Abstract
Background
Two prospective studies that were performed before the era of highly sensitive solid-phase assays have shown a lower incidence of acute rejection in highly sensitized kidney-transplant patients given polyclonal antibodies compared with those given anti-CD25 monoclonal antibodies.
Methods
This prospective pilot randomized French multicenter study aimed to compare anti–T-lymphocyte Ig (ATLG) (n = 32) and basiliximab (n = 27) in highly sensitized kidney-transplant patients without preformed donor-specific antibodies (pDSAs) as assessed by a Luminex Single-Antigen flow bead assay. Only patients with a calculated panel reactive antibody ≥50%, with at least 1 antibody with a mean fluorescence intensity ≥5000 and without a historical pDSA and without a pDSA on the day of transplantation were included.
Results
Treatment failure as defined by biopsy-proven acute rejection, patient lost to follow-up, graft loss, and death was observed in 18.8% (95% confidence interval [CI], 8.9%–37.1%) and 18.8% (95% CI, 8.9%–37.1%) in patients who received ATLG and 14.8% (95% CI, 5.8%–34.8%) and 28.2% (95% CI, 14.2%–51.2%) of patients who received basiliximab, respectively at 6 (P = 0.66) and 12 (P = 0.62) months post-transplantation. One T cell–mediated rejection was observed in ATLG-treated patients (3.1%). One antibody-mediated rejection due to a de novo donor-specific antibody (DSA) occurred in basiliximab-treated patients (3.7%). Patient survival, graft survival, kidney parameters, and infection rate were similar in the 2 groups.
Conclusion
This pilot study indicates that in highly sensitized kidney-transplant patients without pDSAs, both ATLG and basiliximab can be used efficiently and safely. However, because of the lack of power, these results should be interpreted with caution.
Keywords: basiliximab, Grafalon, highly sensitized, DSA, induction therapy, kidney transplantation
Graphical abstract
Induction therapy is frequently used after kidney transplantation.1 In highly anti–human leukocyte antigen (HLA)-sensitized kidney-transplant patients, induction therapy with polyclonal antibodies is recommended.2,3 These recommendations are based on the results of 2 prospective randomized trials.4,5 Brennan and colleagues4 compared the use of rabbit anti-thymocyte globulins (rATG) with basiliximab in patients at high risk of acute rejection and/or of delayed graft function and reported a significantly higher risk of acute rejection in patients given basiliximab. Noël et al.5 subsequently reported the results of the TAXI study that compared rATG with daclizumab in patients at high risk of acute rejection and also observed a significantly higher risk of acute rejection in patients who received daclizumab. However, both studies included patients with relatively low panel reactive antibody (PRA) levels and both were performed before the era of single-antigen bead assays. In addition, anti-DQ, DP, and Cw antibodies were not assessed. It therefore cannot be definitively asserted that patients with pDSAs were indeed excluded from either trial. More recently, in a retrospective study, Goumard et al.6 reported a significantly higher incidence of acute rejection in anti-HLA–sensitized kidney-transplant patients without preformed DSAs given basiliximab when compared with patients given rATG. However, characteristics between the 2 groups differed significantly, specifically in relation to variables that can influence the acute rejection rate, such as the number of HLA mismatches and calculated PRA rates. The aim of the present prospective multicenter randomized pilot study was to compare the efficacy and safety of induction therapy by ATLGs or basiliximab in highly sensitized patients without preformed DSAs when assessed by Luminex assay.
Patients and Methods
Sixty patients from 5 French kidney-transplant centers were included in this pilot prospective multicenter randomized trial (NCT02377193) after having given their written informed consent between January 10, 2013, and December 1, 2016: Toulouse University Hospital (n = 25), Bordeaux University Hospital (n = 10), Nice University Hospital (n = 10), Kremlin Bicêtre University Hospital (n = 9), and Montpellier University Hospital (n = 6). Only adults undergoing an isolated ABO-compatible kidney transplant from a deceased or living donor and responding to the following inclusion criteria were included in the study: (i) a calculated PRA ≥50% at the last available serum test, performed less than 3 months before transplantation; (ii) detectable anti-HLA antibodies with at least 1 antibody having a mean fluorescence intensity >5000; (iii) no historical test result positive for pDSA; (iv) no pDSA detected on the day of transplantation; and (v) negative complement-dependent cytotoxicity crossmatch on historical sera and on the day of transplantation.
Patients were randomized (1:1 in each center) to receive either ATLG (Grafalon; Neovii, Rapperswil, Switzerland, 9 mg/kg at day 0, and then 3 mg/kg at days 1, 3, and 5) or basiliximab (Simulect; Novartis, Basel, Switzerland, 20 mg on days 0 and 4). The randomization sequence was prepared by the research department of Toulouse University Hospital who allocated patient numbers. Treatment allocation was stratified according to study center. Maintenance immunosuppression was based on tacrolimus (Prograf started at the dose of 0.1 mg/kg per day targeting a trough level between 6 and 10 ng/mL during the first year, post-transplantation), mycophenolic acid (Myfortic, 720 mg twice per day for 1 month and then 360 mg twice per day and steroids (500 mg pretransplant and then tapered to 20 mg/d at month 1, 5 mg/d at month 3, and maintained at 5 mg/d thereafter). All patients were given Pneumocystis jirovecii prophylaxis (trimethoprim-sulfamethoxazole 400 mg/80 mg, 3 times a week) for 6 months. Patients at risk of cytomegalovirus (CMV) infection, that is, seropositive recipients or seronegative recipients receiving a kidney from a seropositive donor, were given valganciclovir for 6 months (dose adapted according to the Cockcroft-Gault formula).
The composite primary endpoint of the study was treatment failure as defined by biopsy-proven acute rejection (BPAR) and/or patient lost to follow-up and/or graft loss, and/or death at 6 months. The secondary endpoints were the analyses of each of these endpoints at 6 and 12 months, as well as histological findings on protocol kidney biopsies that were performed at 3 and 12 months post-transplantation, the incidence of de novo DSAs were systematically assessed at 3 and 12 months post-transplantation and in case of kidney function impairment. Safety of both regimens was also analyzed, including viral complications (mainly CMV infection/replication and BK virus [BKV] replication) and hematological parameters. CMV DNAemia was systematically assessed at 6, 9, and 12 months post-transplantation. BKV DNAemia was also systematically assessed at 1, 3, 6, 9, and 12 months post-transplantation and every time a patient presented with impaired kidney function. Patients were followed for 12 months or until they were withdrawn from the study or were lost to follow-up.
Immunological Analyses
The presence of anti-HLA DSAs was tested using Labscreen Single-Antigen technology (One Lambda, Canoga Park, CA). The Labscreen Single-Antigen was used to determine the specificity of class I HLAs in A/B/Cw and class II in DR/DQ/DP IgG antibodies present in the recipients' sera (after centrifugation at 10,000g for 10 minutes, according to the manufacturer’s instructions). The presence and specificity of antibodies was subsequently confirmed with the Labscan 100, and the mean fluorescence (baseline value) of individual samples quantified for each type of bead. A baseline mean fluorescence intensity value of >500 was considered to be positive.
Pathological Analyses
All kidney biopsies were locally read and classified according to the 2015 Banff classification.7 Borderline lesions were not considered as rejections for the primary endpoint.
Statistical Analyses
Because of the lack of available data on the incidence of acute rejection in this selected population when the study was designed, we decided to perform a pilot study. For this purpose, we applied the general rule of thumb of 30 patients by arm to estimate the outcome means and proportions in each treatment arm.8 We performed an intention-to-treat analysis, and applied the principles of a “complete case analysis” to any patients with missing data. The cumulative proportions of treatment failure at 6 and 12 months post-transplantation were determined by Kaplan-Meier estimation. Adverse event rates (per person-month) were estimated by Poisson regression, using ln(time of participation) as an offset. Variables are expressed as the number of events and as percentages, mean ± SD, or median (minimum–maximum). We estimated 95% CIs in each treatment group for cumulative failure proportions, adverse event rates, proportions of BPAR, death, graft loss, and proportions of patients with infections or cancer. Survival functions were compared between the treatment groups using log-rank tests. We compared the distribution of categorical variables with χ2 or Fisher’s exact tests, and the distribution of quantitative variables using Wilcoxon rank-sum tests. Statistical analyses were computed using Stata SE 14.2 (StataCorp, College station, TX).
Results
Patients’ Characteristics
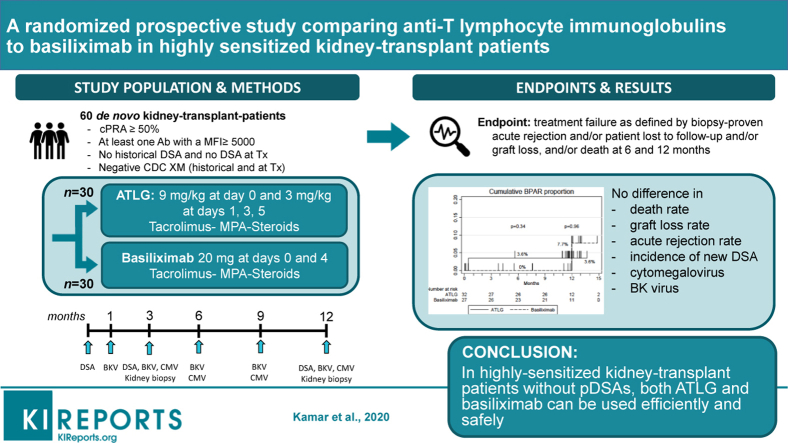
Sixty patients were included in the study. One of these patients did not undergo kidney transplantation and was therefore excluded from the final analysis (Figure 1). Among the 59 remaining patients, 32 patients received ATLG and 27 patients received basiliximab. Patient characteristics are presented in Table 1.
Figure 1.
Chart flow. ATLG, anti–T-lymphocyte Ig; SAE, serious adverse event.
Table 1.
Baseline characteristics
| Variables | Anti–T-lymphocyte Igs n = 32 | Basiliximab n = 27 | P value |
|---|---|---|---|
| Recipient | |||
| Age, yr | 56.8 ± 8.5 | 53.0 ± 13.6 | 0.40a |
| Gender, male/female | 13/19 | 9/18 | 0.56b |
| BMI, kg/m2 | 23.9 ± 3.9 | 24.6 ± 4.5 | 0.51c |
| Donor | |||
| Age, yr | 58.8 ± 12.7 | 57.1 ± 15.6 | 0.65c |
| Gender, male/female | 18/14 | 12/15 | 0.37b |
| Deceased/living | 31/1 | 25/2 | 0.59d |
| Immunology | |||
| HLA class I mismatches (0 to 8) | 5.94 ± 1.01 | 5.89 ± 1.05 | 0.77a |
| HLA class II mismatches (0 to 8) | 5.28 ± 0.96 | 5.40 ± 1.19 | 0.77a |
| Presence anti-HLA Ab (%) | 100 | 100 | - |
| cPRA at transplantation, %, median [IQR] | 89 [81–94.5] | 90 [73–96] | 0.98a |
| Negative CDC T-lymphocyte crossmatch, % | 100 | 100 | - |
| Negative CDC B-lymphocyte crossmatch, % | 93.5 | 100 | 0.49d |
| Transplantation | |||
| Cold ischemia time, h | 18.3 ± 6.8 | 16.1 ± 6.5 | 0.22 |
| Rank of transplantation | 1.6 ± 0.70 | 1.59 ± 0.69 | 0.70 |
| Cytomegalovirus status, n (%) | 0.53d | ||
| Donor + / recipient – | 8 (25) | 5 (18.5) | |
| Recipient + | 19 (59.4) | 20 (74.1) | |
| Donor – / recipient – | 5 (15.6) | 2 (7.4) |
BMI, body mass index; CDC, complement-dependent cytotoxicity; cPRA, calculated panel reactive antibodies; HLA, human leukocyte antigen; IQR, interquartile range.
Wilcoxon rank-sum test.
χ2 test.
Student’s t test.
Fisher’s exact test.
Efficacy Endpoints
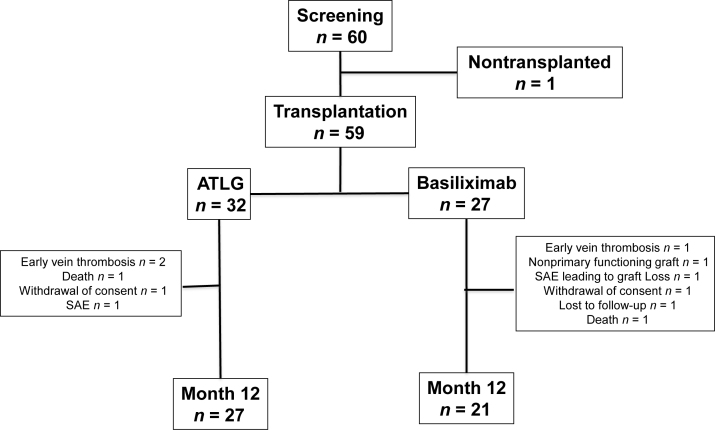
Treatment failure (BPAR, patient lost to follow-up, graft loss, and death) was observed in 18.8% (95% CI, 8.9%–37.1%) and 18.8% (95% CI, 8.9%–37.1%) of patients receiving ATLG and 14.8% (95% CI, 5.8%–34.8%) and 28.2% (95% CI, 14.2%–51.2%) of patients in the basiliximab arm of the study, respectively at 6 (P = 0.66) and 12 (P = 0.62) months post-transplantation (Figure 2). A comparison of efficacy endpoints between the 2 groups is presented in Table 2.
Figure 2.
Primary endpoint: Incidence of biopsy-proven acute rejection (BPAR), graft loss, death, or patient lost to follow-up (FU), in patients receiving anti–T-lymphocyte Ig (ATLG) or anti-CD25 monoclonal antibodies.
Table 2.
Efficacy endpoints
| Variables | Anti–T-lymphocyte Ig n = 32 | Basiliximab n = 27 |
P value |
|---|---|---|---|
| Cumulative failure proportion (BPAR, graft loss, death or loss of follow-up) | |||
| Month 6, % [95% CI] | 18.8 [8.9–37.1] | 14.8 [5.8–34.8] | 0.66a |
| Month 12, % [95% CI] | 18.8 [8.9–37.1] | 28.2 [14.2–51.2] | 0.62a |
| Cumulative failure proportion of BPAR at 12 mo, n events (%) [95% CI] | 1 (3.6) [0.5– 22.8] | 1 (7.7) [1.1–43.4] | 0.96a |
| TCMR, n | 1 | 0 | |
| ABMR, n | 0 | 1 | |
| Treated BPAR, n | 1 | 1 | |
| De novo DSAs, n | 0 | 1 | |
| Cumulative failure proportion of death at 12 months, n events (%) [95% CI] | 1 (3.3) [0.5–21.4] | 1 (4.2) [0.6–26.1] | 0.92a |
| Death with functioning graft, n | 0 | 1 | |
| Cumulative failure proportion of Graft losses at 12 months, n events (%) [95% CI] | 3 (9.6) [3.2–26.8] | 3 (12.1) [4.0–33.0] | 0.85a |
| Early vein thrombosis, n | 2 | 1 | |
| Non primary functioning graft, n | 0 | 1 | |
| Chronic dysfunction, n | 0 | 1 | |
| Death, n | 1 | 0 | |
| MDRD eGFR (ml/min per 1.73 m2), mean ± SD | |||
| Month 3 (among n = 28 and n = 26 patients) | 52.1 ± 23.5 | 50.5 ± 22.1 | 0.89b |
| Month 6 (among n = 27 and n = 26 patients) | 51.8 ± 19.3 | 46.8 ± 22.2 | 0.47b |
| Month 12 (among n = 28 and n = 26 patients) | 50.8 ± 20.1 | 56.3 ± 21.8 | 0.29b |
| Histological findings, median [min–max] | |||
| Month 3 (among n = 28 and n = 26 patients) | |||
| T | 0 [0–0] | 0 [0–2] | 0.03b |
| I | 0 [0–1] | 0 [0–2] | 0.81b |
| G | 0 [0–1] | 0 [0–0] | 0.41b |
| Ptc | 0 [0–2] | 0 [0–1] | 0.18b |
| V | 0 [0–0] | 0 [0–0] | — |
| Ah | 0 [0–3] | 0 [0–1] | 0.38b |
| Cg | 0 [0–0] | 0 [0–1] | 0.23b |
| Ci | 0 [0–1] | 0 [0–1] | 0.72b |
| Ct | 0 [0–1] | 0 [0–1] | 0.20b |
| Cv | 0.5 [0–2] | 0 [0–1] | 0.29b |
| Month 12 (among n = 27 and n = 21 patients) | |||
| T | 0 [0–2] | 0 [0–0] | 0.39b |
| I | 0 [0–2] | 0 [0–1] | 0.52b |
| G | 0 [0–2] | 0 [0–1] | 0.41b |
| Ptc | 0 [0–2] | 0 [0–0] | 0.37b |
| V | 0 [0–0] | 0 [0–0] | — |
| Ah | 0 [0–2] | 0 [0–2] | 0.89b |
| Cg | 0 [0–0] | 0 [0–0] | — |
| Ci | 1 [0–2] | 1 [0–3] | 0.48b |
| Ct | 1 [0–2] | 1 [0–3] | 0.32b |
| Cv | 0 [0–2] | 0.5 [0–1] | 0.87b |
| Proteinuria (g/24 h), median [IQR] | |||
| Month 3 (among n = 18 and n = 10) | 0.20 [0.10–0.30] | 0.15 [0.10–0.20] | 0.35b |
| Month 6 (among n = 20 and n = 17) | 0.20 [0.10–0.40] | 0.22 [0.12–0.50] | 0.78b |
| Month 12 (among n = 17 and n = 10) | 0.10 [0.10–0.30] | 0.19 [0.10–0.30] | 0.70b |
ABMR, B-cell–mediated rejection; ah, arteriolar hyalinosis; BPAR, biopsy-proven acute rejection; cg, glomerular basement membrane double contours; ci, interstitial fibrosis; CI, confidence interval; ct, tubular atrophy; cv, vascular fibrous intimal thickening; DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; i, interstitial inflammation; IQR, interquartile range; g, glomerulitis; MDRD, Modification of Diet in Renal Disease; ptc, peritubular capillaritis; t, tubulitis; TCMR, T cell–mediated rejection; v, intimal arteritis.
Log-rank test.
Wilcoxon rank-sum test.
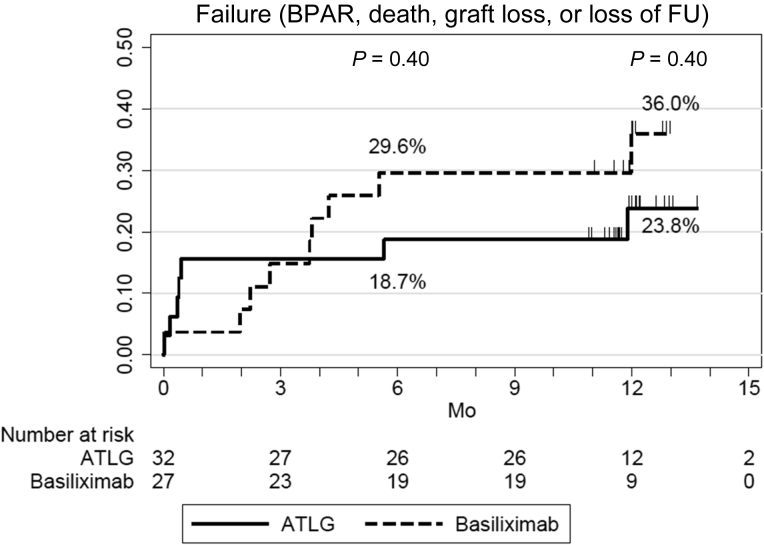
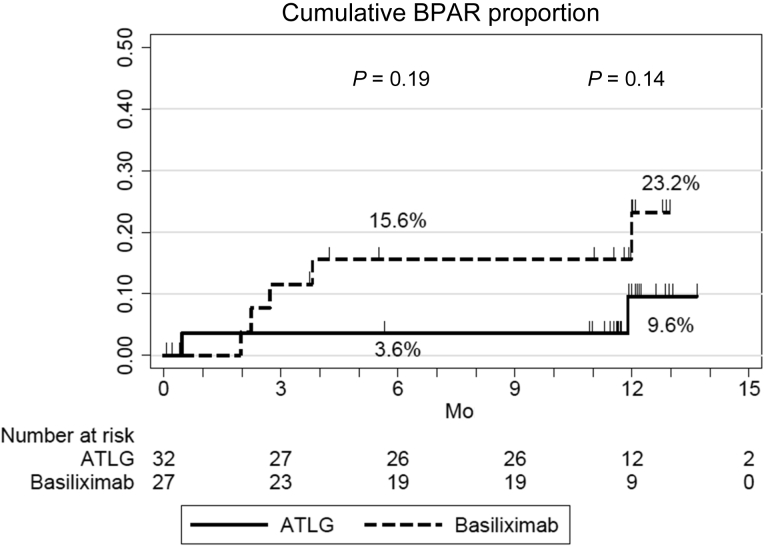
At 6 and 12 months post-transplantation, respectively, BPAR was observed in 3.6% (95% CI, 0.5%–22.8%) and 3.6% (95% CI, 0.5%–22.8%) of patients treated with ATLG compared with 0.0% (P = 0.34) and 7.7% (95% CI, 1.1%–43.4%) (P = 0.96) of patients in the basiliximab arm of the study (Figure 3). In the ATLG arm, 1 patient experienced an acute rejection episode during the first year post-transplantation (3.1%), a T cell–mediated rejection that occurred 15 days post-transplantation and that was successfully treated with steroid pulses. In the basiliximab arm, 1 patient experienced an acute rejection episode during the study period (3.7%): 1 patient developed de novo DSA and experienced an antibody-mediated rejection at 12 months and was treated with steroid pulses, plasmapheresis, and rituximab. Hence, the incidence of treated BPAR was 3.1% (1 of 32) in the ATLG arm and 3.7 % (1 of 27) in the basiliximab arm, P = 1.00. No significant difference in tacrolimus trough levels and mycophenolic acid doses were observed between the 2 groups (Supplementary Figure S1). Four patients from the ATLG arm were converted from mycophenolic acid to everolimus (2 because of BKV replication, 1 for CMV replication, and the last 1 to reduce tacrolimus doses). Three patients from the basiliximab arm were converted from tacrolimus to belatacept because of impaired kidney function. None of these patients experienced an acute rejection.
Figure 3.
Incidence of biopsy-proven acute rejection (BPAR) in patients receiving anti–T-lymphocyte Ig (ATLG) or anti-CD25 monoclonal antibodies.
One patient who received ATLG presented with mesenteric ischemia, lost his graft, and died at day 7 post-transplant. Another patient from the basiliximab group died from an unknown cause with a functioning graft at 6 months post-transplantation. Three grafts were lost in each treatment arm. In the ATLG arm, in addition to the patient described previously who presented with a mesenteric ischemia, 2 patients experienced early vein allograft thrombosis. In the basiliximab arm, 1 patient experienced a vein allograft thrombosis, another one manifested with a nonprimary functioning graft, with the third patient losing his graft at 11 months post-transplantation after several infectious complications (flu, gastroenteritis, and pulmonary infection) that were associated with acute kidney injuries and that led to graft loss. Pathological examination of allograft nephrectomies did not reveal any features of acute rejection. Kidney function did not differ significantly between the 2 arms (Table 2). Histological features observed on protocol kidney biopsies performed at 3 and 12 months post-transplantation were also similar in both arms, with the exception of tubulitis, which occurred more frequently in basiliximab-treated patients at 3 months (Table 2).
Safety Endpoints
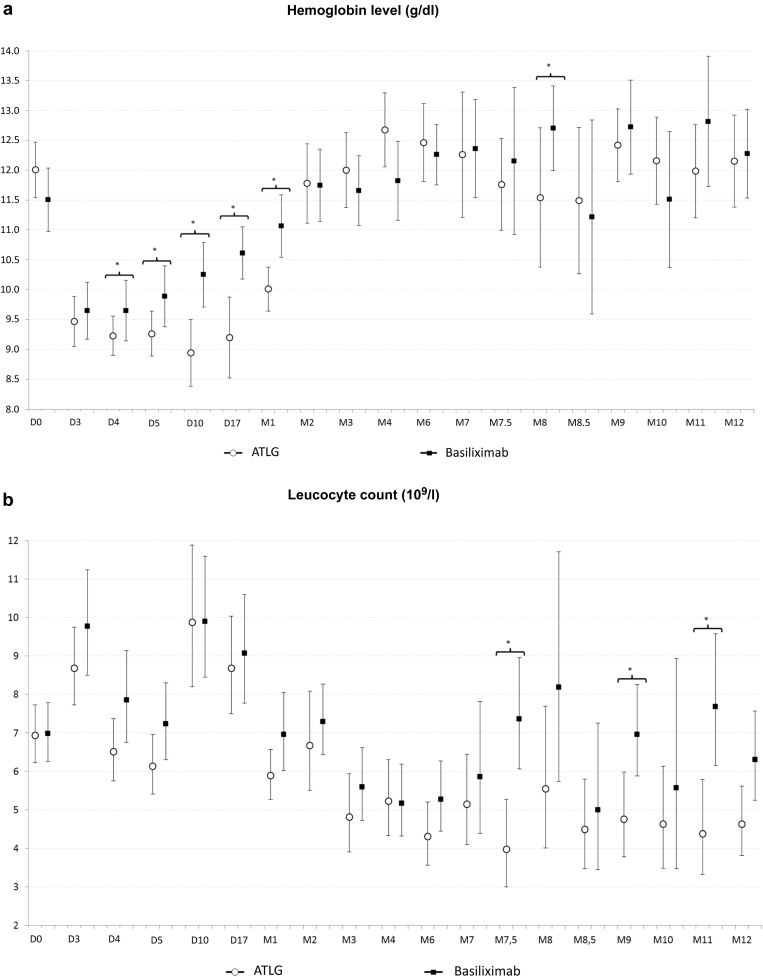
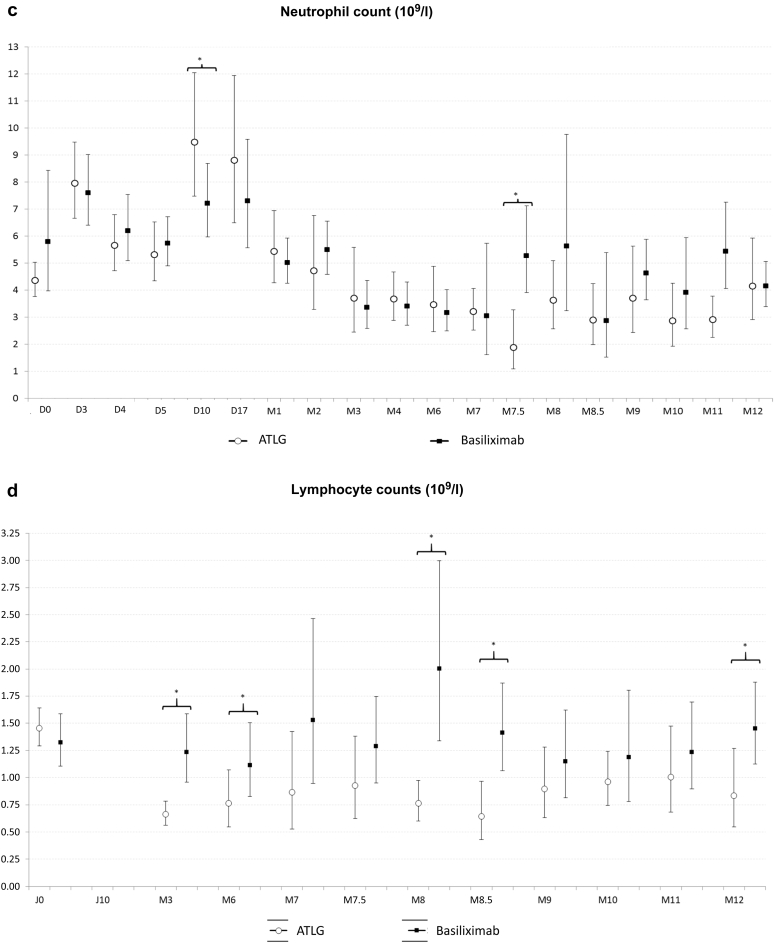
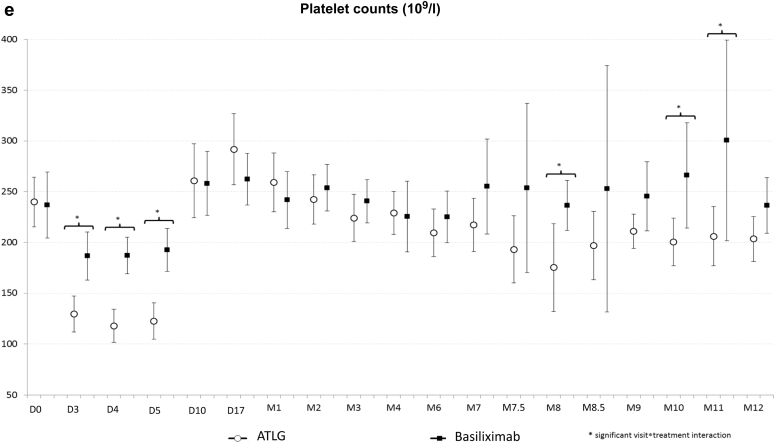
The number and rate of adverse events and serious adverse events were similar in the 2 treatment groups (Table 3). One patient in the ATLG group experienced anemia and thrombocytopenia at day 2 post-transplantation and did not receive subsequent rATG doses. Another patient from the basiliximab group presented several infections that lead to graft loss at 11 months post-transplantation. Overall, the proportions of infections were also similar in both groups. Although more patients presented with a BKV replication in blood samples at 1, 3, and 6 months post-transplantation in the ATLG arm when compared with the basiliximab treatment group, the difference was not statistically significant. It is noteworthy that 2 patients were converted from mycophenolic acid to everolimus because of BKV replication in the ATLG arm, but that only 1 patient who received ATLG developed a polyomavirus-associated nephropathy. With respect to CMV replication, the incidence was statistically higher at 9 months and numerically higher at 12 months in patients receiving ATLG. One patient in the ATLG group developed a CMV disease. Two patients developed a cancer during the study period. Both were in the ATLG group: 1 developed a nonmelanoma skin cancer and the other was diagnosed with a renal carcinoma of the native kidney. No case of post-transplant lymphoma disease or progressive multiple leukoencephalopathy was diagnosed. In the early period post-transplantation, hemoglobin levels and platelet counts were significantly lower in the ATLG group compared with the basiliximab treatment arm. However, thereafter there was no difference between the 2 groups (Figure 4). Leucocyte and neutrophil counts were similar in both groups. Finally, as expected, lymphocyte count was significantly lower in the ATLG group.
Table 3.
Safety endpoints
| Variables | Anti–T-lymphocyte Igs n = 32 | Basiliximab n = 27 | P value |
|---|---|---|---|
| No. of AEs per patient over 12 mo of follow-up, median [IQR] | 10.5 [5.5–16] | 10 [5–15] | |
| AE rate over 12 mo (number of AEs/person-month [95% CI]) | 1.07 [0.97–1.19] | 0.99 [0.88–1.11] | 0.29a |
| No. of SAEs per patient at 12 mo, median [IQR] | 2 [1–3] | 1 [0–2] | |
| SAE rate at 12 mo (number of SAEs/person-month [95% CI]) | 0.21 [0.17–0.27] | 0.16 [0.12–0.21] | 0.15a |
| Patients with ≥1 infection, n (%) [95% CI] (among n = 32 and n = 27 patients) | 23 (71.9) [53.3–86.3] | 18 (66.7) [46.0–83.5] | 0.67b |
| BK virus replication, n (%) | |||
| Month 1 (among n = 28 and n = 26 patients) | 0 (0) | 0 (0) | — |
| Month 3 (among n = 28 and n = 26 patients) | 3 (10.7) | 1 (3.8) | 0.61c |
| Month 6 (among n = 28 and n = 24 patients) | 3 (10.7) | 0 (0) | 0.24c |
| Month 12 (among n = 27 and n = 21 patients) | 1 (3.7) | 1 (3.8) | 1.00c |
| Polyomavirus nephropathy, n (%) | 1 (3.1) | 0 (0) | 0.54c |
| Cytomegalovirus replication, n (%)d | |||
| Month 6 (among n = 28 and n = 24 patients) | 2 (7.1) | 2 (8.3) | 1.00c |
| Month 9 (among n = 27 and n = 21 patients) | 6 (22.2) | 0 (0) | 0.03c |
| Month 12 (among n = 27 and n = 21 patients) | 4 (14.8) | 1 (4.7) | 0.37c |
| Cytomegalovirus disease, n (%) (among n = 32 and n = 27 patients) |
1 (3.1) | 0 (0) | 0.54c |
| Cancer, n (%) [95% CI] (among n = 32 and n = 27 patients) |
2 (6.3) [0.8–20.8] |
0 (0) [0–12.8] |
0.50c |
AE, adverse event; CI, confidence interval; IQR, interquartile range; SAE, serious AE.
Poisson regression (adjusted for an offset of ln[time of follow-up over 12 mo]);
χ2 test.
Fisher’s exact test.
Among the 8 patients who presented with a cytomegalovirus replication in the anti-lymphoglobulin group, 5 were seronegative at transplantation and had received a kidney from a seropositive donor, whereas the 3 remaining patients were seropositive at transplantation. All 3 patients who presented a cytomegalovirus replication in the basiliximab group were seropositive at transplantation.
Figure 4.
Outcome of hemoglobin level (a), leukocyte (b), neutrophil (c), lymphocyte (d), and platelet (e) counts in patients receiving anti–T-lymphocyte Ig (ATLG) or anti-CD25 monoclonal antibodies. ∗Significant difference between both treatments.
Discussion
The optimal induction therapy for highly sensitized patients, without pDSAs determined by using a very sensitive assay, remains to be established. Two multicenter prospective studies have previously compared rATG with anti-CD25 monoclonal antibodies in patients at increased risk of acute rejection or at high immunological risk.4,5 Both studies reported a significantly higher incidence of acute rejection in patients receiving anti-CD25 monoclonal antibodies.4,5 Current guidelines, based on the results of these 2 studies, therefore recommend the use of polyclonal antibodies in highly sensitized patients.2,3 These 2 studies were, however, performed before the era of Luminex assays, and it therefore cannot be definitively asserted that patients with pDSAs were indeed excluded from either of these previous studies.9 The Brennan et al.4 study included patients at high risk of delayed graft function and/or acute rejection, as determined by cold ischemia times and donor characteristics. Analysis of the characteristics of patients included in the Brennan et al.4 study highlights that recipients were not highly sensitized. Indeed, the PRA levels at transplantation were 6.3% ± 19% in the rATG arm and 5.7% ± 17.1% in the basiliximab arm. The historical peaks of PRA were 14% ± 28.2% in the rATG arm and 13.5% ± 27.7% in the basiliximab arm.4 In the TAXI study, the PRA levels at transplantation were higher: 33% ± 30% in the rATG arm and 37% ± 32% in the daclizumab arm.5 The peaks of PRA were 69% ± 25% and 74% ± 22% in the rATG and daclizumab groups, respectively. It is noteworthy that the proportion of patients with a PRA >80% was relatively low: 8.8% in the rATG group and 11.4% in the daclizumab arm.5 The objective of the present study was to compare ATLG and basiliximab treatments in a highly sensitized population without pDSAs as assessed by Luminex assay. The calculated PRA levels were much higher than in the 2 studies quoted previously: 89% (81–94.5) in the ATLG arm and 90% (73–96) in the basiliximab arm. None of the patients included in the study had been identified to be positive for pDSA before or at transplantation.
In a recent retrospective study that compared rATG with basiliximab in sensitized kidney-transplant patients without pDSAs, Goumard et al.6 found that the composite endpoint, including confirmed acute rejection, graft loss, and death, occurred more frequently in patients receiving basiliximab. In the present study, treatment failure defined by BPAR, patient lost to follow-up, graft loss, and death at 6 or at 12 months did not differ between the 2 groups. The incidence of BPAR was 3.1% in the ATLG arm (1 TCMR) and 3.7% in the basiliximab arm (1 ABMR). Interestingly, most acute rejection episodes were borderline rejections that were diagnosed from protocol biopsies and were predominantly left untreated. Only 1 patient in the ATLG arm experienced a TCMR and another in the basiliximab arm developed a de novo DSA that lead to an episode of ABMR. Hence, the incidence of treated BPAR was 3.1% in the ATLG arm and 3.7 % in the basiliximab arm, P = 1.00. These rates are much lower than those reported at 12 months post-transplantation by Brennan et al.4 (i.e., 15.6% in the rATG arm and 25.5% in the basiliximab arm) and by Noël et al.5 (i.e., 15% in the rATG arm and 27.2 % in the daclizumab arm). Neither the Brennan et al.4 nor the Noël et al.5 study assessed the presence of de novo DSAs. In a retrospective study by Goumard et al.,6 the incidence of acute rejection (i.e., TCMR grade ≥1 or ABMR) was higher than ours, that is, 8.2% in patients receiving rATG and 25% in patients receiving basiliximab. Surprisingly, the incidence of de novo DSA was high in their study: 15.8% in patients receiving rATG and 21.7% in patients receiving basiliximab.6 The difference between our results and the Goumard et al.6 study may be explained by differences in the immunosuppression maintenance protocols as well as the levels and doses of immunosuppressants used in the 2 studies. The high incidences of acute rejection and de novo DSA in the Goumard et al.6 study can be related to a low maintenance immunosuppression. Interestingly, the 5-year follow-up of patients enrolled in the TAXI study indicates that despite the higher incidence of acute rejection in the daclizumab treatment arm, there was no significant difference in graft survival at the 5-year time point between the 2 treatment arms.10 Goumard et al.6 reported a similar 4-year survival with induction therapies. In our study, patient survival and graft survival did not differ between the 2 treatment arms. Our findings are also consistent with those of the Brennan et al.4 and Noël et al.,5 because we found no significant difference in kidney function between the 2 treatment arms. Protocol kidney biopsies, performed at 3 and 12 months, also found no significant difference between the 2 treatment groups, with the exception of mild tubulitis at 3 months in the basiliximab arm that in some patients was considered as a borderline rejection. However, in most of these patients no specific treatment was given.
The use of T cell–depleting agents after kidney transplantation is associated with an increased risk of infection, particularly of bacterial and viral infections, such as CMV and BKV infections.11, 12, 13 This prompted us to perform a systematic screen for CMV and BKV after transplantation. In the present study, no difference in the overall infection rate was observed between the 2 treatment arms.
With respect to viral infections, the proportion of patients with CMV and/or BKV replication was nevertheless higher in the ATLG group. This difference was only statistically significant for CMV replication at 9 months. This observation may be related to the small sample size of patients affected by viral disease, with only 1 patient presenting with CMV disease and another patient developing polyoma virus–associated nephropathy. Both patients were in the ATLG group. It is noteworthy that 3 patients in the ATLG treatment arm were converted from mycophenolic acid to everolimus because of CMV or BKV replication to facilitate viral clearance. Recent studies have confirmed the protective effect of the everolimus against viral replication.14,15 Finally, as previously reported, T cell–depleting agents have a hematological toxicity.16 In the present study, hemoglobin levels and platelet counts were lower in the ATLG treatment arm within the first month post-transplantation, but no difference between the 2 groups was observed thereafter. As expected, lymphocyte count was significantly lower in the ATLG group. Leukocyte and neutrophil counts were comparable in both treatment arms during the study period.
The main limitation of the current study is the small number of patients enrolled. Our study does, however, serve as a pilot study of a population that has not been previously examined in the literature. Its main strength is the homogeneity of patient follow-up and the systematic DSA, viral, and histological screens performed.
In conclusion, this pilot study shows that in highly sensitized kidney-transplant patients without pDSAs, both ATLG and basiliximab can be used efficiently and safely. Clinicians can therefore choose the induction agent according to the recipients’ history and comorbidities. Finally, because of the lack of power, these results should be interpreted with caution and a large prospective study is required to confirm our data.
Disclosure
NK received speakers’ fees and participated in advisory boards for AbbVie, Amgen, Astellas, Chiesi, Gilead, Fresenius Medical Care, MSD, Neovii, Novartis, Roche, Sanofi, and Shire. LC received speakers’ fees from Astellas, Chiesi, and Novartis and participated in advisory boards for Astellas, BMS, Biotest, Chiesi, and Novartis. LE participated in advisory boards for Astellas, Sanofi, Chiesi, and Novartis. PM received travel and research grants from Astellas, Fresenius Medical Care, Novartis, Biotest, Chiesi, and BMS. ADB received speakers’ fees from Astellas and Novartis. All the other authors declared no competing interests.
Acknowledgments
This study was promoted by the Centre Hospitalier de Toulouse and funded by Novartis Pharma and Neovii.
Author Contributions
NK designed the study, enrolled patients, analyzed the data, and wrote the paper. BL performed the statistical analyses and reviewed the paper. LC, LA, ADu, VP, LE, ALH, ADa, ML, EC, PM, and ADB enrolled patients, participated in their follow-up, and reviewed the paper. NC contributed to the study design, performed the immunology work-up, and reviewed the paper.
Footnotes
Figure S1. Tacrolimus trough levels (A) and mycophenolic acid doses (B) in patients receiving polyclonal antibodies or anti-CD25 monoclonal antibodies.
CONSORT Statement.
Supplementary Material
References
- 1.Opelz G., Unterrainer C., Susal C. Efficacy and safety of antibody induction therapy in the current era of kidney transplantation. Nephrol Dial Transplant. 2016;31:1730–1738. doi: 10.1093/ndt/gfw086. [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 3.Heemann U., Abramowicz D., Spasovski G. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: a European Renal Best Practice (ERBP) position statement. Nephrol Dial Transplant. 2011;26:2099–2106. doi: 10.1093/ndt/gfr169. [DOI] [PubMed] [Google Scholar]
- 4.Brennan D.C., Daller J.A., Lake K.D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 5.Noël C., Abramowicz D., Durand D. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goumard A., Sautenet B., Bailly E. Increased risk of rejection after basiliximab induction in sensitized kidney transplant recipients without pre-existing donor-specific antibodies - a retrospective study. Transplant Int. 2019;32:820–830. doi: 10.1111/tri.13428. [DOI] [PubMed] [Google Scholar]
- 7.Loupy A., Haas M., Solez K. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies:recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 9.Tait B.D., Hudson F., Cantwell L. Review article: Luminex technology for HLA antibody detection in organ transplantation. Nephrology (Carlton) 2009;14:247–254. doi: 10.1111/j.1440-1797.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 10.Hellemans R., Hazzan M., Durand D. Daclizumab versus rabbit antithymocyte globulin in high-risk renal transplants: five-year follow-up of a randomized study. Am J Transplant. 2015;15:1923–1932. doi: 10.1111/ajt.13191. [DOI] [PubMed] [Google Scholar]
- 11.Padiyar A., Augustine J.J., Hricik D.E. Induction antibody therapy in kidney transplantation. Am J Kidney Dis. 2009;54:935–944. doi: 10.1053/j.ajkd.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Kotton C.N., Kumar D., Caliendo A.M. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch H.H., Randhawa P.S., AST Infecious Diseases Community of Practice BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13528. doi: 10.1111/ctr.13528. [DOI] [PubMed] [Google Scholar]
- 14.Pascual J., Berger S.P., Witzke O. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol. 2018;29:1979–1991. doi: 10.1681/ASN.2018010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommerer C., Suwelack B., Dragun D. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019;96:231–244. doi: 10.1016/j.kint.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Mourad G., Rostaing L., Legendre C. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004;78:584–590. doi: 10.1097/01.tp.0000129812.68794.cc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.