Abstract
In 2018, Kidney Disease: Improving Global Outcomes (KDIGO) published a clinical practice guideline on the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in chronic kidney disease (CKD). The guideline synthesized recent advances, especially in HCV therapeutics and diagnostics, and provided clinical recommendations and suggestions to aid healthcare providers and improve care for CKD patients with HCV. To gain insight into the extent that the 2018 guideline has been adopted in Asia, KDIGO convened an HCV Implementation Summit in Hong Kong. Participants included nephrologists, hepatologists, and nurse consultants from 8 Southeast Asian countries or regions with comparable high-to-middle economic ranking by the World Bank: mainland China, Hong Kong, Japan, Malaysia, Singapore, South Korea, Taiwan, and Thailand. Through presentations and discussions, meeting participants described regional practice patterns related to the KDIGO HCV in CKD guideline, identified barriers to implementing the guideline, and developed strategies for overcoming the barriers in Asia and around the world.
Keywords: chronic kidney disease, dialysis, direct-acting antiviral, infection control, kidney transplantation
People with CKD have an elevated risk of developing chronic HCV infection.1, 2, 3, 4 Chronic HCV infection in the setting of CKD can accelerate decline of kidney function, increase the risk of kidney failure,5, 6, 7, 8 and increase the likelihood of mortality in dialysis patients.9 Fortunately, effective treatment for HCV decreases the incidence of CKD8 and improves kidney and cardiovascular outcomes in patients with diabetes.10 In 2008, KDIGO published the first comprehensive clinical practice guideline on the diagnosis, prevention, and treatment of HCV in CKD.11 At that time, treatment options for HCV in CKD G4 and G5 were limited to interferon with or without ribavirin. Because both interferon and ribavirin are eliminated by the kidney, dose reductions are necessary in patients with advanced CKD. Additionally, interferon and ribavirin have poor efficacy and tolerability, especially in patients undergoing dialysis.9
In the past decade, oral antiviral therapies that directly target HCV replication have become available, with high efficacy rates and good tolerability profiles. Several regimens without interferon and ribavirin have been approved for use in patients with HCV infection and an estimated glomerular filtration rate <30 ml/min per 1.73 m2, including those on dialysis.12,13 Diagnostic methods for determining severity of liver disease have also expanded, and the extent of liver fibrosis can now be assessed using non-invasive measures such as transient elastography.12,13 Given the advances in diagnostics and therapeutics, a comprehensive update of the KDIGO HCV in CKD guideline was undertaken and published in 2018.14
Multiple factors influence the extent of guideline incorporation into clinical practice, such as government health policies, drug availability, reimbursement policies, local economic determinants, and regulatory decisions. KDIGO, in collaboration with the Chinese University of Hong Kong and the Prince of Wales Hospital, Hong Kong, organized the first HCV Guideline Implementation Summit in May 2019 in Hong Kong. The goal of the summit was to understand practice patterns for preventing and managing HCV in CKD in 8 Southeast Asian countries or regions with comparable high or middle economic ranking by the World Bank.15 Nephrologists, hepatologists, and nurse consultants from mainland China, Hong Kong, Japan, Malaysia, Singapore, South Korea, Taiwan, and Thailand attended the meeting and contributed to discussion. Meeting participants shared information related to the availability and reimbursement of direct-acting antiviral (DAA) therapies in each country or region, the current uptake and implementation of the KDIGO HCV in CKD guideline, as well as specific barriers to implementation. Finally, strategies aimed at overcoming these barriers and improving quality of care were developed.
HCV Incidence and Prevalence in Asia
Globally, approximately 70 million people have chronic infection with HCV.16 Prevalence rates vary throughout the world, as well as throughout East and Southeast Asia.17 Gower et al. reported in 2014 an estimated prevalence of HCV RNA positivity of 3.3% in Taiwan, 1.7% in Thailand, 1.1% in Japan and Malaysia, 0.8% in mainland China, 0.4% in the Republic of Korea, and 0.3%–0.5% in Hong Kong.17 HCV genotype distribution also varies throughout Asia. For example, HCV genotype 3 infection is more prevalent in Malaysia (59%) and Thailand (44%) compared with other regions in Asia and with the global population (22%).17
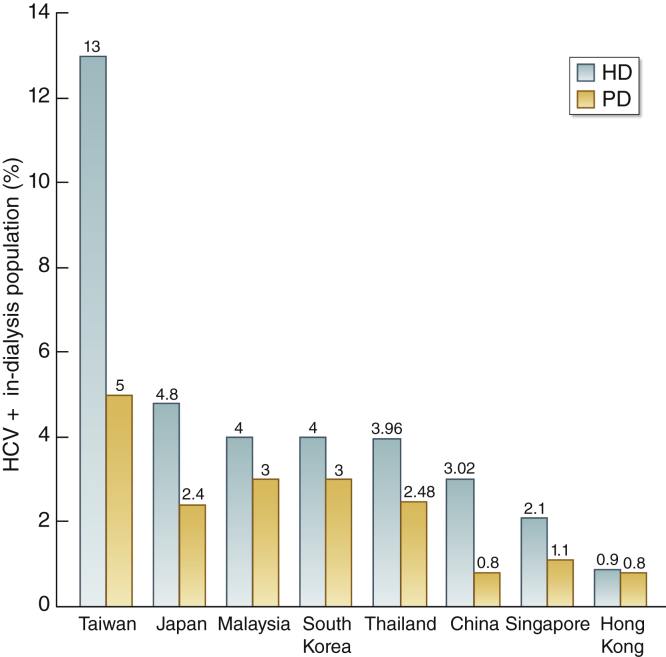
In patients with kidney failure, dialysis modality is an independent predictor of the likelihood of acquiring HCV infection, with hemodialysis (HD) being associated with a higher risk of HCV infection than peritoneal dialysis.18 Internationally, HCV seroprevalence and seroconversion rates among dialysis patients vary widely, suggesting a need for consistent, rigorous local infection control measures. We have updated HCV prevalence in the 8 Southeast Asian countries and regions participating in the summit (Figure 1). Overall, the HCV prevalence in the HD population as determined by anti-HCV antibody testing ranges from 0.9% in Hong Kong to 13% in Taiwan. The HCV prevalence in peritoneal dialysis patients is lower than that in their HD counterparts, ranging from 0.8% in Hong Kong and China to 5% in Taiwan (Figure 1). Since the HCV prevalence data were largely based on anti-HCV antibody positivity rather than HCV RNA positivity, some of the anti–HCV-positive patients may in fact have been cured of HCV after DAA therapy despite harboring anti-HCV antibodies (e.g., Taiwan).
Figure 1.
Prevalence of hepatitis C virus (HCV) in hemodialysis (HD) and peritoneal dialysis (PD) in Southeast Asian countries and regions. China data (2019) are based on estimates of data from 6 provinces and 7 major cities; Hong Kong data (2019) are based on Hong Kong Renal Registry; Japan data (2018) are based on the Japanese Society for Dialysis Therapy Registry; Malaysia data (2017) are based on the Malaysian Dialysis and Transplant Registry; Singapore data (2018) are based on the Renal Registry of Singapore; South Korea data (2018) are based on the Korean Society of Nephrology Registry; and Taiwan data (2017) are based on the Taiwan Renal Registry. Thailand data (2019) are based on the Renal Registry of Thailand. HCV prevalence data were largely based on anti-HCV antibody positivity rather than HCV RNA positivity. Therefore, some of the anti–HCV-positive patients may in fact have been cured of HCV after direct-acting antiviral therapy despite harboring anti-HCV antibodies (e.g., Taiwan).
Current Uptake of HCV in CKD Guideline
In a pre-meeting survey, approximately 75% of 20 respondents indicated that their country or region has local guidelines on treating HCV in patients with CKD or on dialysis. Fewer than 20% indicated that there are critical differences between the local guidelines and the KDIGO HCV in CKD guideline. Indeed, participants indicated that all regions follow most of the guidance put forth by the KDIGO HCV in CKD guideline, as described below.
Detection and Evaluation of HCV in CKD
Method of Detection
Consistent with the KDIGO HCV in CKD guideline, all participating regions reported screening for HCV using an anti-HCV enzyme-linked immunosorbent assay followed by nucleic acid testing if the assay was positive. Because of its relatively low cost, this anti-HCV assay will likely remain the initial screening test for patients on HD in most regions. In Malaysia, where polymerase chain reaction analysis is available in only a few central laboratories, the HCV core antigen test is being considered as an alternative to nucleic acid testing because HCV core antigen testing costs less and allows confirmatory evaluation to be decentralized. Given that HCV core antigen has a detection threshold of at least 3000 IU/ml, and most patients with HCV have high levels of viremia, good concordance between HCV core antigen and polymerase chain reaction has been demonstrated.19, 20, 21 Currently, HCV core antigen is not yet available in Hong Kong and is relatively expensive in Singapore and mainland China.
Frequency of Testing
For HD patients, all of the participating regions except 2 perform HCV screening tests at 6-month intervals, consistent with the KDIGO HCV in CKD guideline. In Taiwan, HCV screening of HD patients is done every 12 months because of reimbursement policies. In Singapore, HD patients are screened every 3 months, a practice initiated in response to an outbreak of 25 cases of acute HCV infection in the renal ward at Singapore General Hospital in 2015.22
Nearly all participating regions reported that nucleic acid testing is performed annually for patients who either are on HD and anti–HCV-positive, have spontaneously cleared HCV, or have achieved sustained virologic response after treatment. An exception is Malaysia, where nucleic acid testing is not repeated except in the context of HCV treatment.
Treatment of HCV Infection in Patients With CKD
Choice of Regimen
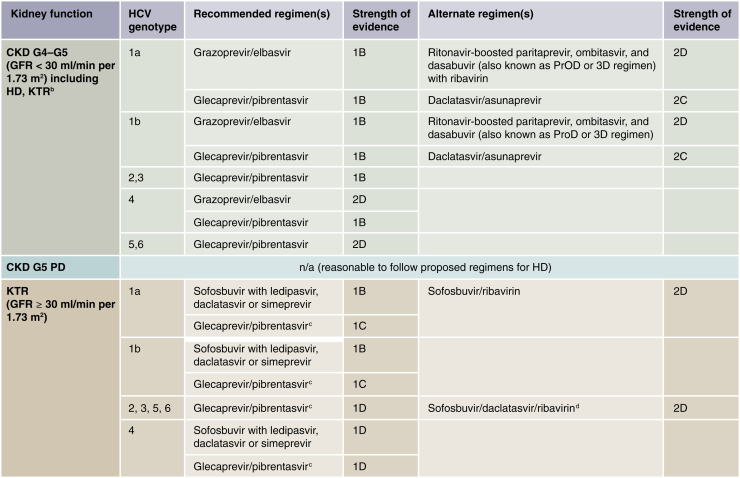
The KDIGO HCV in CKD guideline recommended that all CKD patients with HCV be considered for treatment with an interferon-free regimen. Recommended first-line treatment for patients with CKD G4 or G5 (including HD patients) is either glecaprevir/pibrentasvir for all HCV genotypes or grazoprevir/elbasvir for HCV genotypes 1 and 4 (Figure 2).14,23
Figure 2.
Recommended direct-acting antiviral (DAA) treatment regimens for patients with chronic kidney disease (CKD) G4–G5D and kidney transplant recipients (KTRs), by hepatitis C virus (HCV) genotype.a Duration of therapy for all these regimens is usually 12 weeks, but readers should consult the American Association for the Study of Liver Diseases (AASLD) or the European Association for the Study of the Liver guidelines for the latest information. Use of daclatasvir/asunaprevir is generally more appropriate for patients with genotype 1b, as it is less efficacious in HCV genotype 1a. The US Food and Drug Administration has recently indicated that no dose adjustments are required for sofosbuvir-based regimens in CKD patients, including those on dialysis based on pharmacokinetic data and several case series rather than on randomized controlled trial data. These regimens may be considered pending their availability in various jurisdictions. However, readers are encouraged to consult https://www.hep-druginteractions.org/ for drug–drug interactions, particularly with immunosuppressants (e.g., cyclosporine, sirolimus, and tacrolimus). aIt was recommended that CKD patients with glomerular filtration rates (GFRs) ≥ 30 ml/min per 1.73 m2 (CKD G1T–G3bT) be treated with any licensed DAA regimen. bThere is little published evidence to guide treatment regimens in KTRs with GFR < 30 ml/min per 1.73 m2 (CKD G4T–G5T). Regimens in KTRs should be selected to avoid drug–drug interactions, particularly with calcineurin inhibitors. cBased on Reau et al.23dAs suggested in AASLD guidelines (https://www.hcvguidelines.org), HCV protease inhibitors that end in “previr,” such as glecaprevir or grazoprevir, should not be used in patients with decompensated cirrhosis.12,13 HD, hemodialysis; n/a, no data/evidence available; PD, peritoneal dialysis.
At the time of HCV in CKD KDIGO guideline publication, and during the HCV in CKD Asia summit, sofosbuvir-based regimens were not recommended for CKD G4 or G5 (estimated glomerular filtration rate < 30 ml/min per 1.73 m2).12,13 However, in November 2019, the US Food and Drug Administration extended the label of sofosbuvir to include CKD G4 and G5 patients and those on dialysis. Although sofosbuvir-based regimens may provide a treatment option in countries where glecaprevir/pibrentasvir and/or grazoprevir/elbasvir are not available, this label extension is as yet limited to the US and is based on pharmacokinetic data and several case series rather than on randomized controlled trial data.
Although daclatasvir plus asunaprevir was listed as a treatment option in HCV genotype 1a and 1b patients with CKD G4 or G5,14,24, 25, 26, 27, 28 evidence from Japan suggests that daclatasvir/asunaprevir has suboptimal efficacy against genotype 1a.29,30 Meeting participants felt that all patients who have chronic HCV infection should be treated, regardless of the extent of liver fibrosis, although in some regions, treatment is currently prioritized based on the extent of liver fibrosis. Availability and accessibility of originator and generic DAA regimens vary greatly among the regions represented (Table 1). Lack of availability and high costs or copays were identified as major barriers to treatment. In some regions, a major barrier for CKD patients is that consultation with a hepatologist is necessary for DAA therapy. Additionally, technology related to assessment of liver fibrosis, such as transient elastography, may be available only to hepatologists. Multiple regions, including Taiwan and Korea, cited patients’ lack of awareness of their HCV status or of treatment efficacy as a major barrier to treatment.
Table 1.
DAA treatments for chronic hepatitis C in Asian regions
| Country/region | Available DAAs | Payment policies | Fibrosis status restrictions | CKD restrictions | Specialist restrictions |
|---|---|---|---|---|---|
| China mainland | All major originator drugs are available. Licensed generics are not available. | Covered under national reimbursement list but policy varies across provinces and cities | No restrictions | No restrictions | For CKD G1–G3, need to refer to hepatologist. For CKD G4 or above, nephrologist can initiate treatment. |
| Hong Kong | Licensed generics are not available. | Covered by the Hospital Authority if reimbursement criteria are met | CKD patients with fibrosis stage F2 would receive DAA. | Treatment coverage to all dialysis patients, irrespective of waitlist status for transplants, can receive DAA. | Need referral to hepatologist |
| Korea | All originator drugs are available. Licensed generics are not available. | Covered by national insurance, although copays can be high | Protease inhibitors are prohibited in decompensated cirrhosis and dasabuvir, GLE, GZR, and voxilaprevir are to be avoided in moderate to severe liver failure. DAA treatment is currently not reimbursed in untreated HCC or relapsed patients. (Reimbursement criteria updated periodically by Health Insurance Review & Assessment Service [HIRA]) | Recommend avoiding sofosbuvir in patients with severe kidney impairment (eGFR <30 ml/min per 1.73 m2) | No restrictions but usually in consultation with hepatologist |
| Japan | Licensed generics are not available. | Covered by national insurance, although patients also pay out-of-pocket | No restrictions | For HCV genotype 1: a. CKD G1–G3: SOF/LDV; GZR/EBR; GLE/PIB b. CKD G4-G5D GZR/EBR; GLE/PIB For HCV genotype 2: a. CKD G1–G3: SOF/RBV; GLE/PIB; SOF/LDV b. CKD G4–G5D: GLE/PIB |
No restrictions but usually in consultation with hepatologist |
| Malaysia | Generic sofosbuvir and daclatasvir are currently available in public hospitals. In private practice, some originators are available (sofosbuvir, ledipasvir/sofosbuvir, sofosbuvir/velpatasvir). |
Generic DAAs covered by Ministry of Health; patients must pay for originators. | No restriction except that protease inhibitors GLE and GZR cannot be used in decompensated cirrhosis | If eGFR <30 ml/min per 1.73 m2, formerly GZR/EBR and 3D could be used for priority for transplant candidates. However, these are not available in the Malaysian market anymore. GLE/PIB are likely to be approved in 2020 but may be restricted in use because of cost. | Primary care is able to treat uncomplicated hepatitis C with generic SOF and daclatasvir. For complicated cases such as patients with CKD, due to the cost of GLE/PIB, hepatologist/gastroenterologist consultation is required. |
| Singapore | All originator drugs are available. Licensed generics are not available. | Copays can limit patient access. | No restrictions | No restrictions | No need for hepatologist referral |
| Taiwan | All originator drugs are available. Licensed generics are not available. | Covered by national health insurance | No restrictions | No restrictions | Need referral to hepatologist |
| Thailand | Generic drugs are available but may be restricted in some circumstances. | Government coverage mostly | No restrictions | Only sofosbuvir-based regimens available. | Treatment decisions mostly by hepatologists; nephrologists cannot prescribe DAAs |
3D, ombitasvir/paritaprevir/ritonavir + dasabuvir; CKD, chronic kidney disease; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; GLE/PIB, glecaprevir/pibrentasvir; GZR/EBR, grazoprevir/elbasvir; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SOF/LDV, sofosbuvir/ledipasvir; SOF/RBV, sofosbuvir/ribavirin.
Drug–Drug Interactions
In line with the KDIGO HCV in CKD guideline, participants commonly referred to the Hepatitis Drug Interactions website from the University of Liverpool (http://www.hep-druginteractions.org) as their primary reference tool for minimizing drug–drug interactions.
Hepatitis B Screening
Most participants indicated that they screen for hepatitis B surface antigen and anti-hepatitis B core antibody (anti-HBc) before starting DAAs. In HCV patients positive for the antigen, the benefits of HCV treatment versus the risk of hepatitis B virus (HBV) reactivation must be considered. Patients with HBV/HCV coinfections who are not undergoing treatment for hepatitis B or who are positive for anti-hepatitis B core antibody must have alanine aminotransferase levels monitored during DAA therapy.
Preventing HCV Transmission in HD Units
Participants felt that physicians are ultimately responsible for ensuring infection control measures and for quality assurance practices in their units. Nursing staff should provide input in creating guidelines for quality assurance and be involved in training staff members on quality assurance practices. In many centers in Hong Kong, patients are encouraged to remind staff of infection control practices, including hand hygiene.
Staff Education
All participants reported training of new staff members, and the majority reported repeat training between once and twice a year. Thailand, Taiwan, Malaysia, and Korea report yearly training; Japan and mainland China report training twice a year. In Hong Kong, most dialysis facilities refer to “Clinical Practice Guidelines for the Provision of Renal Service in Hong Kong” published by the Hong Kong College of Physicians, Central Renal Committee of Hospital Authority and Hong Kong Society of Nephrology.31 This guideline was recently updated following its first publication in 1999.
All HD staff should attend infection control refresher training at least once every 24 months. In mainland China, routine nurse training programs are provided twice per year, and nurses from satellite units meet in central cities for lectures on infection control measures. Taiwan has yearly continuing medical education programs, and the renal society offers accreditation (funded by insurance). In Korea, infection control guidelines from the Korean Society of Nephrology are implemented. HD quality practices, including screening for anti-HCV antibodies, are assessed annually by a national health insurance review. In Singapore, all new staff members review written protocols for infection control and subsequently are tested on their knowledge of the material. Clinical practice improvement projects such as Rapid Improvement Events (RIEs) are used to identify shortfalls and implement continuous quality improvement initiatives. In cases of lapses, corrective measures are taken. In Malaysia, infection control measures are covered in national HD quality standards, and nephrologists and senior paramedics lead annual refresher courses on infection control in dialysis. In Thailand, staff must be accredited (i.e., have received training for 6 months, plus have 4 years of experience in HD centers, with a minimum of 1000 HD sessions to qualify for a state license examination) prior to working in dialysis units, with infection control a part of the curriculum. Refresher courses are required annually.
Other Organizational Aspects
Participants reported that auditing measures are in place in most dialysis units in their regions. All regions report having reasonable infrastructure (e.g., adequate space for dialysis stations) and staffing ratios (1:1 for intensive care, 1:3 for HD units in Hong Kong). Taiwan will soon embark on a microelimination program in which all HCV patients in the same unit are treated at the same time, to prevent transmission32; an early treatment program for new HCV patients is underway there.
Care Bundles for Preventing Nosocomial Infections
Care bundles for preventing blood-borne virus transmission should incorporate careful hygienic precautions (especially handwashing and changing gloves between patients), periodic testing of patients, and cleaning of dialysis machines (including their surface) and blood- or body-fluid contaminated environmental areas with 1% sodium hypochlorite solutions or another disinfecting agent as recommended by the manufacturer of the HD machines. For patients with hepatitis B, isolation is practiced, and dialyzers are not reused. A clustered randomized controlled trial could be used to evaluate the effectiveness of care bundles, but a sufficient number of centers would be needed to have adequate statistical power.
Segregation of HCV-Positive Individuals Within Dialysis Units
The KDIGO HCV in CKD guideline recommended not using dedicated dialysis machines for HD patients with HCV and suggested not isolating HD patients with HCV.14 Indeed, although there have been no high-quality randomized trials, there is ample evidence that the transmission of HCV, whose infectivity is much lower than that of HBV, can be avoided if hygienic precautions are correctly followed, and isolation practices may entail the risk of reducing the emphasis on optimal hygienic practices.14 Thailand was the only country/region to report following these recommendations.
Thailand Hemodialysis Clinical Practice Recommendation 2014 does not recommend using dedicated dialysis machines for patients with blood-borne infections and suggests isolating only those HD patients who have HBV. It is notable that there has been no outbreak of HBV or HCV infections in the renal units in Thailand in the past 5 years. The remaining regions felt that stigmatization of HCV-positive patients is not a major problem, and in areas with high HCV prevalence, it is necessary to isolate patients. Reported isolation practices vary by region, with all except Thailand using designated machines. Participants from Singapore reported not isolating patients physically. Other regions isolate patients within the same room (Hong Kong, Taiwan, Japan, Malaysia) or within the same room behind a glass wall or curtains (China, Korea).
Management of CKD Patients With HCV Before and After Kidney Transplantation
Transplantation Waiting Time and HCV Treatment
Among participating regions, the waiting time for deceased-donor kidney transplantation is more than 5 years, and therefore patients with HCV are likely to be treated with DAAs as indicated before transplantation. In nearly all participating regions, in living-donor situations, anti-HCV treatment is not necessarily done prior to transplant, so patient preferences become more important. However, in Taiwan, hepatologists support HCV treatment as soon as possible in living-donor situations because it reduces the potential drug–drug interactions with post-transplant immunosuppressive regimens.
Anti–HCV-Positive Kidney Donors
Because the prevalence of HCV is low in many Asian countries, anti–HCV-positive kidney donors are generally uncommon. Thailand is the only region where HCV-positive organs can be transplanted into uninfected recipients if deemed acceptable by recipients. In South Korea, Taiwan, and Hong Kong, kidneys from anti–HCV-positive donors are accepted for transplantation into HCV-positive recipients. In Japan, if deceased donors are HCV RNA–positive, HCV genotype is tested, and transplantation may be done if the recipient is HCV RNA–positive and has the same HCV genotype. In Malaysia, anti–HCV-positive donors are currently not permitted based upon Ministry of Health guidelines, but these guidelines will be reviewed in the near future. In mainland China, there is no formal policy on this issue.
Assessment of Liver Cirrhosis in Kidney Transplant Candidates
Meeting participants indicated that transient elastography is commonly used to assess extent of liver fibrosis. Liver biopsy is generally not performed unless non-invasive assessments are inconclusive or uncertain. However, in regions where patients with compensated cirrhosis cannot be a candidate for kidney transplantation, liver biopsy may be needed to confirm a diagnosis.
Diagnosis and Management of Kidney Diseases Associated With HCV Infection
Kidney Biopsy and Cryoglobulin Testing
All participants agreed that kidney biopsy is necessary for assessing histological activity of HCV-related glomerulonephritis. In Thailand, not all nephrologists perform biopsies, and availability of renal pathologists is a barrier to assessing histological activity. In mainland China and Japan, nephrologists, in addition to renal pathologists, are involved in pathological interpretation. In Korea, there are generally no limitations on access to or interpretation of kidney biopsies.
For patients suspected to have HCV-related glomerulonephritis, cryoglobulin testing is still performed in most participating regions in Asia. However, false negatives are problematic, and results are not always used in treatment decisions. Evidence is needed to clarify the role of cryoglobulin testing for managing HCV-related glomerulonephritis.
Treatment
Participants agreed with current KDIGO HCV in CKD guideline recommendations that patients with severe cryoglobulinemia or severe glomerular disease induced by HCV should be treated with immunosuppressive agents with or without plasma exchange in addition to DAA therapies.14 Although rituximab is the preferred immunosuppressive agent, it may not be reimbursed in some countries, and further research is needed on how to stratify or identify patients for early rituximab therapy.
In all cases, careful monitoring of HCV RNA levels, changes in kidney function, evolution of proteinuria, and side effects from antiviral therapy is necessary.
Approaches for Improving Guideline Adherence
Awareness
Approaches that could increase regional awareness of the KDIGO HCV in CKD guideline include incorporating its recommendations within local guidelines, developing shortened or simplified guideline summaries, translating the guideline into languages beyond English and Chinese, and developing an app for more widespread accessibility. Participants also felt that the algorithms for HCV testing and management could be simplified, which could in turn increase the understanding of the steps needed to achieve individual cure and monitoring of HCV. Broadening screening recommendations could increase awareness of HCV and access to testing and treatment. To this end, in early 2020, the US Preventive Services Task Force expanded HCV screening recommendations to one-time, universal screening for all adults aged 18 to 79 years.33
Microelimination strategies23,32,34,35 in specific regions or within dialysis units could serve as models to demonstrate that elimination is possible, providing more evidentiary support for adhering to the KDIGO HCV in CKD guideline and realizing the goal by the World Health Organization to eliminate HCV as a public health problem by 2030.36
DAA Availability and Cost
Having more widely available licensed generic DAA regimens would increase the numbers of patients who can be treated. WHO has promoted driving increased access to DAA-based treatment through steep price reductions which have occurred due largely to increased competition, especially from generic manufacturers.37 A voluntary license is an agreement between an originator manufacturer and a generic manufacturer that allows the production and sale of a patented drug in certain countries, subject to licensing terms. It has been reported that voluntary licensing initiatives appeared to substantially improve HCV treatment uptake in eligible countries.38 This evidence supports the expansion of licensing strategies to include more countries and more treatments. However, the number of countries and regions in Southeast Asia with available generic DAA regimens seems unlikely to change in the near future. More interventions through multiple parties, including governments, are required to speed up the expanded access to generic DAA regimens in the region. Reducing patient copays would also make regimens more accessible to patients.
KDIGO’s Role in Assisting Guideline Implementation
Cost and reimbursement policies are among the key barriers to implementing the HCV in CKD guideline. KDIGO could consider analyzing and reporting health economics related to HCV infection and its treatment in CKD. Such cost-effectiveness data are important for developing health policies and could help bridge the gap between guideline recommendations and implementation. Incentivizing payer funding for DAA treatments may result in lower healthcare costs in the long run. General formulas for calculating the incremental cost-effectiveness ratio could be developed, and calculations could be individualized based on actual costs in specific regions or countries. KDIGO also may consider developing web-based education and training materials, such as podcasts, regionally relevant management toolkits, and decision-support aids to assist clinicians in providing structured HCV care. KDIGO is ready to partner with local professional organizations or national nephrology societies on efforts toward guideline education, training, and implementation. Preventing HCV transmission within HD units is a reachable goal if auditing and appropriate education of staff are implemented and prioritized by national and local leaders.
Conclusions
Limited availability and high costs of DAA regimens are major barriers to HCV treatment in CKD populations in many Asian countries. In regions where DAAs are more available and affordable, limited patient awareness of HCV status and treatment options is a common barrier. Infection control practices in dialysis units vary among regions, with most still either physically isolating patients with HCV or using designated dialysis machines. The strategy of microelimination of HCV from specific CKD populations, such as dialysis patients, may be an achievable goal in some countries and regions. Using the HCV in CKD KDIGO guideline as a tool to aid in moving toward successful microelimination could lead to further awareness and uptake of the guideline in the CKD population.
Disclosure
PK-TL declared having received speaker honoraria from FibroGen. C-CS declared having received consultancy fees from Baxter Healthcare and Gilead Sciences. VW-SW declared having received consultancy fees from 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, the Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns; speaker honoraria from AbbVie, Bristol-Myers Squibb, Echosens, and Gilead Sciences; and research support from Gilead Sciences. Y-YD declared having received consultancy fees from Gilead Sciences and Novartis; and research support from Norvo Nordisk and Perspectum Diagnostics. C-LL declared having received consultancy fees from Arrowhead Pharmaceuticals; speaker honoraria from AbbVie, Gilead Sciences, and Novartis; and a patent on Hepatitis B variants with reduced sensitivity to therapeutic compounds, their detection, and uses thereof. GL-HW declared having received consultancy fees from Gilead Sciences and Janssen; speaker honoraria from Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche; and research support from Gilead Sciences. TK declared having expected to receive fees from Visterra and received research support from the National Research Council of Thailand. AY-MW declared having received speaker honoraria from Fresenius Kabi; and research support from Sanofi Renal. M-LY declared having received consultancy fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, and MSD; speaker honoraria from Abbott, AbbVie, Bristol-Myers Squibb, Gilead Sciences, and MSD; and research support from Bristol-Myers Squibb, Gilead Sciences, and MSD. MJ declared having received consultancy fees from Amgen, AstraZeneca, Mundipharma, MSD, and Vifor Fresenius Medical Care; speaker honoraria from AbbVie, Amgen, Menarini, MSD, and Vifor Fresenius Medical Care; and research support from Amgen, MSD, and Otsuka. All the other authors declared no competing interests.
Acknowledgments
The conference was sponsored by KDIGO and supported in part by Fresenius Medical Care and Merck, Sharp & Dohme, Corp. Organizational and logistical support was also provided by The Chinese University of Hong Kong, the Hong Kong Association for the Study of Liver Diseases, the Hong Kong College of Physicians, the Hong Kong Society of Nephrology, and Prince of Wales Hospital.
The authors acknowledge the following for the provision of prevalence of hepatitis C virus data in hemodialysis and peritoneal dialysis patients: China: through Dr. WH Lu; Hong Kong: Hong Kong Renal Registry through Drs. CB Leung and SK Mak; Japan: Japanese Society for Dialysis Therapy through Drs. K Iseki and Y Ito; Malaysia: Malaysian Dialysis and Transplant Registry of Malaysian Society of Nephrology through Drs. G Ahmad and S Bavanandan; Singapore: Singapore Renal Registry, National Registry of Diseases Office through Dr. BW Teo; South Korea: Renal Registry of Korean Society of Nephrology through Dr. HC Park; Taiwan: Renal Registry of Taiwan through Dr. CC Huang; Thailand: Renal Registry of Thailand through Dr. T Kanjanabuch. We also thank Jennifer King, PhD, for assistance with manuscript preparation.
Contributor Information
Philip Kam-Tao Li, Email: philipli@cuhk.edu.hk.
Michel Jadoul, Email: michel.jadoul@uclouvain.be.
References
- 1.Fabrizi F., Verdesca S., Messa P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta-analysis. Dig Dis Sci. 2015;60:3801–3813. doi: 10.1007/s10620-015-3801-y. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H., Xu H., Wu R. Association of hepatitis C and B virus infection with CKD and impact of hepatitis C treatment on CKD. Sci Rep. 2019;9:1910. doi: 10.1038/s41598-018-36437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel A., Bhadauria D.S., Aggarwal R. Hepatitis C virus infection and chronic renal disease: a review. Indian J Gastroenterol. 2018;37:492–503. doi: 10.1007/s12664-018-0920-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.C., Chiou W.Y., Hung S.K. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol. 2013;14:187. doi: 10.1186/1471-2369-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.J., Lin M.Y., Chang J.S. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molnar M.Z., Alhourani H.M., Wall B.M. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H., Adeyemi A., Henry L. A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat. 2015;22:897–905. doi: 10.1111/jvh.12413. [DOI] [PubMed] [Google Scholar]
- 8.Park H., Chen C., Wang W. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD. Hepatology. 2018;67:492–504. doi: 10.1002/hep.29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabrizi F., Takkouche B., Lunghi G. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y.C., Lin J.T., Ho H.J. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes HCV Work Group KDIGO Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int Suppl. 2008:S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 13.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) HCV guidance: recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org/ Available at:
- 14.Kidney Disease: Improving Global Outcomes Hepatitis C Work Group KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int Suppl (2011) 2018;8:91–165. doi: 10.1016/j.kisu.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad N., Jha V. Hemodialysis in Asia. Kidney Dis (Basel) 2015;1:165–177. doi: 10.1159/000441816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Hepatitis C fact sheet. http://www.who.int/news-room/fact-sheets/detail/hepatitis-c Available at:
- 17.Gower E., Estes C., Blach S. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Johnson D.W., Dent H., Yao Q. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598–1603. doi: 10.1093/ndt/gfn684. [DOI] [PubMed] [Google Scholar]
- 19.Freiman J.M., Tran T.M., Schumacher S.G. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016;165:345–355. doi: 10.7326/M16-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevaliez S., Soulier A., Poiteau L. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. J Clin Virol. 2014;61:145–148. doi: 10.1016/j.jcv.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Miedouge M., Saune K., Kamar N. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. J Clin Virol. 2010;48:18–21. doi: 10.1016/j.jcv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Singapore Ministry of Health Independent Review of a Hepatitis C Cluster in Singapore General Hospital Renal Ward. https://www.moh.gov.sg/docs/librariesprovider5/pressroom/current-issues/the-independent-review-committee-report-executive-summary.pdf Available at:
- 23.Reau N., Kwo P.Y., Rhee S. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection. Hepatology. 2018;68:1298–1307. doi: 10.1002/hep.30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suda G., Nagasaka A., Yamamoto Y. Safety and efficacy of daclatasvir and asunaprevir in hepatitis C virus-infected patients with renal impairment. Hepatol Res. 2017;47:1127–1136. doi: 10.1111/hepr.12851. [DOI] [PubMed] [Google Scholar]
- 25.Suda G., Kudo M., Nagasaka A. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol. 2016;51:733–740. doi: 10.1007/s00535-016-1162-8. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda H., Kumada T., Tada T. Safety and efficacy of dual direct-acting antiviral therapy (daclatasvir and asunaprevir) for chronic hepatitis C virus genotype 1 infection in patients on hemodialysis. J Gastroenterol. 2016;51:741–747. doi: 10.1007/s00535-016-1174-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee B.S., Song M.J., Kwon J.H. Efficacy and safety of daclatasvir and asunaprevir in patients with hepatitis C virus genotype 1b infection on hemodialysis. Gut Liver. 2019;13:191–196. doi: 10.5009/gnl18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suda G., Furusyo N., Toyoda H. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: a nationwide retrospective study in Japan. J Gastroenterol. 2018;53:119–128. doi: 10.1007/s00535-017-1353-y. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki F., Hatanaka N., Bando E. Safety and effectiveness of daclatasvir and asunaprevir dual therapy in patients with genotype 1 chronic hepatitis C: results from postmarketing surveillance in Japan. Hepatol Int. 2018;12:244–253. doi: 10.1007/s12072-018-9872-z. [DOI] [PubMed] [Google Scholar]
- 30.Huang C.F., Yu M.L. Daclatasvir plus asunaprevir in the treatment of uremic patients with chronic hepatitis C genotype 1b infection. Kidney Int. 2020;97:615. doi: 10.1016/j.kint.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Li P.K., Kwan B.C., Wong A.K. Clinical practice guidelines for the provision of renal service in Hong Kong: accreditation of renal unit. Nephrology (Carlton) 2019;(suppl 1):130–132. doi: 10.1111/nep.13496. [DOI] [PubMed] [Google Scholar]
- 32.Huang C.F., Chiu Y.W., Yu M.L. Patient-centered outreach treatment toward micro-elimination of hepatitis C virus infection in hemodialysis patients. Kidney Int. 2020;97:421. doi: 10.1016/j.kint.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 33.US Preventive Services Task Force. Owens D.K., Davidson K.W. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323:970–975. doi: 10.1001/jama.2020.1123. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus J.V., Safreed-Harmon K., Thursz M.R. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38:181–192. doi: 10.1055/s-0038-1666841. [DOI] [PubMed] [Google Scholar]
- 35.Devresse A., Delire B., Lazarus J.V. Eliminating hepatitis C virus from a prevalent kidney transplant recipient population: a single-center study in Belgium in the direct-acting antivirals era. Transplantation Proc. 2020;52:815–822. doi: 10.1016/j.transproceed.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Thomas D.L. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041–2050. doi: 10.1056/NEJMra1810477. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization Progress report on access to hepatitis C treatment: focus on overcoming barriers in low- and middle-income countries. https://apps.who.int/iris/handle/10665/260445 Available at:
- 38.Simmons B., Cooke G., Miraldo M. Effect of voluntary licences for hepatitis C medicines on access to treatment: a difference-in-differences analysis. Lancet Glob Health. 2019;7:e1189–e1196. doi: 10.1016/S2214-109X(19)30266-9. [DOI] [PubMed] [Google Scholar]