
Patient Selection
Among 360 patients who underwent renal biopsy from 2012 to 2018 at Toranomon Hospital, 7 patients who fit the diagnostic criteria for TAFRO syndrome3 were analyzed retrospectively. In 1 patient, both autopsy and biopsy were performed. This study was approved by our institutional review board and the patients gave written informed consent.
All patients were Japanese. The median age at diagnosis was 53 years (range: 34–84 years) and the male:female ratio was 4:3.
Laboratory and Clinical Data
All demographic, clinical, and laboratory data at the time of renal biopsy were retrieved from the electronic database of our hospital.
Laboratory tests included measurement of the following: serum creatinine, hemoglobin, platelet count, rheumatoid factor, cryoglobulin, IgG, IgA, IgM, 50% hemolytic complement, C3, C4, antinuclear antibody, anti-double-stranded DNA antibody, anti-SS-A antibody, platelet-associated IgG antibody, anti-neutrophil cytoplasmic antibody (myeloperoxidase/proteinase 3), anti-GBM antibody, ADMATS13 activity, HIV antigen and antibody, human herpesvirus 8–polymerase chain reaction,IL-6, VEGF, and soluble IL-2 receptor. Urine tests included measurement of 24-hour protein excretion and assessment of hematuria (red blood cells per high-power field) in resuspended sediment. The estimated glomerular filtration rate (ml/min per 1.73 m2) was calculated with the formula for Japanese patients devised by Matsuo et al.6
Histological Evaluation
Renal biopsy samples were processed for light microscopy, immunofluorescence microscopy, and electron microscopy by standard techniques according to the published methods.7 In addition, immunohistochemical analysis was conducted with immunoperoxidase staining for CD34 (vascular endothelium), CD68 (macrophages), CD61 (platelets), CD3 (T cells), and CD20 (B cells), and pathological findings were evaluated by the semiquantitative way: 1+ (mild), 2+ (moderate), and 3+ (severe).
Statistical Analysis
Analyses were performed by using the JMP software package (version 10.0.0; SAS Institute, Cary, NC). Continuous variables were expressed as the mean ± SD or the median and interquartile range according to their distribution.
Results
Clinical Features
The clinical features of the 7 patients are summarized in (Table 1).
Table 1.
Clinical data
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Median | Min | Max | Normal value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Sex | Female | Male | Male | Female | Male | Male | Female | ||||
| Age at diagnosis, yr | 80 | 84 | 53 | 60 | 34 | 50 | 38 | 53 | 34 | 84 | |
| Initial symptom | Anasarca | Anasarca, appetite loss | Anasarca, lowering urine output | Anasarca, body weight gain | Anasarca, dyspnea | Anasarca, appetite loss, abdominal swelling | Anasarca, fever, abdominal pain | ||||
| Pleural effusion | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Acites | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Underlying disorders | HT | HT, DM, PMR | HT | no | UC | DM | DM, UC | ||||
| Body weight gain, kg | 13.3 | 26.4 | 22.2 | 8.3 | 23.7 | 26.8 | NA | 22.95 | 8.3 | 26.8 | |
| Blood pressure, mm Hg | 164/80 | 108/79 | 147/91 | 158/100 | 113/77 | 147/88 | 159/98 | 147/88 | 108/77 | 164/98 | |
| Heart rate, beats/min | 97 | 93 | 93 | 85 | 108 | 100 | 130 | 97 | 85 | 130 | |
| Body temperature, °C | 37.5 | 35.9 | 36.9 | 36.9 | 37.8 | 36.8 | 37.4 | 36.9 | 35.9 | 37.8 | |
| Laboratory Test | |||||||||||
| Hb, g/dl | 11.1 | 9.3 | 7.6 | 9.6 | 5.6 | 12.7 | 11.9 | 9.6 | 5.6 | 12.7 | 11.3–15.0 |
| Platelet count (minimum value), 103/μl | 9 | 12 | 21 | 46 | 45 | 28 | 32 | 28 | 9 | 46 | 155–350 |
| Albumin (on admission), g/dl | 2.8 | 2.4 | 2.9 | 3.1 | 1.5 | 2.5 | 2.7 | 2.7 | 1.5 | 3.1 | 4.1–5.1 |
| ALP, IU/l | 1438 | 640 | 570 | 534 | 641 | 1181 | 1179 | 641 | 534 | 1438 | 0.3–11 |
| Total bilirubin, mg/dl | 1.5 | 0.7 | 0.7 | 0.6 | 1.7 | 0.9 | 0.6 | 0.7 | 0.6 | 1.7 | 0.3–1.0 |
| Cre (maximum value), mg/dl | 1.98 | 2.68 | 5 | 1.85 | 6.28 | 2.68 | 5.64 | 2.68 | 1.85 | 6.28 | 0.6–1.0 |
| Na, mEq/l | 136 | 141 | 145 | 138 | 136 | 139 | 137 | 138 | 136 | 145 | 138–145 |
| K, mEq/l | 4.3 | 3.7 | 5.5 | 3.4 | 5.1 | 4.2 | 3.9 | 4.2 | 3.4 | 5.5 | 3.6–4.8 |
| Cl, mEq/l | 106 | 103 | 109 | 100 | 99 | 106 | 104 | 104 | 99 | 109 | 101–108 |
| BNP, pg/ml | 182 | 113.8 | 44.1 | 80.9 | 181.7 | 99.7 | 15.5 | 99.7 | 15.5 | 182 | <18.4 |
| CRP, mg/dl | 7.3 | 8.3 | 14.3 | 2.1 | 12.5 | 11.9 | 21.5 | 11.9 | 2.1 | 21.5 | <0.3 |
| IL-6, ng/l | 21.3 | 12.3 | 17.5 | 1.9 | 6.2 | 3 | 103 | 12.3 | 1.9 | 103 | <4.0 |
| VEGF, pg/ml | 454 | 177 | 58.3 | 48.8 | 552 | 133 | 288 | 177 | 48.8 | 552 | <38 |
| Urine dipstick test | |||||||||||
| Gravity | 1.013 | 1.014 | 1.028 | 1.026 | 1.025 | 1.035 | 1.006 | 1.025 | 1.006 | 1.035 | |
| Urine sedimentation test, /HPF | |||||||||||
| Red blood cell | 10 | 30 | 4 | 100 | Many | 5–9 | <1 | ||||
| Weight blood cell | 1–5 | 11–30 | 1–4 | 1–4 | Many | 5–9 | Many | ||||
| Laboratory data (urine), g/d | |||||||||||
| Urine protein | 0.86 | 0.28 | 0.3 | 3.71 | 0.45 | 0.43 | 0.59 | 0.45 | 0.28 | 3.71 | |
| Diagnosis criteria | |||||||||||
| Thrombocytopenia | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Anasarca | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Fever or elevation of CRP | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Renal insufficiency | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Reticulin fibrosis | No | Yes | Yes | No | Yes | Yes | Yes | ||||
| Organomegaly | Yes | No | Yes | Yes | Yes | Yes | Yes | ||||
| Lymphadenopathy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| Lymphadenopathy (histology) | Not sampled | HV | HV | Atypical | HV | Atypical | HV |
ALP, alkaline phosphatase; BNP, brain natriuretic protein; Cre, creatinine; CRP, C-reactive protein; DM, diabetes mellitus; Hb, hemoglobin; HPF, high-power field; HT, hypertension; HV, hyaline vascular type; IL, interleukin; NA, not available; PMR, polymyalgia rheumatica; UC, ulcerative colitis; VEGF, vascular endothelial growth factor.
Underlying Disorders
The following underlying disorders were noted: hypertension (cases 1, 2, and 3), diabetes mellitus (cases 2, 6, and 7), and ulcerative colitis (cases 5 and 7).
Presenting Symptoms
The patients were all admitted with anasarca and associated symptoms, including leg edema, weight gain, low urine output, loss of appetite, exertional dyspnea, fever, and abdominal swelling.
The median weight gain (difference between the maximum and minimum body weight) was 22.95 kg (8.3–26.8 kg). Five patients had hypertension at admission and all 7 patients had fever or elevation of serum C-reactive protein. All 7 patients also had hepatosplenomegaly (Table 1).
Laboratory Findings
The median platelet count was 36 ×103/μl (28–115 ×103/μl) on admission, but subsequently declined to a minimum of 28 ×103/μl (9–46 ×103/μl). Schistocytes were not detected and lactate dehydrogenase was within normal range in all cases. Serum albumin was 2.7 g/dl (1.5–3.1 g/dl). Serum creatinine was 1.17 mg/dl (0.48–5.81 mg/dl) on admission and increased to a maximum of 2.68 mg/dl (1.85–6.28 mg/dl). C-reactive protein was 11.9 mg/dl (2.1–21.5 mg/dl), IL-6 was 12.3 ng/l (1.9–103 ng/l; normal range, <4.0 ng/l), and VEGF was 177 pg/ml (48.8–552 pg/ml; normal range, <38.3 pg/ml). Serum IgG was 835 mg/dl (684–1449 mg/dl), and alkaline phosphatase was 641 IU/l (534–1438 IU/l). IgM-kappa type monoclonal protein was only found in case 1. Antinuclear antibody (speckled type) was detected only in case 4, who was also positive for anti-SS-A antibody. Platelet-associated IgG antibody was positive in 6 of the 7 patients (except for case 2), but other specific autoimmune antibodies were negative, including proteinase 3–anti-neutrophil cytoplasmic antibody, myeloperoxidase–anti-neutrophil cytoplasmic antibody, and anti-GBM antibody. Complement levels (C3, C4, and C1q) and ADMATS13 activity were within normal limits. HIV antigen and antibody and human herpesvirus 8–polymerase chain reaction were negative in all 7 patients.
Urinary protein excretion was <1 g/d or 1 g/g creatinine in 6 patients, although it was 3.71 g/d in case 4. Urinary sediment showed 5 patients had microscopic hematuria (>4 erythrocytes per high-power field).
Pathological Findings
Bone marrow biopsy revealed myelofibrosis in 5 patients, but not in 2 patients (cases 1 and 4). All 7 patients had mild to moderate lymphadenopathy. Lymph node biopsy showed typical hyaline vascular MCD in 4 patients (cases 2, 3, 5, and 7), but this type of MCD was not detected in 2 patients (cases 4 and 6). Lymph nodes could not be sampled in case 1 (Table 1).
Diagnosis of TAFRO Syndrome
Based on the criteria for diagnosis of Castleman-Kojima disease (TAFRO syndrome),1 all 7 patients fulfilled all of the 3 major categories (anasarca, thrombocytopenia, and systemic inflammation) and at least 2 of the 4 minor categories (Castleman’s disease–like features on lymph node biopsy, reticulin myelofibrosis, organomegaly, and progressive renal insufficiency) (Table 1).
Renal Histology
Table 2 shows the details of renal histology in the 7 patients.
Table 2.
Renal histology of TAFRO syndrome
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Renal biopsy | |||||||
| Diagnosis | Endotheliopathy | Endotheliopathy | Endotheliopathy | Endotheliopathy | Endotheliopathy | Endotheliopathy | Endotheliopathy |
| Light microscopy | |||||||
| Swelling of endothelial cells | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ |
| Mesangiolysis | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ |
| Double contour structure | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ |
| Mytosis of endothelial cell | — | 1+ | — | 1+ | — | — | — |
| IF | |||||||
| IgG | — | — | — | — | — | 1+ (GBM linear) | 1+ (GBM linear) |
| IgA | — | — | — | — | — | 1+ | — |
| IgM | 1+ (mesangial area) | 1+ (GBM) | — | 1+ (GBM) | — | 1+ (GBM) | minute |
| C3 | — | — | — | — | — | minute | — |
| C1q | — | — | — | — | — | — | |
| C4 | — | — | — | — | — | — | — |
| Electron microscopy | |||||||
| Electron-dense deposit | — | — | — | — | — | — | — |
| Swelling of endothelial cells with expansion of subendothelial space | 2+ | 2+ | 2+ | 2+ | 1+ | 2+ | 2+ |
| Loss of the mesangial architecture | 2+ | 1+ | 2+ | 2+ | 1+ | 1+ | 1+ |
| Loss of endothelial cell fenestration | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ |
| Treatment | |||||||
| Induction therapy | TCZ, GC | GC pulse, PEX, TCZ | GC (pulse), TCZ | GC | GC pulse, TCZ | TCZ, RTX, GC | GC pulse, TCZ |
| Thrombopoietin receptor agonist | Eltrombopag | Romiplostim | — | — | Romiplostim | Eltrombopag | — |
| Hemodialysis | No | Once | 3 times | No | 6 times | No | Once |
| Prognosis | Alive | Death | Alive | Alive | Alive | Alive | Alive |
GBM, glomerular basement membrane; GC, glucocorticoid; GC pulse, methylprednisolone pulse therapy; IF, immunofluorescence; PEX, plasma exchange; RTX, rituximab; TCZ, tocilizumab.
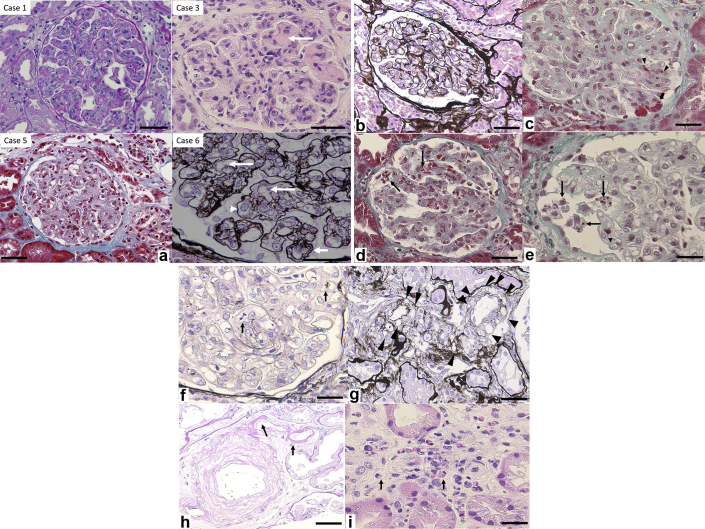
On light microscopy, all patients displayed glomerular endotheliopathy that was characterized by endothelial cell swelling (resulting in obstruction and/or narrowing of glomerular tufts), mesangiolysis or mesangial loosening (loss of mesangial matrix staining), and GBM double contour and thickening (Figure 1a). These changes diffusely and globally affected the glomeruli. None of the biopsies showed thrombi. Segmental tuft prolapse into the proximal tubule were noted in 6 of 7 patients (Figure 1b). Extravasation of erythrocytes from the glomerular capillaries into the surrounding tissues and/or sludging of erythrocytes were seen in 6 of 7 patients (Figure 1c). Fragmentation of erythrocytes (indicating hemolysis) was noted in the glomerular capillaries in only 1 patient (Figure 1d). Massive hyaline degeneration of podocytes was also seen in only 1 patient (Figure 1e). Endothelial cell mitosis was found in 2 of 7 patients (Figure 1f). Vacuolization of arteriolar myocytes was detected in all 7 patients (Figure 1g) and arteriolar hyalinosis was noted 4 of 7 patients (Figure 1h). On the other hand, there were no fibrin thrombi or fibrinoid necrosis of the glomerular capillaries or arterioles and small renal arteries in any patient and no mucoid intimal thickening of small arteries. Peritubular capillaritis was noted in 2 of 7 patients, and eosinophil infiltration was definite in case 3 (Figure 1i).
Figure 1.
(a) Light microscopy showed glomerular endotheliopathy characterized by endothelial cell swelling. Large arrows: mesangial loosening, arrowhead: swelling of glomerular endothelial cells, small arrow: double contour structure of the GBM. Periodic acid–Schiff staining, original magnification ×400 (case 1); hematoxylin and eosin staining, original magnification ×400 (case 3); Masson trichrome staining, original magnification ×200 (case 4); periodic acid–methenamine silver staining, original magnification ×600 (case 6). Bar = 40 μm. (b) Glomerular tip lesion (arrows). Periodic acid–methenamine silver staining, original magnification ×200. Bar = 40 μm (case 5). (c) Extravasation of erythrocytes (arrowheads), sludging of erythrocytes (arrows). Masson trichrome staining, original magnification ×400. Bar = 30 μm (case 4). (d) Fragmentation of erythrocytes (arrows), Masson trichrome staining, original magnification ×400. Bar = 30 μm (case 5). (e) Massive hyaline degeneration of podocytes (arrows). Masson trichrome staining, original magnification ×600. Bar = 20 μm (case 4). (f) Endothelial cell mitosis (arrow). Periodic acid–methenamine silver staining, original magnification ×600. Bar = 20 μm (case 4). (g) Arteriolar myocyte vacuolization (arrowheads). Periodic acid–methenamine staining, original magnification ×400. Bar = 35 μm (case 6). (h) Arteriolar hyalinosis (arrow). Hematoxylin and eosin staining, original magnification ×400. Bar = 25 μm (case 1). (i) Peritubular capillaritis, including infiltration of eosinophils (arrows). Hematoxylin and eosin staining, original magnification ×400. Bar = 20 μm (case 3).
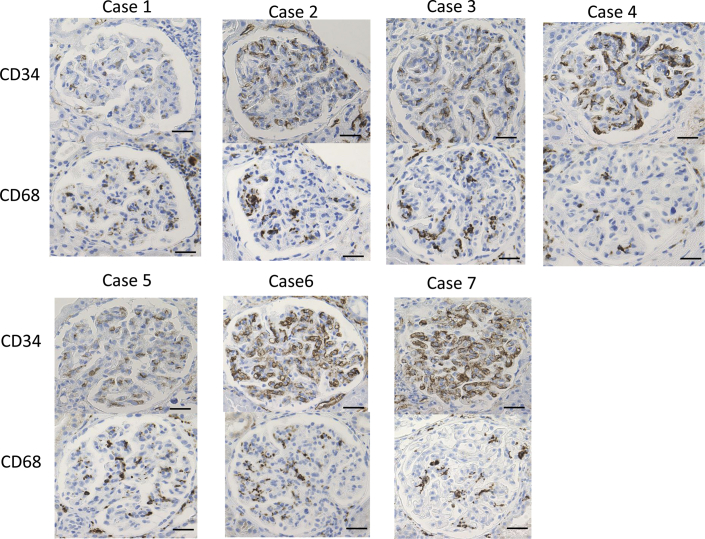
Immunoperoxidase staining for CD34 and CD68 was weakly to strongly positive in all 7 patients (Figure 2), but there was no significant staining for CD61, CD3, and CD20.
Figure 2.
Immunoperoxidase staining for CD34 and CD68 ranged from slightly to strongly positive in all 7 patients. Bar = 30 μm.
Immunofluorescence detected linear staining along the GBM in 2 of 7 patients, probably due to underlying diabetes mellitus. Granular IgA staining along the GBM was noted in only 1 case. Although IgM was positive in 4 of 7 patients, it was considered to be false-positive because of the electron microscopy findings discussed as follows.
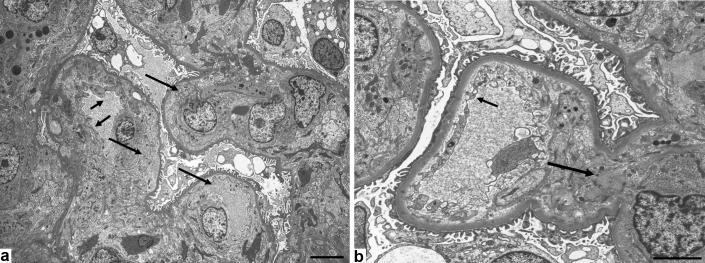
Electron microscopy did not reveal electron-dense deposits in any of the 7 patients. On the other hand, endothelial cell swelling with expansion of the subendothelial space, loss of mesangial architecture (increased electron lucency of the mesangial matrix, or mesangiolysis), and loss of endothelial cell fenestration (Figure 3a) were detected in all 7 patients. In case 7, these findings were noted in most of the glomeruli, although degeneration of endothelial cell fenestrations and mesangial matrix was seen in some glomeruli (Figure 3b). Changes of the mesangial matrix were also detected in cases 2 and 6 with diabetes mellitus. Effacement of foot processes was noted in 3 of 7 patients. The thickness of the glomerular GBM was >380 nm in the 3 patients with a history of diabetes mellitus, but <229 nm in the 4 patients without a history of diabetes mellitus.
Figure 3.
(a) Electron microscopy shows endothelial cell swelling with expansion of the subendothelial space, resulting in narrowing and/or obstruction of glomerular tufts, loss of mesangial architecture, and loss of endothelial cell fenestration. Loss of endothelial cell fenestrations (small arrows) and swelling of endothelial cells with expansion of the subendothelial space (large arrows). Bar = 6.7 μm. (b) degeneration of endothelial cell fenestrations (small arrow) and mesangial matrix (large arrow) were confirmed in parts of the glomeruli. Bar = 3.3 μm.
Outcome
Glucocorticoid (GC) therapy was chosen initially in all 7 patients, but GC monotherapy was effective in only 1 patient (case 4). Tocilizumab was added in the other 6 patients with GC-resistant anasarca. It was effective for 5 patients (cases 1, 2, 3, 5, and 7), but not for 1 patient (case 6). Tocilizumab was switched to rituximab in case 6 and it proved effective.
In 4 patients (cases 2, 3, 5 and 7), hemodialysis was initiated due to diuretic-resistant congestion with massive pleural effusion, but it was discontinued after urine output recovered with treatment such as GC or GC plus tocilizumab. In case 2, plasma exchange was performed because atypical hemolytic uremic syndrome was initially suspected, but this was stopped after TAFRO syndrome was diagnosed.
Another serious complication was GC-resistant, IgG-positive thrombocytopenia, and 4 patients had bleeding complications, including rectal, renal, intracranial, subdural, extradural, and intramuscular hemorrhage. Thrombopoietin receptor analogues, eltrombopag (cases 1 and 6) and romiplostim (cases 2 and 5), were effective for thrombocytopenia in these patients. Posterior reversible encephalopathy syndrome occurred in case 5 after starting induction therapy. Case 2 died of aspiration pneumonia after developing intracranial hemorrhage. The other 6 patients are doing well.
Discussion
The renal histology of patients with typical TAFRO syndrome has only been reported in a few papers. Tanaka et al.8 reported a 70-year-old man with TAFRO syndrome who had acute kidney injury with minute proteinuria and anasarca. Renal biopsy revealed membranoproliferative glomerulonephritis–like changes with endothelial injury. Light microscopy showed a double contour of the GBM and narrowing or occlusion of the glomerular capillaries due to endothelial cell swelling. In addition, electron microscopy demonstrated glomerular endothelial swelling and electron-lucent widening of the subendothelial space. Methylprednisolone pulse therapy was initially effective, but a thrombopoietin receptor agonist was required for steroid-resistant thrombocytopenia. After esophageal cancer surgery, these treatments could be discontinued.8 Ito et al.9 reported a 76-year-old woman with TAFRO syndrome. She presented with nephrotic syndrome, and renal biopsy showed membranoproliferative glomerulonephritis–like glomerulopathy with massive infiltration of macrophages. Glomerular endothelial cell injury was suggested by decreased immunostaining for CD34. Long-term steroid therapy was effective.9 Moreover, Ozeki et al.10 reported a 51-year-old woman with TAFRO syndrome who had similar renal histology. They performed immunostaining for VEGF, but there were no glomerular abnormalities. Steroid therapy was effective.10
Thrombotic microangiopathy (TMA) is a condition that presents with thrombocytopenia, anemia, purpura, and renal failure, and its pathological basis is thrombosis in the capillaries and arterioles due to endothelial injury. The classic conditions that cause TMA are hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Other conditions associated with TMA include atypical hemolytic uremic syndrome, disseminated intravascular coagulation, scleroderma renal crisis, malignant hypertension, antiphospholipid antibody syndrome, and drug toxicities such as calcineurin inhibitor toxicity.11 TAFRO syndrome is another possible cause of this type of TMA, but it differs from the other diseases in several respects, since there are no fibrin thrombi or fibrinoid necrosis in the glomerular capillaries and small renal arteries, mucoid intimal thickening of small arteries, or erythrocyte fragmentation in the glomerular capillaries, which are usually seen in TMA.
Similar to the histopathological findings to previously reports of TAFRO syndrome, preeclampsia causes severe glomerular endotheliopathy,12 which sometimes could lead to HELLP syndrome,13 characterized by multi-organ failure, such as hemolysis, elevated liver enzymes, and low platelet, but there has been no pregnant case with TAFRO syndrome.
In our 7 patients with TAFRO syndrome, the following findings were observed by light microscopy: segmental tuft prolapse into the proximal tubule (in 6 of 7 patients), extravasation and/or sludging of erythrocytes (in 6 of 7 patients),14 and arteriolar myocyte vacuolization (in all 7 patients).15 These may be characteristics of renal histology in TAFRO syndrome, although none of these findings were described in previous reports. Although it was only for case 4 with nephrotic-range proteinuria, massive hyaline degeneration of podocytes16 was found in this study, which had not been mentioned by previous reports. In addition, immunoperoxidase staining for CD34 and CD68 was slightly to strongly positive in all 7 patients. This may indicate that CD34 expression is closely related to endothelial cell injury in the kidneys of patients with TAFRO syndrome,17 whereas CD 68 expression is related to macrophage or monocyte infiltration.18
The limitations of this study were that it was a small case-series from a single center, as well as referral bias and indication bias for renal biopsy. However, TAFRO syndrome is a rare disease and renal biopsy is avoided by many institutions because of thrombocytopenia, so our report is important despite the small sample size.
In conclusion, TAFRO syndrome has the following 4 distinctive characteristics.
-
(i)
Renal biopsy shows glomerular endotheliopathy, that is endothelial cell swelling, mesangiolysis or mesangial loosening (loss of mesangial matrix staining), and GBM double contour and thickening. Electron microscopy shows loss of mesangial architecture and endothelial cell swelling with expansion of the subendothelial space, as well as loss of endothelial cell fenestrations.
-
(ii)
Clinically, it presents with severe diuretic-resistant anasarca, mild proteinuria, and renal dysfunction that sometimes requires dialysis.
-
(iii)
Tocilizumab is effective for steroid-resistant TAFRO syndrome, and rituximab might be an option for tocilizumab-resistant disease.
-
(iv)
Treatment with a thrombopoietin receptor analogue, such as eltrombopag (cases 1 and 6) or romiplostim (cases 2 and 5), may be an option for steroid-resistant thrombocytopenia.
In patients with TAFRO syndrome, endothelial injury mediated via the VEGF/IL-6 axis might contribute to anasarca due to vascular hyperpermeability and water leakage, because molecular-targeting therapy for IL-6 or VEGF improved renal dysfunction and severe endothelial damage.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was funded by the Okinaka Memorial Institute for Medical Research. It did not play any role in this research.
We thank Kazuho Honda (Department of Anatomy, Showa University School of Medicine), Yukiko Kanetsuna (Department of Pathology, International University of Health and Welfare), and Akinori Hashiguchi (Department of Pathology, Keio University School of Medicine) for valuable discussion on the diagnostic and therapeutic strategy.
References
- 1.Masaki Y., Kawabata H., Takai K. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103:686–692. doi: 10.1007/s12185-016-1979-1. [DOI] [PubMed] [Google Scholar]
- 2.Fajgenbaum D.C., Uldrick T.S., Bagg A. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646–1657. doi: 10.1182/blood-2016-10-746933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwaki N., Fajgenbaum D.C., Nabel C.S. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91:220–226. doi: 10.1002/ajh.24242. [DOI] [PubMed] [Google Scholar]
- 4.Noda-Narita S., Sumida K., Sekine A. TAFRO syndrome with refractory thrombocytopenia responding to tocilizumab and romiplostim: a case report. CEN Case Rep. 2018;7:162–168. doi: 10.1007/s13730-018-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno H., Sekine A., Oguro M. Renal histology in a patient with TAFRO syndrome: a case report. Hum Pathol. 2018;82:258–263. doi: 10.1016/j.humpath.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo S., Imai E., Horio M. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu R., Hoshino J., Suwabe T. Membranoproliferative glomerulonephritis and circulating cryoglobulins. Clin Exp Nephrol. 2014;18:88–94. doi: 10.1007/s10157-013-0810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M., Tsujimoto H., Yamamoto K. Clinicopathological features of progressive renal involvement in TAFRO syndrome: a case report and literature review. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S., Uchida T., Itai H. Serial manifestation of acute kidney injury and nephrotic syndrome in a patient with TAFRO syndrome. Intern Med. 2018;57:3129–3133. doi: 10.2169/internalmedicine.0806-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozeki T., Tsuji M., Yamamoto J. Thrombotic microangiopathy on kidney biopsy in a patient with TAFRO syndrome. CEN Case Rep. 2018;7:243–247. doi: 10.1007/s13730-018-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benz K., Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens. 2010;19:242–247. doi: 10.1097/MNH.0b013e3283378f25. [DOI] [PubMed] [Google Scholar]
- 12.Packham D.K., Mathews D.C., Fairley K.F. Morphometric analysis of pre-eclampsia in women biopsy in pregnancy and post-partum. Kidney Int. 1998;34:704–711. doi: 10.1038/ki.1988.236. [DOI] [PubMed] [Google Scholar]
- 13.Stone J.H. HELLP syndrome; hemolysis, elevated liver enzymes, and low platelets. JAMA. 1998;280:559–562. doi: 10.1001/jama.280.6.559. [DOI] [PubMed] [Google Scholar]
- 14.Caggiati A.1, Franceschini M., Heyn R. Skin erythrodiapedesis during chronic venous disorders. J Vasc Surg. 2011;53:1649–1653. doi: 10.1016/j.jvs.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Horike K., Takeda A., Yamaguchi Y. Is arteriolar vacuolization a predictor of calcineurin inhibitor nephrotoxicity? Clin Transplant. 2011;25(Suppl 23):23–27. doi: 10.1111/j.1399-0012.2011.01474.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagata M., Yamaguchi Y., Toki D. Complex glomerular pathology of thrombotic microangiopathy and focal segmental glomerulosclerosis forms tumor-like mass in a renal transplant donor with severe renovascular hypertension. CEN Case Rep. 2017;6:12–17. doi: 10.1007/s13730-016-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusano T., Takano H., Kang D. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum Pathol. 2016;49:135–144. doi: 10.1016/j.humpath.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Kowalewska J., Okoń K., Szynaka B. Expression of macrophage markers in cryoglobulinemic glomerulonephritis - a possible role of CXCL9. Adv Med Sci. 2013;58:394–400. doi: 10.2478/ams-2013-0030. [DOI] [PubMed] [Google Scholar]