Introduction
Polycystic kidney disease (PKD) is a hereditary disease that is usually caused by PKD1 or PKD2 pathogenic mutation and that can be divided into 2 types by heredity. When symptoms present neonatally, the prevalence of autosomal recessive polycystic kidney disease (ARPKD) is from 1/10,000 to 1/40,000.1 Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease,1 which is also an adult-onset type. The incidence rate of ADPKD is estimated to be 1/400 to 1/1000.2 It is characterized by progressive enlargement of cysts.
When it comes to the differential diagnosis of PKD, especially ADPKD, tuberous sclerosis complex (TSC) is mentioned. Tuberous sclerosis complex affects numerous organ systems and is known as an autosomal dominant inherited disorder that is usually caused by TSC1 (OMIM ID: 191100) or TSC2 (OMIM ID: 191115) gene mutation. The birth incidence of TSC is 1 in 5800.3 Triad syndrome, which includes skin lesions, mental retardation, and seizure, is the typical feature of this rare disease, but disease manifestations may vary significantly among individuals. Moreover, TSC can present as PKD.
For an adult with renal injury, regardless of the confirmed family history, the diagnosis of PKD or TSC should not simply be satisfied with ultrasonography because of the similiar images of bilaterally dark cysts. Thus, further imaging examinations (computed tomography [CT] and magnetic resonance imaging) and genetic detection are quite helpful for diagnosis. Here we report a case that had been initially misdiagnosed as PKD by abdominal ultrasound but that turned out to be clinically diagnosed as TSC later and was finally diagnosed as TSC2/PKD1 contiguous gene syndrome (PKDTS) by unique deletion mutation. To the best of our knowledge, it is a rare report demonstrating the heterozygous deletion mutation in TSC2 and neighboring PKD1 (www.LOVD.nl/TSC2). TSC2 gene and its downstream adjacent PKD1 gene are on the same chromosome (16p13.3); when the deletions include both genes and cause TSC accompanied by ADPKD, PKDTS can be diagnosed.4 Looking back on this case, we find that it is a typical teaching case that consists of many important points of diagnosis.
Case Presentation
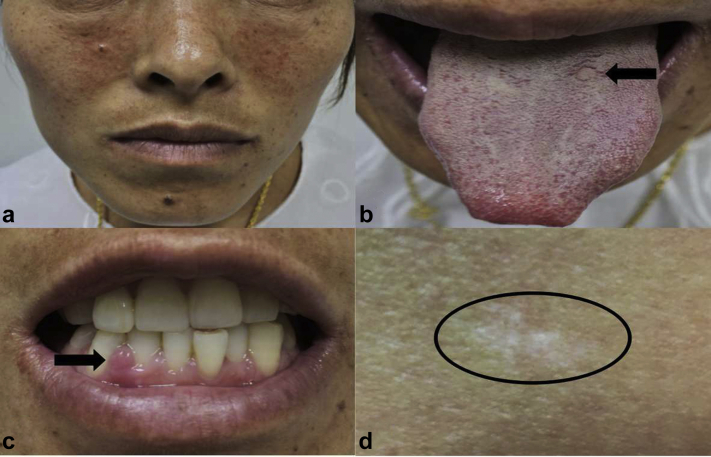
A 33-year-old woman presented to the hospital to treat infertility after 10 years of marriage without contraception, and then was admitted to our hospital for the treatment of PKD with high serum creatinine (Scr). Looking back on the past 2 months, she had experienced increased urinary frequency (>10 times/d), nocturia, and dark urine. According to testing by another hospital, her physical signs were stable, and the laboratory investigation revealed only a little bit of abnormality. Serum creatinine was 1.71 mg/dl, estimated glomerular filtration rate was 41.66 ml/min, and hemoglobin was 10.6 g/dl. Urinalysis showed no hematuria, proteinuria, or pyuria. Ultrasound of the abdomen revealed densely liquid dark areas, indicating PKD. Also, electroencephalography showed no obvious abnormality but the sporadic presence of peak waves. The patient, who had a history of epilepsy controlled with some antiepileptic drugs since childhood, had had no epileptic seizure without medication in the past 10 years. In addition, the patient had no family history of seizure or PKD. Physical examination was otherwise unremarkable, except for multiply facial angiofibromas (Figure 1a), intraoral (lingual and gingival) fibromas (Figure 1b and c), a hypomelanotic macule measuring 2 × 1 cm on the right hip (Figure 1d), and clearly enlarged abdominal masses.
Figure 1.
Remarkable physical examination findings. (a) Multiple facial angiofibromas. (b) Lingual fibroma (black arrows). (c) Gingival fibroma (black arrows). (d) Hypomelanotic macule measuring 2 × 1 cm on the right hip (black circle).
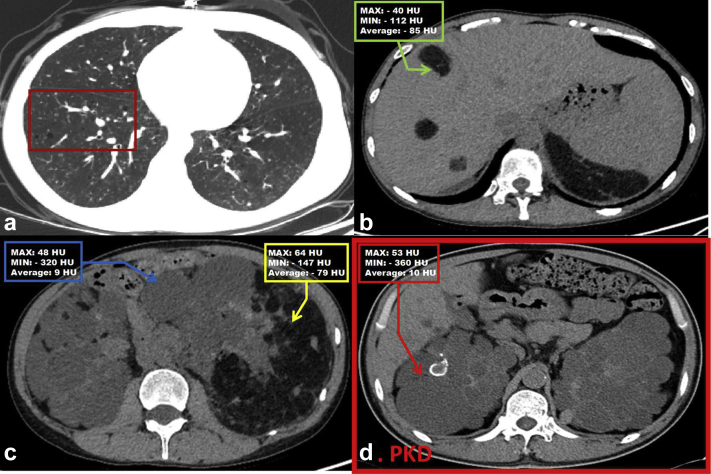
An abdominal CT scan showed multiple disseminated small cyst shadows in both lungs, revealing pulmonary lymphangioma5,6 (Figure 2a) and liver hamartomas consisting of pleomorphic niduses with uneven density (Figure 2b). Besides PKD, CT demonstrated fat-containing tumors in bilateral kidneys (Figure 2c), which were quite distinct from the typical cysts of PKD (Figure 2d) and finally confirmed as kidney angiomyolipomas.
Figure 2.
Abdominal computed tomography (CT). Abdominal CT demonstrated the following: (a) multiple scattered cystic shadows (dark red rectangle) in both lungs; (b) liver hamartomas (green arrows); and (c) multiple renal cysts (blue arrow) and darker areas that illustrate fat-containing bilateral renal angiomyolipomatous tissue (yellow arrow). (d) Typical image from a patient who is diagnosed with polycystic kidney disease (PKD); red arrow shows one of the cysts and the Hounsfield unit (HU).
Besides considering the history of epilepsy, other auxiliary inspections of brain were carried out. Cranial computed tomography demonstrated a slightly high-density nodular shadow with a clear boundary and without invasion of the skull in the left temporal lobe that measured 14 × 10mm (Supplementary Figure S1A and B) and multiple small calcified nodules (Supplementary Figure S1C and D). Furthermore, a cranial magnetic resonance imaging scan was arranged for the patient. The images of the case showed nodular foci and calcified nodules in the left lateral ventricle and subcortical demyelination of bilateral parietal, frontal, and left temporal lobes (Supplementary Figure S1E and F). Taken together, both cranial computed tomography and magnetic resonance imaging demonstrated subependymal nodules.
Discussion
The imaging examinations illustrate that this was not a simple case of PKD. To figure out the exact diagnosis, we sought the help of a multidisciplinary team. Finally, the rashes were confirmed to be angiofibromas, the intraoral fibromas were confirmed, and the cognitive neuropsychological test scale showed a slight reduction of memory function, language function, attention function, and executive ability. To figure out the potential causes of infertility, transvaginal ultrasound, which showed hysteromyoma and hysterosalpingography (HSG), which demonstrated occlusion of bilateral tubals, were performed.
To summarize, seizure history, facial angiofibromas (>2), hypomelanotic macule (only 1 macule 2 × 1 cm), intraoral fibromas7 (≥2), PKD, kidney angiomyolipomas, liver hamartomas, pulmonary lymphangiom, and subependymal nodules can definitely help in eventually determining a clinical diagnosis of TSC3 (Supplementary Table S1).
Tuberous sclerosis complex is known as a hereditary disease. Also, the patient had apparent infertility issues, but her brother was healthy. Therefore, we performed genetic analysis and genetic diagnosis (tested by Beijing MyGenostics Medical Laboratory). Heterozygous deletion mutations in TSC2 (Supplementary Figure S2) and adjacent PKD1 (Supplementary Figure S3) were found, but there was no change in the patient’s family members’ genes (father, mother, and brother). The contiguously whole-gene deletion is rarely reported in the Tuberous Sclerosis Database (https://www.LOVD.nl/TSC2).
Renal problems in TSC are the second leading cause of premature death.3 Differential diagnosis of TSC and other similar diseases, especially PKD1 (Supplementary Table S2), is particularly important. Tuberous sclerosis complex is listed in the first batch of rare diseases in China with a low rate of cognition. Owing to atypical clinical manifestations, it is easy for TSC to be misdiagnosed. Here are some suggestions to make a rapid face-to-face diagnosis. First, physical examination (angiofibromas, fibrous cephalic plaque, ungual fibromas, and mental decline) is of great importance. Second, past history (TSC family history, seizure, and mental retardation) should be taken into account. Thirdly, it is critical that the average Hounsfield unit (HU) of cysts in TSC is usually lower than that in PKD (about −79 HU vs. 10 HU) (Figure 2c and d). Finally, genetic diagnosis can provide key evidence to clarify a diagnosis, especially in atypical cases.
When comes to genetic diagnosis, PKDTS should be mentioned. Because TSC2 and PKD1 are immediately neighboring in a “tail-to-tail” orientation, the deletions of both genes can lead to the clinical manifestations of TSC and ADPKD at the same time and may cooperate in cyst development.4 The quantity, size, and severity of cysts in PKDTS are more than those in TSC or ADPKD.4 Also, compared with TSC and ADPKD, reported cases of PKDTS usually appear as the early-onset type, with a serious condition and poor prognosis.
Currently, there is no effective therapy for PKDTS. Although mammalian target of rapamycin (an mTOR inhibitor) has been reported to be effective in controlling TSC, the level of evidence is low and the treatment guidelines are still unclear.8,9 Considering that this patient’s extrarenal manifestations of both TSC and ADPKD were mild, we provided only symptomatic treatment. In addition, we recommended that blood pressure as well as affected organs such as the brain, kidney, lung, eye, and skin should be monitored for the patient’s entire life.1, 2, 3 In view of the fact that she had bilateral tubal obstruction and heterozygous deletion of both TSC2 and PKD1 genes, pregnancy will be difficult, requiring a recanalization of the fallopian tube and with a chance of conceiving a healthy infant only 50%. In addition, the estimated glomerular filtration rate of this patient was only about 41.66 ml/min, and pregnancy may accelerate the deterioration of renal function. All things considered, we do not recommend pregnancy for this patient.
In conclusion, this case involved PKD, seizure history, skin lesions, intraoral fibromas, kidney angiomyolipomas, liver hamartomas, pulmonary lymphangioma, subependymal nodules, and large deletions involving both PKD1 and TSC2 genes. Comprehensive consideration of family history, characteristic physical examination, imaging examinations such as computed tomography, and genetic detection is extremely important for the correct differential diagnosis of TSC and PKD, as well as the definitive diagnosis of PKDTS (Table 1).
Table 1.
Teaching points
|
|
|
|
|
PKD, polycystic kidney disease; PKDTS, TSC2/PKD1 contiguous gene syndrome; TSC, tuberous sclerosis complex.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (8170061) and the Natural Science Foundation of Jiangsu Province (BK20181487) to Bin Wang and the National Key Research Program (2018YFC130046, 2018YFC1314000) to Bi-Cheng Liu.
Footnotes
Table S1. Diagnostic criteria for TSC.
Table S2. Differential diagnosis of tuberous sclerosis complex (TSC) and polycystic kidney disease (PKD).
Figure S1. Cranial computed tomography (CT) and magnetic resonance imaging (MRI).
Figure S2. Using multiplex ligation-dependent probe amplification to perform TSC2 genetic detection (normal control: 18C041695, patient sample: 19C138323).
Figure S3. Using multiplex ligation-dependent probe amplification to do PKD1 genetic detection (normal control: 19C006415, patient sample: 19C148195).
Supplementary Material
References
- 1.Horie S., Mochizuki T., Muto S. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol. 2016;20:493–509. doi: 10.1007/s10157-015-1219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai B., Mei C.L. Research on autosomal dominant polycystic kidney disease in China. Chin Med J. 2006;119:1915–1924. [PubMed] [Google Scholar]
- 3.Darcy A.K., Hope N., Hope N. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisceglia M., Galliani C., Carosi I. Tuberous sclerosis complex with polycystic kidney disease of the adult type: the TSC2/ADPKD1 contiguous gene syndrome. Int J Surg Pathol. 2008;16:375–385. doi: 10.1177/1066896908319578. [DOI] [PubMed] [Google Scholar]
- 5.Cordier J.F., Lazor, Johnson S.R. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N., Finlay G.A., Kotloff R.M. Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An official American Thoracic Society/Japanese Respiratory Society Clinical Practice guideline. Am J Resp Crit Care. 2017;196:1337–1348. doi: 10.1164/rccm.201709-1965ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng M.C., Cowen E.W., Wataya-Kaneda M. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:95–110. doi: 10.1001/jamadermatol.2014.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEneaney L.J., Tee A.R. Finding a cure for tuberous sclerosis complex: from genetics through to targeted drug therapies. Adv Genet. 2019;103:91–118. doi: 10.1016/bs.adgen.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Li M., Zhou Y., Chen C.Y. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: a meta-analysis. Orphanet J Rare Dis. 2019;14:1. doi: 10.1186/s13023-019-1012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.