Abstract
Introduction
Greater reduction in estimated glomerular filtration rate (eGFR) after specific treatment for primary aldosteronism (PA) reflects improvement in glomerular hyperfiltration associated with PA and leads to better patient outcomes. However, little is known regarding the mechanisms underlying eGFR reduction after treatment for PA.
Methods
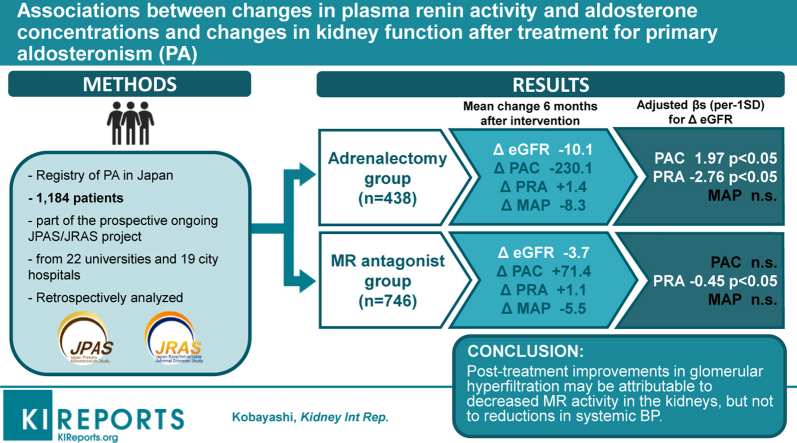
We analyzed data from the nationwide PA registry in Japan. Patients were assigned to adrenalectomy (n = 438) and mineralocorticoid receptor (MR) antagonist (n = 746) groups. We assessed associations between changes in blood pressure (BP), plasma renin activity (PRA) and plasma aldosterone concentrations (PAC), and eGFR before and 6 months after treatment for both groups.
Results
In a multivariable linear regression, the adjusted β values (95% confidence interval [CI]) for change in eGFR after treatment were −2.76 (−4.29, −1.22) ml/min per 1.73 m2 for PRA (per 3.2 ng/ml per hour), and 1.97 (1.08, 2.85) ml/min per 1.73 m2 for PAC (per 236.1 pg/ml) in the adrenalectomy group; and −0.45 (−0.89, −0.01) ml/min per 1.73 m2 for PRA and −0.72 (−1.62, 0.18) ml/min per 1.73 m2 for PAC in the MR antagonist group. Change in mean arterial pressure after treatment was not significantly associated with change in eGFR in either group. Changes in PRA and PAC but not BP before and 6 months after treatment for PA were associated with greater reductions in eGFR.
Conclusion
Post-treatment improvements in glomerular hyperfiltration may be attributable to decreased MR activity in the kidneys, but not to reductions in systemic BP.
Keywords: aldosterone, blood pressure, glomerular hyperfiltration, mineralocorticoid receptor activity, primary aldosteronism, renin activity
Graphical abstract
Primary aldosteronism (PA), characterized by autonomous aldosterone overproduction independently of renin secretion, is the most common cause of secondary hypertension, with an estimated prevalence of 5% to 10% in persons with hypertension.1 Aldosterone adversely affects the kidneys2 by increasing inflammation and fibrosis,3 vascular disease, and podocyte injury.4 Patients with PA have a higher risk of kidney diseases compared to those with essential hypertension.2 This suggests that kidney injury associated with PA may be independent of BP.
Aldosterone stimulates sodium reabsorption in the kidneys and volume expansion in vivo, which results in increased renal perfusion and glomerular hyperfiltration.5,6 Treatment options for PA include use of an MR antagonist and surgery (i.e., adrenalectomy). These treatments have been shown to reduce eGFR within 6 months after treatment, which is supposed to reflect improvement of glomerular hyperfiltration by treatment. We recently reported that greater reduction in eGFR 6 months after treatment for PA was linked to less reduction in eGFR from the 6-month follow-up examination through the 5-year follow-up examination.7 This suggests that improvement in glomerular hyperfiltration within 6 months after treatment contributes to better kidney outcomes in later life among PA patients. Surgical treatment has been shown to be better in preventing kidney dysfunction compared to MR antagonist treatment among patients with PA.8,9 However, it is not clear whether a reduction in eGFR within 6 months after treatment is associated with changes in circulating aldosterone levels and BP due to treatments, and these associations differ by treatment type. Clarification factors associated with reductions in eGFR within 6 months after treatment after treatment for PA could help scientists and physicians to better identify therapeutic targets to improve kidney outcomes among PA patients.
Using data from a nationwide PA registry (the Japan Primary Aldosteronism Study [JPAS] and Japan Rare/Intractable Adrenal Diseases Study [JRAS]),7,10,11 we assessed whether changes in circulating aldosterone levels and BP were associated with changes in eGFR after surgical or MR antagonist treatment, respectively.
Methods
Study Design and Patients
This study was conducted as a part of the prospective ongoing JPAS/JRAS project, a nationwide PA registry of patients from 22 universities and 19 city hospitals.7,10,11 Both JPAS and JRAS enrolled patients with PA 20 to 90 years of age between January 2006 and January 2019. Based on the guidelines of the Japan Endocrine Society12 and the Japan Society of Hypertension,13,14 all participants underwent confirmatory testing, completing the captopril challenge test, furosemide upright test, and/or saline infusion test. Participants underwent adrenal venous sampling to determine whether they had a lateralized form of PA (i.e., aldosterone-producing adenoma) or bilateral adrenocortical hyperplasia (i.e., idiopathic hyperaldosteronism). According to their subtype of PA, patients were categorized into the surgical treatment group or the MR antagonist treatment group. For the current analyses, we selected records of individuals (n = 1184) that included assessments of BP inside of the clinic, measures of kidney function, circulating levels of aldosterone, and covariates before and 6 months after treatment.
The study was conducted according to Declaration of Helsinki guidelines and the guidelines for clinical studies published by the Ministry of Health and Labor, Japan, and was approved by the ethics committee of each participating center or hospital. The study also was registered with the University Hospital Medical Information Network (UMIN ID 18756 and 32525). Informed consent was obtained using an opt-out method at each center or hospital.
Measurements
BP was measured at each local medical institution according to the recommendations of the Japanese Society of Hypertension Guidelines for the Management of Hypertension,13,14 by medical staff using a standard sphygmomanometer or an automated device. BP measurements were collected on the same day when eGFR measurements were obtained. Mean arterial pressure was defined as follows: (systolic BP + 2 × diastolic BP)/3. Other data collected before and 6 months after treatment included height, weight, smoking, medication use, history of hypertension, diabetes, or cardiovascular disease (i.e., coronary heart disease, stroke, and congestive heart failure), and fasting laboratory values. The diagnostic criteria for diabetes followed the recommendation from the Japan Diabetes Guidelines.15 Serum creatinine was assayed using an enzymatic method. eGFR was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation modified by a Japanese coefficient (see online-only Data Supplement of Horio et al.).16 PAC was determined by radioimmunoassay (SPAC-S Aldosterone kit; Fuji Rebio, Co., Ltd, Tokyo, Japan), and PRA by radioimmunoassay or enzyme immunoassay.
Kidney Outcome
eGFR measurements were obtained before and 6 months after treatment. Changes in eGFR were calculated by subtracting eGFR at baseline from eGFR at the 6-month follow-up after treatment.
Statistical Analyses
All analyses were performed for participants who underwent surgery or took MR antagonists, separately. Descriptive statistics are reported as mean (SD) or median (interquartile range) for skewed variables, and proportions where appropriate. The unpaired t test was used to compare the means of eGFR reduction between the surgical and MR antagonist treatment groups. Multivariable linear regression was used to assess associations between changes in PAC, PRA, and mean arterial pressure with changes in eGFR before and 6 months after treatment. We also assessed whether change in PAC/PRA ratio was associated with changes in eGFR before and 6 months after treatment. Results were reported as standardized regression coefficients for each SD higher level for each exposure. Possible violations of the assumptions of multiple linear regression were examined by visual inspection of the distribution of residuals through both histograms and normal probability plots. We further checked for homoscedasticity and deviations from linearity by visually inspecting scatterplots of standardized residuals by standardized predicted values. Standardized regression coefficients were calculated in an unadjusted model, and after adjustment for age, sex, and other characteristics before the treatments (smoking status, body mass index, diabetes, lipid lowering medication use, history of cardiovascular disease, and use of calcium-channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, or β-blockers). Covariates were selected a priori because they may be associated with circulating renin–angiotensin–aldosterone levels and kidney function.17, 18, 19, 20, 21, 22, 23 Assuming that missing data for covariates occurred independently of missing measures of eGFR (i.e., arbitrary missing patterns), all variables with missing data (<8%) were imputed with 10 data sets using chained equations.21 We tested for heterogeneity in the associations between changes in PAC or PRA and changes in eGFR by antihypertensive medication use and each class of antihypertensive medication (i.e., calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and β-blockers) via the inclusion of multiplicative interaction terms. We also tested for heterogeneity by prevalent diabetes because it can affect PAC, PRA, and eGFR.24,25 Stratified analyses were considered when an interaction was observed (P < 0.05). We conducted 3 sensitivity analyses. First, we adjusted for eGFR and mean arterial pressure at baseline. Second, we adjusted for eGFR at baseline, and defined the outcome as eGFR at the 6-month follow-up examination rather than as change in eGFR before and 6 months after treatment. Third, we excluded participants taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, or β-blockers at baseline.
All statistical analyses were performed with R version 3.4.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/). Statistical significance was defined as a P value <0.05 using 2-sided tests.
Results
Participant characteristics are shown in Table 1. Among the 438 participants who underwent surgical treatment, the mean age ± standard deviation was 51 ± 11 years, 50% were female, and 93% were taking antihypertensive medication when PA treatment was initiated. Among the 746 participants who took an MR antagonist, the mean age ± standard deviation was 54 ± 11 years, 53% were female, and 86% were taking antihypertensive medication when PA treatment was initiated.
Table 1.
Characteristics of JRAS participants (n = 1,184)
| Characteristic | Overall (N = 1184) | Surgery (N = 438) | Mineralocorticoid receptor antagonist (N = 746) |
|---|---|---|---|
| Age, yr | 52.8 ± 11.0 | 51.3 ± 11.4 | 53.6 ± 10.7 |
| Women | 611 (51.6) | 218 (49.8) | 393 (52.7) |
| BMI, kg/m2 | 24.9 ± 4.1 | 24.3 ± 4.1 | 25.2 ± 4.1 |
| Current smoker | 430 (36.3) | 159 (36.3) | 270 (36.2) |
| Lipid-lowering therapy | 182 (15.4) | 67 (15.3) | 114 (15.3) |
| Antihypertensive medication use | 1045 (88.3) | 405 (92.5) | 640 (85.8) |
| Calcium channel blockers | 990 (83.6) | 380 (86.8) | 610 (81.8) |
| ACE inhibitors | 8 (0.7) | 3 (0.7) | 5 (0.7) |
| Angiotensin receptor blockers | 52 (4.4) | 26 (5.9) | 26 (3.5) |
| Diuretics | 7 (0.6) | 4 (0.9) | 3 (0.4) |
| β-Blockers | 48 (4.1) | 25 (5.7) | 23 (3.1) |
| History of diabetes | 149 (12.6) | 66 (15.1) | 83 (11.1) |
| History of CVD | 79 (6.7) | 40 (9.1) | 39 (5.2) |
| eGFR, ml/min per 1.73 m2 | 80.8 ± 13.7 | 81.8 ± 15.2 | 80.2 ± 12.7 |
| Plasma aldosterone concentration, pg/ml | 243.3 ± 186.3 | 343.1 ± 247.4 | 184.7 ± 99.7 |
| Plasma renin activity, ng/ml per h | 0.4 ± 0.3 | 0.3 ± 0.3 | 0.4 ± 0.3 |
| Systolic blood pressure, mm Hg | 141.4 ± 18.0 | 142.0 ± 19.3 | 141.1 ± 17.2 |
| Diastolic blood pressure, mm Hg | 87.1 ± 12.5 | 87.4 ± 12.5 | 86.9 ± 12.5 |
| Mean arterial pressure, mm Hg | 105.2 ± 13.1 | 105.6 ± 13.5 | 105.0 ± 12.8 |
ACE, angiotensin-converting enzyme; BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; JRAS, Japan Rare/Intractable Adrenal Diseases Study.
Data are expressed as mean ± SD or as n (%).
In both the surgical and MR antagonist groups, pPRA significantly increased, and eGFR, systolic BP, diastolic BP, and mean arterial pressure were reduced after the treatments (Table 2). PAC significantly increased in the MR antagonist group after treatment but was reduced in the surgical group. Reduction in eGFR after treatment was greater in the surgical compared to the MR antagonist treatment group (median, −8.6; 95% CI, −9.5 to −7.8 ml/min per 1.73 m2; vs. median, −3.2; 95% CI, −3.6 to −2.8 ml/min per 1.73 m2; P < 0.001).
Table 2.
Changes in kidney function, plasma aldosterone concentration, plasma renin activity, and blood pressure after intervention
| Characteristic | Surgery (N = 438) |
Mineralocorticoid receptor antagonist (N = 746) |
||
|---|---|---|---|---|
| Baseline | 6 mo | Baseline | 6 mo | |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 81.8 ± 15.2 | 71.7 ± 19.0a | 80.2 ± 12.7 | 76.5 ± 13.9a |
| Plasma aldosterone concentration, pg/ml | 343.1 ± 247.4 | 113.0 ± 74.9a | 184.7 ± 99.7 | 256.1 ± 156.1a |
| Plasma renin activity, ng/ml per h | 0.3 ± 0.3 | 1.7 ± 2.1a | 0.4 ± 0.3 | 1.5 ± 3.8a |
| Systolic blood pressure, mm Hg | 142.0 ± 19.3 | 129.2 ± 14.0a | 141.1 ± 17.2 | 132.9 ± 13.8a |
| Diastolic blood pressure, mm Hg | 87.4 ± 12.5 | 81.4 ± 10.6a | 86.9 ± 12.5 | 82.8 ± 10.8a |
| Mean arterial pressure, mm Hg | 105.6 ± 13.5 | 97.3 ± 10.5a | 105.0 ± 12.8 | 99.5 ± 10.6a |
Data are expressed as mean ± SD. P values were calculated by the paired Student t test.
Statistical significance was defined as P < 0.05.
In unadjusted models, the adjusted β values (95% CI) for change in eGFR after treatment for each standard deviation higher level of PAC (per 236.1 pg/ml) and PRA (per 3.2 ng/ml per hour) were 1.95 ml/min per 1.73 m2 (1.07, 2.82 ml/min per 1.73 m2) and −2.12 ml/min per 1.73 m2 (−3.60, −0.64 ml/min per 1.73 m2) in the surgery group, respectively, and −0.81 ml/min per 1.73 m2 (−1.70, 0.09 ml/min per 1.73 m2) for PAC and −0.39 ml/min per 1.73 m2 (−0.83, 0.05 ml/min per 1.73 m2) for and PRA in the MR antagonist group, respectively (Table 3). After multivariable adjustment including change in mean arterial pressure, the adjusted β values (95% CI) for change in eGFR after the treatments for each standard deviation higher level of PAC and PRA were 1.97 ml/min per 1.73 m2 (1.08, 2.85 ml/min per 1.73 m2) and −2.76 ml/min per 1.73 m2 (−4.29, −1.22 ml/min per 1.73 m2) in the surgery group, respectively, and −0.72 ml/min per 1.73 m2 (−1.62, 0.18 ml/min per 1.73 m2) and −0.45 ml/min per 1.73 m2 (−0.89, −0.01 ml/min per 1.73 m2) in the MR antagonist group, respectively. In the adjusted models, changes in mean arterial pressure were not associated with changes in eGFR after surgical or MR antagonist treatments (Table 3). In the adjusted models, higher age and use of calcium channel blockers at baseline were associated with greater reduction in eGFR after surgical or MR antagonist treatments (Supplementary Tables S1 and S2). No evidence was found to suggest that diabetes or use of antihypertensive medication interacted in the associations between changes in either PAC or PRA and change in eGFR after treatment (all P for interaction >0.07).
Table 3.
Associations between changes in each exposure and changes in eGFR before and 6 months after intervention
| Characteristic | Surgery (N = 438) |
Mineralocorticoid receptor antagonist (N = 746) |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Δ Plasma aldosterone concentration, pg/ml | 1.95 (1.07, 2.82)a | 1.97 (1.08, 2.85)a | –0.81 (–1.70, 0.09) | –0.72 (–1.62, 0.18) |
| Δ Plasma renin activity, ng/ml per h | –2.12 (–3.60, –0.64)a | –2.76 (–4.29, –1.22)a | –0.39 (–0.83, 0.05) | –0.45 (–0.89, –0.01)a |
| Δ Mean arterial pressure,b mm Hg | –0.80 (–1.73, 0.13) | –0.41 (–1.35, 0.52) | 0.24 (–0.28, 0.75) | 0.34 (–0.19, 0.86) |
| Δ Mean arterial pressure,c mm Hg | –0.59 (–1.53, 0.35) | 0.32 (–0.20, 0.84) | ||
Adjusted β (95% confidence interval) associated with 1-SD increases in plasma aldosterone concentration and plasma renin activity after surgical and pharmacological treatments are shown. The 1-SD increment for each exposure measure is as follows: plasma aldosterone concentration, 236.1 pg/ml; plasma renin activity, 3.2 ng/ml per hour; and mean arterial pressure, 15.1 mm Hg. In unadjusted models, each exposure measure was analyzed in a separate model. Adjusted models include adjustment for age, sex, characteristics at baseline (smoking status, body mass index, diabetes, lipid lowering medication use, history of cardiovascular disease, and use of calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, or β-blockers), and change in mean arterial pressure. eGFR, estimated glomerular filtration rate.
P < 0.05.
Separate model including change in mean arterial pressure was produced for change in plasma aldosterone concentration.
Separate model including change in mean arterial pressure was produced for change in plasma renin activity.
Sensitivity Analyses
Results were similar when we adjusted for eGFR and mean arterial pressure at baseline (Supplementary Table S3). Results were similar when we adjusted for eGFR at baseline, and eGFR at the 6-month follow-up examination defined as an outcome (Supplementary Tables S4 and S5). The eGFR at baseline accounted for 54% to 61% of the variance in eGFR at the 6-month follow-up examination in both the surgical and MR antagonist groups. Changes in PRA or PAC accounted for an additional 2.9% to 4.7% of variance in the surgical group and 0.3% to 0.6% of variance in the MR antagonist group, respectively. There were 88 participants taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, or β-blockers at baseline. Results were similar regardless of whether these 88 participants were excluded or included in our analyses (Supplementary Table S6). We used the PAC/PRA ratio instead of PAC and PRA (Supplementary Table S7). A greater reduction in the PAC/PRA ratio was associated with a greater reduction in eGFR after adrenalectomy, whereas there was no significant association between changes in the PAC/PRA ratio and changes in eGFR after MR antagonist treatment.
Discussion
In this nationwide registry sample of PA patients, greater reduction in PAC was associated with greater reduction in eGFR in patients in the surgical group but not in the MR antagonist treatment group. Greater increase in PRA after treatment was associated with greater reduction in eGFR among both the surgical and MR antagonist treatment groups. Change in mean arterial pressure after treatment was not associated with change in eGFR in either group.
Previous studies have noted that patients with PA had glomerular hyperfiltration that was reversed after adrenalectomy and MR antagonist treatments.5,6,26,27 The improvement of glomerular hyperfiltration occurs within 6 months and has been presumed to be due to decreased systemic BP, postoperative aldosterone suppression, and a rise in PRA.27, 28, 29 A rise in PRA after treatment for PA reflects suppression of MR activity and volume contraction.30 However, no human studies have comprehensively assessed changes in mean arterial pressure, PAC, and PRA after treatments for PA, or how these changes relate to changes in eGFR. The current study extends existing knowledge by demonstrating that postoperative aldosterone suppression and a rise in PRA, but not decreased systemic BP, are associated with a greater reduction in eGFR after treatment. However, changes in systemic BP after treatments may not reflect changes in effective renal plasma flow among patients with PA.28 Therefore, it is unclear how hemodynamic change within the kidneys affects change in eGFR after adrenalectomy or MR antagonist treatment.
Catena et al. demonstrated that among 56 patients with PA, higher PAC and lower PRA before surgical and MR antagonist treatments were associated with a greater reduction in creatine clearance over 6 months after the treatments.28 Iwakura et al.31 have shown that among 213 patients with PA, lower PRA before surgical and MR antagonist treatments were associated with greater reduction in eGFR over 1 month after the treatments.28 Limitations of these studies include the following: (i) associations of changes in PAC and PRA with changes in eGFR after treatments were not assessed; (ii) analyses were not conducted in PA patients who had surgical or MR antagonist treatments, separately; and (iii) and sample sizes were small. These limitations have been addressed in the current study.
Mineralocorticoid receptor antagonist treatment suppresses MR activation,32 expressed as increased PAC and PRA in the current study. Conversely, adrenalectomy reduces PAC by removing tumors that produce aldosterone autonomously, expressed in the current study as reduced PAC but increased PRA. Nakano et al. have noted that PA patients experienced a reduction in eGFR after taking a high-dose MR antagonist (which reflects improvement of glomerular hyperfiltration after treatment), but 82% of them experienced a further reduction in eGFR after surgical adrenalectomy.33 This suggests that even a high-dose MR antagonist treatment may not adequately suppress MR activation within the kidneys, and that further improvement in glomerular hyperfiltration33 may be possible via surgery. Hundemer et al. reported that surgical treatment was associated with a lower risk of kidney dysfunction compared to MR antagonist treatment among patients with PA.8,9 In the current study, reduction in eGFR after treatment was greater in the surgical treatment group than among those in the MR antagonist treatment group. This suggests that (i) surgical treatment may improve glomerular hyperfiltration better than MR antagonist treatment and (ii) MR antagonist treatment may not adequately suppress MR activation within the kidneys. The MR antagonist dose was not available for consideration in the current study. Therefore, it remains uncertain whether improvement in glomerular hyperfiltration by a MR antagonist treatment differs by the drug dose.
Strengths of this study include the large, well-characterized, nationwide sample of Japanese adults. This study has several limitations. Because the findings are based on a cross-sectional analysis, we are unable to determine the causal relationships of the associations observed. The inference of an effect of antihypertensive medication use on PAC and PRA may be a confounder. Some classes of antihypertensive drugs (e.g., renin–angiotensin–aldosterone inhibitors, β-blockers, diuretics) can affect PAC and PRA and eGFR,12,29 and the use of these medications may be a confounder due to their possible effects on PAC and PRA. However, we adjusted for each class of antihypertensive medication and when participants taking angiotensin receptor blockers, diuretics, or β-blockers at baseline were excluded from models. We did not confirm whether standardized BP measurement techniques were used and met the recommendations in the Japanese Society of Hypertension Guidelines for obtaining accurate BP measurements in the clinic. Therefore, it remains uncertain whether accurate BP measurements were obtained for all registry participants. Dietary sodium intake affects PAC and PRA. In clinical practice, salt restriction is recommended for PA patients. However, we did not assess how PA patients may have changed their dietary sodium intake after treatment for PA. Furthermore, body mass index was measured only at baseline. Therefore, it is unknown whether body mass index changed after treatment for PA. These factors could confound the associations between PAC and PRA and eGFR.34 Our results may not be generalizable to other racial and ethnic groups (e.g., white, African American, and Hispanic).
In conclusion, this nationwide study of PA patients suggested that changes in PAC and PRA were associated with eGFR decline after surgical and MR antagonist treatments. Post-treatment improvements in glomerular hyperfiltration among patients with PA may be attributable to suppressed MR activity within the kidneys, not to reductions in systemic BP. It is important for clinicians not only to treat PA, but also to confirm adequately suppressed MR activity after treatment for correction of glomerular hyperfiltration.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by research grants from the Japan Agency for Medical Research and Development (AMED) (grant numbers AMED JP17ek0109122 and JP20ek0109352). This study was also supported by a grant from the National Center for Health and Medicine, Japan (27-1402, 30-1008). We thank the JPAS/JRAS Study members for collecting the clinical data.
Footnotes
Table S1. Associations between each covariate at baseline and changes in eGFR 6 months after intervention (with adjusted models including change in plasma aldosterone concentration).
Table S2. Associations between each covariate at baseline and changes in eGFR 6 months after intervention (with adjusted models including change in plasma renin activity).
Table S3. Associations between changes in each exposure and changes in eGFR before and 6 months after intervention.
Table S4. Characteristics associated with eGFR at the 6-month follow-up examination.
Table S5. Characteristics associated with eGFR at the 6-month follow-up examination.
Table S6. Associations between changes in each exposure and changes in eGFR before and 6 months after intervention among participants not taking angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, diuretics, or β-blockers at baseline.
STROBE Statement.
Supplementary Material
References
- 1.Käyser S.C., Dekkers T., Groenewoud H.J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101:2826–2835. doi: 10.1210/jc.2016-1472. [DOI] [PubMed] [Google Scholar]
- 2.Rossi G.P., Bernini G., Desideri G. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 3.Blasi E.R., Rocha R., Rudolph A.E. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 4.Shibata S., Nagase M., Yoshida S. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 5.Sechi L.A., Novello M., Lapenna R. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. doi: 10.1001/jama.295.22.2638. Erratum in: JAMA. 2006;296:1842. [DOI] [PubMed] [Google Scholar]
- 6.Ribstein J., Du Cailar G., Fesler P. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16:1320–1325. doi: 10.1681/ASN.2004100878. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H., Abe M., Nakamura Y. Association between acute fall in estimated glomerular filtration rate after treatment for primary aldosteronism and long-term decline in renal function. Hypertension. 2019;74:630–638. doi: 10.1161/HYPERTENSIONAHA.119.13131. [DOI] [PubMed] [Google Scholar]
- 8.Hundemer G.L., Curhan G.C., Yozamp N. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–666. doi: 10.1161/HYPERTENSIONAHA.118.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.Y., Lin Y.H., Huang W.C. Adrenalectomy improves the long-term risk of end-stage renal disease and mortality of primary aldosteronism. J Endocr Soc. 2019;3:1110–1126. doi: 10.1210/js.2019-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katabami T., Fukuda H., Tsukiyama H. Clinical and biochemical outcomes after adrenalectomy and medical treatment in patients with unilateral primary aldosteronism. J Hypertens. 2019;37:1513–1520. doi: 10.1097/HJH.0000000000002070. [DOI] [PubMed] [Google Scholar]
- 11.Akehi Y., Yanase T., Motonaga R. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a large, multicenter cohort study in Japan. Diabetes Care. 2019;42:938–945. doi: 10.2337/dc18-1293. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T., Omura M., Satoh F. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 13.Ogihara T., Kikuchi K., Matsuoka H. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 14.Shimamoto K., Ando K., Fujita T. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 15.Seino Y., Nanjo K., Tajima N. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horio M., Imai E., Yasuda Y. Modification of the CKD Epidemiology Collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 17.Kramers B.J., Kramers C., Lenders J.W. Effects of treating primary aldosteronism on renal function. J Clin Hypertens (Greenwich) 2017;19:290–295. doi: 10.1111/jch.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai W.C., Wu H.Y., Peng Y.S. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor K.S., Mclellan J., Verbakel J.Y. Effects of antihypertensives, lipid-modifying drugs, glycaemic control drugs and sodium bicarbonate on the progression of stages 3 and 4 chronic kidney disease in adults: a systematic review and meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall M.E., Wang W., Okhomina V. Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thethi T., Kamiyama M., Kobori H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep. 2012;14:160–169. doi: 10.1007/s11906-012-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregg L.P., Hedayati S.S. Management of traditional cardiovascular risk factors in CKD: what are the data? Am J Kidney Dis. 2018;72:728–744. doi: 10.1053/j.ajkd.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garofalo C., Borrelli S., Minutolo R. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91:1224–1235. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Underwood P.C., Adler G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15:59–70. doi: 10.1007/s11906-012-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Retnakaran R., Cull C.A., Thorne K.I. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 26.Sechi L.A., Di Fabio A., Bazzocchi M. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94:1191–1197. doi: 10.1210/jc.2008-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo C.C., Wu V.C., Tsai C.W. Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:113–122. doi: 10.1177/1470320310391331. [DOI] [PubMed] [Google Scholar]
- 28.Catena C., Colussi G., Nadalini E. Relationships of plasma renin levels with renal function in patients with primary aldosteronism. Clin J Am Soc Nephrol. 2007;2:722–731. doi: 10.2215/CJN.00050107. [DOI] [PubMed] [Google Scholar]
- 29.Schlueter W.A., Batlle D.C. Renal effects of antihypertensive drugs. Drugs. 1989;37:900–925. doi: 10.2165/00003495-198937060-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hundemer G.L., Curhan G.C., Yozamp N. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. doi: 10.1016/S2213-8587(17)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwakura Y., Morimoto R., Kudo M. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99:1593–1598. doi: 10.1210/jc.2013-2180. [DOI] [PubMed] [Google Scholar]
- 32.Sato A. Mineralocorticoid receptor antagonists: their use and differentiation in Japan. Hypertens Res. 2013;36:185–190. doi: 10.1038/hr.2012.182. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y., Yoshimoto T., Fukuda T. Effect of eplerenone on the glomerular filtration rate (GFR) in primary aldosteronism: sequential changes in the GFR during preoperative eplerenone treatment to subsequent adrenalectomy. Intern Med. 2018;57:2459–2466. doi: 10.2169/internalmedicine.0438-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborn J.L. Relation between sodium intake, renal function, and the regulation of arterial pressure. Hypertension. 1991;17(1 suppl):I91–I96. doi: 10.1161/01.hyp.17.1_suppl.i91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.