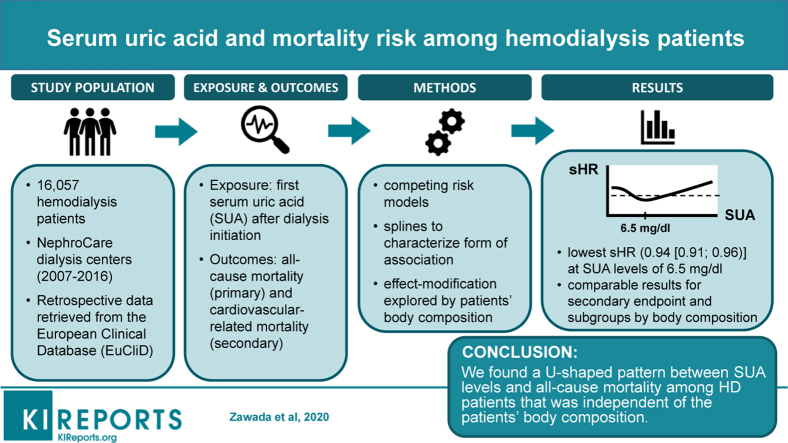
Abstract
Introduction
Although high serum uric acid (SUA) has been consistently associated with an increased risk of death in the general population and in persons with nondialysis chronic kidney disease (CKD), studies in patients undergoing dialysis are conflicting. It has been postulated that low SUA simply reflects poor nutritional status in dialysis patients. We here characterize the association between SUA and the risk of death in a large dialysis cohort and explore effect modification by underlying nutritional status as reflected by body composition.
Methods
In this retrospective cohort study, we included 16,057 hemodialysis (HD) patients treated during 2007 to 2016 in NephroCare centers as recorded in the European Clinical Database (EuCliD). The association between SUA, all-cause, and cardiovascular (CV)−related mortality was evaluated with competing risk models and characterized with splines. Effect modification was explored by lean tissue index (LTI) and fat tissue index (FTI).
Results
During a mean of 1.8 years of follow-up, 2791 patients (17.4%) died. We found a multivariable-adjusted U-shaped pattern between SUA and all-cause mortality. Patients with SUA levels of 6.5 mg/dl (387 μmol/l) were at the lowest risk of death (subdistribution hazard ratio = 0.94 [confidence interval {CI} 0.91; 0.96]). The form of association was not meaningfully affected by underlying LTI and FTI.
Conclusion
We found a U-shaped pattern between SUA levels and all-cause mortality among HD patients, which was independent of the patients’ body composition.
Keywords: body composition, hemodialysis, lean tissue index, mortality risk, nutrition, uric acid
Graphical abstract
Uric acid is the end product of purine metabolism and is eliminated by renal (60%−70%) and intestinal (30%−40%) excretion.1,2 Consequently, declining renal function is associated with elevations in SUA, and 40% to 80% of patients with end-stage renal disease (ESRD) have hyperuricemia, typically defined as SUA levels ≥7 mg/dl (416 μmol/l).2 In patients treated with hemodialysis, SUA is efficiently removed from blood, given its clearance pattern and sieving coefficient (1.01) similar to that of urea; thus, during 1 hemodialysis session on average 1 g uric acid is eliminated.2
Typically, hyperuricemia is the hallmark of gout. Moreover, detrimental pathophysiological effects have been attributed to this compound and linked to the pathogenesis of cardiovascular disease, the main cause of mortality in dialysis patients.3 Despite its antioxidant properties, uric acid was found to activate inflammatory pathways in the body such as the NALP3 inflammasome, leading to secretion of interleukin-1β and reactive oxygen species. In addition, uric acid triggers endothelial dysfunction and stimulates the renin−angiotensin−aldosterone system, thus contributing to vascular smooth muscle cell growth and arterial function impairment.4, 5, 6, 7, 8, 9, 10, 11, 12 In line with this, hyperuricemia has been associated with conditions associated with cardiovascular disease, such as hypertension, diabetes mellitus, and insulin resistance.2,13, 14, 15, 16, 17, 18
Although there is agreement on the association between high SUA levels and the risk of all-cause as well as cardiovascular-related mortality in the general population,19, 20, 21, 22, 23 studies exploring the role of SUA in the context of high risk of mortality and cardiovascular disease of ESRD patients are inconclusive, reporting direct, inverse, or different forms of associations.2,24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 The 4 largest cohort studies, including data from the Dialysis Outcomes and Practice Patterns Study (DOPPS; n = 5827),32 DaVita Inc (n = 4298),35 Korean Society of Nephrology registry (n = 7333),31 and Taiwan Society of Nephrology dialysis registry (n = 27,229)28 found high SUA levels to be associated with lower risk for all-cause mortality. In another large cohort study from Asia with 1738 patients,24 a U-shaped association between SUA and all-cause mortality was found, whereas further smaller studies found J-shaped associations.27,36 Discrepancies in study results may be explained by differences in cohort characteristics, low power, as well as the possibility of residual confounding. Regarding the latter, and in line with SUA’s role in protein metabolism, recent studies pinpointed that SUA may be considered as a marker of the nutritional status among patients undergoing hemodialysis,25,26,35 and suggested that a better nutritional status and not a high SUA is likely to explain survival associations.
A better understanding of the reasons behind these paradoxical findings is fundamental to determine target SUA levels for hemodialysis patients. In the present study, we characterized the association between SUA and all-cause as well as CV-related mortality among a large cohort of hemodialysis patients, and explored effect modification by underlying body composition, taken here as a marker of nutritional status of the patients.
Methods
Data Source
Pseudonymized data have been retrieved from EuCliD. As a clinical information system, EuCliD is implemented in NephroCare clinics from Fresenius Medical Care, and all clinical data are collected according to standardized clinical protocols and procedures of the clinics.37,38 Routinely collected medical information includes demographics, comorbidities, laboratory data, and medication, as well as information on underlying kidney disease, vascular access, dialysis treatments, and clinical outcome (hospitalization and mortality). International Classification of Diseases, 10th Revision (ICD-10) codes are used to classify disease information.
Study Population and Design
We included data from 16,057 hemodialysis patients treated in 564 NephroCare centers in EMEA (Europe, Middle East, and Africa; n = 15,127) and Latin America (n = 930) into this retrospective cohort study. Patients were treated in the following 22 countries: Argentina (n = 274), Bosnia (n = 93), Brazil (n = 263), Chile (n = 8), Colombia (n = 384), Croatia (n = 162), Czech Republic (n = 446), Ecuador (n = 1), France (n = 771), Hungary (n = 389), Italy (n = 1 077), Poland (n = 794), Portugal (n = 749), Romania (n = 427), Russia (n = 3 471), Serbia (n = 114), Slovakia (n = 836), Slovenia (n = 18), South Africa (n = 1), Spain (n = 3607), Turkey (n =2150), and the United Kingdom (n = 22).
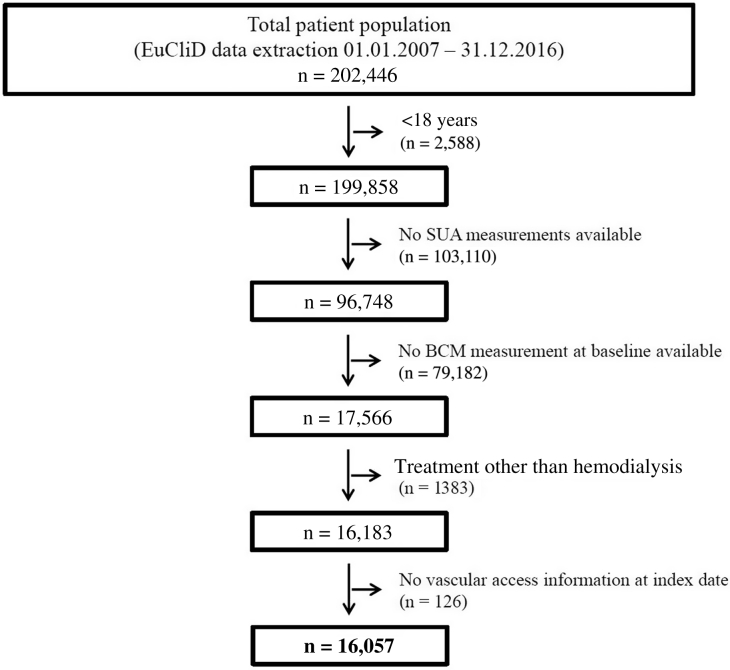
The flow chart displaying patient selection is presented in Figure 1. Data from adult patients on hemodialysis who were treated between January 1, 2007 and December 31, 2016 and who provided their written informed consent to secondary use of their clinical data for research purposes were eligible for study inclusion. At least 1 SUA measurement with adjacent body composition monitor (BCM) examination (maximum 3 months before the SUA measurement) had to be available for each included patient. Patients with missing vascular access information at index date were additionally excluded.
Figure 1.
Flow chart with patient numbers. BCM, body composition monitor; EuCliD, European Clinical Database; SUA, serum uric acid.
For each patient, the first available SUA measurement after renal replacement therapy initiation was defined as the index date. All patients were followed from the index date until death, kidney transplantation, center change, treatment change (peritoneal dialysis, home hemodialysis, treatment stop, spontaneous recovery), loss to follow-up, other unspecified reasons, and end of data extraction (December 31, 2016).
The analysis was performed in adherence to the Declaration of Helsinki.
Baseline Patient and Treatment Characteristics
All demographic information of the patients is presented as of the index date. By definition, SUA was measured at the index date. For the determination of SUA levels, blood samples taken before the dialysis session were used. For all other laboratory, treatment, and BCM information, the last assessment within the last 3 months before the index date was used, if not available at the index date. Dialysis vintage was calculated as the time from initiation of renal replacement therapy until the index date. Body mass index (BMI) was calculated based on postdialysis body weight. The LTI and FTI represent the respective tissue masses normalized to height squared and were determined with the body composition monitor (BCM), which applies the bioimpedance spectroscopy technique; measurements are routinely performed in NephroCare clinics. Presenting blood pressure was measured before dialysis. Use of SUA-modulating medication was collected for the 4 different medication groups: medications inhibiting uric acid production (including allopurinol, tisopurine, and febuxostat); medications increasing uric acid excretion (including probenecid, sulfinpyrazone, benzbromarone, isobromindione, and lesinurad); other antigout medications (including urate oxidase and pegloticase); and preparations that were reported to increase SUA levels (diuretics, β-blockers, aspirin, pyrazinamide, ethambutol, nicotinic acid, lactic acid, cyclosporin, tacrolimus, fructose, xylitol, theophylline, levodopa, filgrastim, ribavirin, interferon, ritonavir, darunavir, didanosine, rituximab, basiliximab, teriparatide, sildenafil, and diazoxide).39
Exposure Definition and Outcome Assessment
The first SUA measurement after renal replacement therapy initiation in the NephroCare clinic was defined as exposure.
Time to all-cause mortality was predefined as the primary outcome, and time to CV-related mortality as secondary outcome. All mortality information including the date of death during follow-up and cause of death is included in EuCliD. Cardiovascular-related mortality was defined as any death for any reason with ICD-10 code I00-I99. For 93% of deaths, information about the underlying cause of death was available in EuCliD.
Statistical Analyses
Baseline Patient and Treatment Characteristics
By descriptive statistics, we calculated summary statistic measures for patient and treatment characteristics as of the index date. All normally distributed variables are presented as mean ± SD; non−normally distributed variables are given as median, with 25th and 75th percentiles.
Outcome Assessment
Primary Outcome Analysis
The association between SUA and all-cause mortality was assessed with competing risk models. Change to peritoneal dialysis (PD), transplantation, and termination of dialysis treatment were considered as competing events; all other dropout reasons were censored. To evaluate the form of association between SUA and mortality, SUA was included in the model in nonparametric form as penalized smoothing spline and fitted with the pspline function within the coxph function in R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Two different models were fitted: 1 unadjusted model, and 1 model adjusted for age, sex, ethnicity, dialysis vintage, comorbidities (diabetes mellitus, cardiovascular disease, liver disease, and cancer), vascular access, LTI, and FTI. Confounders were a priori defined based on literature review. In addition, a cluster term for country was included in the model to consider the correlation between subjects within the same country. This correlation was estimated by adding a working correlation matrix (generalized estimating equations).
Secondary Outcome Analysis
The association between SUA and CV-related mortality was assessed with the same models as described for the primary outcome. In the secondary analysis, death due to other reasons was considered as a competing event besides the above-mentioned reasons.
In addition, for the primary outcome, we calculated cumulative mortality incidence, taking competing risks into account. The cumulative incidence function is the sum of all-cause mortality incidences up to the follow-up time point tj and can be interpreted as the probability of dying up to time tj, accounting for competing risks. The same competing and censoring events were considered as described above for the primary outcome analysis.
Subgroup Analyses
Outcome assessment for the primary endpoint was also performed for lean tissue index (LTI) and fat tissue index (FTI) subgroups. Because of the lack of established cut-off values for LTI and FTI, we stratified patients according to the median LTI and FTI values, respectively. In addition, we performed a subgroup analysis regarding geographic regions (EMEA vs. Latin America). For each subgroup, separate competing risk models were fitted as described above.
SAS statistical software version 9.4 (SAS Institute, Cary, NC) and R software version 3.5.1 were used in the present study.
Results
A total of 16,057 hemodialysis patients were included in the present study (Figure 1). Demographic characteristics as of the index date for the total study population as well as for the cohort stratified by SUA quartiles are displayed in Table 1.
Table 1.
Baseline characteristics of the total study population and stratified by SUA quartiles
| Total n = 16,057 | SUA quartiles, mg/dl |
P | ||||
|---|---|---|---|---|---|---|
| ≤5.0 (n = 4060) | >5.0−6.0 (n = 4128) | >6.0−7.1 (n = 3948) | >7.1 (n = 3921) | |||
| Age, yr | 61.6 ± 15.1 | 66.9 ± 13.9 | 63.4 ± 14.6 | 59.9 ± 14.6 | 55.7 ± 14.8 | <0.001 |
| Men, % | 59.8 | 54.4 | 57.6 | 62.1 | 65.5 | <0.001 |
| Dialysis vintage, mo | 3.6 (1.2; 16.4) | 3.1 (1.1; 16.4) | 3.5 (1.2; 19.5) | 3.6 (1.3; 15.0) | 4.2 (1.4; 15.2) | <0.001 |
| Comorbidities, % | ||||||
| Cardiovascular diseasea | 31.4 | 31.9 | 30.4 | 31.3 | 32.2 | 0.335 |
| Diabetes mellitus | 32.7 | 37.0 | 36.8 | 31.5 | 25.4 | <0.001 |
| Hypertension | 64.5 | 61.3 | 62.8 | 63.9 | 70.3 | <0.001 |
| Cancer | 7.9 | 10.4 | 8.1 | 6.6 | 6.2 | <0.001 |
| Liver disease | 9.4 | 5.8 | 7.5 | 9.7 | 14.7 | <0.001 |
| Treatment modality, % | 0.005 | |||||
| Hemodialysis | 65.2 | 63.9 | 64.7 | 65.0 | 67.1 | |
| Hemodiafiltration | 34.1 | 35.5 | 34.7 | 34.3 | 31.9 | |
| Other | 0.7 | 0.6 | 0.6 | 0.7 | 1.0 | |
| Treatment parameter | ||||||
| OCM Kt/V | 1.27 ± 0.45 | 1.31 ± 0.46 | 1.31 ± 0.44 | 1.26 ± 0.44 | 1.19 ± 0.44 | <0.001 |
| Intradialytic weight loss, kg | 1.1 (0.5; 2.0) | 1.0 (0.5; 1.9) | 1.2 (0.5; 2.0) | 1.1 (0.5; 2.0) | 1.2 (0.4; 2.2) | <0.001 |
| Convection volume, Lb | 18.8 ± 8.7 | 18.4 ± 8.7 | 18.9 ± 9.2 | 18.9 ± 8.5 | 19.1 ± 8.2 | 0.193 |
| Effective treatment time, min | 240.0 (176.0; 240.0) | 232.0 (177.0; 240.0) | 240.0 (180.0; 240.0) | 240.0 (176.0; 240.0) | 240.0 (150.0; 241.0) | <0.001 |
| Treatments/wk, % | <0.001 | |||||
| <3 | 2.5 | 1.7 | 2.4 | 3.3 | 2.7 | |
| 3 | 96.0 | 96.8 | 96.0 | 95.4 | 95.6 | |
| >3 | 1.6 | 1.6 | 1.6 | 1.3 | 1.8 | |
| Vascular access, % | <0.001 | |||||
| Fistula | 62.7 | 55.5 | 61.1 | 65.0 | 69.6 | |
| Graft | 2.3 | 2.5 | 2.4 | 2.1 | 2.3 | |
| Catheter | 34.9 | 42.0 | 36.5 | 32.9 | 28.1 | |
| Blood pressure, mm Hg | ||||||
| Systolic | 145.6 ± 25.8 | 143.3 ± 26.1 | 144.9 ± 25.3 | 146.1 ± 25.8 | 148.1 ± 25.6 | <0.001 |
| Diastolic | 75.2 ± 15.1 | 72.0 ± 14.6 | 74.4 ± 14.7 | 76.2 ± 15.0 | 78.6 ± 15.3 | <0.001 |
| Medication, % | ||||||
| Allopurinol | 12.1 | 20.4 | 13.1 | 8.8 | 5.9 | <0.001 |
| Febuxostat | 0.5 | 1.5 | 0.1 | 0.2 | 0 | <0.001 |
| Statins | 24.0 | 29.8 | 26.8 | 22.8 | 16.2 | <0.001 |
| Medications increasing SUA levelsc |
51.9 | 57.6 | 54.6 | 51.5 | 43.6 | <0.001 |
| Diuretics | 24.6 | 30.0 | 26.5 | 24.1 | 17.4 | <0.001 |
| β-Blocker | 31.3 | 32.3 | 32.6 | 31.1 | 29.1 | 0.003 |
| Body composition | ||||||
| Body mass index, kg/m2 | 26.9 ± 5.6 | 26.5 ± 5.5 | 26.7 ± 5.4 | 27.0 ± 5.6 | 27.4 ± 6.0 | <0.001 |
| Lean tissue index, kg/m2 | 12.7 ± 3.0 | 12.0 ± 2.9 | 12.4 ± 2.9 | 12.9 ± 3.0 | 13.5 ± 3.0 | <0.001 |
| Fat tissue index, kg/m2 | 13.3 ± 6.1 | 13.2 ± 5.9 | 13.3 ± 6.1 | 13.2 ± 6.2 | 13.3 ± 6.4 | 0.930 |
| Overhydration, L | 2.8 ± 2.3 | 3.3 ± 2.4 | 2.9 ± 2.2 | 2.6 ± 2.2 | 2.2 ± 2.2 | <0.001 |
| Laboratory values | ||||||
| Serum uric acid, mg/dl | 6.1 ± 1.7 | 4.2 ± 0.9 | 5.6 ± 0.3 | 6.6 ± 0.3 | 8.3 ± 1.2 | <0.001 |
| Hemoglobin, g/dl | 10.5 ± 1.7 | 10.5 ± 1.6 | 10.6 ± 1.6 | 10.6 ± 1.7 | 10.4 ± 1.7 | <0.001 |
| Phosphate, mg/dl | 4.7 ± 1.5 | 4.1 ± 1.3 | 4.6 ± 1.4 | 4.9 ± 1.4 | 5.4 ± 1.6 | <0.001 |
| Albumin, g/dl | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.9 ± 0.5 | <0.001 |
| Creatinine, mg/dl | 7.1 ± 2.7 | 5.8 ± 2.1 | 6.6 ± 2.2 | 7.4 ± 2.4 | 8.6 ± 3.1 | <0.001 |
| Total cholesterol, mg/dl | 168.7 ± 46.2 | 160.7 ± 44.8 | 164.7 ± 44.6 | 171.6 ± 46.7 | 178.6 ± 46.9 | <0.001 |
| Triglycerides, mg/dl | 136.7 (96.0; 195.6) | 121.0 (86.0; 170.0) | 131.0 (94.0; 184.0) | 141.6 (101.0; 203.0) | 157.0 (110.6; 231.0) | <0.001 |
| nPCR, g/kg per day | 1.01 ± 0.28 | 0.91 ± 0.25 | 0.98 ± 0.24 | 1.04 ± 0.26 | 1.10 ± 0.30 | <0.001 |
| PTH, pg/ml | 239.0 (126.6; 432.4) | 193.0 (103.0; 363.0) | 226.0 (121.7; 406.0) | 256.0 (136.3; 443.8) | 295.3 (155.6; 521.6) | <0.001 |
| C-reactive protein, mg/L | 5.3 (2.0; 14.5) | 5.6 (2.0; 15.5) | 5.1 (1.8; 14.8) | 5.1 (2.0; 13.6) | 5.3 (2.0; 14.3) | 0.106 |
nPCR, normalized protein catabolic rate; OCM, Online Clearance Monitoring; PTH, parathyroid hormone; SUA, serum uric acid.
Data are presented as mean ± SD or median (quartile 1; quartile 3) or percentage, as appropriate. Statistical analysis was performed with 1-way analysis of variance for normally distributed continuous variables, the Wilcoxon rank-sum test for non−normally distributed continuous variables, and the Pearson χ2 test for dichotomous variables. Information was missing for following parameters in the total cohort: OCM Kt/V, 1686; intradialytic weight loss, 125; convection volume, 157; body mass index, 142; hemoglobin, 46; phosphate, 48; albumin, 541; creatinine, 496; total cholesterol, 2966; triglycerides, 3153; nPCR, 5326; PTH, 1473; and C-reactive protein, 2270.
Cardiovascular disease was defined as prevalence of myocardial infarction, congestive heart failure, peripheral vascular disease, or cerebrovascular disease.
Information on convection volume is presented for patients treated with hemodiafiltration (HDF).
Medications that increase SUA levels were defined according to Moriwaki.39
In the total population, the mean age was 61.6 years, and 59.8% of the patients were men. The median dialysis vintage was 3.6 months, and 62% (n = 9951) of all patients had a dialysis vintage of <6 months. The mean SUA of the total population was 6.1 mg/dl (363 μmol/l), and the percentage of patients with SUA levels ≥6.8 mg/dl (404 μmol/l), 7.0 mg/dl (416 μmol/l), and 8.0 mg/dl (476 μmol/l) were 31.4%, 27.5%, and 12.7%, respectively, following the different definitions of hyperuricemia in the literature. Of all patients, 13.9% received drugs inhibiting uric acid production (M04AA), and 12.1% were on allopurinol. No patient received preparations that increase uric acid excretion (M04AB) or other antigout preparations (M04AX). In contrast, 51.9% of the total population received at least 1 medication that was associated with an increase in SUA levels (according to Moriwaki39).
When patients were stratified according to SUA quartiles, patients with higher SUA levels were significantly younger and more often men. Patients in lower SUA quartiles had a higher prevalence of diabetes mellitus and cancer, whereas patients in higher SUA quartiles had a higher prevalence of hypertension and liver disease. A higher percentage of patients in the lower SUA quartiles were treated with hemodiafiltration and had a catheter as vascular access as compared to patients in the higher SUA quartiles. Patients with higher SUA levels had higher BMI and LTI, whereas FTI was comparable between patients in the SUA quartiles. Moreover, patients in the lower SUA quartiles were more overhydrated than patients with higher SUA levels. Regarding laboratory parameters, patients with higher SUA levels also had higher values of phosphate, albumin, creatinine, total cholesterol, triglycerides, normalized protein catabolic rate (nPCR), and parathyroid hormone.
During a mean of 21.8 months of follow-up, there were 2791 patient deaths (17.4%), 1203 (7.5%) of which were attributed to CV disease. Moreover, 179 patients (1.1%) changed to PD, 1464 (9.1%) underwent transplantation, 216 (1.3%) terminated dialysis treatment, 1670 (10.4%) changed the dialysis center, 79 (0.5%) changed to home hemodialysis, 51 (0.3%) recovered, 142 (0.9%) could not be followed until the end of data extraction due to unspecified reasons, and 119 (0.7%) were lost to follow-up. Thus, 9346 patients (58.2%) were followed until the end of the data extraction. The number of events across the SUA quartiles is presented in Supplementary Table S1. Patients with the lowest SUA levels had the highest crude mortality rates (Supplementary Table S1 and Supplementary Figure S1). Transplantation and center change rates were higher in patients with higher SUA levels, and the frequencies of the other events did not differ substantially between the SUA quartiles.
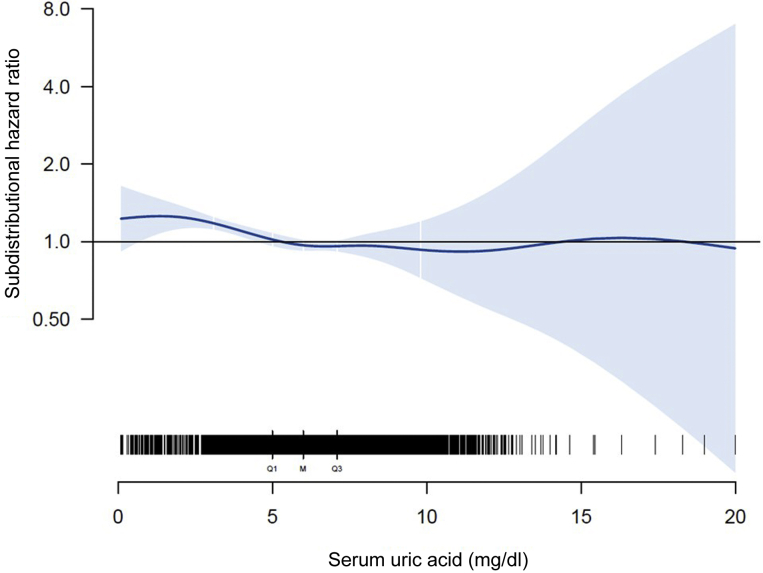
Results of our adjusted spline analyses are presented in Figure 2, which characterize the form of association between SUA levels and all-cause mortality. We found a U-shaped pattern between SUA and all-cause mortality, with a survival benefit for patients with SUA levels between 5.5 and 7.7 mg/dl (327 and 458 μmol/l). The lowest subdistribution hazard ratio (sHR) was found at a SUA level of 6.5 mg/dl (387 μmol/l; sHR, 0.94 [CI, 0.91; 0.96]) (Figure 2); lower and higher SUA levels were associated with a higher sHR, respectively. The U-shaped pattern was also found when stratifying patients according to geographic regions (Supplementary Figure S2). For CV-related mortality (Figure 3), the form of association was comparable for SUA levels <8 mg/dl (476 μmol/l), with the lowest sHR at a SUA level of 6.7 mg/dl (399 μmol/l; sHR, 0.96 [0.92; 0.99]). For SUA levels >8 mg/dl (476 μmol/l), no definite conclusion could be drawn regarding the form of association due to the low number of events and the large CIs.
Figure 2.
Adjusted spline analysis for the association between serum uric acid (SUA) and all-cause mortality. Subdistribution hazard ratio and confidence intervals across different SUA levels are displayed. Black bars indicate the number of patients for different SUA levels (Q1, first quartile; M, median; Q3, third quartile). Adjustment was performed for age, sex, ethnicity, dialysis vintage, comorbidities (diabetes mellitus, cardiovascular disease, liver disease, and cancer), vascular access, lean tissue index (LTI), and fat tissue index (FTI).
Figure 3.
Adjusted spline analysis for the association between serum uric acid (SUA) and cardiovascular (CV)−related mortality. Subdistributional hazard ratio and confidence intervals across different SUA levels are displayed. Black bars indicate the number of patients for different SUA levels (Q1, first quartile; M, median; Q3, third quartile). Adjustment was performed for age, sex, ethnicity, dialysis vintage, comorbidities (diabetes mellitus, CV disease, liver disease, and cancer), vascular access, lean tissue index (LTI), and fat tissue index (FTI).
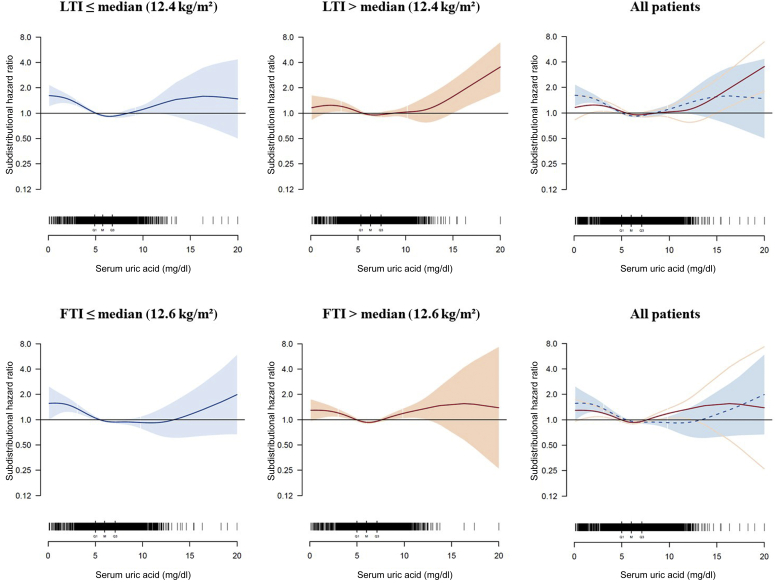
Finally, we analyzed the form of association between SUA levels and all-cause mortality in LTI and FTI subgroups (Figure 4). Patients with LTI below the median had a mean SUA level of 5.9 mg/dl (351 μmol/l), whereas patients with LTI above the median had a mean SUA level of 6.4 mg/dl (381 μmol/l) (Supplementary Table S2). In both subgroups, the U-shaped pattern between SUA and all-cause mortality was still present, with the lowest sHR at SUA levels of 6.5 and 6.7 mg/dl (387 and 399 μmol/l) for the patients with LTI below the median and for patients with LTI above the median, respectively. A slightly lower sHR was found for hypouricemic patients with LTI above the median (maximal sHR, 1.24 [1.02; 1.50]) as compared to hypouricemic patients with LTI below the median (maximal sHR, 1.61 [1.21; 2.15]); however, this difference was not significant, given the overlap of the CIs. A similar pattern was found for FTI subgroups (Figure 4).
Figure 4.
Adjusted spline analysis for the association between serum uric acid (SUA) and all-cause mortality in lean tissue index (LTI) and fat tissue index (FTI) subgroups. Subdistribution hazard ratios and confidence intervals across different SUA levels are displayed for patients with LTI at or below the median (12.4 kg/m2; blue) and LTI above the median (red) as well as for patients with FTI at or below the median (12.6 kg/m2; blue) and FTI above the median (red). Overlapping splines are displayed in the right plots (LTI/FTI at or below the median: dashed blue line indicates hazard ratios, and solid blue line indicates confidence intervals; LTI/FTI above the median: red line indicates hazard ratios, and light red lines indicate confidence intervals). Black bars indicate the number of patients for different SUA levels (Q1, first quartile; M, median; Q3, third quartile). Adjustment was performed for age, sex, ethnicity, dialysis vintage, comorbidities (diabetes mellitus, cardiovascular disease, liver disease, and cancer), vascular access, FTI (for LTI subgroups), and LTI (for FTI subgroups).
Discussion
In this retrospective study from a large cohort of patients undergoing hemodialysis, we found a U-shaped pattern between SUA levels and all-cause mortality, with a survival benefit for patients with SUA levels between 5.5 and 7.7 mg/dl (327 and 458 μmol/l) and the lowest sHR for SUA levels at 6.5 mg/dl (387 μmol/l). This form of association was independent of the patients’ body composition and was found for patients treated in EMEA and Latin America. A similar pattern was found for CV-related mortality for SUA levels <8 mg/dl (476 μmol/l); because of the low number of events, no definite conclusions regarding the form of association could be drawn for this secondary endpoint for SUA levels >8 mg/dl (476 μmol/l).
Our results contribute to ongoing debates regarding the clinical implications of SUA disturbances in persons with CKD.40 Currently, no consensus exists regarding target SUA levels or the use of SUA-lowering drugs in persons with CKD to delay progression or to reduce cardiovascular/mortality risk.35,41 Especially for hemodialysis patients, current evidence is conflicting; all sorts of associations between SUA and mortality risk have been described for this patient population, which were nicely summarized in a recent report.2 For instance, the largest study to date included 27,229 hemodialysis patients from the Taiwan Society of Nephrology dialysis registry.28 When stratifying patients into quartiles (<6.2, 6.2−7.1, 7.1−8.1, >8.1 mg/dl), the authors found that lower SUA levels were associated with higher risk for all-cause and CV-related mortality. Although differences in study designs, study populations, sample sizes and ascertainment of outcomes can explain this evidence inconsistency, an additional explanation may lie in the post hoc assumption of linearity in the relation between SUA and health. The use of penalized smoothing splines in our analysis showed a U-shaped pattern and allowed evaluation of risks at extreme SUA values, although the large CIs may limit our conclusions here. This is also an advantage compared to most of the previous studies who used internal cutoffs (such as quartiles of distribution) that preclude comparison between studies.
Inconsistency across studies may be also attributed to the fact that SUA has different functional properties, depending on the physiological context. First, uric acid may have both pro-oxidant as well as antioxidant effects: on 1 hand, uric acid is a potent radical scavenger and antioxidant and reduces oxidative stress in the human body, a condition implicated in the pathogenesis of cardiovascular disease2,42,43; in this context, uric acid was characterized to prevent oxidative inactivation of endothelial enzymes and to preserve the ability of the endothelium to mediate vascular dilatation during oxidative stress.2,42 Therefore, it has been discussed that elevated SUA levels may be a result of the bodies’ compensatory mechanism to counteract oxidative damage in the context of atherosclerosis.27,44 On the other hand, in certain situations, uric acid may become a pro-oxidant, and both clinical and experimental studies have demonstrated such a capability of this compound; this is especially the case when SUA exceeds supranormal levels in the blood.2,40,45, 46, 47 Thus, depending on its concentrations in the human body, SUA may act as a potent antioxidant at physiological levels, or as a pro-oxidant at high, supraphysiological levels.40
Second, high levels of SUA have been associated with several severe clinical conditions including hypertension, diabetes mellitus, insulin resistance and metabolic syndrome, CV disease, and chronic kidney disease.2,13, 14, 15, 16, 17, 18,48, 49, 50, 51 However, low SUA levels have been proposed as a surrogate of protein energy wasting in hemodialysis patients as a consequence of inadequate protein intake, given that SUA levels are strongly associated with the consumption of purine-rich meals.25,26,35 Serum uric acid has been shown to be associated with the nutritional status of patients on hemodialysis, as it correlates with laboratory nutritional markers, with parameters of anthropometry and with health-related quality of life scoring.25,26,35 Moreover, it was shown that the association between SUA levels and mortality was modified by nPCR.35 Our study is in line with such previous publications, as patients with higher SUA levels also had a better nutritional profile, as indicated by several laboratory parameters such as higher albumin and nPCR values. Moreover, patients with higher SUA levels had also higher LTI values, taken here as a surrogate of better nutritional status and greater muscle mass. We also investigated the FTI in the present study; a higher FTI, as marker of higher fat mass, may also indicate better energy stores in dialysis patients. Interestingly, we found that the U-shaped pattern between SUA levels and all-cause mortality was similar and not modified by the patient’s body composition. Of note, hypouricemic patients with high LTI/FTI values had a lower sHR than hypouricemic patients with low LTI/FTI values. However, the results were not significantly different between the 2 groups, and the large CIs at extreme SUA levels may limit our conclusions here. Nonetheless, our findings are partly in agreement with a recent population-based cohort study from the Taipei City Elderly Health Examination Program.52 First, also for this elderly population (≥65 years of age), the authors found a U-shaped association between SUA and all-cause mortality, with SUA levels <4 and ≥8 mg/dl (<238 and ≥476 μmol/l) independently predicting mortality. Second, when stratifying patients according to their malnutrition status as assessed by the Geriatric Nutritional Risk Index (GNRI), the authors observed lower hazard ratios in hypouricemic subjects without malnutrition as compared to those with proven malnutrition. Based on these findings, it is tempting to speculate that low SUA levels may not be as detrimental for patients with a good nutritional status as for malnourished patients.
Certainly, we need clinical evidence as to whether treatment of asymptomatic hyperuricemia is beneficial for patients with CKD, but also for CV disease protection and mortality risk reduction. Currently, no consensus exists regarding the treatment of asymptomatic hyperuricemia in dialysis patients. A recent meta-analysis that included 832 CKD patients from 12 randomized controlled trials found that the risk for CKD progression and mortality was lower in patients who received uric acid−lowering therapy.53 In our study, we observed that patients on allopurinol treatment had a higher sHR for SUA levels >8 mg/dl (476 μmol/l) than patients who were not receiving allopurinol treatment, which may be linked to potential adverse effects of allopurinol, such as fatal hypersensitivity syndrome (data not shown).14
Our study has several strengths. First, this is the largest study to analyze the association among SUA, mortality risk, and markers of malnutrition in hemodialysis patients. Body composition was measured with the same medical device and according to prespecified protocols, which reduces variability in the measurement results between different dialysis centers. We included hemodialysis patients from 22 countries, which enhances the generalizability of our results. Analysis was performed with competing risk models, and the form of association was evaluated with spline analyses. Finally, information on SUA-lowering medication was available in the present study. This being said, our study also has limitations. First, because of our observational design, we cannot draw any conclusions regarding the causality of our results. We lacked information on residual renal function and thus could not account for this; it is important to note that even low renal function may substantially contribute to SUA removal.54 Moreover, some selection bias may be present in our study, given that, by design, at least 1 SUA and BCM measurement had to be available in our study cohort, and patients had to survive until the index date. However, the dialysis vintage of our study population is very low (median, 3.6 months), and we do not expect a meaningful effect from this. Nonetheless, as SUA testing is not a clinical routine, the results obtained in our present cohort may not be generalized to the total dialysis population. Moreover, when comparing our results with those of other studies, it must be considered that according to the statistical analyses performed, we present sHRs and not hazard ratios (HRs), as in some other publications. Because of the different models and the different interpretations thereof, sHR cannot be equated with HR.55 Finally, in our analyses, we considered single SUA measurements and did not perform trajectory analyses. Of note, as most previous studies used single SUA measurements, we applied the same design for comparability reasons. In addition, previous studies that analyzed single SUA measurements and SUA trajectories found that both measurements are well associated with the nutritional status and clinical outcomes of the patients, and show that SUA levels are quite stable over time.25,26,56
In conclusion, we found a U-shaped pattern between SUA levels and all-cause mortality among hemodialysis patients. This form of association between SUA levels and all-cause mortality appears to be independent of the patients’ body composition.
Disclosure
AMZ, MW, AF, SS, AG, ACW, RR, and BC are employees of Fresenius Medical Care and may hold stock in the company. JJC has received speaker fees from Abbott Laboratories. DF has received lecture fees from Sanofi, Vifor Pharma, Fresenius Kabi, and a research grant from Fresenius Medical Care.
Acknowledgments
The authors thank all physicians and nurses working at the NephroCare dialysis centers for their efforts in handling the EuCliD data, which made this project possible. The European Renal Nutrition working group is an initiative of and supported by the European Renal Association–European Dialysis Transplant Association (ERA-EDTA). No funding was received for this work. Results of the present study have been presented as a free communication at the 56th ERA-EDTA congress, June 13–16, 2019, Budapest, Hungary.
Author Contributions
Conception and design of the study was performed by AMZ, JJC, SS, AG, ACW, RR, DF, and BC. Statistical analyses were conducted by MW and AF. Interpretation of the data was done by all authors. The manuscript was drafted by AMZ and BC and revised critically for important intellectual content by all authors. The final version of the manuscript was approved by all authors.
Footnotes
Table S1. Mean follow-up time and number [percentage] of events in the total study population and stratified by SUA quartiles.
Table S2. Analyzed subgroups in the present study.
Figure S1. Plot showing the cumulative all-cause mortality incidences of patients in different SUA quartiles and table showing the number of patients at risk during follow-up.
Figure S2. Adjusted spline analysis for the association between SUA and all-cause mortality in patients treated in EMEA or Latin America.
Supplementary Material
References
- 1.Bobulescu I.A., Moe O.W. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chron Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murea M., Tucker B.M. The physiology of uric acid and the impact of end-stage kidney disease and dialysis. Semin Dial. 2019;32:47–57. doi: 10.1111/sdi.12735. [DOI] [PubMed] [Google Scholar]
- 3.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Corry D.B., Eslami P., Yamamoto K. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 5.Mazzali M., Hughes J., Kim Y.G. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 6.Rabadi M.M., Kuo M.C., Ghaly T. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. Am J Physiol Renal Physiol. 2012;302:F730–F741. doi: 10.1152/ajprenal.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Lozada L.G., Lanaspa M.A., Cristobal-Garcia M. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Lozada L.G., Tapia E., Santamaria J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y., Fang L., Jiang L. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoccali C., Maio R., Mallamaci F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17:1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson D., Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14:164. doi: 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muiesan M.L., Agabiti-Rosei C., Paini A. Uric acid and cardiovascular disease: an update. Eur Cardiol. 2016;11:54–59. doi: 10.15420/ecr.2016:4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silbernagel G., Hoffmann M.M., Grammer T.B. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23:46–52. doi: 10.1016/j.numecd.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos M.J., Koudstaal P.J., Hofman A. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi N., Okamoto M., Yoshida H. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 17.Cirillo P., Sato W., Reungjui S. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17:S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 18.Masuo K., Kawaguchi H., Mikami H. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad M.I., Dutta A., Anees M.A. Interrelations Between Serum Uric Acid, Silent Myocardial Infarction, and Mortality in the General Population. Am J Cardiol. 2019;123:882–888. doi: 10.1016/j.amjcard.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Niskanen L.K., Laaksonen D.E., Nyyssonen K. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 21.Fang J., Alderman M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 22.Freedman D.S., Williamson D.F., Gunter E.W. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson C., Lapidus L., Stendahl C. Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand. 1988;224:549–555. [PubMed] [Google Scholar]
- 24.Bae E., Cho H.J., Shin N. Lower serum uric acid level predicts mortality in dialysis patients. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beberashvili I., Erlich A., Azar A. Longitudinal Study of Serum Uric Acid, Nutritional Status, and Mortality in Maintenance Hemodialysis Patients. Clin J Am Soc Nephrol. 2016;11:1015–1023. doi: 10.2215/CJN.10400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beberashvili I., Sinuani I., Azar A. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition. 2015;31:138–147. doi: 10.1016/j.nut.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Hsu S.P., Pai M.F., Peng Y.S. Serum uric acid levels show a 'J-shaped' association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant. 2004;19:457–462. doi: 10.1093/ndt/gfg563. [DOI] [PubMed] [Google Scholar]
- 28.Hsu W.L., Li S.Y., Liu J.S. High uric acid ameliorates indoxyl sulfate-induced endothelial dysfunction and is associated with lower mortality among hemodialysis patients. Toxins (Basel) 2017;9:20. doi: 10.3390/toxins9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon J.S., Chung S.H., Han D.C. Mortality predictive role of serum uric acid in diabetic hemodialysis patients. J Ren Nutr. 2014;24:336–342. doi: 10.1053/j.jrn.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Jiang M.Y., Hwang J.C., Lu Y.H. Clinical implications and outcome prediction in chronic hemodialysis patients with lower serum potassiumxuric acid product. Eur J Intern Med. 2015;26:646–651. doi: 10.1016/j.ejim.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Kim C.S., Jin D.C., Yun Y.C. Relationship between serum uric acid and mortality among hemodialysis patients: retrospective analysis of Korean end-stage renal disease registry data. Kidney Res Clin Pract. 2017;36:368–376. doi: 10.23876/j.krcp.2017.36.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latif W., Karaboyas A., Tong L. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6:2470–2477. doi: 10.2215/CJN.00670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.M., Lee A.L., Winters T.J. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol. 2009;29:79–85. doi: 10.1159/000151292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muela H.C., De Lima J.J., Gowdak L.H. Prognostic value of serum uric acid in patients on the waiting list before and after renal transplantation. Int J Nephrol. 2015;2015:375606. doi: 10.1155/2015/375606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park C., Obi Y., Streja E. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol Dial Transplant. 2017;32:1750–1757. doi: 10.1093/ndt/gfw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suliman M.E., Johnson R.J., Garcia-Lopez E. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48:761–771. doi: 10.1053/j.ajkd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Marcelli D., Kirchgessner J., Amato C. EuCliD (European Clinical Database): a database comparing different realities. J Nephrol. 2001;14(suppl 4):S94–S100. [PubMed] [Google Scholar]
- 38.Marcelli D., Moscardo V., Steil H. Data management and quality assurance for dialysis network. Contrib Nephrol. 2002;137:293–299. doi: 10.1159/000060251. [DOI] [PubMed] [Google Scholar]
- 39.Moriwaki Y. Effects on uric acid metabolism of the drugs except the antihyperuricemics. J Bioequiv Availab. 2014;6:1. [Google Scholar]
- 40.Dousdampanis P., Trigka K., Musso C.G. Hyperuricemia and chronic kidney disease: an enigma yet to be solved. Ren Fail. 2014;36:1351–1359. doi: 10.3109/0886022X.2014.947516. [DOI] [PubMed] [Google Scholar]
- 41.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 42.Becker B.F. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 43.Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and Oxidative Stress. J Clin Med. 2017;6:22. doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieto F.J., Iribarren C., Gross M.D. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148:131–139. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 45.Kang D.H., Park S.K., Lee I.K. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 46.Sautin Y.Y., Nakagawa T., Zharikov S. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 47.Yu M.A., Sanchez-Lozada L.G., Johnson R.J. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 48.Hsu C.Y., Iribarren C., McCulloch C.E. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan E., Akhras K.S., Sharma H. Serum urate and incidence of kidney disease among veterans with gout. J Rheumatol. 2013;40:1166–1172. doi: 10.3899/jrheum.121061. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava A., Kaze A.D., McMullan C.J. Uric Acid and the Risks of Kidney Failure and Death in Individuals With CKD. Am J Kidney Dis. 2018;71:362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner D.E., Tighiouart H., Elsayed E.F. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng W.C., Chen Y.T., Ou S.M. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: the role of malnourishment. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X., Zhai T., Ma R. Effects of uric acid-lowering therapy on the progression of chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2018;40:289–297. doi: 10.1080/0886022X.2018.1456463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morduchowicz G., Winkler J., Zabludowski J.R. Effects of residual renal function in haemodialysis patients. Int Urol Nephrol. 1994;26:125–131. doi: 10.1007/BF02768252. [DOI] [PubMed] [Google Scholar]
- 55.Andersen P.K., Geskus R.B., de Witte T. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai C.W., Chiu H.T., Huang H.C. Uric acid predicts adverse outcomes in chronic kidney disease: a novel insight from trajectory analyses. Nephrol Dial Transplant. 2018;33:231–241. doi: 10.1093/ndt/gfx297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.