Abstract
Renal toxicities have been increasingly recognized as complications of the immune checkpoint inhibitors (ICIs). Recent studies have outlined the incidence and potential risk factors for nephrotoxicity. For clinicians, the key question is how to manage patients who develop these adverse renal effects. This is of paramount importance to providers as ICI use for cancer therapy becomes more widespread and nephrotoxicity increasingly develops. As clinicians encounter ICI-associated nephrotoxicity, an appropriate approach to management is required to facilitate the best outcomes in patients with cancer. Importantly, ICI rechallenge in patients who developed ICI-related acute kidney injury (AKI) is unclear and represents a conundrum for providers. Clinicians struggle with the “if, when, and how to” questions related to ICI rechallenge in this subset of patients. In addition, ICI use in the transplant population raises concerns for promoting acute rejection when treating cancer in these patients. We herein review current information on these various topics.
Keywords: acute intertitial nephritis, CTLA-4, immune checkpoint inhibitors, immunotherapy, PD1, PD-L1, renal immune-related adverse effect
ICIs are a new class of immunotherapy drugs that have revolutionized cancer treatment by substantially improving the overall prognosis of several types of malignancies.1, 2, 3 These monoclonal antibodies act by blocking intrinsic downregulators of the immune system, so-called “immune checkpoints.” These “immune checkpoints” consist of 2 receptors: cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed death 1 pathway (PD-1/PD-Ligand-1 [PD-L1]).4,5 They are localized on immune system cells, such as T cells and other cells, but also can be found on cancer cells where they are selectively upregulated to evade immune cells. As such, they are prime targets for ICI blockade, particularly combination ICI therapy approaches.6 By boosting tumor-directed immune responses, ICIs facilitate immune cells to fight the cancer; however, this increased immune system activity can also cause inflammatory adverse effects, which are called immune-related adverse events (iRAEs). Skin, gastrointestinal tract, and the endocrine system are most commonly affected.7 Kidney toxicity from these agents is relatively uncommon; however, the incidence can be 5% (or potentially higher), especially with the use of combination ICI therapy.8, 9, 10, 11, 12 Herein, we discuss the mechanisms of action and kidney injury associated with the ICIs, the evolving incidence and types of renal iRAEs, and risk factors for nephrotoxicity and management of kidney injury. In addition, we discuss rechallenge with these drugs after the development of AKI in the setting of ICI therapy and their use in kidney transplant recipients.
Mechanisms of Immune Checkpoint Inhibition and Associated Adverse Renal Effects
Immune checkpoints have the crucial role of maintaining physiological modulation of immune responses to avoid collateral immune damage and maintain self-tolerance. ICIs exert inhibitory signals to costimulatory receptors, targeting the lymphocyte receptors or their ligands to unleash the anti-tumor immune response. CTLA-4 and PD-1/PD-L1 receptor blockade regulates immune responses at different levels and by different mechanisms. CTLA-4 regulates the activation of antigen-specific T cells in lymph nodes, whereas PD-1 is present on peripheral antigen-specific T cells and is activated following antigen presentation by antigen-presenting cells in the tumor microenvironment.6 In addition, PD-1 receptors also can be activated by upregulated PD-L1 on tumor cells, thereby evading immune detection.6,12
The mechanism by which ICIs induce AKI is not well established. PD-1 is expressed after activation on T cells, B cells, natural killer T cells, activated monocytes, and dendritic cells,13 whereas its ligand PD-L1 is expressed on kidney tubules, especially the proximal tubular segments.14 In preclinical studies, PD-1 knockout mice spontaneously developed chronic systemic inflammatory responses and a kidney lesion similar to lupus glomerulonephritis,15,16 supporting an adverse immune effect. Once PD-1/CTLA-4 blockade is initiated, it breaks immune tolerance by unleashing quiescent tissue-specific self-reactive T cells, which may lead to development of drug-specific antibodies after drug exposure that engage in an immune reaction such that cells of the proximal tubule may hydrolyze and metabolize exogenous antigens and present them to antigen-presenting cells in the kidney.17 Furthermore, another potential mechanism by which ICI-AKI may occur is through haptenization, when low-molecular-weight drug compounds bind tubular antigens, thus creating a hapten that can be trapped in the parenchyma, leading to an immune response and tubular damage. This latter hypothesis is supported by recent studies showing the association of biopsy-proven acute interstitial nephritis (AIN) in ICI-treated patients who had previous exposure to other AIN-associated drugs, such as proton pump inhibitors or nonsteroidal anti-inflammatory drugs.10,18
Currently, the U.S. Food and Drug Administration has approved 1 CTLA-4 inhibitor and 6 PD-1/PD-L1 inhibitors for several types of malignancies (Table 1), and additional clinical trials are currently under way to expand the indication for ICIs.19
Table 1.
Food and Drug Administration–approved immune checkpoint inhibitors
| Drug | Target | Indication |
|---|---|---|
| Ipilimumab | CTLA-4 | Melanoma, MSI-colorectal cancer, renal-cell carcinoma |
| Cemiplimab | PD-1 | Cutaneous squamous cell cancer |
| Nivolumab | PD-1 | Melanoma, non–small/small-cell lung cancer, renal-cell carcinoma, classic Hodgkin’s lymphoma, head and neck squamous cell carcinoma, urothelial carcinoma, MSI-colorectal, hepatocellular carcinoma |
| Pembrolizumab | PD-1 | Melanoma, non–small-cell lung cancer, classic Hodgkin’s lymphoma, primary mediastinal large B-cell lymphoma, head and neck squamous cell carcinoma, urothelial carcinoma, gastric cancer, cervical cancer, solid tumors with high microsatellite instability or mismatch-repair deficiency |
| Atezolizumab | PD-L1 | Non–small-cell lung cancer, urothelial carcinoma |
| Avelumab | PD-L1 | Merkel-cell carcinoma, urothelial carcinoma |
| Durvalumab | PD-L1 | Urothelial carcinoma, non–small-cell lung cancer |
CTLA-4, cytotoxic T-lymphocyte antigen 4; MSI, microsatellite instability; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Incidence, Clinical, and Pathological Features of ICI-Associated Adverse Renal Effects
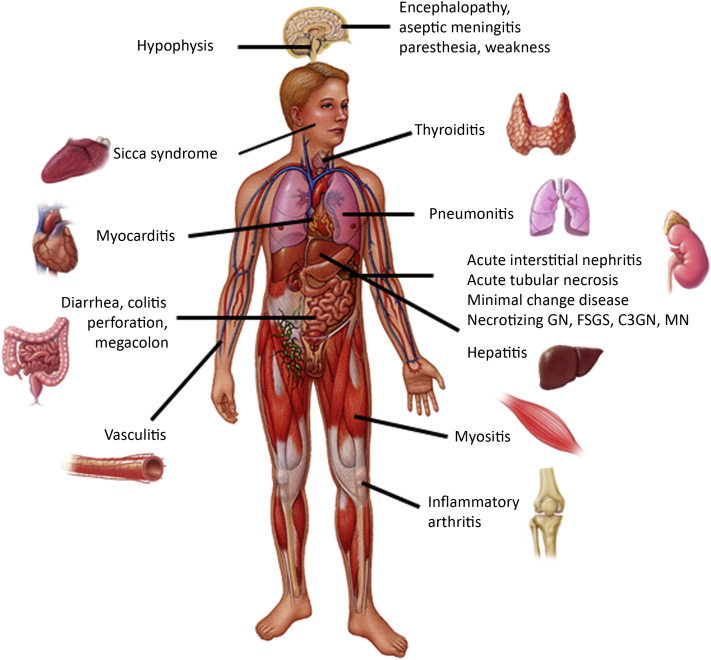
A number of organs can be affected by iRAEs (Figure 1) in patients receiving ICIs and the incidence can be as high as 59% to 85%, depending on the use of single-agent or combination immune checkpoint inhibitor therapy.7,20 The overall incidence of AKI in all patients treated with ICI is approximately 17% defined by an increase in serum creatinine of at least 1.5 times the baseline,10 but the estimated incidence of AKI directly related to ICI varies from 2.2% to close to 5.0% in recent retrospective studies.8, 9, 10,18 In case reports and series, the most common histopathological finding in patients who develop AKI-ICI is AIN8,21; however, acute tubular injury also has been described in a significant number of patients. Glomerular disorders including minimal change disease, IgA nephropathy, focal segmental glomerulosclerosis, Goodpasture’s disease, vasculitis, and immune complex–mediated glomerulonephritis were also reported.22, 23, 24, 25, 26, 27, 28, 29, 30 These findings were confirmed by a recent multicenter study by Cortazar and colleagues18 in which AIN was observed in 93% of the 60 patients with a kidney biopsy. ICI-associated AKI usually occurs at 12 to 14 weeks after ICI initiation. Importantly, AKI can develop earlier, especially when CTLA-4 and PD-1 signaling blockade are combined or after several months to more than a year after ICI treatment discontinuation.18,24,31 The severity of AKI varies, but most of the cases described were Kidney Disease: Improving Global Outcomes AKI stage 2 and stage 3 at the time of diagnosis.10,32 The clinical and laboratory features of ICI-associated AKI are similar to those observed with AKI caused by other drug-induced AINs with essentially “bland urinalysis,” subnephrotic range proteinuria, and sterile pyuria, unless a vascular and glomerular lesion is present.8,24,32 An important finding is that 40% to 87% of patients who develop ICI-associated AKI had a prior or concomitant history of an extrarenal iRAE, such as skin rash, thyroiditis, or colitis; however, the presence or absence of an extrarenal iRAE does not confirm or exclude the existence of AIN.10,18,32
Figure 1.
Immune-related adverse events. Various organs can be affected in immune-mediated injury caused by immune checkpoint inhibitors. C3GN, C3 glomerulopathy; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; MN, membranous nephropathy. Copyright © Mayo Clinic. Used with permission.
Although less common than AIN, glomerular diseases are an important renal complication of ICIs, as seen in Table 2.22, 23, 24, 25, 26, 27, 28, 29, 30 Four cases of ICI-associated glomerulonephritis and renal vasculitis were recently reported by Gallan et al.25 Three patients had small- to medium-vessel vasculitis in the kidneys and 1 had crescentic pauci-immune glomerulonephritis. Three patients presented with AKI, and 1 presented with nephrotic syndrome and hematuria. Interestingly, 3 patients had antineutrophil cytoplasmic antibody testing that was negative.25 On another study, Izzedine et al.33 reported a case series of 12 patients who developed either AKI or nephrotic proteinuria after pembrolizumab therapy. Acute tubular injury/acute tubular necrosis (ATN) occurred in 5 cases; followed by AIN in 4 and minimal change disease in 2 patients. Cassol and colleagues34 described 15 patients treated with ICIs who developed AKI and underwent kidney biopsy. Notably, 9 had AIN and 6 had ATN. Clinical and laboratory parameters (including extrarenal iRAEs) were unhelpful in differentiating AIN from ATN in these cases.34
Table 2.
Immune checkpoint inhibitor–associated glomerular diseases
| Glomerular disease | Drug | Reference |
|---|---|---|
| Minimal change disease | Ipilimumab Pembrolizumab |
Kidd et al., 201626 Kitchlu et al., 201723 Kitchlu et al., 2017 |
| Membranous nephropathy | Nivolumab | Mamlouk et al., 201924 |
| Lupus nephritis | Ipilimumab Nivolumab |
Fadel et al., 200922 Mamlouk et al., 201924 |
| Pauci-immune glomerulonephritis | Nivolumab Tremelimumab Nivolumab and ipilimumab Ipilimumab + pembrolizumab Pembrolizumab |
Mamlouk et al., 201924 Mamlouk et al., 201924 Mamlouk et al., 201924 Van den Brom et al., 201627 Gallan et al., 201925 |
| IgA nephropathy | Pembrolizumab Nivolumab and ipilimumab Nivolumab |
Mamlouk et al., 201924 Mamlouk et al., 201924 Jung et al., 201628 Kishi et al., 201829 |
| C3 glomerulopathy | Pembrolizumab | Mamlouk et al., 201924 |
| Focal segmental glomerulosclerosis | Nivolumab | Mamlouk et al., 201924 Daanen et al., 201730 |
| Renal vasculitis | Nivolumab | Gallan et al., 201925 |
AKI is the most common renal iRAE associated with ICI therapy reported by the Food and Drug Administration Adverse Events Reporting System, followed by the electrolyte abnormality hyponatremia.35 Hyponatremia is seen commonly in patients with cancer and it is important to recognize the underlying process. ICI-related autoimmune adrenalitis/hypophysitis may cause primary or secondary adrenal insufficiency, respectively, as well as thyroiditis. These autoimmune-related injuries are occurring more frequently as a result of increasing use of ICI in patients with various malignancies. These endocrinopathies can cause hyponatremia in up to 50% of patients in this setting, which usually improves after hormonal replacement (Figure 2).36, 37, 38 It is also important to remember that ICIs may induce renal tubular damage with acid-base/electrolyte abnormalities without significant change in kidney function. As an example, a few reports of distal renal tubular acidosis have emerged in the setting of AIN.39,40 Mechanistically, this is believed to be an autoimmune process causing an alteration of H+-ATPase or Cl−/HCO3− in type A intercalated cells.40 Therefore, vigilance for disturbances in kidney function, development of proteinuria, and acid-base and electrolyte abnormalities is warranted.
Figure 2.
Immune checkpoint inhibitor (ICI)–related hyponatremia. Endocrinopathies involving the pituitary, thyroid, and adrenal glands may develop in patients treated with ICIs, which can lead to hyponatremia. ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone.
Risk Factors for Development of ICI-Associated Adverse Renal Effects
Several retrospective studies, including the largest retrospective multicenter study with 138 patients with ICI-associated AKI, identified potential risk factors for development of AKI.10,18,21,32 In these studies, patients with AIN were on several drugs: proton pump inhibitors were most common followed by nonsteroidal anti-inflammatory drugs and a few other drugs. As such, exposure to any drug that has been associated with AIN could potentially lead to the initial cascade of events with drug-specific T cells triggering initiation of the immune reaction. Similar phenomenon has been described by others in the development iRAEs, such as ICI-mediated diarrhea and/or colitis. When comparing patients who received antibiotic therapy before ICI therapy initiation, those receiving antibiotics after ICI therapy had a higher ICI-mediated diarrhea and/or colitis rate and more often needed immunosuppressive therapy and hospitalization for ICI-mediated diarrhea and/or colitis.41 In addition to medications, other identified risk factors for ICI-associated AKI include lower median baseline estimated glomerular function rate and combination ICI therapy.18 Increased nephrotoxicity attributed to the combination of ICIs therapy is logical; however, decreased estimated glomerular filtration rate as a risk for AKI likely represents that AKI becomes more obvious in the setting of chronic kidney disease due to larger increases in serum creatinine despite smaller changes in true glomerular filtration rate and lack of renal reserve. Because the ICIs undergo proteolytic degradation and not renal excretion, lower glomerular filtration rate would not increase risk as seen with potentially nephrotoxic drugs. As an example, a study published recently suggests that ICIs can be safely administered to patients with end-stage kidney disease without dose adjustments. Furthermore, the rate of iRAE development appears similar to patients without end-stage kidney disease.42 Also, the incidence of AKI does not appear to be related to the type of malignancy, but studies have been underpowered to analyze this effect.10
Recommendations for Diagnosis of ICI-Associated Adverse Renal Effects
Patients who are candidates for ICI therapy normally undergo routine baseline laboratory tests before therapy. Surprisingly, urinalysis is not part of the initial baseline testing along with complete blood count and chemistry panel. We recommend baseline urinalysis, with quantification of proteinuria or microalbuminuria if present, before ICI therapy. With this information, physicians would have a better picture of patients’ baseline renal characteristics that can be used for comparison during therapy. For example, this may allow early capture of subtle renal changes, such as transition from bland urinalysis to active sediment. This would promote earlier investigation of cases where kidney function may not have changed significantly (e.g., serum creatinine elevation ≤0.3 mg/dl, or presence of proteinuria alone with or without electrolyte abnormalities).
C-reactive protein also appears to be of potential value in this population. Abolhassan et al.43 demonstrated that C-reactive protein elevation may serve as a predictor of onset of iRAEs in patients treated with ICIs. During the development of iRAEs, the C-reactive protein level is usually elevated before development of clinical symptoms. Therefore, it may serve as an adjuvant biomarker for diagnosis and follow-up response for immunosuppressive therapy in the setting of renal iRAE. This is an area of potential study.
Imaging studies, such as positron emission tomography–computed tomography scan has been reported as a potential diagnostic tool for ICI-associated AIN.44,45 Although this diagnostic imaging test may be useful in cases with significant AKI from AIN, it may not be helpful in the mild AIN cases, especially if a baseline positron emission tomography–computed tomography scan before ICI treatment is not available for comparison. Importantly, mild 18F-flourodeoxyglucose uptake has been observed in other non-AIN causes of AKI from ICIs.45,46 Therefore, clinicians should use this test judiciously with familiarity of its limitations. For those patients who have kidney biopsy contraindication, positron emission tomography–computed tomography scan may assist with a presumptive diagnosis of ICI-associated AIN when other causes of AKI have been ruled out.
Another issue of intense debate is the role of kidney biopsy in patients who developed AKI while undergoing treatment with ICIs. The initial approach before kidney biopsy includes clinical evaluation to rule out alternative etiologies for the AKI, such as volume depletion, contrast-enhanced nephropathy, and obstructive uropathy. The American Society of Clinical Oncology guidelines recommend the following: if an alternative cause of AKI is not identified, clinicians should proceed directly with immunosuppressive therapy without a kidney biopsy.47 Similarly, the National Comprehensive Cancer Network does not advocate for kidney biopsy in the evaluation of patients with ICI-associated AKI, unless a moderate/severe life-threatening renal iRAE defined as Common Terminology Criteria for Adverse Events Grade 2–3 or higher is present.48
Although the previously noted approach makes therapy easy, it may expose patients without AIN to unnecessary corticosteroids. It is important for clinicians to realize that clinical findings and laboratory tests are often not reliable in predicting the underlying kidney lesion.12,34,49, 50 In addition, patients with cancer are at very high risk for AKI that is unrelated to the ICIs, such as AKI from ischemic or chemotherapy-related renal tubular injury.51 Therefore, in addition to a thorough nephrology evaluation, kidney biopsy may be necessary to definitely diagnose the precise kidney lesion (AIN vs. acute tubular injury/ATN vs. other) and provide optimal treatment (corticosteroids vs. supportive care). With this approach, this will allow appropriate therapy for AKI and improve survival in this population.51,52 Others argue that because AIN appears to be the most common cause of AKI, empiric corticosteroids are reasonable in patients without an obvious alternative etiology for AKI to avoid the potential complications of kidney biopsy,18 especially in those who are at risk for potential complications (e.g., solitary kidney or patients with coagulopathy or on anticoagulant therapy). This approach may be reasonable for patients who have an extrarenal iRAE that would otherwise require immunosuppression.
Recommendations for Management of ICI-Associated Adverse Renal Events
Effective management of iRAEs hinges on early recognition and prompt intervention with appropriate immunomodulatory strategies depending on the severity of nephrotoxicity. Patients who develop Kidney Disease: Improving Global Outcomes stage 1 AKI should be evaluated for the causes of AKI frequently observed in patients with cancer, such as obstructive uropathy,
prerenal azotemia, or drug-induced nephrotoxicity from chemotherapeutic agents or other nephrotoxic drugs (e.g., aminoglycosides, contrast, bisphosphonates). Immunotherapy should be temporarily discontinued until further clarification of the cause of AKI is determined and preferably until AKI has resolved. Patients with sustained stage 1 AKI, and especially those with stage 2 or 3 AKI, should be promptly referred to nephrology for consultation and consideration of kidney biopsy. Once the diagnosis of ICI-associated AKI is made, management of AIN includes withholding ICI therapy and treating with corticosteroids, if no contraindication exists. This initial approach is associated with partial or complete remission in approximately 90% of cases.18,32 Immunosuppression with prednisone at a starting dosage of 0.8 to 1.0 mg/kg (or equivalent) with maximal dosage of 60 to 80 mg daily for stage 1 to 2 AKI, has resulted in excellent response. Pulse-dose i.v. corticosteroids for 2 to 3 days are usually reserved for patients with stage 3 AKI, followed by oral prednisone treatment. An important point is that corticosteroid therapy should be longer in these patients with a slow taper due to frequent relapse. Eight to 12 weeks may be required depending on the response to immunosuppression and/or recurrence of AKI as the steroid is being tapered. This is in part because of the long half-life of ICIs, which range from 6.1 days (avelumab) to up to 27.3 days (pembrolizumab).53 Because of the long half-life and unique immune response that may be quite prolonged over several months after drug discontinuation, an extended time of immunosuppressive therapy may be required.
In addition to corticosteroid duration, the initial dose may also play a role in the recovery. Manohar et al.32 noted that patients who had a complete response received higher corticosteroid dosages than those who had only a partial response (median 2.79 [1.45–3.2] mg/kg per month vs. 1.74 [0.8–3.2] mg/kg per month). Although comparisons of taper regimens were not able to be performed, there was a trend to suggest that higher initial steroid doses may improve recovery of kidney function.32 However, when patients present with corticosteroid-refractory or significant steroid-dependent side effects, prompt escalation of immunosuppression by switching or adding other immunosuppressant drugs, such as mycophenolate mofetil, infliximab, or rituximab, should be considered.24,47
Another unsettled question is whether immunosuppression therapy for iRAEs can counteract the therapeutic effects of ICIs and interfere with overall cancer outcomes. Despite this concern, most of the studies have not shown worsening of outcomes.54, 55, 56 Survivorship bias may exist in these analyses, as some studies have indicated that higher doses of corticosteroids may reduce survival, with prednisone doses ≥10 mg (or equivalent) have been associated with poorer outcome in patients with non–small-cell lung cancer treated with PD-L1 inhibitors.57,58 Therefore, cautious use of corticosteroids is recommended, supporting definitive diagnosis of the kidney lesion (AIN vs. other). Overall, it appears that the inability to use another effective chemotherapy agent or to rechallenge with same or alternative ICI therapy may be the main factor affecting outcomes.
Recommendations for ICI Rechallenge After ICI-Associated AKI
In some patients with cancer, ICIs may be the only therapeutic option available to effectively treat the cancer and maintain tumor remission. The big question that exists is the safety of ICI rechallenge after an episode of AKI, in particular stage 2 or 3 AKI. We suggest the following approach to ICI rechallenge. Once kidney function improves after an episode of ICI-associated AKI, and corticosteroid taper is complete or nearly complete, rechallenge can be attempted. Before starting, an in-depth discussion of risk-benefit with patient and the oncology/nephrology team is required. Cortazar and colleagues18 rechallenged 31 patients with the same ICI; 40% received corticosteroids at the time of rechallenge. Recurrence of ICI-associated AKI occurred in 23% of rechallenged patients, with a shorter latency period between the initial AKI episode and rechallenge. Importantly, all but 1 patient had complete or partial recovery of kidney function after rechallenge.18 Similarly, Manohar et al.32 described 4 patients who underwent rechallenge after removing AIN-associated drugs in 3 of the 4 patients. Rechallenge was done along with low dosage of corticosteroid therapy (prednisone 10–20 mg daily) in 3 of 4 patients. Only 1 patient developed recurrent AKI, but this patient also had an extrarenal iRAE and cancer-associated partial obstructive uropathy.32
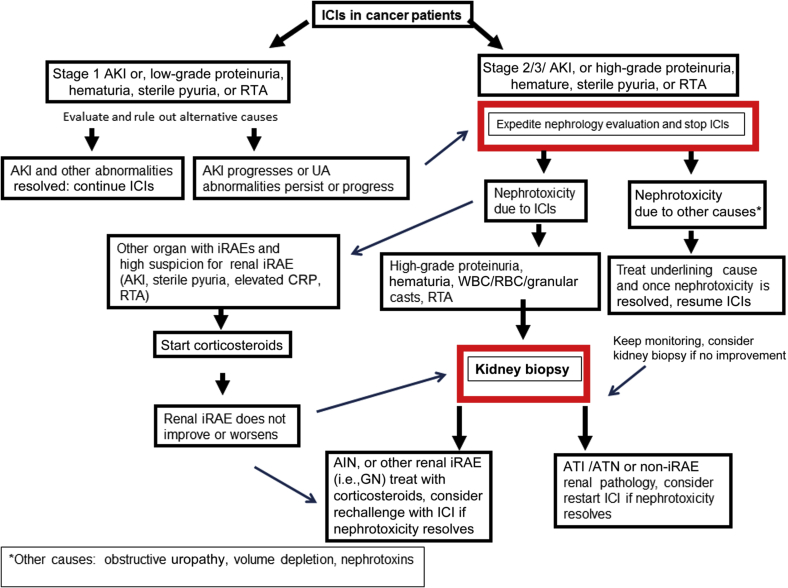
It is important to note that in both studies in which ICI-associated AKI occurred, patients were receiving a concomitant AIN-inducible drug. These patients had a greater probability of complete kidney recovery with corticosteroid therapy.18,32 This result may reflect decreased immunologic activity to the drug (proton pump inhibitor, nonsteroidal anti-inflammatory drug, or antibiotic) once discontinued rather than the presence of a self-antigen.59 As a result, this information may help guide the decision in favor of ICI rechallenge in patients who had a potential drug trigger for the ICI-associated AIN. Once rechallenge is pursued, close monitoring for AKI recurrence is important for early recognition and prompt intervention with immune suppression and/or immunomodulatory strategies. Figure 3 outlines an algorithm of our approach on how to proceed in cases of ICI-associated nephrotoxicity and rechallenge.
Figure 3.
Approach to immune checkpoint inhibitor (ICI) nephrotoxicity and rechallenge. AIN, acute interstitial nephritis; AKI, acute kidney injury; ATN, acute tubular necrosis; CRP, C-reactive protein; GN, glomerulonephritis; iRAE, immune-related adverse event; RBC, red blood cell; RTA, renal tubular acidosis; UA, urinalysis; WBC, white blood cell.
Kidney Transplant and ICI Therapy
Kidney transplant recipients were excluded from initial clinical cancer trials of ICIs, because of the concern of allograft rejection/failure among this population. Therefore, limited data exist on the safety and efficacy of these agents in kidney transplant recipients. The CTLA-4 and PD-1/PD-L1 pathways are essential for self-tolerance by downregulating the immune system during T-cell activation to prevent autoimmunity. Both pathways are implicated in transplant organ tolerance, and interference of these pathways may lead to transplant rejection.60 The limited data available on the efficacy of these drugs in treating cancer and risk for acute rejection is reviewed.
Previous reports on transplant recipients identified patients who experience kidney allograft graft rejection after combination therapy or PD-1 monotherapy.31,61 Ipilimumab monotherapy in patients who received kidney transplantation appeared to have more favorable results than PD-1 pathway blockade.62 In 1 small series, 6 patients with minimized immunosuppression for cancer were treated with ipilimumab.58 At median follow-up of 4.5 months, 1 patient achieved partial response, 1 had disease stabilization, and 4 had progression of the malignancy. Of these, 1 patient had acute T-cell–mediated rejection after the first injection of ipilimumab. The authors concluded that immunosuppression should not be minimized, as the impact on metastatic disease control is probably small.63 Abdel-Wahab and colleagues64 demonstrated early rejection episodes at median time of 21 days in 41% of patients in solid organ transplants. Kidney transplant recipients had the highest indices of allograft rejection. Mortality was reported in 46% of patients who received solid organ transplantation, which included 4 renal transplantation recipients, primarily due to allograft rejection or rejection complications.64 Similarly, De Bruyn et al.61 reported rejection rates of 45% in kidney transplant recipients treated with ICI. In 2 studies, patients were receiving corticosteroids as single-agent maintenance immunosuppression therapy at the time of ICI therapy, which was associated with a higher rate of graft rejection.61,64 Currently, there is no evidence that weaning off all immunosuppression would result in better outcomes and may just risk rejection.
We performed a systematic review of ICI therapy in kidney transplant recipients. A total of 18 of 44 kidney transplant recipients treated with ICIs developed acute rejection. Median time from ICIs to acute rejection diagnosis was 24 days. Fifteen (83%) had allograft failure and 8 (44%) died. Three patients had partial cancer remission (17%), 1 patient achieved cancer response (6%), and 5 patients had stable disease (28%).31 Similar to other studies, the PD-1 pathway appears to be more commonly associated with the graft rejection/failure, suggesting that this pathway may play a more critical role in allograft tolerance.
The optimal immunomodulatory strategies to maximize the outcomes in solid organ transplanted patients while on ICIs are unknown. Some approaches include either reduced doses of calcineurin inhibitors and prednisone or switching to mammalian target of rapamycin inhibitors and using higher-dose prednisone.65,66 Large series are required to more clearly understand the pathogenesis of ICIs in this population. Important information includes data on transplant duration and the time to initiation of immunotherapy, history of donor-specific antibodies or previous rejection, and the strength of correlation of maintenance of immunosuppressive therapy with outcomes. These data may provide insight into the pathogenesis and allow optimal balanced therapy that helps sustain graft tolerance while effectively treating cancer. Despite high risk of rejection, immunotherapy may offer some kidney transplant recipients the last option to treat their malignancy. In this setting, dialysis could be used as renal replacement therapy should the kidney allograft fail. This will ultimately require discussion with the patient and clinicians to reach a consensus decision.
Conclusion
ICI-related nephrotoxicity is uncommon, but the incidence is likely to rise as the use of these efficacious drugs continues to increase. Diagnosis can be a challenge, and at times kidney biopsy may be required. Therapy depends on diagnosis: AKI from AIN benefits from drug withdrawal and corticosteroid therapy, whereas acute tubular injury/ATN should not prevent continued ICI therapy. In patients who would benefit from immunotherapy, rechallenge with an ICI after an episode of AKI can be attempted with close monitoring. ICI therapy in solid organ transplant patients is tricky and more information is required to develop the best immunosuppressive regimen to use during ICI therapy. Importantly, collaborative management by the nephrology and oncology team is paramount, as this population often requires highly individualized management. Further studies will be important for understanding the underlining mechanisms of AKI and identifying predictive biomarkers for treatment outcomes and adverse events to improve treatment efficacy in addition to avoid unnecessary toxicity.
Disclosure
All the authors declared no competing interests.
Acknowledgments
SMH is supported by National Institute of Diabetes and Digestive and Kidney Diseases (grant K08 DK118120) and by Mary Kathryn and Michael B. Panitch Career Development. Both authors had a role in writing the manuscript.
References
- 1.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi L., Rodriguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Hamid O., Robert C., Daud A. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 8.Cortazar F.B., Marrone K.A., Troxell M.L. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manohar S., Kompotiatis P., Thongprayoon C., Cheungpasitporn W., Herrmann J., Herrmann S.M. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34(1):108–117. doi: 10.1093/ndt/gfy105. [DOI] [PubMed] [Google Scholar]
- 10.Seethapathy H., Zhao S., Chute D.F. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14:1692–1700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandhya Manohar R.G., Chengappa M., Bacik Goksu B.N. Acute interstitial nephritis and checkpoint inhibitor therapy: single center experience of management and drug rechallenge. Kidney. 2020;360(1):16–24. doi: 10.34067/KID.0000152019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sury K., Perazella M.A., Shirali A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol. 2018;14:571–588. doi: 10.1038/s41581-018-0035-1. [DOI] [PubMed] [Google Scholar]
- 13.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding H., Wu X., Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–191. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Menke J., Lucas J.A., Zeller G.C. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol. 2007;179:7466–7477. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura H., Nose M., Hiai H. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 17.Marco T., Anna P., Annalisa T. The mechanisms of acute interstitial nephritis in the era of immune checkpoint inhibitors in melanoma. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919875549. 1758835919875549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortazar F.B., Kibbelaar Z.A., Glezerman I.G. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirali A.C., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Fadel F., El Karoui K., Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361:211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 23.Kitchlu A., Fingrut W., Avila-Casado C. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70:581–585. doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Mamlouk O., Selamet U., Machado S. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallan A.J., Alexander E., Reid P., Kutuby F., Chang A., Henriksen K.J. Renal vasculitis and pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis. 2019;74:853–856. doi: 10.1053/j.ajkd.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Kidd J.M., Gizaw A.B. Ipilimumab-associated minimal-change disease. Kidney Int. 2016;89:720. doi: 10.1016/j.kint.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 27.van den Brom R.R.H., Abdulahad W.H., Rutgers A. Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology. 2016;55:1143–1145. doi: 10.1093/rheumatology/kew063. [DOI] [PubMed] [Google Scholar]
- 28.Jung K., Zeng X., Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016;17:188. doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishi S., Minato M., Saijo A. IgA nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med. 2018;57:1259–1263. doi: 10.2169/internalmedicine.9814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daanen R.A., Maas R.J.H., Koornstra R.H.T., Steenbergen E.J., van Herpen C.M.L., Willemsen A. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: a case report. J Immunother. 2017;40:345–348. doi: 10.1097/CJI.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 31.Manohar S., Thongprayoon C., Cheungpasitporn W., Markovic S.N., Herrmann S.M. Systematic review of the safety of immune checkpoint inhibitors among kidney transplant patients. Kidney Int Rep. 2020;5:149–158. doi: 10.1016/j.ekir.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manohar S., Ghamrawi R., Chengappa M. Acute interstitial nephritis and checkpoint inhibitor therapy. Single center experience of management and drug rechallenge. Kidney 360. 2020;1:16–24. doi: 10.34067/KID.0000152019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izzedine H., Mathian A., Champiat S. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassol C., Satoskar A., Lozanski G. Anti-PD-1 immunotherapy may induce interstitial nephritis with increased tubular epithelial expression of PD-L1. Kidney Int Rep. 2019;4:1152–1160. doi: 10.1016/j.ekir.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanchoo R., Karam S., Uppal N.N. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–169. doi: 10.1159/000455014. [DOI] [PubMed] [Google Scholar]
- 36.Girotra M., Hansen A., Farooki A. The Current Understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr. 2018;2:pky021. doi: 10.1093/jncics/pky021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trainer H., Hulse P., Higham C.E. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinol Diabetes Metab Case Rep. 2016;2016 doi: 10.1530/EDM-16-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faje A.T., Sullivan R., Lawrence D. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078–4085. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 39.El Bitar S., Weerasinghe C., El-Charabaty E., Odaimi M. Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med. 2018;2018:8408015. doi: 10.1155/2018/8408015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charmetant X., Teuma C., Lake J. A new expression of immune checkpoint inhibitors’ renal toxicity: when distal tubular acidosis precedes creatinine elevation. Clin Kidney J. 2019;13:42–45. doi: 10.1093/ckj/sfz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Sbeih H., Herrera L.N., Tang T. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J Immunother Cancer. 2019;7:242. doi: 10.1186/s40425-019-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsch J.S., Wanchoo R., Ng J.H. Use of immune checkpoint inhibitors in end stage kidney disease patients, single center experience and review of the literature. Kidney360. 2020;1:399–402. doi: 10.34067/KID.0000422020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abolhassani A.-R., Schuler G., Kirchberger M.C., Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145:2625–2631. doi: 10.1007/s00432-019-03002-1. [DOI] [PubMed] [Google Scholar]
- 44.Manohar S., Albright R.C., Jr. Interstitial nephritis in immune checkpoint inhibitor therapy. Kidney Int. 2019;96:252. doi: 10.1016/j.kint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Qualls D., Seethapathy H., Bates H. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J Immunother Cancer. 2019;7:356. doi: 10.1186/s40425-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams M.C., Turkington T.G., Wilson J.M., Wong T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 47.Brahmer J.R., Lacchetti C., Schneider B.J. Management of Immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson J.A., Schneider B.J., Brahmer J. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Cancer Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 49.Perazella M.A., Shirali A.C. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29:2039–2052. doi: 10.1681/ASN.2018050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann S., Cornell L. SP037PD-L1 staining does not distinguish interstitial nephritis secondary to immune checkpoint inhibitors. Nephrol Dial Transplant. 2018;33(suppl_1):i358–i. [Google Scholar]
- 51.Rosner M.H., Perazella M.A. Acute kidney injury in patients with cancer. N Engl J Med. 2017;376:1770–1781. doi: 10.1056/NEJMra1613984. [DOI] [PubMed] [Google Scholar]
- 52.Pannu N., James M., Hemmelgarn B. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perazella M.A., Shirali A.C. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020;97:62–74. doi: 10.1016/j.kint.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 54.Horvat T.Z., Adel N.G., Dang T.O. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson D.B., Friedman D.L., Berry E. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunol Res. 2015;3:464–469. doi: 10.1158/2326-6066.CIR-14-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toi Y., Sugawara S., Sugisaka J. Profiling preexisting antibodies in patients treated with anti–PD-1 therapy for advanced non–small cell lung cancer. JAMA Oncol. 2019;5:376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faje A.T., Lawrence D., Flaherty K. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124:3706–3714. doi: 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]
- 58.Arbour K.C., Mezquita L., Long N. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 59.Koda R., Watanabe H., Tsuchida M. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. 2018;19:48. doi: 10.1186/s12882-018-0848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kittai A.S., Oldham H., Cetnar J., Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40:277–281. doi: 10.1097/CJI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 61.De Bruyn P., Van Gestel D., Ost P. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol. 2019;31:54–64. doi: 10.1097/CCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 62.Lipson E.J., Bodell M.A., Kraus E.S., Sharfman W.H. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32:e69–e71. doi: 10.1200/JCO.2013.49.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zehou O., Leibler C., Arnault J.-P. Ipilimumab for the treatment of advanced melanoma in six kidney transplant patients. Am J Transplant. 2018;18:3065–3071. doi: 10.1111/ajt.15071. [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Wahab N., Safa H., Abudayyeh A. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106. doi: 10.1186/s40425-019-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnett R., Barta V.S., Jhaveri K.D. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med. 2017;376:191–192. doi: 10.1056/NEJMc1614298. [DOI] [PubMed] [Google Scholar]
- 66.Esfahani K., Al-Aubodah T.-A., Thebault P. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. 2019;10:4712. doi: 10.1038/s41467-019-12628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]