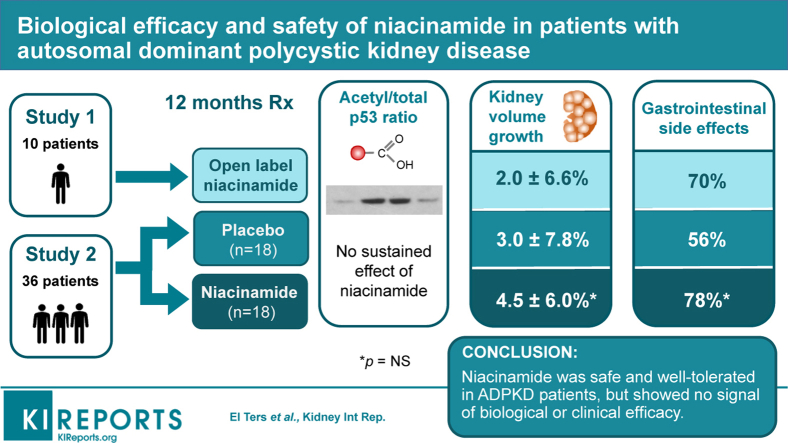
Abstract
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive cyst enlargement, leading to kidney failure. Sirtuin-1 is upregulated in ADPKD and accelerates disease progression by deacetylating p53. Niacinamide is a dietary supplement that inhibits sirtuins at high doses.
Methods
We conducted an open-label, single-arm intervention trial (study 1, N = 10), and a randomized, double blinded, placebo-controlled trial (study 2, N = 36) to assess the biological activity and safety of niacinamide. Patients with ADPKD were given 30 mg/kg oral niacinamide or placebo, for 12 months. The primary endpoint was the ratio of acetylated p53 to total p53 protein in peripheral blood mononuclear cells (PBMCs).
Results
There was no sustained effect of niacinamide on acetylated/total p53 in either study and no difference between placebo and niacinamide arms. There was no difference in the change in height-adjusted total kidney volume over 12 months between niacinamide and placebo. Niacinamide was generally well tolerated. The most common adverse effects were nausea, diarrhea, gastroesophageal reflux, headache, and acneiform rash but there was no difference in their incidence between niacinamide and placebo.
Conclusions
In conclusion, niacinamide is safe and well-tolerated in patients with ADPKD. However, we were unable to detect a sustained inhibition of sirtuin activity over 12 months of treatment, and there was no signal to suggest a beneficial effect on any efficacy measure.
Keywords: clinical trial, niacinamide, polycystic kidney disease, p53, sirtuin
Graphical abstract
ADPKD is a rare disease with a diagnosed prevalence of approximately 4 in 10,000,1, 2, 3 so that approximately 140,000 patients are believed to be affected in the United States. It is characterized by the early development and unrelenting growth of bilateral renal cysts, leading to progressive increase in renal volume and decline in glomerular filtration rate (GFR). End-stage renal disease typically occurs in the fifth or sixth decade of life. ADPKD is predominantly caused by mutations in 2 genes, PKD14,5 and PKD2.6 Its pathogenesis is believed to involve hyperproliferation, dedifferentiation, and fluid secretion by renal tubular epithelial cells, leading to cyst formation and growth. The abnormal, cell-autonomous proliferation of cyst epithelial cells in ADPKD is associated with cellular dedifferentiation and upregulation of numerous pro-proliferative factors (e.g., EGF, Src, Erk, AKT, Myc, Rb, Raf),7,8 and resembles a neoplastic process.9 The only approved therapy is tolvaptan, a vasopressin V2 receptor antagonist.10,11 However, tolvaptan causes profound and sustained aquaresis, increasing the urine output to a mean of 6 liters per day12 and leading to side effects that include polyuria, nocturia, thirst, and polydipsia. It also causes significant elevation of liver enzymes in 4% to 6% of treated patients, as compared with 1% on placebo.10,11,13 In TEMPO3:4, 15% of patients permanently discontinued the drug because of adverse events.10 There is therefore an unmet need for new drugs that have greater efficacy and/or are better tolerated.
We recently described a novel pathway for cyst growth.14 Sirtuin 1 (SIRT1) belongs to the sirtuin family of nicotinamide adenine dinucleotide–dependent protein deacetylases, which target proteins involved in metabolism regulation, stress response and DNA repair. Zhou et al.14 found that PKD1-mutant renal epithelial cells and tissues overexpress SIRT1, and that targeted deletion of the SIRT1 gene delayed cyst formation and normalized kidney function in mutant mice with PKD1 deletion. The study provided evidence that increased SIRT1 expression in PKD1 mutant mice drives cystic epithelial cell proliferation. This is mediated by deacetylation and increased phosphorylation of retinoblastoma (Rb) protein (at residue S780), which becomes inactive, in turn enabling transcription of genes that mediate entry into the S-phase of the cell cycle15 and also by inhibition of apoptosis via deacetylation of p53,16 an important tumor suppressor protein, at residue K382.
Zhou et al.14 found that niacinamide (also known as nicotinamide), a noncompetitive inhibitor of sirtuins,17 was able to decrease proliferation and increase apoptosis of cystic epithelial cells by inhibiting the deacetylation of Rb and p53. Niacinamide administration to pregnant mice ameliorated cystic disease in PKD embryos, whereas its administration to neonatal pups in 2 different mouse PKD1 models led to delayed cyst growth, decreased kidney weight–to–body weight ratio, and decreased blood urea nitrogen levels compared with controls.14 Importantly, niacinamide did not delay cyst growth in PKD1 mice that lacked SIRT1, proving that niacinamide exerted its beneficial effect specifically by inhibiting SIRT1. Based on this initial evidence, we hypothesized that niacinamide might delay progression of ADPKD.
Niacinamide is a water-soluble amide derivative of nicotinic acid (niacin), and together these represent the 2 major forms of vitamin B3. The recommended dietary allowance of vitamin B3 in adults is 14 to 16 mg daily.18 In mice, the niacinamide dose that was shown to be effective at inhibiting cyst growth in PKD was 0.25 mg/g daily i.p.,14 which would be equivalent to approximately 20 mg/kg per day in humans.19 Niacinamide has been tested in humans at doses ranging from 1 g/m2 to 6 g daily as an antioxidant, anti-inflammatory, and immune modulator in many disease conditions, including type 1 diabetes mellitus, schizophrenia, Alzheimer disease, neurodegenerative disorders, hyperphosphatemia in dialysis patients, and as a radiosensitizer in cancer, and it has an excellent safety profile.20 Knip et al.20 reviewed the experience before 1999 in almost 2000 patients treated with doses of 1 to 6 g/d in 18 independent trials and concluded that it is a safe therapy to use when given to adults at doses of no more than 3 g/d. Although the precursor of niacinamide, nicotinic acid, has occasionally been associated with abnormalities of liver function tests when used in large doses, modern preparations of niacinamide are pure and seem to be relatively free of this toxicity. There is a single report of severe but reversible hepatotoxicity in a patient taking 9 g/d of niacinamide.21 In the ENDIT study, 276 patients with type 1 diabetes were treated with 1.2 g/m2 niacinamide daily (30 mg/kg for an average adult) up to a maximum of 3 g/d for 5 years, with no excess of adverse events compared with the placebo group.22 Niacinamide has also been used in dialysis patients to treat hyperphosphatemia. In this population, it lowers serum phosphate levels by 1.0 to 1.5 mg/dl and can cause diarrhea and reversible thrombocytopenia.23, 24, 25, 26, 27 None of these effects have been reported in any other patient population. Niacinamide also potentiates the level or effect of some antiepileptic drugs, including carbamazepine and sodium valproate.28,29
Niacinamide is an inexpensive dietary supplement that is already widely available to the public and can be taken without need for Food and Drug Administration approval, so if it can be proven to be safe or effective, patients would have immediate access to therapy. However, whether niacinamide has pharmacological activity as a SIRT1 inhibitor in humans is unknown. We report here the results of 2 pilot clinical trials of niacinamide in patients with ADPKD: an open-label, single-arm study (study 1), and a randomized, placebo-controlled trial (study 2). The goal of these studies was to establish whether niacinamide has biological activity at the maximum safe dose, determine its tolerability, and to detect any signal suggestive of clinical efficacy.
Methods
Study Design
Study 1 (NIAC-PKD1) was a single-center, open-label, single-arm phase IIa study. Patients were enrolled from August 2014 to January 2015 at the University of Kansas Medical Center. After screening and baseline assessments, patients were given standard-release niacinamide (Douglas Laboratories, Pittsburgh, PA) at a dosage of 30 mg/kg day p.o. up to a maximum of 3 g/d in 2 divided daily doses, to be taken for 12 months. Patients had a total of 4 study visits (baseline, 1 month, 6 months, and 12 months). Blood was drawn at each visit for standard clinical laboratory evaluations (complete blood count and comprehensive metabolic panel including magnesium and phosphorus), and for isolation of PBMCs by Ficoll-Paque PLUS (GE Healthcare, Little Chalfont, UK) density gradient fractionation. Urine was collected at each visit for protein/creatinine ratio and assay of monocyte chemoattractant protein-1. Pain and quality of life (QOL) were assessed at baseline, and 6 and 12 months. Patients also underwent magnetic resonance imaging scans of the kidneys (head-first, supine) at the baseline and 12-month visits in a 3.0T Siemens (Munich, Germany) Skyra scanner, using a phased-array surface coil. T1-weighted volume-interpolated gradient echo (VIBE, repetition time = 4.0 ms, echo time =1.66 ms, flip angle = 9°) and fat-suppressed T2-weighted half-Fourier acquisition single-shot turbo spin echo (HASTE, repetition time = 1400 ms, echo time = 101 ms, flip angle = 90°) images were acquired during multiple breath-holds at 4-mm slice thickness in the coronal plane (1.56 × 1.56 mm in-plane resolution, field of view = 256 × 256). Pill counts were performed at each visit to assess compliance with the study drug.
Study 2 (NIAC-PKD2) was a single-center, randomized, double-blind, placebo-controlled trial. Patients were enrolled from September 2015 to November 2016 and randomly assigned in a 1:1 ratio to niacinamide or identical matching placebo capsules according to a blocked randomization schedule that was generated by a statistician and shared only with the research pharmacy. The study drug dose (30 mg/kg per day p.o. in 2 divided daily doses), study duration (12 months), and study visit schedule and assessments were identical to those for NIAC-PKD1.
Study Participants
Participants had to have a diagnosis of ADPKD confirmed by magnetic resonance, computed tomography, or ultrasound imaging based on Ravine’s criteria.30 The inclusion criteria for study 1 was 18 to 55 years of age and an estimated GFR (eGFR) >60 ml/min per 1.73 m2 as determined from the serum creatinine by the Chronic Kidney Disease–Epidemiology Collaboration equation.31 For study 2, we included patients 18 to 60 years old with an eGFR >55 ml/min per 1.73 m2. The key exclusion criteria for both studies were history of liver disease, abnormal liver function test, heavy alcohol usage, chronic diarrhea, thrombocytopenia, hypophosphatemia, pregnancy or lactation, treatment with tolvaptan within the prior 2 months, concomitant use of antiepileptic drugs, and partial nephrectomy, total nephrectomy, or renal cyst reduction within a year before screening.
Study Outcomes and Assessments
The primary outcome for both studies was the ratio of acetylated p53 (at K382) to total p53 protein abundance in PBMCs. Because the abundance of p53 was too low to detect by enzyme-linked immunosorbent assay, we used quantitative Western blotting. The isolated PBMCs were lysed in RIPA buffer (Millipore, Bedford, MA). Whole cell extracts were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. After blocking with 5% nonfat milk in TBST, the membrane was incubated with antibodies against acetyl-p53 (Lys379) (#2570, 1:1000; Cell Signaling Technology, Danvers, MA), and total p53 (SC-126, 1:1000; Santa Cruz Biotechnology, Dallas, TX). Donkey-anti-rabbit IgG-horseradish peroxidase and donkey-anti-mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) were used as secondary antibodies. Blots were developed with ECL Western blot substrate (Pierce). The band density was quantified by ImageJ software (National Institutes of Health, Bethesda, MD). A prespecified co-primary outcome, the ratio of phosphorylated (S780) to total Rb protein in PBMCs, could not be assessed by either enzyme-linked immunosorbent assay or Western blot because both proteins were below the limits of detection, and so was abandoned.
Height-adjusted total kidney volume (htTKV) from baseline to 12 months was assessed as a secondary outcome. Total kidney volume was determined by manual stereology at University of Kansas Medical Center based on methods previously described,32 using a 100 mm2 grid overlaid on the coronal T2 images (ImageJ 1.48v, National Institutes of Health), and then normalized to height. Three investigators independently measured the same set of images. Raters were blinded to intervention group and also timepoint, to mitigate observer bias. Interrater reliability of TKV measurements across patients, as determined by the intra-class correlation coefficient, was 0.988 indicating excellent interrater agreement. The expected rate of growth of htTKV (Supplementary Figure S1) was estimated from baseline values of htTKV (V) and age (T) by (V/150)1/T − 1, as described by Irazabal et al.33 and categorized according to the Mayo Imaging Classification. For corroboration, TKV was also determined using semiautomated segmentation software (MIROS).34 MIROS showed minimal bias and good precision compared with manual stereology (Supplementary Figure S2A).
Individual and overall pain and QOL scores were determined from a 9-question survey (see footnotes in Supplementary Table S1). This was based on a questionnaire originally devised by Eiji Higashihara of Kyorin University School of Medicine (PKD-8) that included 8 questions assessing the frequency and intensity of symptoms of pain and QOL over the prior 3 months. We modified this by adding a ninth question about what, if any, treatment was required for their pain, which was designed to be a more objective and more practical assessment of the everyday impact of the pain. Other biomarkers of efficacy included eGFR, determined by the Chronic Kidney Disease–Epidemiology Collaboration equation from serum creatinine, C-reactive protein, urine protein/creatinine ratio, and urine monocyte chemoattractant protein-1 concentration, which was determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) and normalized to urine creatinine.
Safety was assessed at each study visit using Common Terminology Criteria for Adverse Events v4.0. Prespecified safety outcomes included change in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, serum phosphorus, and platelet count.
Statistics
Linear mixed models were used to model the acetyl-p53/total p53 protein ratio, and all secondary efficacy measures except htTKV, over time in study. Each patient provided up to 4 measures over time, and random intercept coefficient models were used to account for the repeated measures within patient. For the outcomes of acetyl-p53/total p53 ratio and C-reactive protein, residual diagnostics indicated that the model assumptions were violated, so these 2 measures were each transformed by taking the natural log of the measure plus 1 (this offset was used because some observations had a ratio of zero). For study 1, a global test of equality over time was conducted. This was followed by pairwise comparisons performed against baseline, and with a comparison of on- versus off-treatment using a linear contrast. For study 2, the model included a linear parameter for treatment effect and an interaction term for this effect over time. Linear contrasts provided the estimated annual change (slope) in each measure, and P values for the comparison of slopes between treatment arms were determined. htTKV was measured at only 2 time points, so the comparison between baseline and 12 months in study 1 was obtained using the Wilcoxon Signed Rank test, and the change between treatment versus control arms in study 2 was assessed by the 2-sample paired t test. Because of the low expected counts, the relative frequencies of adverse events between treatment arms in study 2 were compared using Fisher’s exact test. Family-wise P values of less than 0.05 were regarded as statistically significant. We used the Bonferroni method to adjust for the multiple pairwise comparisons between time points and baseline in study 1 (i.e., P values of less than 0.0166 were regarded as statistically significant). The internal consistency of the QOL questionnaire was assessed using data from the placebo-treated patients in study 2 at baseline, using the standardized Cronbach’s alpha test.
Study Approval
Both studies were approved by the University of Kansas Medical Center Institutional Review Board. All participants provided written informed consent before participating in the study. The studies were registered with ClinicalTrials.gov (NCT02140814, NCT02558595).
Results
Patient Characteristics and Follow-Up
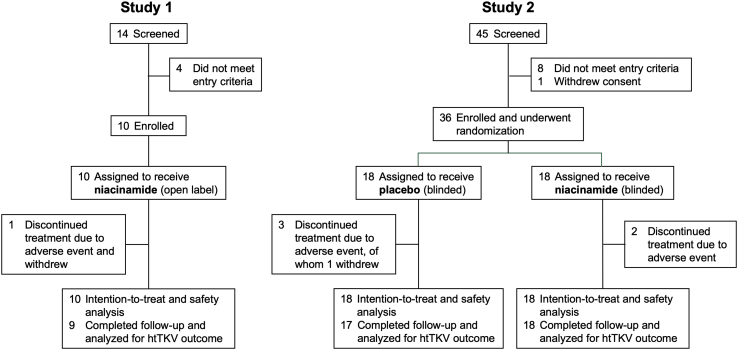
Ten patients were enrolled in study 1 and were given open-label niacinamide, of whom 9 completed the study per protocol and 1 withdrew at 8 months after completing 3 of 4 study visits (Figure 1). The baseline characteristics are shown in Table 1. Thirty-six patients were enrolled in study 2 and randomized to niacinamide (18) or placebo (18) (Figure 1). The patients were slightly older than in study 1, with larger kidneys and lower eGFR (Table 1). There were no statistically significant differences in baseline characteristics between the treatment arms, but the niacinamide group had numerically more men (56% vs. 33% in placebo group), greater frequency of hypertension (100% vs. 78%), higher baseline htTKV (1210 ± 772 vs. 1021 ± 434, mean ± SD in ml/m), and more patients with advanced Irazabal class (28% vs. 6% in Class 1E). In study 2, all patients in the niacinamide group completed the study; 1 patient in the placebo group withdrew due to adverse events.
Figure 1.
CONSORT flow diagram showing numbers of patients screened, enrolled, completed, and available for analysis of outcomes. htTKV, height-adjusted total kidney volume.
Table 1.
Baseline characteristics of enrolled participants
| Baseline characteristic | Study 1 |
Study 2a |
|
|---|---|---|---|
| Niacinamide (N = 10)b | Niacinamide (N = 18) | Placebo (N = 18) | |
| Age, mean (SD), yr | 34.4 (11.3) | 40.1 (10.9) | 44.7 (9.1) |
| Male sex, n (%) | 8 (80) | 10 (56) | 6 (33) |
| Race, n (%) | |||
| Caucasian, non-Hispanic | 9 (90) | 18 (100) | 18 (100) |
| Hispanic | 1 (10) | 0 (0) | 0 (0) |
| Hypertension, n (%) | 9 (90) | 18 (100) | 14 (78) |
| ACEI/ARB, n (%) | 6 (60) | 16 (89) | 13 (72) |
| htTKV, mean (SD), ml/m | 729 (319) | 1210 (772) | 1021 (434) |
| Irazabal class, n (%) | |||
| 1A | 0 (0.0) | 0 (0) | 0 (0) |
| 1B | 1 (11) | 3 (17) | 1 (6) |
| 1C | 3 (33) | 5 (28) | 10 (56) |
| 1D | 4 (44) | 5 (28) | 6 (33) |
| 1E | 1 (11) | 5 (28) | 1 (6) |
| 2 | 0 (0) | 0 (0) | 0 (0) |
| eGFR, mean (SD), ml/min per 1.73 m2 | 102.1 (16.7) | 78.1 (18.6) | 68.1 (12.0) |
ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume.
In study 2, continuous measures were compared between treatment groups using the 2-sample t test. htTKV violated the assumption of normality, so this measure was log-transformed for inferential comparison. For categorical measures, Pearson’s χ2 test was used when expected cell counts were at least 5 in at least 80% of the cells; otherwise, Fisher’s exact test was used. No significant difference between treatment groups was detected.
N = 9 for htTKV and Mayo class.
Primary Outcome
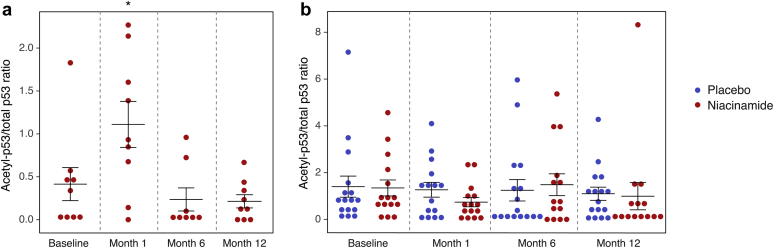
In study 1, 8 of the 9 patients who completed the study had p53 detectable at baseline by Western blot, and were therefore evaluable. The ratio of acetyl-p53 to total p53 showed significant variation over time (P = 0.001 for overall test), with a significant increase at 1 month compared with baseline (P = 0.003 for the pairwise test), but not at 6 and 12 months (Figure 2). In study 2, there were 5 subjects with missing data due to undetectable total p53, 3 from the treatment arm and 2 from the placebo arm. In the evaluable patients, no difference between groups was detected (P = 0.51) and, in contrast to study 1, the 1-month value actually decreased compared with baseline in the niacinamide arm (Figure 2).
Figure 2.
Ratio of acetyl-p53 to total p53 determined by Western blotting in peripheral blood mononuclear cells over the course of the study. (a) Values at each time point in study 1. ∗P = 0.003 for pairwise comparison to baseline values. (b) Values in each treatment arm at each time point in study 2. No difference between groups with respect to time or treatment arm was detected.
Secondary Efficacy Outcomes
The secondary outcomes of efficacy are reported in Tables 2 and 3. Individual components of the QOL score are detailed in Supplementary Table S1. In study 1, the expected rate of kidney growth based on the Mayo Image Classification was 4.6% ± 1.4% per year (mean ± SD). The observed htTKV increased from 729 ± 319 ml/m at baseline to 732 ± 287 ml/m at 12 months (P = 0.73, Table 2). The average growth rate was 2.0% ± 6.6% (Supplementary Figure S1), which was lower than expected, but the difference was not statistically significant (1-sided P = 0.16). One patient ruptured a large cyst and had a 1.2% reduction in htTKV, but, excluding this patient, did not significantly affect the outcome. Likewise, for the other efficacy measures (eGFR, C-reactive protein, urine monocyte chemoattractant protein-1, urine protein, and aggregate QOL score) reported in Table 2, there were no significant changes over time, and no significant differences were detected between the values on-treatment (1, 6, and/or 12 months) compared with off-treatment (baseline).
Table 2.
Changes in measures of efficacy over the course of study 1
| Efficacy measure | Baseline | 1 mo | 6 mo | 12 mo | P valuea |
|---|---|---|---|---|---|
| htTKV, ml/m | 729 ± 319 | — | — | 732 ± 287 | 0.73 |
| eGFR, ml/min per 1.73 m2 | 102.1 ± 16.7 | 94.7 ± 15.7 | 101.1 ± 15.6 | 98.9 ± 16.3 | 0.13 |
| C-reactive protein mg/dl | 0.18 ± 0.19 | 0.20 ± 0.24 | 0.38 ± 0.81 | 0.23 ± 0.37 | 0.46 |
| Urine monocyte chemoattractant protein-1/creatinine, pg/mg | 354 ± 240 | 268 ± 186 | 265 ± 172 | 371 ± 206 | 0.17 |
| Urine protein/creatinine, mg/g | 69.7 ± 37.4 | 86.0 ± 76.6 | 63.3 ± 61.6 | 94.9 ± 91.6 | 0.78 |
| Aggregate QOL score | 25.5 ± 9.6 | — | 22.7 ± 11.1 | 20.4 ± 9.0 | 0.06 |
eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; QOL, quality of life.
The P value for the comparison of htTKV between baseline and 12 months is obtained using the Wilcoxon Signed Rank test. All other P values are obtained from linear contrast on versus off test of linear mixed effect model fitting results.
Table 3.
Efficacy outcomes by treatment arm in study 2
| Efficacy measure | Baseline | 1 mo | 6 mo | 12 mo | Annual change (%) | P valuea |
|---|---|---|---|---|---|---|
| htTKV, ml/m | ||||||
| Placebo | 938 ± 261 | — | — | 963 ± 254 | 24.9 (2.66) | 0.32 |
| Niacinamide | 1210 ± 772 | — | — | 1256 ± 790 | 46.9 (3.88) | |
| eGFR, ml/min per 1.73 m2 | ||||||
| Placebo | 68.1 ± 12.0 | 65.2 ± 17.4 | 70.2 ± 16.4 | 66.3 ± 14.5 | 0.032 (0.05) | 0.55 |
| Niacinamide | 78.1 ± 18.6 | 79.7 ± 17.5 | 76.4 ± 18.7 | 77.5 ± 19.7 | −1.589 (−2.02) | |
| C-reactive protein, mg/dl | ||||||
| Placebo | 0.27 ± 0.27 | 0.28 ± 0.30 | 0.28 ± 0.26 | 0.32 ± 0.26 | 0.029 (13.45) | 0.04 |
| Niacinamide | 0.15 ± 0.08 | 0.28 ± 0.29 | 0.48 ± 1.09 | 0.77 ± 1.32 | 0.245 (144.42) | |
| Urine monocyte chemoattractant protein-1/creatinine, pg/mg | ||||||
| Placebo | 405.5 ± 267.3 | 409.1 ± 244.2 | 336.9 ± 208.3 | 331.7 ± 188.4 | 82.7 (−20.53) | 0.29 |
| Niacinamide | 379.8 ± 179.4 | 437.8 ± 236.5 | 475.4 ± 516.3 | 402.7 ± 238.0 | 9.0 (2.14) | |
| Urine protein/creatinine, mg/g | ||||||
| Placebo | 104.7 ± 38.8 | 102.1 ± 42.3 | 137.1 ± 93.1 | 128.2 ± 58.6 | 26.2 (24.85) | 0.21 |
| Niacinamide | 95.1 ± 54.3 | 97.4 ± 54.7 | 86.0 ± 34.1 | 107.4 ± 53.1 | 2.7 (2.85) | |
| Aggregate QOL score | ||||||
| Placebo | 24.5 ± 9.2 | — | 24.5 ± 9.9 | 24.6 ± 9.8 | 0.056 (0.23) | 0.35 |
| Niacinamide | 23.7 ± 10.0 | — | 22.4 ± 10.2 | 21.4 ± 8.9 | −2.333 (−9.86) |
eGFR, estimated glomerular filtration rate; htTKV, height-adjusted total kidney volume; QOL, quality of life.
n = 12–18/group as some patients were missing some measures.
P values for the difference in annual change between treatment arms. For htTKV this was based on the 2-sample t test, and for all other outcomes from linear mixed models. C-reactive protein was also modeled as ln(1+C-reactive protein) because residual analysis indicated the underlying assumptions were violated.
In study 2, there was no significant difference in the annual rate of htTKV growth between the placebo (24.9 ± 61.6 ml/m, 3.0% ± 7.8%) and niacinamide (46.9 ± 65.7 ml/m, 4.5% ± 6.0%) arm (P = 0.32, Table 3). Measurement of htTKV using a semiautomated method (MIROS) showed similar results to manual stereology (Supplementary Figure S2B). Differences in the baseline htTKV and predicted growth rate were minimal and insufficient to explain the negative result. Likewise, there were no differences between treatment groups in any of the other measures of efficacy. In a post hoc analysis, the niacinamide-treated patients in both studies were combined to increase the sample size. Analysis of the combined group showed no significant treatment effects (Supplementary Table S2). The reliability of the QOL questionnaire was determined from the standardized Cronbach’s alpha, which was 0.84.
Safety
Overall, 89% of patients in both studies experienced an adverse event. Table 4 summarizes the events that occurred in at least 10% of patients. None of the adverse events differed in frequency between placebo- and niacinamide-treated patients. Gastrointestinal events, including diarrhea, nausea, dyspepsia, and gastroesophageal reflux disease, were the most common adverse events, occurring in study 1 in 70% of patients, and in study 2 in 56% of patients in the placebo arm and 78% of patients in the niacinamide arm. Three patients in study 1, but none of the patients in study 2, developed an acneiform facial rash. The rash resolved spontaneously in 2 patients and resolved shortly after stopping niacinamide in the third patient. There were no grade 3 or 4 laboratory abnormalities. One patient in the placebo arm of study 2 developed an increase in transaminases to 2 times the upper limit of normal. None of the patients developed hypophosphatemia. The only effect of niacinamide on the laboratory values that was consistent across both studies was a minor decrease in the serum potassium level (Supplementary Tables S3 and S4).
Table 4.
Adverse events during both studies
| Adverse eventa | Study 1 |
Study 2b |
||
|---|---|---|---|---|
| No. patients | No. likely or possibly related to study drug | No. of patients assigned to placebo | No. of patients assigned to niacinamide | |
| Gastrointestinal disorders | ||||
| Diarrhea | 5 | 2 | 2 | 4 |
| Gastroesophageal reflux disease | 2 | 2 | 3 | 7 |
| Nausea | 1 | 1 | 5 | 4 |
| Dyspepsia | 0 | 0 | 2 | 4 |
| Abdominal pain | 1 | 3 | ||
| Nervous system disorders | ||||
| Dizziness | 2 | 1 | 0 | 0 |
| Headache | 3 | 2 | 3 | 3 |
| Peripheral motor neuropathy | 1 | 0 | 0 | 0 |
| Infections and infestations | ||||
| Upper respiratory, bronchial & sinus infection | 4 | 0 | 3 | 3 |
| Small intestine infection | 0 | 0 | 2 | 4 |
| Kidney, urinary tract infection | 0 | 0 | 1 | 2 |
| Vascular disorders | ||||
| Flushing | 2 | 2 | 2 | 2 |
| Hypertension | 0 | 0 | 0 | 2 |
| Blood and lymphatic system disorders | ||||
| Anemia | 0 | 0 | 2 | 1 |
| Eye disorders | ||||
| Floaters | 0 | 0 | 2 | 0 |
| General disorders and administration site conditions | ||||
| Fatigue | 0 | 0 | 0 | 2 |
| Musculoskeletal and connective tissue disorders | ||||
| Flank pain | 0 | 0 | 3 | 2 |
| Back pain | 0 | 0 | 2 | 2 |
| Psychiatric disorders | ||||
| Insomnia | 0 | 0 | 1 | 4 |
| Renal and urinary disorders | ||||
| Urinary frequency | 0 | 0 | 2 | 2 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 1 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rash acneiform | 3 | 3 | 0 | 0 |
| Other (contact dermatitis) | 1 | 0 | 0 | 0 |
All adverse events in study 1, and any adverse events that occurred in ≥10% of patients in any study arm of study 2.
None of the events in study 2 differed in frequency between the study arms (P > 0.05 by Fisher’s exact test).
There were no serious adverse events in study 1. In study 2 there were 5 serious adverse events. Four were considered unlikely to be related to the study treatment (flank pain, ruptured kidney cyst, acute appendicitis, and acute bronchitis) and 1 was thought to be possibly related (worsening gastroesophageal reflux disease in a patient on niacinamide). One patient in study 1 withdrew due to facial rash. One patient in the placebo arm of study 2 withdrew due to gastrointestinal side effects.
Discussion
The goal of this study was to determine whether oral niacinamide has biological activity at its maximum safe dose, determine its tolerability, and to detect any signal suggestive of clinical efficacy. Niacinamide is an inhibitor of sirtuins that have been shown to deacetylate p53,16 so the ratio of acetylated to total p53 was used as a measure of an on-target effect. Niacinamide is a polar small molecule that is freely filtered by the glomerulus, is not secreted by organic cation transporters,10 and does not accumulate in urine. Thus, we expect levels in kidney and other well-perfused tissues to be similar to the levels in circulating blood cells. Indeed, acetylated and total p53 have been measured in PBMCs in mice and appear similar to tissue levels in solid organs, such as the brain.35 In study 1, the acetyl-p53/total p53 ratio increased after 1 month of treatment, but decreased back to baseline by 6 and 12 months. We confirmed that this was not simply due to patient non-compliance by checking pill counts. The diminution in effect over time was unexpected and may represent a compensatory response to the effect of the drug. In study 2, there was no effect of niacinamide on the acetyl-p53/total p53 ratio, and no difference in this measure between niacinamide- and placebo-treated patients. The reason for the different results between the 2 studies is unclear. In general, the patients in study 2 were older and had more advanced disease, but whether and how this might affect their response to niacinamide is unknown. Alternatively, imprecision in the assay of acetyl-p53/total p53 in PBMCs may have limited our ability to detect small differences.
Our study was not designed or powered to determine the clinical efficacy of niacinamide. The rate of growth of htTKV in the 9 patients who completed study 1 was slower than would be predicted based on their Mayo Image Classification, but this was not statistically significant. In study 2, there was no difference in the change in htTKV between the treatment arms. Patients in the group randomized to niacinamide had baseline markers suggestive of a worse prognosis compared with the placebo group (a greater proportion were male and hypertensive, mean htTKV was larger, and more were in advanced Irazabal classes), which could have masked a small efficacy signal. Further pooled analysis of both studies to increase the power did not reveal any signal of efficacy. Given the lack of effect on htTKV, it is not surprising that there were no effects of niacinamide on any of the other secondary efficacy outcomes.
In general, niacinamide was well-tolerated. Diarrhea, nausea, gastroesophageal reflux disease, acne, and headache were the most common side effects but were mostly mild and manageable. However, these also appear to be common symptoms in patients with ADPKD and we were unable to demonstrate a significant difference in their incidence between placebo and niacinamide-treated patients. Importantly, there was no signal for niacinamide that was suggestive of hepatotoxicity.
This is the first clinical study to assess the pharmacodynamic efficacy of niacinamide as a sirtuin inhibitor. The strengths of the 2 studies were the inclusion of relatively young patients with rapidly progressive disease, good compliance with drug administration, and the careful evaluation of pharmacological and clinical endpoints. The weaknesses were those inherent to any pilot study, namely the small sample size, short duration of intervention, and (for study 1) uncontrolled study design. In addition, the accuracy and reliability of Western blotting as a quantitative assay for acetyl- and total p53 is unknown. Finally, the questionnaire used for assessing QOL has not been validated, other than our determination that it is internally consistent.
In conclusion, oral niacinamide at a dose of 30 mg/kg daily appears to be safe and well-tolerated in patients with ADPKD. However, this pilot clinical trial did not detect a sustained inhibition of sirtuin activity over 12 months of treatment at this dose, and there was no signal to suggest a beneficial effect on any efficacy measure. It is possible that higher doses of niacinamide might achieve the desired pharmacodynamic effect, but the safety of such high doses is unknown. In addition, because niacinamide acts by inhibiting cell proliferation and human ADPKD is significantly determined by early cyst formation and rapid growth occurring in utero and early childhood, it is conceivable that it may be effective if given to children and adolescents. Given its excellent safety profile, as demonstrated in the current studies and in prior studies in children,22 a trial in this age group is potentially feasible. Further studies will be needed to validate SIRT1 as a target for clinical therapy in ADPKD.
Disclosure
ASLY is a consultant for Regulus and has served on an advisory board for Otsuka Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
We thank Cathy Creed, RN, for coordinating the study and for her incredible work ethic, and Prof. Eiji Higashihara of Kyorin University School of Medicine for allowing us to modify and use his pain and quality of life questionnaire.
This study was supported by a pilot grant through the Frontiers Pilot and Collaborative Studies Funding Program funded by a National Institutes of Health (NIH) Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179) awarded to the University of Kansas Medical Center, by the internal clinical pilot grant program of the KUMC Research Institute, by a Pilot and Feasibility Clinical Research Grants in Kidney or Urologic Diseases grant (R21 DK104086), by the Kansas PKD Research and Translation Core Center (P30 DK106912), and by the Kansas Institute of Precision Medicine (P20 GM130423). The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund and funding from NIH (P30 DK106912, S10 RR29577, UL1 TR000001).
Footnotes
Table S1. Pain and quality of life assessment.
Table S2. Analysis of efficacy outcomes with combined study 1 and 2.
Table S3. Blood test values over the course of study 1.
Table S4. Blood test values over the course of study 2.
Figure S1. Observed versus expected change in htTKV. The observed annual rate of increase of htTKV in each study according to treatment assignment is compared with the rate predicted from the baseline htTKV using the Irazabal method. (A) Scatter plot of individual patient data. (B) Summary plot of means and standard errors for each group. The dotted line represents the line of identity (observed rate = predicted rate).
Figure S2. Comparison of manual stereology with a semiautomated method of htTKV determination. (A) Bland-Altman plot comparing measurements of htTKV from all MRI images in this study (n = 71) performed by stereology or by semiautomated software (MIROS). The y-axis represents the difference between the 2 methods, expressed as a percentage of the stereology htTKV. Mean bias was −0.2% and the 95% confidence intervals (−1.96 SD to +1.96 SD), shown on the plot by dotted lines, was ±8%. (B) Change in htTKV over 12 months of the study, as determined by stereology or MIROS (mean and SEM).
Supplementary Material
References
- 1.Solazzo A., Testa F., Giovanella S. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): a meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willey C., Kamat S., Stellhorn R., Blais J. Analysis of nationwide data to determine the incidence and diagnosed prevalence of autosomal dominant polycystic kidney disease in the USA: 2013–2015. Kidney Dis (Basel) 2019;5:107–117. doi: 10.1159/000494923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willey C.J., Blais J.D., Hall A.K. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32:1356–1363. doi: 10.1093/ndt/gfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consortium TEPKD The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 5.Consortium IPKD Polycystic kidney disease: The complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki T., Wu G., Hayashi T. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 7.Cowley B.D., Jr., Smardo F.L., Jr., Grantham J.J., Calvet J.P. Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc Natl Acad Sci U S A. 1987;84:8394–8398. doi: 10.1073/pnas.84.23.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards W.G., Sweeney W.E., Yoder B.K. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clinical Invest. 1998;101:935–939. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grantham J.J. Polycystic kidney disease: neoplasia in disguise. Am J Kidney Dis. 1990;15:110–116. doi: 10.1016/s0272-6386(12)80507-5. [DOI] [PubMed] [Google Scholar]
- 10.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 12.Kramers B.J., van Gastel M.D.A., Boertien W.E. Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist tolvaptan. Am J Kidney Dis. 2019;73:354–362. doi: 10.1053/j.ajkd.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Watkins P.B., Lewis J.H., Kaplowitz N. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–1113. doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X., Fan L.X., Sweeney W.E., Jr. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clinical Invest. 2013;123:3084–3098. doi: 10.1172/JCI64401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong S., Weber J.D. Deacetylation of the retinoblastoma tumour suppressor protein by SIRT1. Biochem J. 2007;407:451–460. doi: 10.1042/BJ20070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J., Nikolaev A.Y., Imai S. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 17.Bitterman K.J., Anderson R.M., Cohen H.Y. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine of the National Academies. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press: Washington, DC; 2000. [PubMed]
- 19.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 20.Knip M., Douek I.F., Moore W.P. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 21.Winter S.L., Boyer J.L. Hepatic toxicity from large doses of vitamin B3 (nicotinamide) N Engl J Med. 1973;289:1180–1182. doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- 22.Gale E.A., Bingley P.J., Emmett C.L., Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y., Tanaka A., Nakamura T. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 24.Rottembourg J.B., Launay-Vacher V., Massard J. Thrombocytopenia induced by nicotinamide in hemodialysis patients. Kidney Int. 2005;68:2911–2912. doi: 10.1111/j.1523-1755.2005.00583_8.x. [DOI] [PubMed] [Google Scholar]
- 25.Delanaye P., Weekers L., Krzesinski J.M. Diarrhea induced by high doses of nicotinamide in dialysis patients. Kidney Int. 2006;69:1914. doi: 10.1038/sj.ki.5000381. [DOI] [PubMed] [Google Scholar]
- 26.Young D.O., Cheng S.C., Delmez J.A., Coyne D.W. The effect of oral niacinamide on plasma phosphorus levels in peritoneal dialysis patients. Perit Dial Int. 2009;29:562–567. [PubMed] [Google Scholar]
- 27.Shahbazian H., Zafar Mohtashami A., Ghorbani A. Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: a double-blind randomized clinical trial. Nefrologia. 2011;31:58–65. doi: 10.3265/Nefrologia.pre2010.Nov.10734. [DOI] [PubMed] [Google Scholar]
- 28.Bourgeois B.F., Dodson W.E., Ferrendelli J.A. Interactions between primidone, carbamazepine, and nicotinamide. Neurology. 1982;32:1122–1126. doi: 10.1212/wnl.32.10.1122. [DOI] [PubMed] [Google Scholar]
- 29.Kryzhanovskii G.N., Shandra A.A. [Effect of diazepam, carbamazepine, sodium valproate and their combinations with vitamin preparations on epileptic activity] Biull Eksp Biol Med. 1985;100:545–547. [PubMed] [Google Scholar]
- 30.Ravine D., Gibson R.N., Walker R.G. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 31.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae K.T., Commean P.K., Lee J. Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr. 2000;24:614–619. doi: 10.1097/00004728-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Irazabal M.V., Rangel L.J., Bergstralh E.J. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kline T.L., Edwards M.E., Korfiatis P. Semiautomated segmentation of polycystic kidneys in T2-weighted MR images. AJR Am J Roentgenol. 2016;207:605–613. doi: 10.2214/AJR.15.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Khanna A., Li N., Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3:985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.