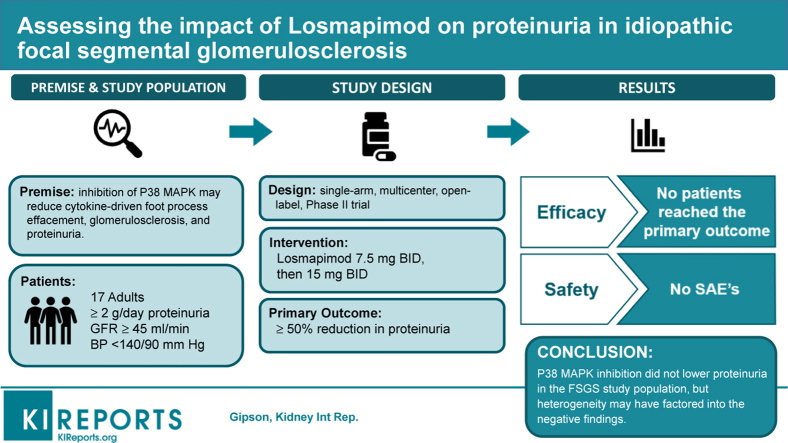
Abstract
Introduction
Idiopathic focal segmental glomerulosclerosis (FSGS) is a leading cause of nephrotic syndrome and end-stage renal disease. In preclinical models and biopsies of human FSGS kidneys, p38 mitogen-activated protein kinase (MAPK) has demonstrated enhanced activity; and p38 MAPK inhibition has improved disease markers. This proof-of-concept trial aimed to assess efficacy, safety, tolerability, and pharmacokinetics of losmapimod, an oral p38 MAPK inhibitor, in humans with FSGS.
Methods
A single-arm, multicenter, open-label, Phase II trial (NCT02000440) was conducted in adults with FSGS; proteinuria ≥2.0 g/d; estimated glomerular filtration rate (eGFR) ≥45 ml/min per 1.73 m2; blood pressure <140/90 mm Hg. Collapsing and genetic forms of FSGS were excluded. The primary endpoint was number of patients with ≥50% proteinuria reduction and eGFR ≥70% of baseline after receiving losmapimod twice-daily for 16 to 24 weeks.
Results
Seventeen patients received ≥1 losmapimod dose. No patients achieved the primary endpoint; therefore, the study was terminated following a prespecified interim analysis. At week 24, proteinuria reductions between 20% and <50% were observed in 4 patients and proteinuria increases >20% in 3 patients. One patient achieved a proteinuria response (≥50% reduction) at week 2 but subsequently relapsed. Losmapimod pharmacokinetics were consistent with prior studies. No serious adverse events (AEs) were reported.
Conclusion
p38 MAPK inhibition with losmapimod did not result in ≥50% reduction of proteinuria in patients with FSGS. However, study population heterogeneity may have contributed to our negative findings and therefore this does not eliminate the potential to demonstrate benefit in a population more sensitive to p38 MAPK inhibition if identifiable in the future by precision-medicine methods.
Key words: clinical trial, FSGS, glomerulosclerosis, nephrotic syndrome, proteinuria
Graphical abstract
FSGS is a leading cause of kidney disease worldwide,1 accounting for approximately 40% of idiopathic nephrotic syndrome and approximately 4% of end-stage renal disease in adults in the United States.2, 3, 4 FSGS is characterized by proteinuria, which commonly presents with hypoalbuminemia, hypercholesterolemia, and peripheral edema,3 in addition to patient-reported fatigue, and reduced emotional and social functioning.5 In more than half of patients who have proteinuria exceeding 3.5 g daily, FSGS results in end-stage renal disease within 5 to 10 years of diagnosis.6 Proteinuria remission is predictive of reducing the decline in kidney function and incidence of end-stage renal disease, but current therapeutic regimens only normalize or improve proteinuria in a minority of patients.7, 8, 9, 10
Losmapimod (GW856553) is an oral, p38 MAPK inhibitor that potently inhibits the production of inflammatory cytokines such as tumor necrosis factor alpha, interleukin-1β, interleukin-6, and interleukin-8.11, 12, 13 It also inhibits the fibrosis-inducing pathway of transforming growth factor-beta14 and decreases plasma fibrinogen levels.15 p38 MAPK activation has been observed in kidney biopsies, including those from patients with idiopathic FSGS.16,17 Normally quiescent, p38 MAPK is activated in podocytes, with the magnitude of activation correlating with the severity of glomerulosclerosis and the degree of proteinuria in both patients with FSGS and animal models.16,17 In animal models, p38 MAPK inhibition, including with losmapimod, leads to diminished podocyte foot process effacement, and a reduction in glomerulosclerosis and proteinuria.12,16,17 This is consistent with the observation that p38 MAPK inhibition preserves podocyte survival and prevents podocyte actin reorganization in vitro after stress stimuli in immortalized mouse cell lines and kidney biopsies.14,17,18 p38 MAPK inhibition may improve podocyte function in patients with FSGS, manifesting clinically as a reduction in proteinuria and fibrosis development, with preservation of kidney function. This proof-of-concept study examined these effects in adults with idiopathic FSGS.
Methods
Study Design
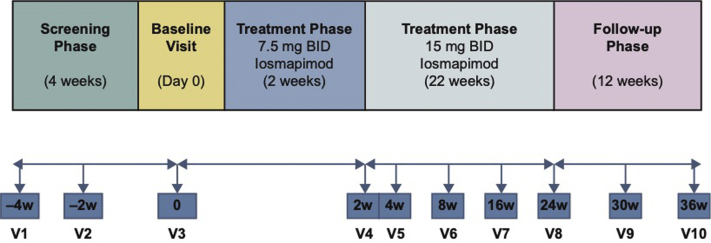
This was a single-arm, multicenter, open-label, Phase II study (NCT02000440; registered December 4, 2013) to evaluate efficacy, safety, tolerability, and pharmacokinetics of twice-daily (BID) oral losmapimod for 24 weeks in patients with idiopathic FSGS (Figure 1). Following informed consent, eligibility was confirmed during the screening period. Eligible patients were treated with losmapimod 7.5 mg BID for 2 weeks. Following review of vital signs, electrocardiogram (ECG), AEs, and symptoms, the dose was increased to 15 mg BID for a further 22 weeks.
Figure 1.
Study design. BID, twice daily; V, visit; w, week.
The study protocol was approved by the institutional review boards at participating centers (Supplementary Table S1), and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. There were 3 amendments to the original protocol, all in response to recruitment challenges (Supplementary Table S2). One amendment was made after recruitment had started, in response to site feedback to broaden eligibility criteria; this included a decrease in the proteinuria eligibility level to 24-hour urine total protein ≥2.0 g/d or spot urine protein-to-creatinine ratio (uPCR) ≥2.0 g/g from the original 3.0 g/d or 3.0 g/g.
Dose Selection
Losmapimod dose selection was based on data from previous trials, including the SOLSTICE study (NCT00910962) in patients with acute coronary syndrome (ACS) (losmapimod 7.5 or 15 mg loading dose followed by 7.5 mg BID for 12 weeks),19 and a study in patients with chronic obstructive pulmonary disease (COPD; NCT01218126) (losmapimod 2.5, 7.5, or 15 mg BID for 24 weeks).15 The 7.5-mg BID dose was used in most prior trials in various patient populations (N = 996), with no observed safety signals, and has shown pharmacodynamic activity on inflammatory markers.11,19, 20, 21 The 15-mg BID dose showed enhanced p38 inhibitory activity in healthy volunteers22 and a similar safety profile to 7.5 mg BID in the 24-week COPD study.15 Evaluation of the higher 15-mg BID dose in this study was warranted because losmapimod is highly protein-bound (95%) in the circulation, suggesting potential for enhanced kidney clearance in patients with severe proteinuria.
Patients
Patients aged 18 to 70 years with idiopathic FSGS were recruited. Qualifying biopsy diagnosis was confirmed by the study’s central pathologist (JCJ) based on a past kidney biopsy pathology report within 6 years of study start, describing light, immunofluorescence, and electron microscopy observations, and review of glass slides and electron micrographs provided by the study sites. Eligibility criteria also included the following: proteinuria of 24-hour urine protein ≥2.0 g/d or a spot uPCR ≥2.0 g/g; eGFR ≥45 ml/min per 1.73 m2 (4-variable modification of diet in renal disease formula23); and blood pressure <140/90 mm Hg.
Patients with collapsing FSGS lesions, secondary forms of FSGS, or a previously identified genetic etiology were excluded. Patients with major systemic disorders (e.g., heart failure, diabetes mellitus, malignancy), chronic or active infections, or with positive serology for HIV or hepatitis were also excluded, as were those with baseline aspartate aminotransferase or alanine aminotransferase ≥2 times, or alkaline phosphatase or bilirubin >1.5 times, the upper limit of normal.
To ensure any treatment effect was not confounded by concomitant medications, patients were limited to treatment with conventional background therapy (including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and diuretics). Patients were required to be on stable doses of background therapy for ≥30 days before screening, continuing for the duration of the trial. Patients were not permitted to receive immunosuppressive FSGS treatments (ISTs) beyond a low-dose corticosteroid (≤10 mg/d prednisone or equivalent) within 30 days of losmapimod initiation. Post hoc retrospective data collection was conducted to assess IST withdrawal (yes/no) within 30 day before this IST-free 30-day window. All available information is reported herein.
Endpoints and Assessments
The primary endpoint was the number of patients with proteinuria response, defined as ≥50% reduction in proteinuria (24-hour total protein) from baseline with preserved kidney function (maintaining ≥70% of baseline eGFR), assessed at the end of treatment in patients who received ≥16 weeks of treatment.
Secondary endpoints included the following: number of patients with proteinuria (24-hour total protein) reduction ≥50% with preserved kidney function at any time during treatment; continuous uPCR change from baseline; incidence of complete remission (defined as 24-hour total protein <0.3 g/d with preserved kidney function); safety; and pharmacokinetics (plasma exposure, area under the concentration-time curve [AUC0-t], area under the concentration-time curve over the dosing interval [AUC0-τ], and maximum observed concentration [Cmax] post-first 7.5-mg dose and AUC0-τ post-first 15-mg dose, as data permit). Samples were taken for pharmacokinetic assessments at baseline (pre-dose, 1, 2, 4, and 6 hours post-dose), week 2 (pre-dose and 2 hours post-dose), and at weeks 4, 8, 16, and 24 (at one of the following post-dose intervals: 0–2, 2–4, 4–6, or 6–8 hours).
Exploratory endpoints included the effect of losmapimod on pharmacodynamic markers: serum high-sensitivity C-reactive protein (hsCRP), fibrinogen and soluble urokinase-type plasminogen activator receptor (suPAR), a potential, putative FSGS-related circulating factor.24 Blood samples to assess these parameters were taken at each study visit (pre-dose at baseline and week 2, with convenience sampling at subsequent visits).
For each patient, 24-hour urine protein and 24-hour uPCR were determined from a single urine sample (collected from the second morning void on the day before each study visit through to first morning void on the day of the study visit); spot uPCR was assessed with a second independent “spot” urine sample collected on-site during the study visit. Serum creatinine was measured by spectrophotometry and used to calculate eGFR, and suPAR was measured by enzyme-linked immunosorbent assay. Patients were followed for 12 weeks after losmapimod discontinuation.
Losmapimod plasma concentrations in patients with FSGS were compared with a population pharmacokinetic model built using historical data obtained in a Phase II trial in patients with ACS (NCT00910962).19 The model was used to simulate concentration-time profiles (median, and 5th and 95th percentiles) at the same doses administered to patients with FSGS.
Losmapimod safety and tolerability were assessed based on the occurrence of AEs, serious AEs, and changes in clinical laboratory values. Patients ceased losmapimod if they met protocol-defined stopping criteria for lack of efficacy (eGFR ≤50% of baseline and/or a doubling of proteinuria compared with baseline, confirmed by 2 and 3 repeat measurements, respectively).
Statistical Analysis
Safety was assessed in all patients who received ≥1 dose of losmapimod. Pharmacokinetics and pharmacodynamics were assessed in all patients who received ≥1 dose of losmapimod and provided ≥1 sample for the respective assessments. The primary endpoint was analyzed in patients who completed ≥16 weeks of treatment. Values from baseline and week 24 were defined as the 2 assay points for percent change comparisons. Patients who withdrew before week 16 were deemed to have not met the defined proteinuria response. Sample size and stopping rules were based on the methodology of Lee and Liu.25 Target enrollment was 24 patients, assuming a 10% drop-out rate. An interim analysis was planned to start when 14 patients had completed 16 weeks of treatment or had withdrawn. A Bayesian predictive probability approach was used for the primary endpoint to determine whether to continue the study or stop for futility. The design was under the hypothesis-testing framework, assuming an 8% untreated versus 35% intervention-arm probability of achieving the primary endpoint and had a type I error rate of 2.7% and 91% overall power.
Data Sharing
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Results
Study Population
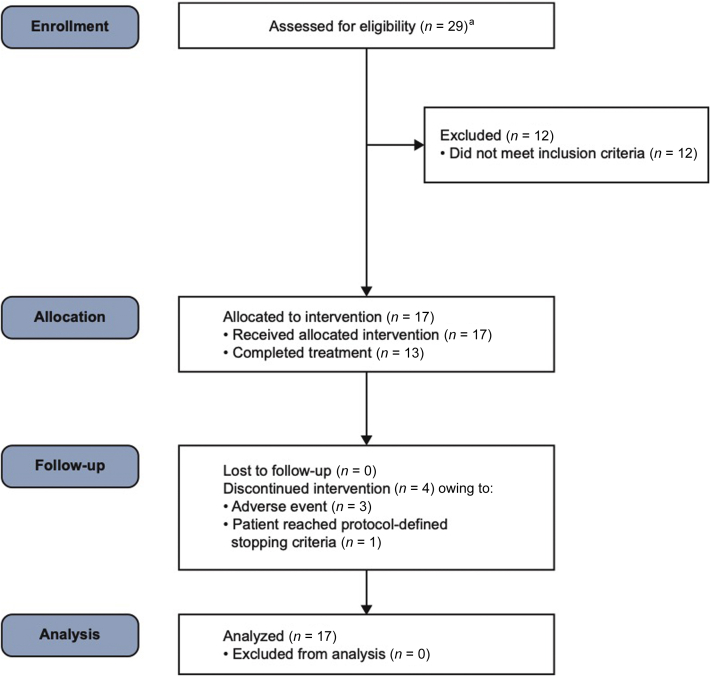
Twelve sites (in Canada and the United States) participated in the study, and 17 patients from 8 of those sites were enrolled from July 31, 2014 (first patient, first visit), to May 11, 2016 (last patient, last visit), and received ≥1 dose of losmapimod (Figure 2). The median number of patients enrolled was 2 (ranging from 1 to 5) per enrolling site. The study was terminated on July 25, 2016, following the planned interim analysis. Following the initial 2-week treatment phase in which patients received oral losmapimod 7.5 mg BID, all patients received losmapimod 15 mg BID, and 13 patients (76%) completed treatment through to week 16; these 13 patients are hereafter referred to as “treatment completers.” Demographics and baseline disease characteristics are shown in Table 1, and renal histopathology information is summarized in Supplementary Table S3.26 The median (range) duration of exposure to losmapimod was 24.0 (3.7–25.0) weeks.
Figure 2.
Patient disposition. aTotal of 27 patients, as 2 of 29 patients were rescreened.
Table 1.
Demographics and baseline disease characteristics
| Characteristic | Losmapimod (N = 17) |
|---|---|
| Age, mean (SD), yr | 40.4 (13.7) |
| Male, n (%) | 9 (53) |
| Race, n (%) | |
| African American | 1 (6) |
| Asian | 5 (29) |
| White | 11 (65) |
| BMI, mean (SD), kg/m2 | 28.7 (6.2) |
| FSGS classification,an (%) | |
| Tip | 4 (24) |
| Perihilar | 2 (12) |
| Not otherwise specified (variant) | 11 (65) |
| Interstitial fibrosis, n (%) | |
| ≤10% | 12 (71) |
| 11%–20% | 1 (6) |
| 21%–30% | 1 (6) |
| 31%–45% | 3 (18) |
| Concurrent medical conditions, n (%) | |
| Hyperlipidemia | 13 (76) |
| Hypertension | 11 (65) |
| Prior immunosuppressive therapy, n (%) | |
| Cyclosporine | 7 (41) |
| Mycophenolic acid | 4 (24) |
| Prednisone | 9 (53) |
| Rituximab | 1 (6) |
| Steroids, not specified | 1 (6) |
| Tacrolimus | 2 (12) |
| Proteinuria | |
| 24-h urine protein, median (min, max) g/d | 6.5 (1.6, 16.3) |
| Serum albumin, median (min, max), g/l | 30.0 (15.0, 42.0) |
| eGFR, median (min, max), ml/min per 1.73 m2 | 72 (36.0, 155.0) |
| Fibrinogen,b median (min, max), mg/dl | 417.5 (162.0, 657.0) |
| suPAR, median (min, max), pg/ml | 2871.3 (1625.0, 4753.0) |
| hsCRP, median (min, max), mg/l | 1.8 (0.3, 8.2) |
| Time since biopsy diagnosis of FSGS | |
| Median (min, max), mo | 10.1 (3.2, 69.2) |
BMI, body mass index; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; hsCRP, high-sensitivity C-reactive protein; suPAR, soluble urokinase-type plasminogen activator receptor.
Columbia FSGS Classification System.26
n = 14.
Efficacy Outcomes
None of the enrolled patients, and none of the 13 treatment completers, met the primary endpoint of ≥50% proteinuria reduction with preserved kidney function. One patient had a transient proteinuria response (≥50% reduction in proteinuria) after 2 weeks of treatment but relapsed within the treatment period. No patients achieved complete proteinuria remission at any timepoint. In treatment completers, median (min, max) change in spot urine uPCR from baseline to week 16 was –27.7% (–47.7, 83.7) and to week 24, was –2.6% (−31.6, 121.7). Four patients had >20% decrease in 24-hour proteinuria (i.e., by 22%, 23%, 40%, and 47%) and 3 had a >20% increase (i.e., 56%, 68%, and 74%) at week 24. Of these 7 subjects, only 2 had reproducible proteinuria changes when measured by uPCR on independent spot urine samples at week 24 (Table 2).26
Table 2.
Patient-level outcomes for primary and secondary endpoints over time (listed by lowest to highest serum albumin at week 0), with week 24 change (%) from baseline
| Patient ID no. | Age/sex/race | FSGS classificationa | Serum albumin (g/l) |
24-h urine protein (g/24 h)b |
24-h urine protein/creatinine ratio (g/g)b |
Spot urine protein/creatinine ratio (g/g)b |
eGFR |
IST distc (Y/N) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 0 Ranked lowest to highest |
Wk 24 %CFBd |
Wk 0 | Wk 16 | Wk 24 %CFBe |
Wk 36 | Wk 0 | Wk 16 | Wk 24 %CFBe |
Wk 36 | Wk 0 | Wk 16 | Wk 24 %CFBe |
Wk 36 | Wk 0 | Wk 16 | Wk 24 %CFBd |
Wk 36 | |||||
| 1f | 60/M/W | Tip lesion | 15 | N/A | 14.8 | N/A | N/A | 17.1 | 6.3 | N/A | N/A | 10.2 | 6.7 | N/A | N/A | 11.8 | 39 | N/A | N/A | 18 | N | |
| 2 | 34/F/W | NOS | 17 | 21 23.5% |
11.1 | 11.1 | 13.1 18.0% |
7.8 | 7.7 | 5.6 | 6.4 −17.9% |
4.8 | 9.5 | 8.1 | 7.5 −21.0% |
7.4 | 94 | 88 | 91 −3.2% |
89 | Y | |
| 3g | 42/F/A | Tip lesion | 18 | 20 11.1% |
2.7 | 2.3 | 2.9 8.3% |
1.8 | 3.2 | 3.8 | 3.4 5.0% |
1.9 | 2.2 | 3.8 | 4.8 121.7% |
2.1 | 72 | 100 | 102 41.7% |
119 | N | |
| 4h | 18/M/W | NOS | 18 | 22 22.2% |
16.2 | 14 | 12.5 −22.9% |
7.9 | 10.9 | 6.5 | 6.5 −39.9% |
5.4 | 9.9 | 7.6 | 6.7 −31.6% |
4.5 | 43 | 59 | 82 90.7% |
101 | Y | |
| 5f | 63/F/W | NOS | 23 | N/A | 12.1 | N/A | N/A | 1.8 | 9.4 | N/A | N/A | 1.7 | 10.8 | N/A | N/A | 1.2 | 45 | N/A | N/A | 51 | Y | |
| 6h | 35/F/A | Tip lesion | 26 | 33 26.9% |
6.5 | 3.8 | 3.5 −46.7% |
3.2 | 4.4 | 2.3 | 2.1 −52.1% |
2.0 | 4.7 | 2.8 | 3.2 −31.4% |
2.6 | 128 | 118 | 118 −7.8% |
120 | N | |
| 7f | 39/M/W | NOS | 29 | N/A | 7.5 | N/A | N/A | N/A | 5.7 | N/A | N/A | 5.1 | 4.4 | N/A | N/A | 5.5 | 72 | N/A | N/A | 75 | N | |
| 8 | 56/F/W | NOS | 30 | 30 0% |
4.7 | 5.6 | 7.9 68.0% |
5.8 | 5.2 | 5.6 | 6.0 15.8% |
5.3 | 5.5 | 6.9 | 6.5 16.7% |
5.6 | 101 | 86 | 86 −14.9% |
88 | N | |
| 9h | 47/F/W | Tip lesion | 30 | 27 −10.0% |
3.1 | 2.1 | 1.9 −40.1% |
2.3 | 2.7 | 1.8 | 1.6 −41.5% |
1.9 | 2.1 | 1.7 | 2.2 5.6% |
3.0 | 126 | 105 | 113 −10.3% |
141 | N | |
| 10 | 27/F/W | NOS | 31 | 37 19.4% |
1.6 | 1.8 | 1.7 9.9% |
1.1 | 1.1 | 1.2 | 1.3 18.5% |
1.0 | 1.3 | 1.3 | 1.3 −2.9% |
0.4 | 155 | 124 | 134 −13.6% |
147 | N | |
| 11f | 51/M/W | NOS | 31 | N/A | 16.3 | N/A | N/A | 11 | 7.8 | N/A | N/A | 7.0 | 9.2 | N/A | N/A | 7.2 | 46 | N/A | N/A | 54 | Y | |
| 12h | 32/M/AA | NOS | 35 | 37 5.7% |
4.4 | 3.7 | 3.7 −16.9% |
3.5 | 1.5 | 0.8 | 1.6 3.5% |
1.4 | 1.5 | 1.1 | 1.7 8.3% |
1.6 | 69 | 67 | 66 −4.4% |
70 | Y | |
| 13i | 60/M/W | NOS | 38 | 37 −2.6% |
4.1 | 3.2 | 3.7 −10.0% |
3.4 | 2.1 | 1.5 | 2.2 4.7% |
1.7 | 2.3 | 1.8 | 2.3 −2.3% |
2.1 | 56 | 52 | 55 −1.8% |
60 | N | |
| 14 | 38/M/W | NOS | 38 | 43 13.2% |
9.1 | 6.3 | 15.8 73.9% |
11.8 | 3.8 | 3.1 | 5.3 39.5% |
6.6 | 4.6 | 5.4 | 5.1 10.4% |
7.3 | 36 | 38 | 34 −5.6% |
35 | N | |
| 15 | 26/F/A | NOS | 39 | 35 −10.3% |
7.2 | 8.9 | 11.2 56.4% |
9.8 | 4.7 | 5.4 | 6.4 35.5% |
6.0 | 5.2 | 5.3 | N/A | 6.7 | 83 | 75 | 67 −19.3% |
71 | N | |
| 16 | 32/M/A | Perihilar | 40 | 43 7.5% |
4.5 | 3.8 | 4.7 4.2% |
6.5 | 2.5 | 1.6 | 2.4 −5.2% |
3.2 | 3.4 | 2.1 | 2.8 −16.0% |
3.5 | 104 | 108 | 110 5.8% |
111 | N | |
| 17 | 26/M/A | Perihilar | 42 | 41 −2.4% |
3.6 | 4.1 | 2.8 −22.4% |
5.0 | 1.2 | 2.2 | 1.5 26.4% |
1.8 | 1.6 | 2.5 | 1.5 −8.7% |
2.0 | 81 | 89 | 85 4.9% |
72 | N | |
| Group (median) %CFB | 7.5% | 4.2% | 4.7% | −2.6% | −4.3%h | |||||||||||||||||
AA, African American/African Heritage; A, Asian; CFB, change from baseline; eGFR, estimated glomerular filtration rate using MDRD equation; F, female; FSGS, focal segmental glomerulosclerosis; IST, immunosuppression therapy; M, male; MDRD, 4-variable Modification of Diet in Renal Disease; N/A, not available; NOS, not otherwise specified (variant); W, White; Wk, week.
Idiopathic FSGS was diagnosed by medical history and histology on renal biopsy using the Columbia FSGS Classification System.26
For each patient, 24-h urine protein and 24-h urine protein-to-creatinine ratios were determined from a single urine sample (collected from the second morning void on the day before each study visit through to first morning void on the day of the study visit); spot urine protein-to-creatinine ratio was assessed with a second independent “spot” urine sample collected on-site during the study visit.
IST, such as prednisone, cyclosporine, tacrolimus, and mycophenolate, discontinued (dist.) within 30 d of screening.
Data shown rounded to whole number; percentage changes based on full reported data.
Data shown rounded to 1 decimal place; percentage changes based on full reported data.
Patient discontinued therapy (corresponding rows also shown in bold); where available, wk 36 is follow-up visit 3 mo after last dose received.
Twenty-four-hour urine protein level decreased from baseline at wk 16 but increased from baseline at wk 24.
Twenty-four-hour urine protein level decreased from baseline.
Twenty-four-hour urine protein level decreased from baseline at wk 16 and then increased at wk 24 but not above baseline value.
Estimated GFR at week 24 (Table 2) remained within 10% of baseline in 8 of the 13 treatment completers; 3 patients experienced a decrease (−14%, −15%, and −19%) versus baseline and 2 patients experienced an increase (+42% and +91%). Compared with baseline, serum albumin increased in 8 and decreased in 4 of the treatment completers.
Pharmacokinetics and Pharmacodynamics
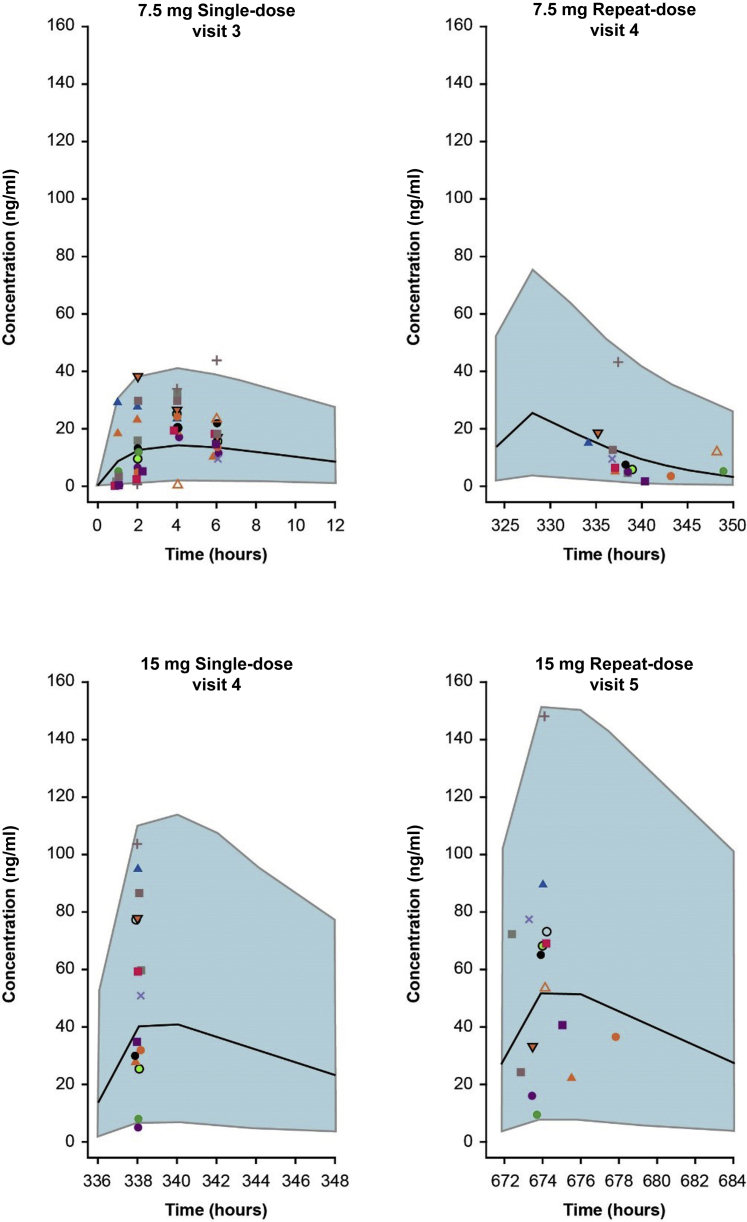
Exposure in patients with FSGS was similar to the modeled exposure levels over the 24-week treatment period (Figure 3). Fibrinogen, suPAR, and hsCRP were measured for pharmacodynamic analysis (Table 3). At baseline, median (min, max) fibrinogen (417.5 [162.0, 657.0] mg/dl; n = 14) and suPAR (2871.3 [1625.0, 4753.0] pg/ml; n = 17) levels were elevated, and hsCRP values were generally in the normal range (Table 1). No clinically significant changes in these markers were noted over 24 weeks of treatment.
Figure 3.
Predicted losmapimod exposures based on a pharmacokinetic model built using historic data from patients with acute coronary syndrome (black line represents median, shaded areas highlight 5th and 95th percentiles), overlaid with actual losmapimod concentrations measured from patients with focal segmental glomerulosclerosis in the current study (represented by individual colored shapes). Panel headings show dose and visit number relevant to focal segmental glomerulosclerosis study patients.
Table 3.
Patient pharmacodynamic outcomes for exploratory biomarkers over time (listed by lowest to highest serum albumin at week 0), with week 24 change (%) from baseline
| Patient ID no. | Age/sex/race | Serum albumin (g/l) |
Fibrinogen (mg/dl) |
suPAR (pg/ml) |
hsCRP (mg/l) |
|||
|---|---|---|---|---|---|---|---|---|
| Wk 0 ranked lowest to highest | Wk 0 | Wk 24 %CFB |
Wk 0 | Wk 24 %CFB |
Wk 0 | Wk 24 %CFB |
||
| 1a | 60/M/W | 15 | 439 | N/A | 4198 | N/A | 2.7 | N/A |
| 2 | 34/F/W | 17 | 642 | 694 8.1% |
4753 | 3846 −19.1% |
4.3 | 6.4 48.8% |
| 3 | 42/F/A | 18 | 441 | 551 24.9% |
3637 | 3907 7.4% |
0.3 | 0.4 33.3% |
| 4 | 18/M/W | 18 | N/A | 669 | 3047 | 3802 24.8% |
1.2 | 7.1 491.7% |
| 5a | 63/F/W | 23 | 657 | N/A | 4240 | N/A | 0.4 | N/A |
| 6 | 35/F/A | 26 | 278 | 299 7.6% |
1638 | 2375 45.0% |
5.3 | 12.8 141.5% |
| 7a | 39/M/W | 29 | 529 | N/A | 4463 | N/A | 2.6 | N/A |
| 8 | 56/F/W | 30 | 573 | 415 −27.6% |
2580 | 1965 −23.8% |
1.4 | 0.5 −64.3% |
| 9 | 47/F/W | 30 | 303 | 165 −45.5% |
4637 | 3477 −25.0% |
0.3 | 0.3 0% |
| 10 | 27/F/W | 31 | N/A | 412 | 1824 | 1683 −7.7% |
0.5 | 0.4 −20.0% |
| 11a | 51/M/W | 31 | 481 | N/A | 3646 | N/A | 8.1 | N/A |
| 12 | 32/M/AA | 35 | 343 | 331 −3.5% |
1625 | 1508 −7.2% |
1.7 | 5.2 205.9% |
| 13 | 60/M/W | 38 | 396 | 274 −30.8% |
2871 | 3000 4.5% |
2.5 | 4 60.0% |
| 14 | 38/M/W | 38 | N/A | 324 | 1891 | 2118 12.0% |
2.9 | 2.3 −20.7% |
| 15 | 26/F/A | 39 | 376 | 342 −9.0% |
2352 | 2156 −8.3% |
1.7 | 0.6 −64.7% |
| 16 | 32/M/A | 40 | 162 | 201 24.1% |
2193 | N/A | 1.8 | 0.4 −77.8% |
| 17 | 26/M/A | 42 | 373 | 335 −10.2% |
2035 | 2185 7.4% |
3.7 | 3.6 −2.7% |
| Group (median) %CFB | — | −6.3% | −1.4% | 48.8% | ||||
| Patients with paired biomarker results (wk 0 and wk 24), n | 13 | 10 | 12 | 13 | ||||
AA, African American/African Heritage; A, Asian; CFB, change from baseline; F, female; hsCRP, serum high-sensitivity C-reactive protein; M, male; N/A, not available; suPAR, soluble urokinase-type plasminogen activator receptor; W, White; Wk, week.
Data shown rounded to whole number; percentage changes based on full reported data.
Patient discontinued therapy (corresponding rows also shown in bold).
Safety
There were no serious AEs among the 17 patients who received ≥1 dose of losmapimod. Most patients (16 of 17; 94%) experienced ≥1 AE. Twelve (71%) patients had AEs considered related to study treatment. The most common AEs reported by >10% of patients were headache (5 of 17; 29%) and fatigue (4 of 17; 24%) (Table 4). Almost all AEs (97%), including all treatment-related AEs, were mild or moderate in intensity. Three AEs (abdominal pain, nausea, and vomiting) experienced by 1 patient were reported as severe, although none were considered treatment-related. There were no significant electrocardiogram abnormalities and no clinically significant changes in vital signs.
Table 4.
Adverse events reported in >10% of patientsa
| Event (MedDRA preferred term) | Patients, n (%) (N = 17) |
|---|---|
| Any event | 16 (94) |
| Headache | 5 (29) |
| Fatigue | 4 (24) |
| Blood creatinine increased | 3 (18) |
| Dizziness | 3 (18) |
| Muscle spasms | 3 (18) |
| Nausea | 3 (18) |
| Oropharyngeal pain | 3 (18) |
| Rash | 3 (18) |
| Vomiting | 3 (18) |
| Abdominal pain | 2 (12) |
| Blood pressure increased | 2 (12) |
| Dyspepsia | 2 (12) |
| Edema peripheral | 2 (12) |
| Upper respiratory tract infection | 2 (12) |
MedDRA, Medical Dictionary for Regulatory Activities.
No serious adverse events were reported during the study. Patients may have had more than 1 event.
Reflecting a progressive decline in kidney function, 4 patients withdrew from the study: 3 due to AEs and 1 due to reaching protocol-defined stopping criteria. Two of the 3 patients who withdrew due to AEs had elevations in blood creatinine; these were not reported as severe or serious and were not considered treatment-related. The third patient who withdrew due to an AE had joint stiffness, considered treatment-related by the investigator. The patient who withdrew due to reaching protocol-defined stopping criteria (treatment-related toxicity/treatment not tolerated) experienced an AE of blood creatinine increase not considered treatment-related.
Discussion
In this open-label, single-arm, proof-of-concept study, losmapimod, a p38 MAPK inhibitor, was evaluated in 17 patients with idiopathic FSGS, proteinuria, and preserved kidney function. None of the patients met the primary endpoint (≥50% proteinuria reduction with preserved kidney function) after ≥16 weeks of treatment. Safety assessments showed that losmapimod was generally well tolerated, with no unexpected safety signals and no serious AEs. Due to the primary endpoint not being met, the study was terminated after the prespecified interim analysis.
The rationale for the single-arm study design was based on the improbability of spontaneous improvement in patients with active FSGS. This anticipated persistence of proteinuria, together with the relative safety of losmapimod observed in other patient populations,15,19,20 negated the need for placebo. This approach is consistent with early exploratory studies performed previously in FSGS with other agents.27, 28, 29 A potential added value of single-arm designs is to improve recruitment based on the preference of patients and their care givers to enroll into open-label versus randomized controlled trials.30
Pharmacokinetic assessments showed that losmapimod exposure in this trial was similar to that predicted by a losmapimod trial in patients with ACS,19 despite the potential for increased losmapimod excretion in the urine of patients with proteinuria, as observed previously with the primary metabolite for rosiglitazone.27,31 Therefore, the lack of efficacy, as assessed by a reduction in proteinuria, in this trial is not related to a substantive decrease in the expected losmapimod exposures at the 15-mg BID dose. The current study dosing paradigm was selected based on experience with losmapimod and the perceived balance of potential efficacy and tolerability. Whether a higher losmapimod dose and/or more sustained exposure (>24 weeks) would have benefited clinical efficacy in these patients remains unknown. Prior losmapimod studies in both patients with ACS and patients with COPD also have failed to provide efficacy based on primary endpoints but have yielded positive findings for the inflammatory biomarkers of hsCRP32 and plasma fibrinogen,20 respectively (both with the 7.5-mg BID dose).
This study required patients to participate in the absence of concurrent IST. Although the disease in patients with FSGS commonly fails to fully respond to IST, discontinuation of partially effective therapy could destabilize a patient, increase the likelihood of rising proteinuria levels and declining eGFR, and contribute to heterogeneity between patient outcomes that is difficult to overcome in small trials. In this study, retrospective chart review conducted by enrolling sites found 5 of 17 patients stopped prior ISTs within 30 days of screening, of whom 1 was recorded as having worsening proteinuria, which may have confounded the results. Furthermore, the requirement to be off other ISTs in the presence of persistent proteinuria for trial eligibility may have impeded trial recruitment. Future trials of novel therapies may benefit from an add-on approach and from the collection of a longer (e.g., 3 months) prior history of proteinuria, eGFR measures, and medications to enable a more informed interpretation of results.
Furthermore, although lack of response to losmapimod may call into question the relevance of p38 MAPK activity inhibition in FSGS, it may be speculated that the population was too heterogeneous, or that secondary counterregulatory activation of a non-p38 MAPK pathway, such as Jun amino-terminal kinase, may have occurred. In clinical trials, changes in inflammatory markers, including reduced hsCRP in patients with hypercholesterolemia and myocardial infarction,11,19 and lower levels of fibrinogen in patients with COPD,20 have been demonstrated with p38 MAPK inhibition. The current trial also assessed the effect of losmapimod on hsCRP and fibrinogen, as well as suPAR, as exploratory endpoints. At baseline, fibrinogen and suPAR levels were elevated, and hsCRP values were generally in the normal range, without change at end of study. This disconnect with previous observations in patients with ACS and patients with COPD may reflect differences in the nature of the inflammatory response in different patient populations. hsCRP levels may be nuanced or difficult to interpret in nephrotic syndrome; for example, previous research has shown a discordance between erythrocyte sedimentation rate and CRP in patients with nephrotic syndrome.33 Unlike in patients with FSGS, hsCRP was elevated in patients with ACS and patients with COPD and was associated with acute events (i.e., angioplasty and exacerbations, respectively). This may suggest a more efficacious effect of losmapimod on inflammatory biomarkers in acute settings versus low-level chronic inflammation and may reflect counterregulatory mechanisms highlighted previously. Alternatively, the lower number of enrolled patients in the current study may have contributed to the lack of detected signals for these inflammatory biomarkers.
Furthermore, the definition of proteinuria response used in this study was ≥50% reduction in proteinuria from baseline with preserved kidney function (≥70% of baseline eGFR); since the time this study was conducted, a novel, data-derived definition of partial proteinuria remission (40% proteinuria reduction and proteinuria <1.5 g/g) has been suggested, with the potential to more accurately predict clinically meaningful outcomes.9
This trial may have benefited from a precision-medicine patient enrichment strategy, determining the p38 MAPK biology of individual patients at enrollment, via a biomarker, biopsy immunostaining, genetic, or gene expression-profiling approach. However, patients were recruited based on kidney biopsy report, proteinuria, and eGFR, which do not specifically identify patients most sensitive to p38 MAPK targeting. In addition, the collapsing variant of FSGS was excluded due to disease severity, potentially making treatment benefit even less attainable. Although assessment of p38 MAPK expression in kidney biopsy is possible, this study did not include a new kidney biopsy as part of screening or eligibility assessment. At the time of the study, no biomarkers were available to allow noninvasive patient selection strategies for p38 MAPK status; these methods are emerging in glomerular disease translational investigations34,35 and are expected to improve drug development initiatives for FSGS and other glomerular diseases involving the p38 MAPK pathway, such as diabetic nephropathy.36,37
Patients with a known secondary cause of FSGS and established genetic forms of the disease were excluded; however, genetic testing was not performed. During the time of the study, few commercial tests were available, and comprehensive genetic assessment was not feasible given the continued identification of new gene associations and limited data on genotype-phenotype correlations.38 Although cohort studies have documented reduced responsiveness to cyclosporine therapy in children with congenital and steroid-resistant nephrotic syndrome, varying responsiveness to p38 MAPK inhibitors by genetic variant status is not available,39,40 although there are no clear data showing an impact of genetic status on response to nonsteroid IST.
As genetic testing was not performed, patients with monogenetic forms of FSGS or with high-risk APOL1 genotypes may have been included. It is unlikely that this factor would have had a significant impact on the results, as adults in North America had a very low likelihood of FSGS-causing monogenic disorders.38 Also, APOL1 risk alleles for renal disease are common in populations of African descent41, 42, 43, 44, 45 but there was only 1 African American patient in this study. In addition, most FSGS variants present with disease in childhood,38,40 whereas patients in the current study were ≥18 years of age.
In summary, in adult patients with FSGS, p38 MAPK inhibition with losmapimod for ≥16 weeks did not result in ≥50% reduction of proteinuria compared with baseline. A lesser reduction in proteinuria (between 20% and <50%) was demonstrated in 4 patients and a >20% worsening of proteinuria was seen in 3 patients. However, it remains possible that the level of p38 MAPK inhibition was too low for this patient population or that benefit could potentially be demonstrated with an alternative dosing paradigm, an alternate concomitant-drug usage design, or if precision-medicine methods are established to select patients more sensitive to p38 MAPK inhibition.
Disclosure
DSG served as a consultant for the trial design. All trial sites received funding for participation in this trial, including University of Michigan, Ann Arbor, Michigan, USA (DSG); Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada (MAH); Stanford University, Stanford, California, USA (RL); Metro Health System Medical Center, Cleveland, Ohio, USA (JRS); Ohio State University Wexner Medical Center, Columbus, Ohio, USA (BHR); University of British Columbia, Vancouver, British Columbia, Canada (SJB); University of Alberta Hospital, Edmonton, Alberta, Canada (AM); University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA (JCJ, PHN). JRS served as a consultant for GSK (data interpretation). MP, FG, and KST are employees of GSK, with stock ownership. RNW, SV, MCH, DLS, and JART were employees of GSK, with stock ownership, at the time the study was conducted.
Acknowledgments
Heather St Michael, of Fishawack Indicia Ltd., provided medical writing support, which was funded by GlaxoSmithKline (GSK). David M. Tenero, who is an employee of GSK, provided assistance with data modeling and simulation.
This work was supported by GlaxoSmithKline (GSK study FSG117283; ClinicalTrials.gov NCT02000440).
Author Contributions
All authors contributed important intellectual content during manuscript drafting or revision, accept accountability for the work, and provided final approval of the submitted manuscript. Individual contributions of each author are as follows: DSG, MAH, RL, JRS, JCJ, BHR, and PHN contributed to the conception or design of the study, patient recruitment, the acquisition of data, and data analysis or interpretation. DLS and MP monitored study protocol, maintained study integrity and facilitated patient recruitment. DLS, MP, KST, FG, JART, SV, and RNW contributed to the conception or design of the study and data analysis or interpretation. Statistical analyses were conducted by FG. MCH contributed to data analysis or interpretation. AM and SJB contributed to the acquisition of data and data analysis or interpretation.
Footnotes
Table S1. Listing of PK concentrations by Iosmapimod 7.5-mg and 15-mg dose.
Table S2. Institutional review boards/independent ethics committees.
Table S3. Summary of protocol amendments.
Supplementary Material
References
- 1.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitiyakara C., Kopp J.B., Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:172–182. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 3.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 5.Mathias S.D., Vallow S., Gipson D. Development of focal segmental glomerulosclerosis patient-reported outcome measures: symptom diary and symptom impact questionnaire. Am J Kidney Dis. 2017;70:532–540. doi: 10.1053/j.ajkd.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 7.Troyanov S., Wall C.A., Miller J.A. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 8.Banfi G., Moriggi M., Sabadini E. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol. 1991;36:53–59. [PubMed] [Google Scholar]
- 9.Troost J.P., Trachtman H., Nachman P.H. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiffel J., Rahimzada Y., Trachtman H. Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis. 2011;18:332–338. doi: 10.1053/j.ackd.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheriyan J., Webb A.J., Sarov-Blat L. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123:515–523. doi: 10.1161/CIRCULATIONAHA.110.971986. [DOI] [PubMed] [Google Scholar]
- 12.Willette R.N., Eybye M.E., Olzinski A.R. Differential effects of p38 mitogen-activated protein kinase and cyclooxygenase 2 inhibitors in a model of cardiovascular disease. J Pharmacol Exp Ther. 2009;330:964–970. doi: 10.1124/jpet.109.154443. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Boehm J., Lee J.C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S., Nagase M., Shibata S. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Exp Nephrol. 2008;108:e57–e68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 15.Watz H., Barnacle H., Hartley B.F. Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:63–72. doi: 10.1016/S2213-2600(13)70200-5. [DOI] [PubMed] [Google Scholar]
- 16.Stambe C., Nikolic-Paterson D.J., Hill P.A. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol. 2004;15:326–336. doi: 10.1097/01.asn.0000108520.63445.e0. [DOI] [PubMed] [Google Scholar]
- 17.Koshikawa M., Mukoyama M., Mori K. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol. 2005;16:2690–2701. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 18.Pengal R., Guess A.J., Agrawal S. Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. Am J Physiol Renal Physiol. 2011;301:F509–F519. doi: 10.1152/ajprenal.00661.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newby L.K., Marber M.S., Melloni C. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial. Lancet. 2014;384:1187–1195. doi: 10.1016/S0140-6736(14)60417-7. [DOI] [PubMed] [Google Scholar]
- 20.Lomas D.A., Lipson D.A., Miller B.E. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2012;52:416–424. doi: 10.1177/0091270010397050. [DOI] [PubMed] [Google Scholar]
- 21.Elkhawad M., Rudd J.H., Sarov-Blat L. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc Imaging. 2012;5:911–922. doi: 10.1016/j.jcmg.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Barbour A.M., Sarov-Blat L., Cai G. Safety, tolerability, pharmacokinetics and pharmacodynamics of losmapimod following a single intravenous or oral dose in healthy volunteers. Br J Clin Pharmacol. 2013;76:99–106. doi: 10.1111/bcp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Wei C., El Hindi S., Li J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J.J., Liu D.D. A predictive probability design for phase II cancer clinical trials. Clin Trials. 2008;5:93–106. doi: 10.1177/1740774508089279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 27.Joy M.S., Gipson D.S., Dike M. Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group. Clin J Am Soc Nephrol. 2009;4:39–47. doi: 10.2215/CJN.02310508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joy M.S., Gipson D.S., Powell L. Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis. 2010;55:50–60. doi: 10.1053/j.ajkd.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trachtman H., Vento S., Gipson D. Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: study design. BMC Nephrol. 2011;12:8. doi: 10.1186/1471-2369-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montaner J.S., Hogg R., Srour L.F. Should we embrace new drugs with open arms? Experience from a community-based, open-arm, randomized clinical trial of combination antiretroviral therapy in advanced HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:422–426. doi: 10.1097/00042560-199612150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration Avandia (rosiglitazone maleate) Prescribing Information. 2007. https://www.fda.gov/media/75754/download Available at:
- 32.O'Donoghue M.L., Glaser R., Cavender M.A. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA. 2016;315:1591–1599. doi: 10.1001/jama.2016.3609. [DOI] [PubMed] [Google Scholar]
- 33.Ferraccioli G.F., Cavalieri F., Mercandanti M. CRP in nephrotic syndrome: relationship with hemostatic abnormalities? Nephron. 1985;41:369–370. doi: 10.1159/000183618. [DOI] [PubMed] [Google Scholar]
- 34.Tuttle K.R., Brosius F.C., III, Adler S.G. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33:1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao J., Mariani L., Eddy S. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 2018;94:795–808. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhikary L., Chow F., Nikolic-Paterson D.J. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–1222. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- 37.Lim A.K., Nikolic-Paterson D.J., Ma F.Y. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–358. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 38.De Vriese A.S., Sethi S., Nath K.A. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29:759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buscher A.K., Kranz B., Büscher R. Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2010;5:2075–2084. doi: 10.2215/CJN.01190210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lepori N., Zand L., Sethi S. Clinical and pathological phenotype of genetic causes of focal segmental glomerulosclerosis in adults. Clin Kidney J. 2018;11:179–190. doi: 10.1093/ckj/sfx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipkowitz M.S., Freedman B.I., Langefeld C.D. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzur S., Rosset S., Shemer R. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsa A., Kao W.H., Xie D. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grams M.E., Rebholz C.M., Chen Y. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27:2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.