PREAMBLE
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, which are based on systematic methods to evaluate and classify evidence, provide a foundation for the delivery of quality cardiovascular care. The ACC and AHA sponsor the development and publication of clinical practice guidelines without commercial support, and members volunteer their time to the writing and review efforts.
Clinical practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease (CVD). The focus is on medical practice in the United States, but these guidelines are relevant to patients throughout the world. Although guidelines may be used to inform regulatory or payer decisions, the intent is to improve quality of care and align with patients’ interests. Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances, and should not replace clinical judgment.
Recommendations for guideline-directed management and therapy, which encompasses clinical evaluation, diagnostic testing, and both pharmacological and procedural treatments, are effective only when followed by both practitioners and patients. Adherence to recommendations can be enhanced by shared decision-making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities.
The ACC/AHA Task Force on Clinical Practice Guidelines strives to ensure that the guideline writing committee both contains requisite expertise and is representative of the broader medical community by selecting experts from a broad array of backgrounds, representing different geographic regions, sexes, races, ethnicities, intellectual perspectives/biases, and scopes of clinical practice, and by inviting organizations and professional societies with related interests and expertise to participate as partners or collaborators. The ACC and AHA have rigorous policies and methods to ensure that documents are developed without bias or improper influence. The complete policy on relationships with industry and other entities (RWI) can be found online.
Beginning in 2017, numerous modifications to the guidelines have been and continue to be implemented to make guidelines shorter and enhance “user friendliness.” Guidelines are written and presented in a modular knowledge chunk format, in which each chunk includes a table of recommendations, a brief synopsis, recommendation-specific supportive text and, when appropriate, flow diagrams or additional tables. Hyperlinked references are provided for each modular knowledge chunk to facilitate quick access and review. More structured guidelines–including word limits (“targets”) and a web guideline supplement for useful but noncritical tables and figures–are 2 such changes. This Preamble is an abbreviated version, with the detailed version available online. The reader is encouraged to consult the full-text guidelineP-1 for additional guidance and details, since the executive summary contains mainly the recommendations.
Keywords: AHA Scientific Statements; Guidelines; biomarkers, coronary artery calcium score; pharmacological; cardiovascular disease; cholesterol, LDL-cholesterol; diabetes mellitus; drug therapy; hydroxymethylglutaryl-CoA reductase inhibitors/statins; hypercholesterolemia; lipids; patient compliance; primary prevention; risk assessment; risk reduction discussion; risk treatment discussion, secondary prevention; ezetimibe; proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9) inhibitors
TOP 10 TAKE-HOME MESSAGES TO REDUCE RISK OF ATHEROSCLEROTIC CARDIOVASCULAR DISEASE THROUGH CHOLESTEROL MANAGEMENT
In all individuals, emphasize a heart-healthy lifestyle across the life course. A healthy lifestyle reduces atherosclerotic cardiovascular disease (ASCVD) risk at all ages. In younger individuals, healthy lifestyle can reduce development of risk factors and is the foundation of ASCVD risk reduction. In young adults 20 to 39 years of age, an assessment of lifetime risk facilitates the clinician–patient risk discussion (see No. 6) and emphasizes intensive lifestyle efforts. In all age groups, lifestyle therapy is the primary intervention for metabolic syndrome.
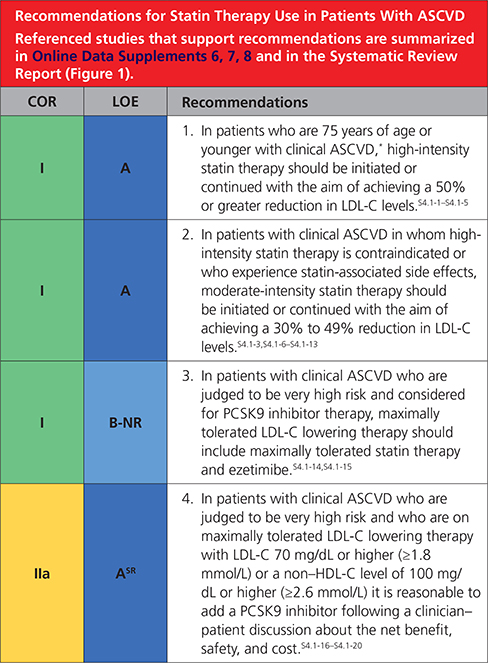
In patients with clinical ASCVD, reduce low-density lipoprotein cholesterol (LDL-C) with high-intensity statin therapy or maximally tolerated statin therapy. The more LDL-C is reduced on statin therapy, the greater will be subsequent risk reduction. Use a maximally tolerated statin to lower LDL-C levels by ≥50%.
In very high-risk ASCVD, use a LDL-C threshold of 70 mg/dL (1.8 mmol/L) to consider addition of nonstatins to statin therapy. Very high-risk includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions. In very high-risk ASCVD patients, it is reasonable to add ezetimibe to maximally tolerated statin therapy when the LDL-C level remains ≥70 mg/dL (≥1.8 mmol/L). In patients at very high risk whose LDL-C level remains ≥70 mg/dL(≥1.8 mmol/L) on maximally tolerated statin and ezetimibe therapy, adding a PCSK9 inhibitor is reasonable, although the long-term safety (>3 years) is uncertain and cost effectiveness is low at mid-2018 list prices.
In patients with severe primary hypercholesterolemia (LDL-C level ≥190 mg/dL [=4.9 mmol/L]), without calculating 10-year ASCVD risk, begin high-intensity statin therapy. If the LDL-C level remains ≥100 mg/dL (≥2.6 mmol/L), adding ezetimibe is reasonable. If the LDL-C level on statin plus ezetimibe remains ≥100 mg/dL (≥2.6 mmol/L) and the patient has multiple factors that increase subsequent risk of ASCVD events, a PCSK9 inhibitor may be considered, although the long-term safety (>3 years) is uncertain and economic value is low at mid-2018 list prices.
In patients 40 to 75 years of age with diabetes mellitus and LDL-C ≥70 mg/dL (≥1.8 mmol/L), start moderate-intensity statin therapy without calculating 10-year ASCVD risk. In patients with diabetes mellitus at higher risk, especially those with multiple risk factors or those 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥50%.
In adults 40 to 75 years of age evaluated for primary ASCVD prevention, have a clinician–patient risk discussion before starting statin therapy. Risk discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, LDL-C, hemoglobin A1C [if indicated], and calculated 10-year risk of ASCVD); the presence of risk-enhancing factors (see No. 8); the potential benefits of lifestyle and statin therapies; the potential for adverse effects and drug–drug interactions; consideration of costs of statin therapy; and patient preferences and values in shared decision-making.
In adults 40 to 75 years of age without diabetes mellitus and with LDL-C levels ≥70 mg/ dL (≥1.8 mmol/L), at a 10-year ASCVD risk of ≥7.5%, start a moderate-intensity statin if a discussion of treatment options favors statin therapy. Risk-enhancing factors favor statin therapy (see No. 8). If risk status is uncertain, consider using coronary artery calcium (CAC) to improve specificity (see No. 9). If statins are indicated, reduce LDL-C levels by ≥30%, and if 10-year risk is ≥20%, reduce LDL-C levels by ≥50%.
In adults 40 to 75 years of age without diabetes mellitus and 10-year risk of 7.5% to 19.9% (intermediate risk), risk-enhancing factors favor initiation of statin therapy (see No. 7). Risk-enhancing factors include family history of premature ASCVD; persistently elevated LDL-C levels ≥160 mg/dL (≥4.1 mmol/L); metabolic syndrome; chronic kidney disease; history of preeclampsia or premature menopause (age <40 years); chronic inflammatory disorders (eg, rheumatoid arthritis, psoriasis, or chronic HIV); high-risk ethnic groups (eg, South Asian); persistent elevations of triglycerides ≥175 mg/dL (≥1.97 mmol/L); and, if measured in selected individuals, apolipoprotein B ≥130 mg/dL, high-sensitivity C-reactive protein ≥2.0 mg/L, ankle-brachial index (ABI) <0.9 and lipoprotein (a) ≥50 mg/dL or 125 nmol/L, especially at higher values of lipoprotein (a). Risk-enhancing factors may favor statin therapy in patients at 10-year risk of 5% to 7.5% (borderline risk).
In adults 40 to 75 years of age without diabetes mellitus and with LDL-C levels ≥70 mg/dL to 189 mg/dL (≥1.8–4.9 mmol/L), at a 10-year ASCVD risk of ≥7.5% to 19.9%, if a decision about statin therapy is uncertain, consider measuring CAC. If CAC is zero, treatment with statin therapy may be withheld or delayed, except in cigarette smokers, those with diabetes mellitus, and those with a strong family history of premature ASCVD. A CAC score of 1 to 99 favors statin therapy, especially in those =55 years of age. For any patient, if the CAC score is ≥100 Agatston units or ≥75th percentile, statin therapy is indicated unless otherwise deferred by the outcome of clinician–patient risk discussion.
Assess adherence and percentage response to LDL-C–lowering medications and lifestyle changes with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed. Define responses to lifestyle and statin therapy by percentage reductions in LDL-C levels compared with baseline. In ASCVD patients at very high-risk, triggers for adding nonstatin drug therapy are defined by threshold LDL-C levels ≥70 mg/dL (≥1.8 mmol/L) on maximal statin therapy (see No. 3).
1. INTRODUCTION
1.1. Methodology and Evidence Review
The recommendations listed in the present guideline are, whenever possible, evidence based. An initial extensive evidence review, which included literature derived from research involving human subjects, published in English, and indexed in MEDLINE (through PubMed), EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to the present guideline, was conducted from May 1980 to July 2017. Key search words included but were not limited to the following: hyperlipidemia, cholesterol, LDL-C, HDL-C, ezetimibe, bile acid sequestrants, PCSK9 inhibitors, lifestyle, diet, exercise, medications, child, adolescent, screening, primary prevention, secondary prevention, cardiovascular disease, coronary artery calcium, familial hypercholesterolemia. ASCVD risk-enhancing factors, statin therapy, diabetes mellitus, women, adherence, Hispanic/Latino, South Asian, African American. Additional relevant studies published through August 2018 during the guideline writing process, were also considered by the writing committee and added to the evidence tables when appropriate. The final evidence tables are included in the Online Data Supplement and summarize the evidence used by the writing committee to formulate recommendations. References selected and published in the present document are representative and not all-inclusive.
As noted in the detailed version of the Preamble, an independent evidence review committee was commissioned to perform a formal systematic review of critical clinical questions related to cholesterol (Table 1), the results of which were considered by the writing committee for incorporation into the present guideline. Concurrent with this process, writing committee members evaluated study data relevant to the rest of the guideline. The findings of the evidence review committee and the writing committee members were formally presented and discussed, and then recommendations were developed. The systematic review for the 2018 Cholesterol Clinical Practice GuidelinesS1.1-1 is published in conjunction with the full-text guideline,S1.1-2 and includes its respective data supplements.
Table 1.
ERC Questions
| Question | Section Number |
|---|---|
| In adults ≥20 years of age with clinical atherosclerotic disease (eg, CHD, peripheral artery disease, or CVD) or at high-risk of ASCVD, what are the magnitude of benefit (absolute reduction; NNT) in individual endpoints and composite ischemic events (eg, fatal cardiovascular event, nonfatal MI, nonfatal stroke, unstable angina/revascularization) and magnitude of harm (absolute increase; NNH) in terms of adverse events (e.g, cancer, rhabdomyolysis, diabetes mellitus) derived from LDL-C lowering in large RCTs (>1 000 participants and originally designed to last >12 months) with statin therapy plus a second lipid-modifying agent compared with statin alone? | 4.1 |
Clinical atherosclerotic cardiovascular disease (ASCVD) includes acutecoronary syndrome (ACS), those with history of myocardial infarction (MI),stable or unstable angina or coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral artery disease (PAD) including aortic aneurysm, all of atherosclerotic origin.
ASCVD indicates atherosclerotic cardiovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease; ERC, Evidence Review Committee; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; NNH, number needed to harm; NNT number needed to treat; and RCT, randomized controlled trial.
Numerical values for triglycerides, total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), and non–HDL-C are given in both mg/ dL and mmol/L. To convert to mmol/L, the values in mg/ dL for TC, LDL-C, HDL-C, and non–HDL-C were divided by 38.6 and for triglycerides, by 88.6. On May 10, 2018 a writing committee member discussed their participation in an industry-supported, multicenter study, which they had thought was not relevant to this prevention guideline. However, when this was reviewed using specific ACC/AHA criteria, it was considered to represent a relevant relationship with industry. Given the current policy that a prevention guideline writing committee member must be free of any relevant relationships with industry, this member was removed from the committee. The 2 sections authored by the writing committee member were removed and replaced by new material written by the guideline chairs, and the revised sections reviewed and approved by all remaining writing committee members. The writing committee member did not participate in any further guideline discussions or review of the manuscript or recommendations.
1.2. Organization of the Writing Committee
The writing committee consisted of medical experts including cardiologists, internists, interventionalists, a nurse practitioner, pharmacists, a physician assistant, a pediatrician, a nephrologist, and a lay/patient representative. The writing committee included representatives from the American College of Cardiology (ACC), American Heart Association (AHA), American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR), American Association Academy of Physician Assistants (AAPA), Association of Black Cardiologists (ABC), American College of Preventive Medicine (ACPM), American Diabetes Association (ADA), American Geriatrics Society (AGS), American Pharmacists Association (APhA), American Society for Preventive Cardiology (ASPC), National Lipid Association (NLA), and Preventive Cardiovascular Nurses Association (PCNA). Appendix 1 of the present document lists writing committee members’ relevant RWI. For the purposes of full transparency, the writing committee members’ comprehensive disclosure information is available online.
1.3. Document Review and Approval
This document was reviewed by 21 official reviewers each nominated by the ACC, AHA, AAPA, ABC, ACPM, ADA, AGS, APhA, ASPC, NLA, and PCNA, as well as 27 individual content reviewers. Reviewers’ RWI information was distributed to the writing committee and is published in this document (Appendix 2).
This document was approved for publication by the governing bodies of the ACC, the AHA, AAPA, ABC, ACPM, ADA, AGS, APhA, ASPC, NLA, and PCNA.
1.4. Scope of the Guideline
The purpose of the present guideline is to address the practical management of patients with high blood cholesterol and related disorders. The writing committee reviewed previously published guidelines, evidence reviews, and related statements. Table S1 in the Web Supplement contains a list of publications and statements deemed pertinent. The primary sources of evidence are randomized controlled trials (RCTs). Most RCTs in this area have been performed with statins as the only cholesterol-lowering drug.S1.4-1–S1.4-3 Since the 2013 ACC/AHA cholesterol guideline,S1.4-4 newer cholesterol-lowering agents (nonstatin drugs) have been introduced and subjected to RCTs. They include ezetimibe and PCSK9 inhibitors, and their use is limited mainly to secondary prevention in patients at very high-risk of new atherosclerotic cardiovascular disease (ASCVD) events. Most other patients with ASCVD are treated with statins alone. In primary prevention, statins are recommended for patients with severe hypercholesterolemia and in adults 40 to 75 years of age either with diabetes mellitus or at higher ASCVD risk. Throughout these guidelines similar to the 2013 guidelines, consistent attention is given to a clinician–patient risk discussion for making shared decisions. Besides major risk factors of the pooled cohort equations (PCE), the clinician–patient risk discussion can include other risk-enhancing factors, and when risk status is uncertain, a coronary artery calcium (CAC) score is an option to facilitate decision-making in adults ≥40 years of age. In children, adolescents, and young adults, identifying those with familial hypercholesterolemia (FH) is a priority. However, most attention is given to reducing lifetime ASCVD risk through lifestyle therapies.
1.5. Class of Recommendation and Level of Evidence
Recommendations are designated with both a class of recommendation (COR) and a level of evidence (LOE). The class of recommendation indicates the strength of recommendation, encompassing the estimated magnitude and certainty of benefit in proportion to risk. The level of evidence rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 2).S1.5-1
Table 2.
Applying Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care (Updated August 2015)
 |
2. HIGH BLOOD CHOLESTEROL AND ASCVD
2.1. Measurements of LDL-C and Non–HDL-C
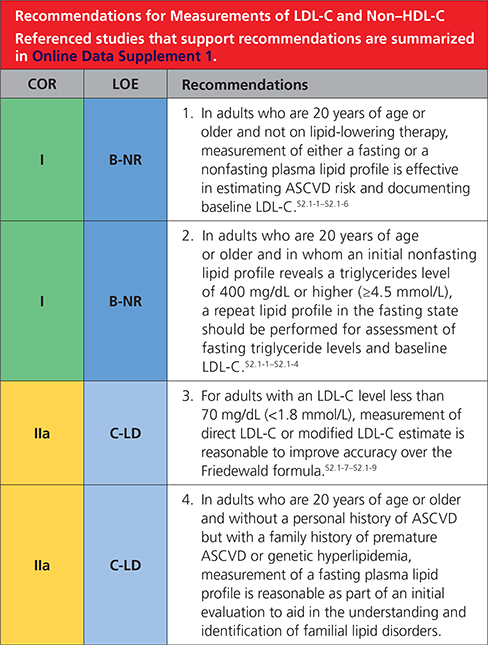 |
3. THERAPEUTIC MODALITIES
3.1. Lipid-Lowering Drugs
Among lipid-lowering drugs, statins are the cornerstone of therapy, in addition to healthy lifestyle interventions. Other LDL-lowering drugs include ezetimibe, bile acid sequestrants, and PCSK9 inhibitors. Triglyceride-lowering drugs are fibrates and niacin; they have a mild LDL-lowering action, but RCTs do not support their use as add-on drugs to statin therapy.S3.1-1 Characteristics of LDL-lowering drugs are summarized in Table S3 in the Web Supplement.
3.1.1. Statin Therapy
The intensity of statin therapy is divided into 3 categories: high-intensity, moderate-intensity, and low-intensity.S3.1.1-1 High-intensity statin therapy typically lowers LDL-C levels by ≥50%, moderate-intensity statin therapy by 30% to 49%, and low-intensity statin therapy by <30% (Table 3). Of course, the magnitude of LDL-C lowering will vary in clinical practice.S3.1.1-2 Certain Asian populations may have a greater response to certain statins.S3.1.1-3 Pharmacokinetic profiles among statins are heterogeneous (Table S4 in the Web Supplement). Statin safety has been extensively evaluated.S3.1.1-4 Statin-associated side effects are discussed in Section 5. Common medications that may potentially interact with statins are listed in Table S5 in the Web Supplement. More information on statin drug–drug interactions can be obtained from the ACC LDL-C Manager.S3.1.1-5
Table 3.
High-, Moderate-, and Low-Intensity Statin Therapy*
| High Intensity | Moderate Intensity | Low Intensity | |
|---|---|---|---|
| LDL-C lowering† | ≥50% | 30%–49% | <30% |
| Statins | Atorvastatin (40 mg‡) 80 mg | Atorvastatin 10 mg (20 mg) | Simvastatin 10 mg |
| Rosuvastatin 20 mg (40 mg) | Rosuvastatin (5 mg) 10 mg | ||
| Simvastatin 20–40 mg§ | |||
| ... | Pravastatin 40 mg (80 mg) | Pravastatin 10–20 mg | |
| Lovastatin 40 mg (80 mg) | Lovastatin 20 mg | ||
| Fluvastatin XL 80 mg | Fluvastatin 20–40 mg | ||
| Fluvastatin 40 mg BID | |||
| Pitavastatin 1–4 mg | |||
Percent LDL-C reductions with the primary statin medications used in clinical practice (atorvastatin, rosuvastatin, simvastatin) were estimated using the median reduction in LDL-C from the VOYAGER database.S3.1.1-2 Reductions in LDL-C for other statin medications (fluvastatin, lovastatin, pitavastatin, pravastatin) were identified according to FDA-approved product labeling in adults with hyperlipidemia, primary hypercholesterolemia, and mixeddyslipidemia.S3.1.1-6 Boldface type indicates specific statins and doses that were evaluated in RCTs, S3.1.1-7–S3.1.1-19 and the Cholesterol Treatment Trialists’ 2010 meta-analysis.S3.1.1-20 All these RCTs demonstrated a reduction in major cardiovascular events.
Percent reductions are estimates from data across large populations. Individual responses to statin therapy varied in the RCTs and should be expected to vary in clinical practice.S3.1.1-2
LDL-C lowering that should occur with the dosage listed below eachintensity.
Evidence from 1 RCT only: down titration if unable to tolerate atorvastatin 80 mg in the IDEAL (Incremental Decrease through Aggressive Lipid Lowering) study.S3.1.1-18
Although simvastatin 80 mg was evaluated in RCTs, initiation of simvastatin 80 mg or titration to 80 mg is not recommended by the FDA because of the increased risk of myopathy, including rhabdomyolysis.
BID indicates twice daily; FDA, US Food and Drug Administration; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial; VOYAGER, an individual patient data meta-analysis Of statin therapy in Atrisk Groups: Effects of Rosuvastatin, atorvastatin and simvastatin; and XL, extended release.
4. PATIENT MANAGEMENT GROUPS
4.1. Secondary ASCVD Prevention
 |
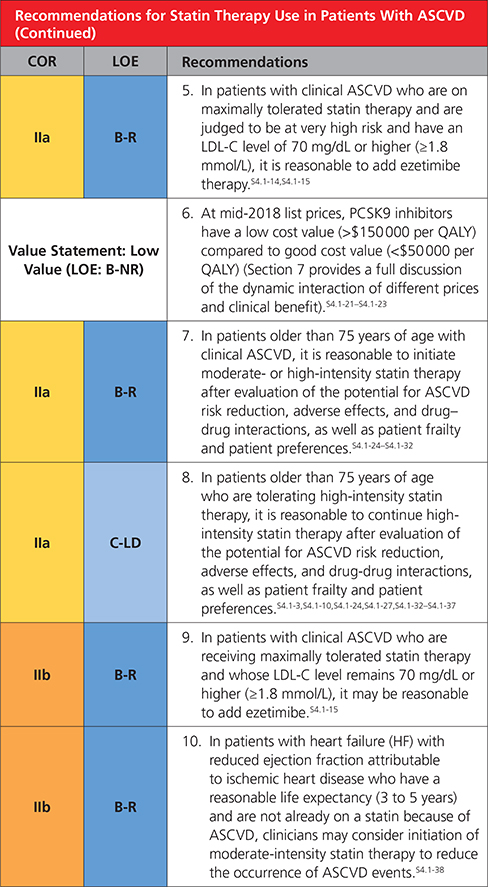 |
Clinical atherosclerotic cardiovascular disease (ASCVD) includes acute coronary syndrome (ACS), those with history of myocardial infarction (MI), stable or unstable angina or coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral artery disease (PAD) including aortic aneurysm, all of atherosclerotic origin.
4.2. Severe Hypercholesterolemia (LDL-C ≥190 mg/dL [≥4.9 mmol/L])
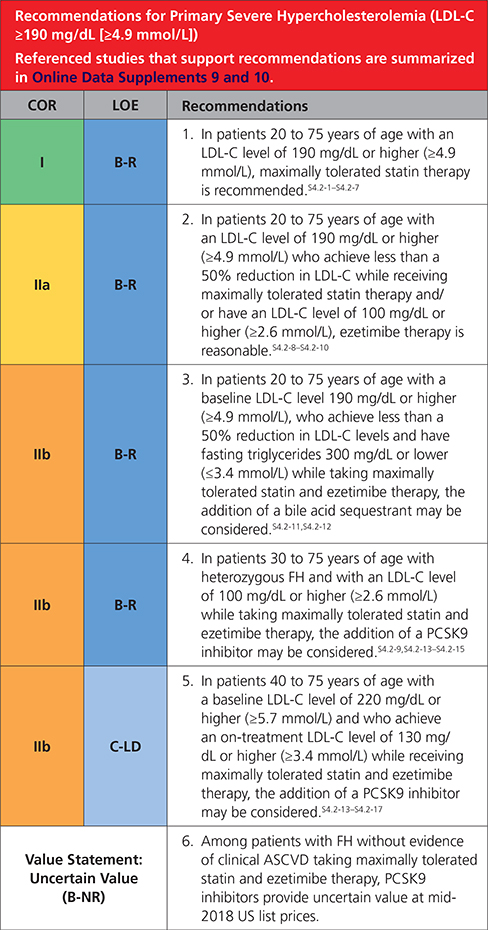 |
4.3. Diabetes Mellitus in Adults
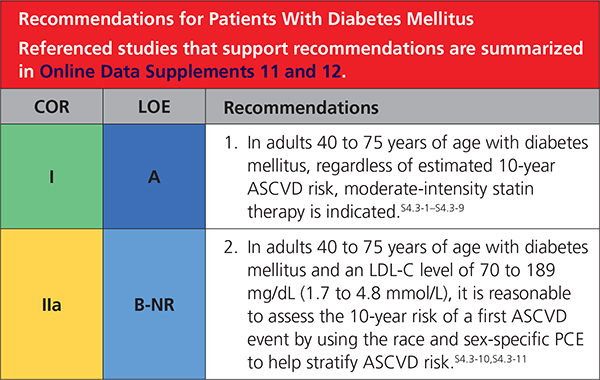 |
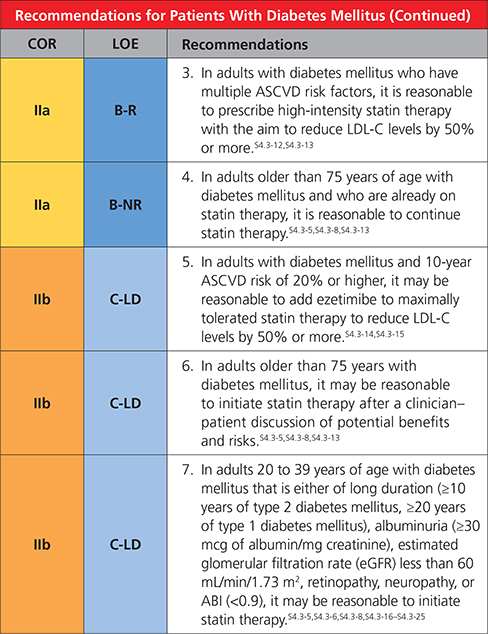 |
Synopsis
Adults 20 to 39 yearsofagearemostly atlow10-year risk, although moderate-intensity statin therapy in those with long-standing diabetes mellitus or a concomitant higher-risk condition may be reasonable (Table 5).S4.3-17,S4.3-20,S4.3-21 It may be reasonable to have a discussion about initiating moderate-intensity statin therapy with patients who have had type 2 diabetes mellitus for at least 10 years or type 1 diabetes mellitus for at least 20 years and with patients with ≥1 major CVD risk factors or complications, such as diabetic retinopathy,S4.3-19 neuropathy,S4.3-16 nephropathy (eGFR <60 mL/min/1.73 m2 or albuminuria ≥30 mcg albumin/ mg creatinine),S4.3-25 or an ABI of <0.9S4.3-22,S4.3-24 (Table 5).
Table 5.
Diabetes-Specific Risk Enhancers That Are Independent of Other Risk Factors in Diabetes Mellitus
| Risk Enhancers |
| Long duration (≥ 10 years for type 2 diabetes mellituS4.3-20 or ≥20 years for type 1 diabetes mellitusS4.3-6 |
| Albuminuria ≥30 meg of albumin/mg creatinineS4.3-25 |
| eGFR <60 mL/min/1.73 m2S4.3-25 |
| RetinopathyS4.3-9 |
| NeuropathyS4.3-16 |
| ABI <0.9S4.3-22,S4.3-24 |
ABI indicates ankle-brachial index; and eGFR, estimated glomerular filtration rate.
4.4. Primary Prevention
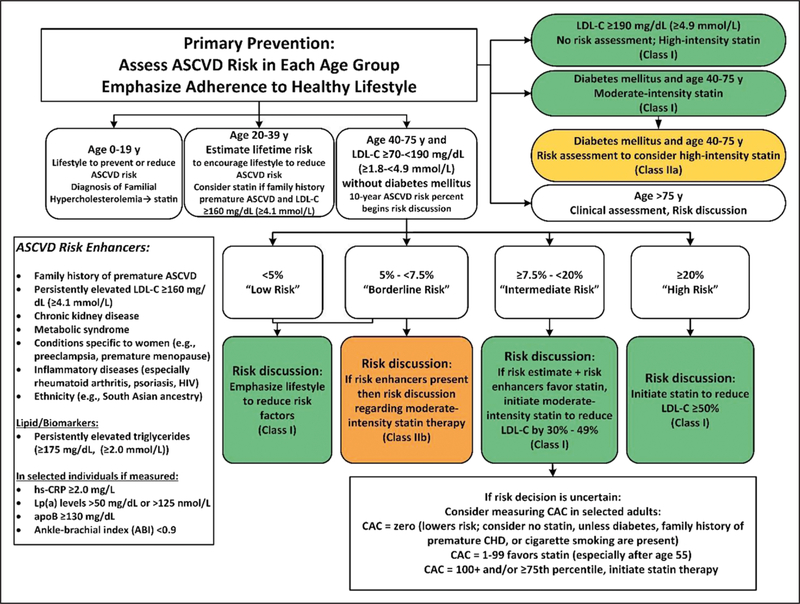
Primary prevention of ASCVD over the life span requires attention to prevention or management of ASCVD risk factors beginning early in life (Figure 2). One major ASCVD risk factor is elevated serum cholesterol, usually identified clinically as measured LDL-C. Screening can be performed with fasting or nonfasting measurement of lipids. In children, adolescents (10 to 19 years of age), and young adults (20 to 39 years of age), priority should be given to estimation of lifetime risk and promotion of lifestyle risk reduction. Drug therapy is needed only in selected patients with moderately high LDL-C levels (≥160 mg/dL [≥4.1 mmol/L]) or patients with very high LDL-C levels (190 mg/dL [4.9 mmol/L]). Three major higher-risk categories are patients with severe hypercholesterolemia (LDL-C levels ≥190 mg/dL [≥4.9 mmol/L]), adults with diabetes, and adults 40 to 75 years of age. Patients with severe hypercholesterolemia and adults 40 to 75 years of age with diabetes mellitus are candidates for immediate statin therapy without further risk assessment. Adults with diabetes mellitus should start with a moderate-intensity statin, and as they accrue multiple risk factors, a high-intensity statin may be indicated. In other adults 40 to 75 years of age, 10-year ASCVD risk should guide therapeutic considerations. The higher the estimated ASCVD risk, the more likely the patient is to benefit from evidence-based statin treatment. The risk discussion should also consider several “risk enhancers” that can be used to favor initiation or intensification of statin therapy. When risk is uncertain or if statin therapy is problematic, it can be helpful to measure CAC to refine risk assessment. A CAC score predicts ASCVD events in a graded fashion and is independent of other risk factors, such as age, sex, and ethnicity.S4.4-1 A CAC score equal to zero is useful for reclassifying patients to a lower-risk group, often allowing statin therapy to be withheld or postponed unless higher risk conditions are present. For patients >75 years of age, RCT evidence for statin therapy is not strong, so clinical assessment of risk status in a clinician–patient risk discussion is needed for deciding whether to continue or initiate statin treatment.S4.4-2–S4.4-21
Figure 2. Primary prevention.
Colors correspond to Class of Recommendation in Table 2. apoB indicates apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; and Lp(a), lipoprotein (a).
4.4.1. . Evaluation and Risk Assessment
4.4.1.1. Risk-Enhancing Factors
Moderate intensity generic statins allow for efficacious and cost-effective primary prevention in patients with a 10-year risk of ASCVD ≥7.5%.S4.4.1.1-1 Since 2013 ACC/ AHA guidelines,S4.4.1.1-2 the HOPE-3 RCTS4.4.1.1-3 provided additional support for this finding. The pooled cohort equation (PCE) is the single most robust tool for estimating 10-year risk in US adults 40 to 75 years of age. Its strength can be explained by inclusion of major, independent risk factors. One limitation on the PCE when applied to individuals is that age counts as a risk factor and dominates risk scoring with advancing age. Age is a powerful population risk factor but does not necessarily reflect individual risk. Another factor influencing risk are baseline characteristics of populations (baseline risk). These characteristics include both genetic and acquired risk factors other than established major risk factors. Variation in baseline risk accounts for difference in risk in different ethnic groups. Absolute risk predictions depend on the baseline risk of a population (eg, the US population). These considerations in patients at intermediate risk leave room in the clinician-patient risk discussion to withhold or delay initiation of statin therapy, depending on age, pattern of risk factors, and patient preferences and values.
In sum, the PCE is a powerful tool to predict population risk, but it has limitations when applied to individuals. One purpose of the clinician patient risk discussion is to individualize risk status based on PCE as well as other factors that may inform risk prediction. Among these other factors are the risk-enhancing factors discussed in this guideline. These risk-enhancing factors are listed in Table 6, and evidence base and strength of association with ASCVD are shown in Table S6 in the Web Supplement. In the general population, they may or may not predict risk independently of PCE. But in the clinician–patient risk discussion they can be useful for identifying specific factors that influence risk. Their presence helps to confirm a higher risk state and thereby supports a decision to initiate or intensify statin therapy. They are useful for clarifying which atherogenic factors are present in a particular patient. And in some patients, certain risk-enhancing factors carry greater lifetime risk than denoted by 10-year risk prediction in the PCE. Finally, several risk-enhancing factors may be specific targets therapy beyond those of the PCE.
Table 6.
Risk-Enhancing Factors for Clinician–Patient Risk Discussion
| Risk-Enhancing Factors |
| Family history of premature ASCVD (males, age <55 y; females, age <65 y) |
| Primary hypercholesterolemia (LDL-C, 160–189 mg/dL [4.1-4.8 mmol/L); non-HDL-C 190–219 mg/dL [4.9–5.6 mmol/L])* |
| Metabolic syndrome (increased waist circumference, elevated triglycerides [>175 mg/dL], elevated blood pressure, elevated glucose, and low HDL-C [<40 mg/dL in men; <50 in women mg/dL] are factors; tally of 3 makes the diagnosis) |
| Chronic kidney disease (eGFR 15–59 mL/min/1.73 m2 with or without albuminuria; not treated with dialysis or kidney transplantation) |
| Chronic inflammatory conditions such as psoriasis, RA, or HIV/AIDS |
| History of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk such as preeclampsia |
| High-risk race/ethnicities (eg, South Asian ancestry) |
| Lipid/biomarkers: Associated with increased ASCVD risk |
| Persistently* elevated, primary hypertriglyceridemia (≥175 mg/dL); |
| If measured: |
| 1. Elevated high-sensitivity C-reactive protein (≥2.0 mg/L) |
| 2. Elevated Lp(a): A relative indication for its measurement is family history of premature ASCVD. An Lp(a) ≥50 mg/dL or ≥125 nmol/L constitutes a risk-enhancing factor especially at higher levels of Lp(a). |
| 3. Elevated apoB ≥130 mg/dL: A relative indication for its measurement would be triglyceride ≥200 mg/dL. A level ≥130 mg/dL corresponds to an LDL-C ≥160 mg/dL and constitutes a risk¬ enhancing factor |
| 4. ABI <0.9 |
Optimally, 3 determinations.
AIDS indicates acquired immunodeficiency syndrome; ABI, ankle-brachial index; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); and RA, rheumatoid arthritis.
A few comments may illustrate the potential usefulness of risk-enhancing factors in the patient discussion. LDL-C ≥160 mg/dL (≥4.1 mmol/L), apoB ≥130 mg/dL (particularly when accompanied by persistently elevated triglycerides), and elevated Lp(a) denote high lifetime risk for ASCVD and favor initiation of statin therapy. The presence of family history of ASCVD, premature menopause, and patients of South Asian race appear to convey a higher baseline risk and are stronger candidates for statin therapy. See Table 7 for a checklist of clinician-patient shared decision making for initiating therapy. Conditions associated with systemic inflammation (chronic inflammatory disorders, metabolic syndrome, chronic renal disease, and elevated hsCRP) appear to predispose to atherothrombotic events, which reasonably justifies statin therapy in intermediate-risk patients.
Table 7.
Checklist for Clinician-Patient Shared Decision-Making for Initiating Therapy
| Checklist Item | Recommendation |
|---|---|
| ASCVD risk assessment | Assign to statin treatment group; use ASCVD Risk Estimator Plus.* |
| In lower-risk primary-prevention adults 40–75 y of age with LDL-C ≥70 mg/dL (≥1.8 mmol/L). | |
| Not needed in secondary prevention, in those with LDL-C ≥190 mg/dL (≥4.9 mmol/L), or in those 40–75 y of age with diabetes mellitus. | |
| Assess other patient characteristics that influence risk. See Risk-Enhancing Factors (Section 4.4.1.3. and Table 6). | |
| Assess CAC (Section 4.4.1.4.) if risk decision is uncertain and additional information is needed to clarify ASCVD risk. | |
| Use decision tools to explain risk (eg, ASCVD Risk Estimator Plus,* Mayo Clinic Statin Choice Decision Aidt). | |
| Lifestyle modifications | Review lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). |
| Endorse a healthy lifestyle and provide relevant advice, materials, or referrals, (eg, CardioSmart‡, AHA Life’s Simple 7§, NLA Patient Tear Sheets||, PCNA Heart Healthy Toolbox¶, cardiac rehabilitation, dietitian, smoking cessation program). | |
| Potential net clinical benefit of pharmacotherapy | Recommend statins as first-line therapy. |
| Consider the combination of statin and nonstatin therapy in selected patients. | |
| Discuss potential risk reduction from lipid-lowering therapy. | |
| Discuss the potential for adverse effects or drug- drug interactions. | |
| Cost considerations | Discuss potential out-of-pocket cost of therapy to the patient (eg, insurance plan coverage, tier level, copayment). |
| Shared decision-making | Encourage the patient to verbalize what was heard (eg, patienťs personal ASCVD risk, available options, and risks/benefits). |
| Invite the patient to ask questions, express values and preferences, and state ability to adhere to lifestyle changes and medications. | |
| Refer patients to trustworthy materials to aid in their understanding of issues regarding risk decisions. | |
| Collaborate with the patient to determine therapy and follow-up plan. | |
ASCVD Risk Predictor Plus is available at: http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/ http://static.heart.org/riskcalc/app/index.html#!/baseline-risk. Accessed September 1, 2018.
Mayo Clinic Statin Decision Aid information is available at: https://statindecisionaid.mayoclinic.org.
CardioSmart health information is available at: https://www.cardiosmart.org/About.
AHA Life’s Simple 7 information is available at: https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check-lifes-simple-7.
NLA Patient Tear Sheets information is available at: https://www.lipid.org/practicetools/tools/tearsheets.
PCNA Heart Healthy Toolbox information is available at: http://pcna.net/clinical-tools/tools-for-healthcare-providers/heart-healthy-toolbox.
AHA indicates American Heart Association; ASCVD, atheroscleroticcardiovascular disease; CAC, coronary artery calcium; CKD, chronic kidneydisease; HIV, human immunodeficiency virus; LDL-C, low-density lipoproteincholesterol; PCNA, Preventive Cardiology Nurses Association and NLA,National Lipid Association.
4.4.2. Primary Prevention Adults 40 to 75 Years of Age With LDL-C Levels 70 to 189 mg/dL (1.7 to 4.8 mmol/L)
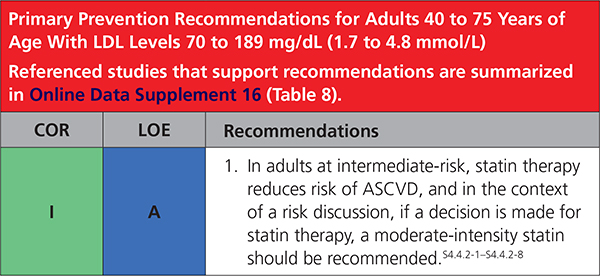 |
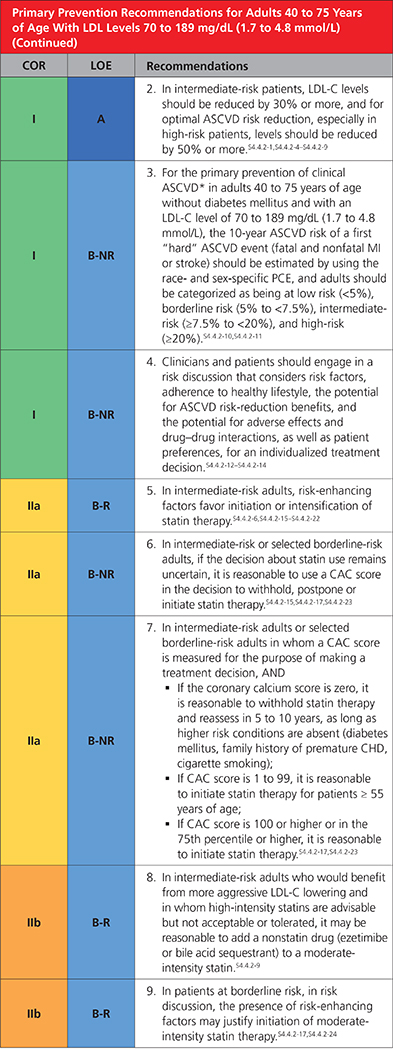 |
Definition of clinical ASCVD includes acute coronary syndrome (ACS), those with history of myocardial infarction (MI), stable or unstable angina or coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral artery disease (PAD) including aortic aneurysm, all of atherosclerotic origin.
4.4.3. Monitoring in Response to LDL-C–Lowering Therapy
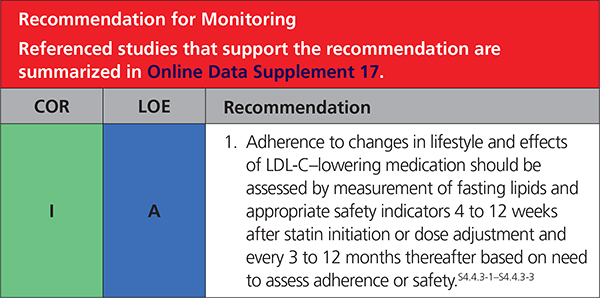 |
4.4.4. Primary Prevention in Other Age Groups
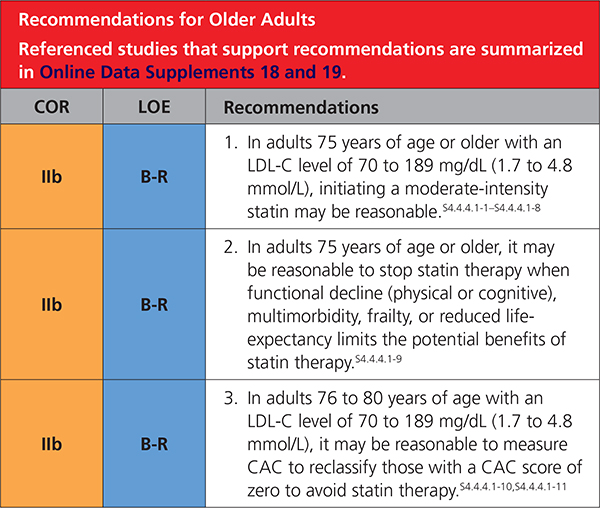
4.4.4.1. Older Adults
Additional recommendations for adults >75 years of age are included in Section 4.1. (Secondary ASCVD Prevention) and Section 4.3. (Diabetes Mellitus in Adults).
 |
4.4.4.2. Children and Adolescents
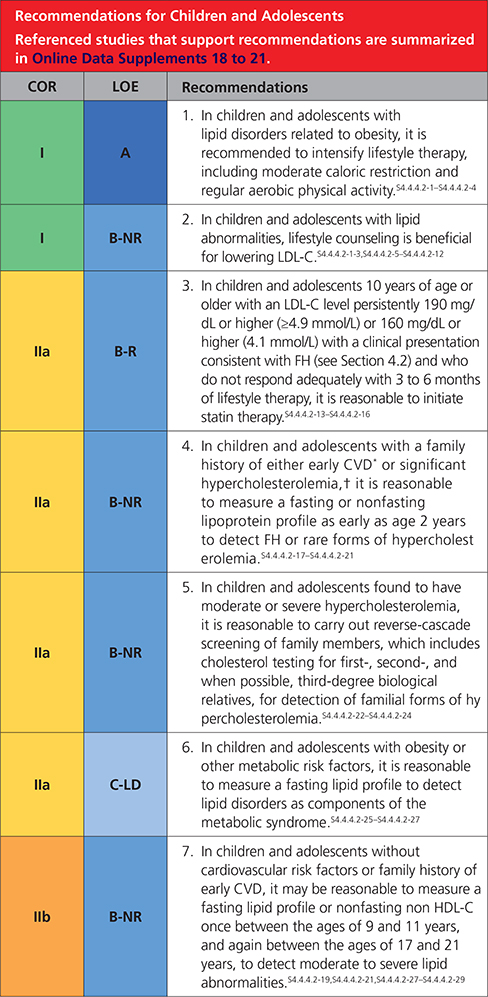 |
Synopsis
Selective screening for lipid disorders on the basis of family history (Recommendation 1) or lifestyle-related factors (Recommendation 2) identifies only a portion of childhood lipid abnormalitiesS4.4.4.2-19,S4.4.4.2-21,S4.4.4.2-26 (Table 9).
Table 9.
| Acceptable, mg/dL | Borderline, mg/dL | Abnormal, mg/dL | |
|---|---|---|---|
| TC | <170 (<4.3 mmol/L) | 170–199 (4.3-5.1 mmol/L) | ≥200 (≥5.1 mmol/L) |
| Triglycerides (0–9 y) | <75 (<0.8 mmol/L) | 75–99 (0.8–1.1 mmol/L) | ≥100 (≥1.1 mmol/L) |
| Triglycerides (10–19 y) | <90 (<1.0 mmol/L) | 90–129 (1.0–1.5 mmol/L) | ≥130 (≥1.4 mmol/L) |
| HDL-C | >45 (>1.2 mmol/L) | 40–45 (1.0–1.2 mmol/L) | <40 (<1.0 mmol/L) |
| LDL-C | <110 (<2.8 mmol/L) | 110–129 (2.8–3.3 mmol/L) | ≥130 (≥3.4 mmol/L) |
| Non-HDL-C | <120 (<3.1 mmol/L) | 120–144 (3.1-3.7 mmol/L) | ≥145 (≥3.7 mmol/L) |
Values given are in mg/dL. To convert to SI units, divide the results for TC, LDL-C, HDL-C, and non-HDL-C by 38.6; for triglycerides, divide by 88.6.
Values for plasma lipid and lipoprotein levels are from the NCEP Expert Panel on Cholesterol Levels in Children. Non-HDL-C values from the Bogalusa Heart Study are equivalent to the NCEP Pediatric Panel cutpoints for LDL-C.
The cutpoints for high and borderline high represent approximately the 95th and 75th percentiles, respectively. Low cutpoints for HDL-C represent approximately the 10th percentile.
HDL-C indicates high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NCEP, National Cholesterol Education Program; SI, Système international d’unitès (International System of Units); and TC, total cholesterol.
4.5. Other Populations at Risk
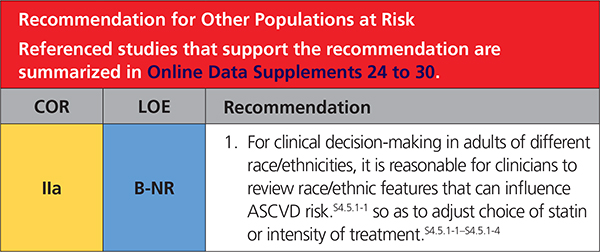
4.5.1. Ethnicity
 |
Synopsis
Race/ethnicity factors can influence estimations of ASCVD risk,S4.5.1-4 intensity of treatmentS4.5.1-1–S4.5.1-4 or even lipid drug use.S4.5.1-5,S4.5.1-6 Important examples include the heightened risk of ASCVD in those who identify as South Asians, the increased sensitivity to statins in those who identify as East Asians, and the increased prevalence of hypertension in blacks. An important issue in management of ASCVD risk in those who identify as Hispanics/Latinos in the United States is the lack of specificity of the term Hispanic/Latino. Race/ethnicity and country of origin, together with socioeconomic status and acculturation level, should be discussed and may explain ASCVD risk factor burden more precisely than the generic term Hispanic/Latino.S4.5.1-6–S4.5.1-11 In addition, those who identify as Native American/Alaskan natives have high rates of risk factors for ASCVD compared to non-Hispanic whites. In many ways, the increase in metabolic risk factors and propensity for diabetes mellitus resembles the risk profiles of those who identify as Mexican Americans.S4.5.1-12 Table 10 reviews these and other racial/ethnic issues that may be useful in clinical management.
Table 10.
Racial/Ethnic Issues in Evaluation, Risk Decisions, and Treatment of ASCVD Risk
| Racial/Ethnic Groupings | ||||
|---|---|---|---|---|
| Asian AmericansS4.5.1-4,S4.5.1-13* | Hispanic/Latino AmericansS4.5.1-7–S4.5.1-11† | Blacks/African AmericansS4.5.1-14 | Comments | |
| Evaluation | ||||
| ASCVD issues informed by race/ethnicity | ASCVD issues informed by race/ ethnicity ASCVD risk in people of South Asian and East Asian origin varies by country of origin; individuals from South Asia (see below) have increased ASCVD risk. | Race/ethnicity and country of origin, together with socioeconomic status and acculturation level, may explain risk factor burden more precisely (eg, ASCVD risk is higher among individuals from Puerto Rico than those from Mexico). | ASCVD risk assessment in black women shows increased ASCVD risk compared with their otherwise similar white counterparts | There is heterogeneity in risk according to racial/ethnic group and within racial/ethnic groups. Native American/ Alaskan populations have high rates of risk factors for ASCVD compared with non-Hispanic whites.S4.5.1-12 |
| Lipid issues informed by race//ethnicityS4.5.1-15,S4.5.1-16 | Asian Americans have lower levels of HDL-C than whites. | Hispanic/Latino women have higher prevalence of low HDL-C compared to Hispanic/ Latino men. | Blacks have higher levels of HDL-C and lower levels of triglycerides than non-Hispanic whites or Mexican Americans. | All ethnic groups appear to be at greater risk for dyslipidemia, but important to identify those with more sedentary behavior and less favorable diet. |
| There is higher prevalence of LDL-C among Asian Indians, Filipinos, Japanese, and Vietnamese than among whites. An increased prevalence of high TG was seen in all Asian American subgroups. | ||||
| Metabolic issues informed by race/ethnicity S4.5.1-3,S4.5.1-17, S4.5.1-1 | Increased MetS is seen with lower waist circumference than in whites. | DM is disproportionately present compared with whites and blacks. There is increased prevalence of MetS and DM in Mexican Americans compared with whites and Puerto Ricans. | There is increased DM and hypertension. | There is increased prevalence of DM. Features of MetS vary by race/ethnicity. Waist circumference, not weight, should be used to determine abdominal adiposity when possible. |
| DM develops at a lower lean body mass and at earlier ages.S4.5.1-19–S4.5.1-21 Majority of risk in South Asians is explained by known risk factors, especially those related to insulin resistance.S4.5.1-13 | ||||
| Risk Decisions | ||||
| PCS4.5.1-22–S4.5.1-25 | No separate PCE is available; use PCE for whites. PCE may underestimate ASCVD risk in South Asians. PCE may overestimate risk in East Asians.S4.5.1-26 | No separate PCE is available; use PCE for non-Hispanic whites. If African-American ancestry is also present, then use PCE for blacks. | Use PCE for blacks.S4.5.1-10 | Country-specific race/ ethnicity, along with socioeconomic status, may affect estimation of risk by PCE. |
| CAC scoreS4.5.1-27-S4.5.1-30 | In terms of CAC burden, South Asian men were similar to non- Hispanic white men, but higher CAC when than blacks, Latinos, and Chinese Americans. South Asian women had similar CAC scores to whites and other racial/ethnic women, although CAC burden higher in older age.S4.5.1-31 | CAC predicts similarly in whites and in those who identify as Hispanic/Latino. | In MESA, CAC score was highest in white and Hispanic men, with blacks having significantly lower prevalence and severity of CAC. | Risk factor differences in MESA between ethnicities did not fully explain variability in CAC. However, CAC predicted ASCVD events over and above traditional risk factors in all ethnicities.S4.5.1-32 |
| Treatment | ||||
| Lifestyle counseling (use principles of Mediterranean and DASH diets) | Use lifestyle counseling to recommend a hearthealthy diet consistent with racial/ethnic preferences to avoid weight gain and address BP and lipids. | Use lifestyle counseling to recommend a hearthealthy diet consistent with racial/ ethnic preferences to avoid weight gain and address BP and lipids. | Use lifestyle counseling to recommend a hearthealthy diet consistent with racial/ ethnic preferences to avoid weight gain and address BP and lipids. | Asian and Hispanic/ Latino groups need to be disaggregated because of regional differences in lifestyle preferences. Challenge is to avoid increased sodium, sugar, and calories as groups acculturate. |
| Intensity of statin therapy and response to LDL-C lowering | Japanese patients may be sensitive to statin dosing. In an open-label, randomized primaryprevention trial, Japanese participants had a reduction in CVD events with low- intensity doses of pravastatin as compared with placebo.S4.5.1-33 In a secondary-prevention trial, Japanese participants with CAD benefitted from a moderate-intensity dose of pitavastatin.S4.5.1-34 | No sensitivity to statin dosage is seen, as compared with non-Hispanic white or black individuals. | No sensitivity to statin dosage is seen, as compared with non-Hispanic white individuals. | Using a lower statin intensity in Japanese patients may give results similar to those seen with higher intensities in non-Japanese patients. |
| Safety | Higher rosuvastatin plasma levels are seen in Japanese, Chinese, Malay, and Asian Indians as compared with whites.S4.5.1-35–S4.5.1-37 FDA recommends a lower starting dose (5 mg of rosuvastatin in Asians vs. 10 mg in whites). Caution is urged as dose is uptitrated | There are no specific safety issues with statins related to Hispanic/Latino ethnicity.S4.5.1-38 | Baseline serum CK values are higher in blacks than in whites.S4.5.1-39 The 95th percentile race/ethnicity-specific and sexspecific serum CK normal levels are available for assessing changes in serum CK. | Clinicians should take Asian race into account when prescribing dose of rosuvastatin (See package insert). In adults of East Asian descent, other statins should be used preferentially over simvastatin.S4.5.1-5 |
The term Asian characterizes a diverse portion of the world’s population. Individuals from Bangladesh, India, Nepal, Pakistan, and Sri Lanka make up most of the South Asian group.S4.5.1-26 Individuals from Japan, Korea, and China make up most of the East Asian group.
The term Hispanics/Latinos in the United States characterizes a diverse population group. This includes white, black, and Native American races. Their ancestry goes from Europe to America, including among these, individuals from the Caribbean, Mexico, Central and South America.
ASCVD indicates atherosclerotic cardiovascular disease; BP, blood pressure; CAC, coronary artery calcium; CAD, coronary artery disease; CK, creatine kinase;CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DM, type 2 diabetes mellitus; FDA, US Food and Drug Administration; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MESA, Multi-Ethnic Study of Atherosclerosis; MetS, metabolic syndrome; and PCE, pooled cohort equations.
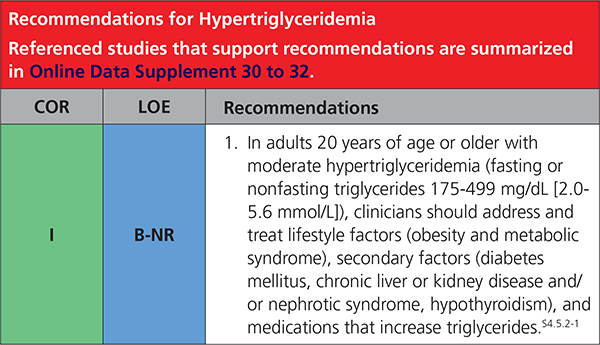
4.5.2. Hypertriglyceridemia
 |
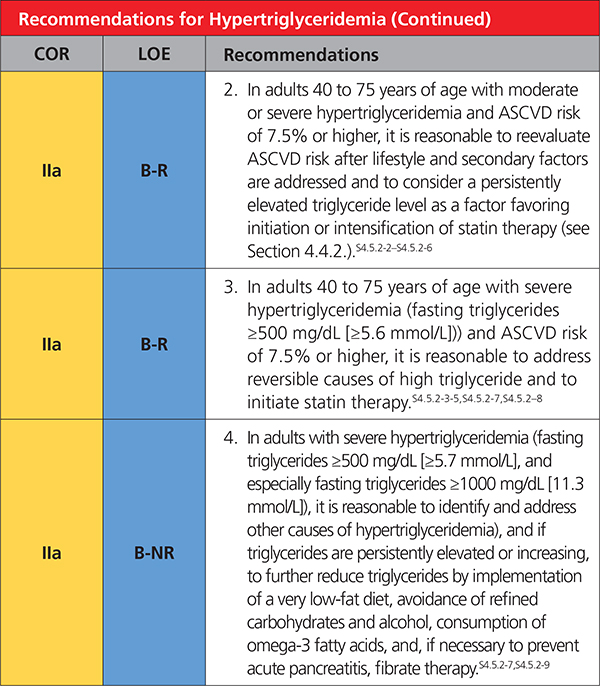 |
4.5.3. Issues Specific to Women
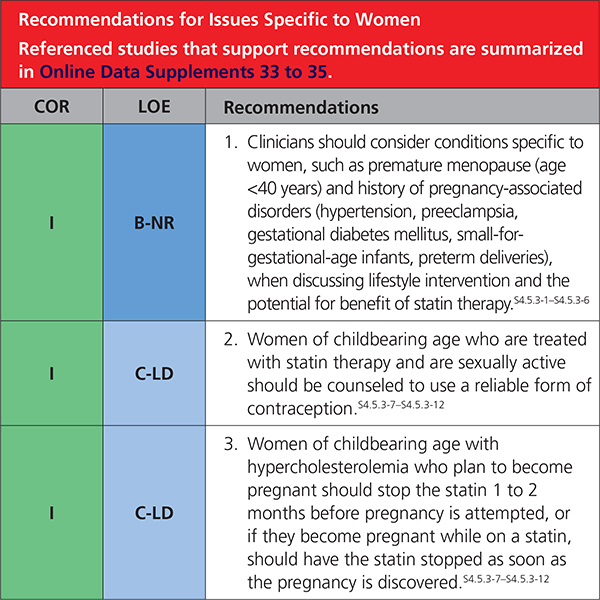 |
4.5.4. Adults With CKD
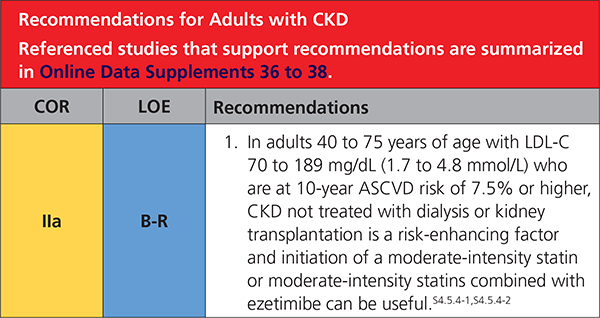 |
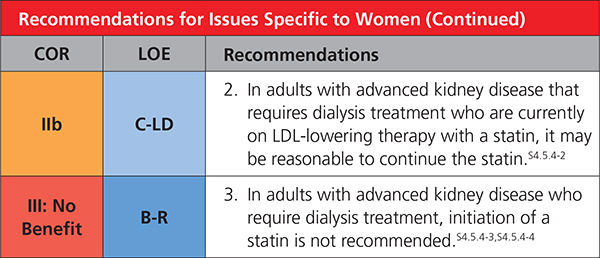 |
4.5.5. Adults With Chronic Inflammatory Disorders and HIV
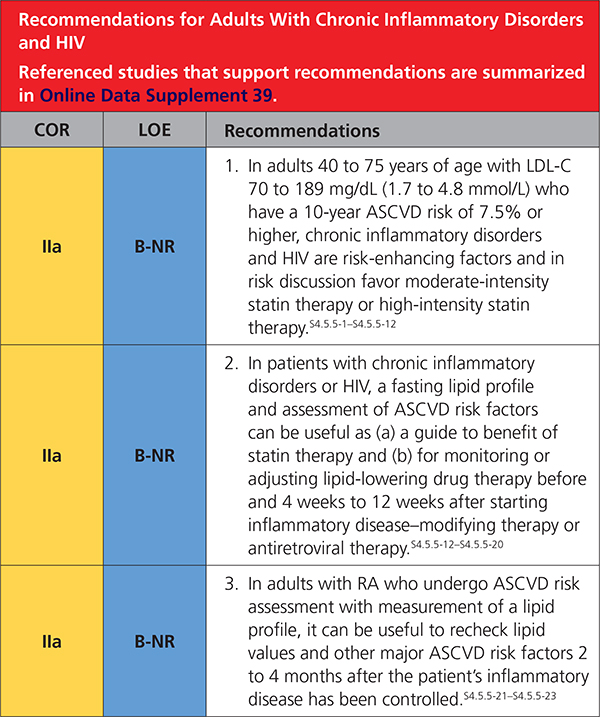 |
5. STATIN SAFETY AND STATIN-ASSOCIATED SIDE EFFECTS
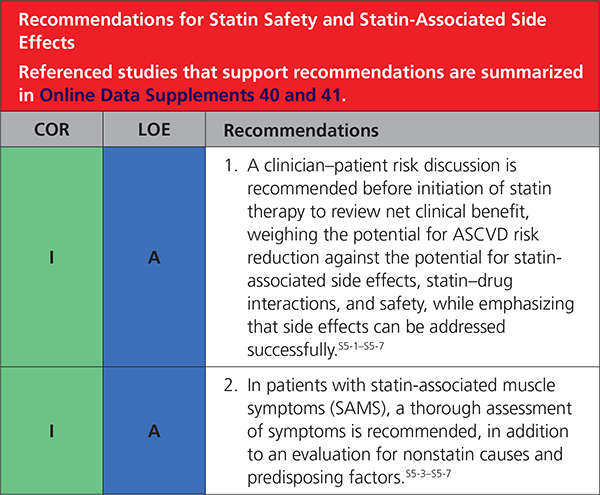 |
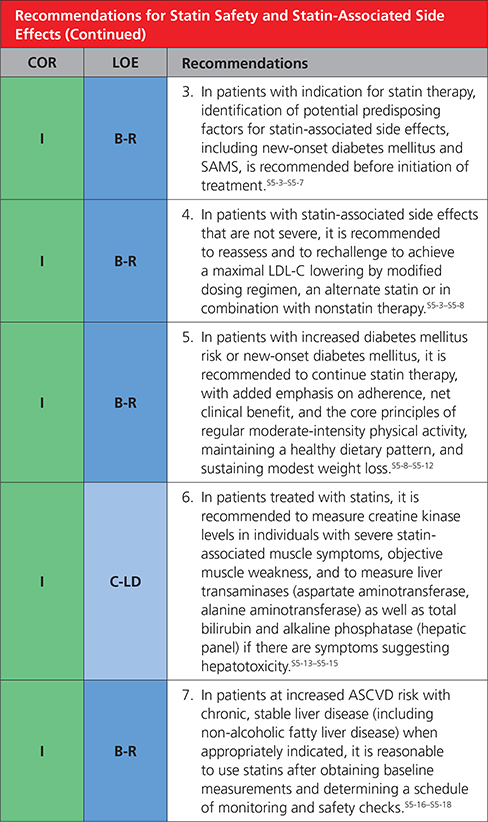 |
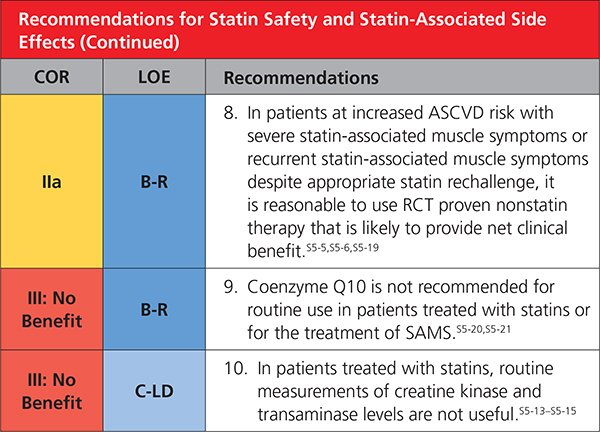 |
Synopsis
Statin therapy is usually well tolerated and safe.S5-1,S5-14,S5-22–S5-24 As with other classes of medications, associated side effects are seen. Instead of the label statin intolerance, the present guideline prefers statin-associated side effects because the large majority of patients are able to tolerate statin rechallenge with an alternative statin or alternative regimen, such as reduced dose or in combination with nonstatins. Although infrequent or rare in clinical trials, statin-associated side effects can be challenging to assess and manage.S5-25,S5-26 The most frequent are SAMS. SAMS usually are subjective myalgia, reported observationally in 5% to 20% of patients.S5-11–S5-14 SAMS often result in nonadherence and can adversely impact ASCVD outcomes.S5-27–S5-29 Statins modestly increase risk of incident diabetes mellitus in susceptible individuals,S5-8–S5-11 but this should not be cause for discontinuation (Table 11).
Table 11.
Statin-Associated Side Effects (SASE)
| Statin-Associated Side Effects | Frequency | Predisposing Factors | Quality of Evidence |
|---|---|---|---|
| Statin-associated muscle symptoms (SAMS) | |||
| Myalgias (CK normal) | Infrequent (1 % to 5%) in RCTs; frequent (5% to 10%) in observational studies and clinical setting | Age, female sex, low body mass index, high-risk medications (CYP3A4 inhibitors, OATP1B1 inhibitors), comorbidities (HIV, renal, liver, thyroid, preexisting myopathy), Asian ancestry, excess alcohol, high levels of physical activity, and trauma | RCTs cohorts/ observational |
| Myositis/myopathy (CK > ULN) with concerning symptoms or objective weakness | Rare | RCTs cohorts/ observational | |
| Rhabdomyolysis (CK >10× ULN + renal injury) | Rare | RCTs cohorts/ observational | |
| Statin-associated autoimmune myopathy (HMGCR antibodies, incomplete resolution) | Rare | Case reports | |
| New-onset diabetes mellitus | Depends on population; more frequent if diabetes mellitus risk factors are present, such as body mass index ≥30, fasting blood glucose ≥100 mg/dL; metabolic syndrome, or A1c ≥6%.S5-8 | Diabetes mellitus risk factors/metabolic syndrome | RCTs/meta-analyses |
| High-intensity statin therapy | |||
| Liver | |||
| Transaminase elevation 3x ULN | Infrequent | RCTs/cohorts/observational | |
| Case reports | |||
| Hepatic failure | Rare | ||
| Central nervous system | |||
| Memory/cognition | Rare | Case reports; no increase in memory/cognition problems in 3 large-scale RCTs | |
| Cancer | No definite association | RCTs/meta-analyses | |
| Other | |||
| Renal function | Unfounded | ||
| Cataracts | Unfounded | ||
| Tendon rupture | Unfounded | ||
| Hemorrhagic stroke | Unfounded | ||
| Interstitial lung disease | Unfounded | ||
| Low testosterone | Unfounded | ||
CK indicates creatine kinase; HIV, human immunodeficiency virus; HMGCR, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; SAAM, statin-associatedautoimmune myopathy; SAMS, statin-associated muscle symptoms; SASE, statin associated side effects; and ULN, upper limit of normal.
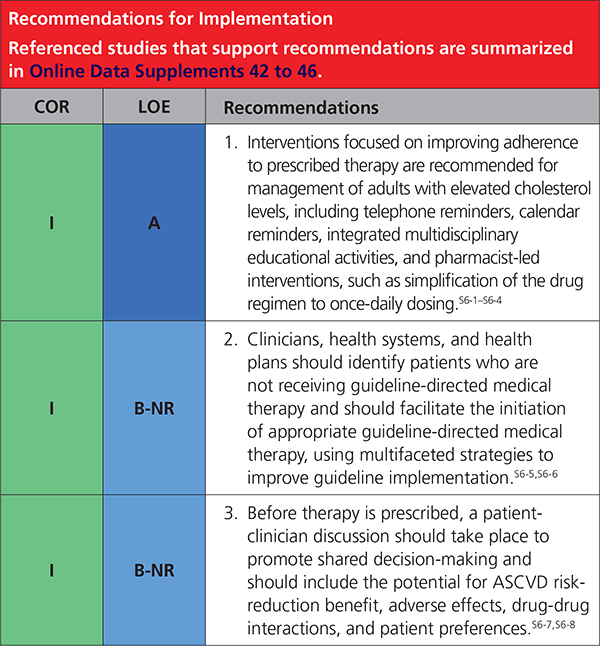
6. IMPLEMENTATION
 |
7. COST AND VALUE CONSIDERATIONS
7.1. Economic Value Considerations: PCSK9 Inhibitors
ACC/AHA clinical guidelines now recognize the importance of considering economic value in making recommendations, in accordance with the principles established by an expert group.S7.1-1 PCSK9 inhibitors further reduce LDL-C when combined with other LDL-lowering drugs, and they reduced composite cardiovascular events in 2 RCTs of high-risk, secondary-prevention patients with clinical ASCVD.S7.1-2 The cost-effectiveness and economic value of PCSK9 inhibitors have been assessed by using simulation models (Online Data Supplements 47 and 48); the published models are based on different sets of assumptions. Compared with statin therapy for secondary prevention, PCSK9 inhibitors have incremental cost-effectiveness ratiosS7.1-3 from $141700 to $450000 per quality-adjusted life-year (QALY) added, at mid-2018 prices. None of the published models report “good value” (<$50000 per QALY added; Table 12), and virtually all indicate “low value” (≥$150000 per QALY added). All models projected mortality benefit by assuming that mortality rate reductions either parallel LDL-C loweringS7.1-4 or parallel RRRs for nonfatal ASCVD events.
Table 12.
Proposed Integration of Level of Value Into Clinical Guideline Recommendations*
| Level of Value |
|---|
| High value: Better outcomes at lower cost or ICER <$50000 per QALY gained |
| Intermediate value: $50000 to <$150000 per QALY gained |
| Low value: ≥$150000 per QALY gained |
| Uncertain value: Value examined, but data are insufficient to draw a conclusion because of absence of studies, low-quality studies, conflicting studies, or prior studies that are no longer relevant |
| Not assessed: Value not assessed by the writing committee |
| Proposed abbreviations for each value recommendation:Level of value:H to indicate high value; I, intermediate value; L, low value; U, uncertain value; and NA, value not assessed. |
Dollar amounts used in this table are based on US GDP data from 2012 and were obtained from WHO-CHOICE Cost-Effectiveness Thresholds.S7.1-9Reproduced from Anderson et al.S7.1-1
GDP indicates gross domestic product; ICER, incremental cost-effectivenessratio; QALY, quality-adjusted life-years; and WHO-CHOICE, World HealthOrganization Choosing Interventions that are Cost-Effective.
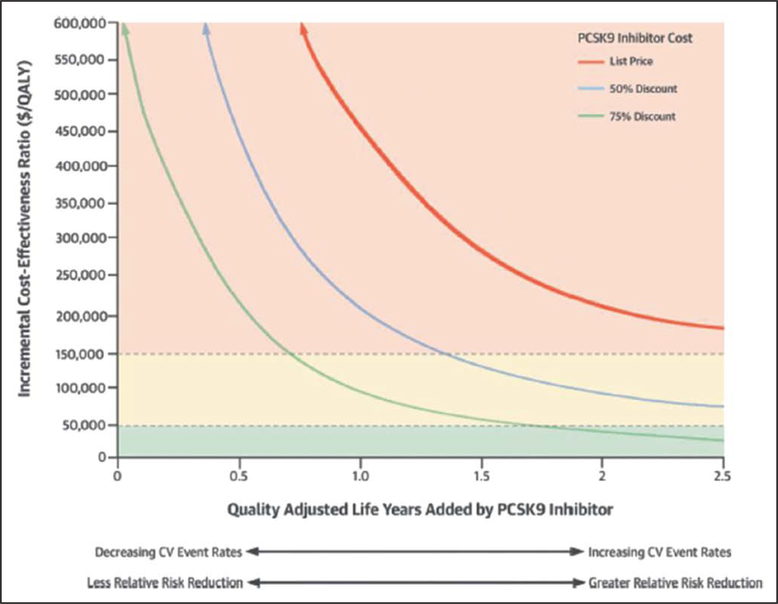
All models project higher lifetime cost from use of PCSK9 inhibitors because the cost will exceed any savings from prevention of cardiovascular events. To be cost-effective by conventional standards, the cost of PCSK9 inhibitors will have to be reduced on the order of 70% to 85% in the United States.S7.1-3 At any given price, the economic value of PCSK9 inhibitors will be improved by restricting their use to patients at very high-risk of ASCVD events, as recommended in the present guidelines. The inverse relationship between improved survival and the incremental cost-effectiveness ratio (Figure 3) indicates that the economic value of PCSK9 inhibitors will be improved by selecting higher-risk patients. One simulation model suggested that restricting the use of PCSK9 inhibitor therapy to patients with baseline LDL-C levels ≥119 mg/dL (≥3 mmol/L), instead of ≥70 mg/dL (≥1.8 mmol/L), would improve their cost-effectiveness to $150000 per QALY added, instead of $268000.S7.1-5 Another study projected a similar improvement in economic value.S7.1-6 Thus, raising the threshold for LDL-C on maximal statin therapy to initiate a PCSK9 inhibitor should improve its cost-effectiveness (Figure 3).
Figure 3. Cost-effectiveness analysis for PCSK9 inhibitors.
Conceptual relationship between the clinical effectiveness of PCSK9 inhibitor therapy, measured in QALYs added compared with statin therapy, on the horizontal axis, and their clinical value, measured in dollars per QALY added, on the vertical axis. The top curve indicates the relationship at full U.S list price of PCSK9 inhibitor therapy ($14000/y), the middle curve indicates the relationship if the price were reduced by 50% (ie, to $7000/y), and the bottom curve indicates the relationship if the price were reduced by 75% (ie, to $3500/y). Reproduced from Hlatky et al.S7.1-3 CV indicates cardiovascular; and QALY, quality-adjusted life-years.
Only 2 economic models have specifically examined the value provided by PCSK9 inhibitors for primary prevention in patients with heterozygous FH (Online Data Supplement 45). One modelS7.1-7 found low value when PCSK9 inhibitors were used for FH ($503000 per QALY added), whereas the second modelS7.1-8 reported intermediate value (incremental cost-effectiveness ratio of $75900 per QALY added). Consequently, the value of PCSK9 inhibitor therapy in FH is uncertain.
8. LIMITATIONS AND KNOWLEDGE GAPS
8.1. Randomized Controlled Trials
ACC/AHA guidelines are based largely on the outcomes of RCTs. Cholesterol guidelines have fortunately benefited from a large number of RCTs of cholesterol-lowering therapies. They have established that greater reductions of LDL-C are accompanied by greater reductions in risk of ASCVD. Robust RCTs exist for both primary and secondary prevention. Most of the data from RCTs have been obtained with statin therapy. Important limited data have also been obtained with nonstatins as add-on drugs to statin therapy. Nevertheless, more data are needed to determine the full scope of the benefit of nonstatin drugs. Several important questions need to be addressed by additional RCTs.
In secondary prevention, does a lower limit for LDL-C attainment exist, beyond which the incremental benefit attained is worth neither the risks nor the cost of additional therapy?
In secondary prevention, what are the indications for adding PCSK9 inhibitors to maximal statin therapy?
In patients with ASCVD who have statin-associated side effects, are PCSK9 inhibitors an effective and safe substitute for high-intensity statins?
In primary prevention for adults 45 to 75 years of age (LDL-C <90 mg/dL [<2.3 mmol/L]) with or without diabetes mellitus, what is the incremental risk reduction imparted by high-intensity statins as compared with moderate-intensity statins?
In primary prevention for adults 45 to 75 years of age (LDL-C <190 mg/dL [<4.9 mmol/L]) with or without diabetes mellitus, what is the incremental risk reduction imparted by moderate-intensity statins plus ezetimibe as compared with moderate-intensity statins alone?
Is statin therapy efficacious and safe in older patients (>75 years of age)? If so, what is a net benefit of statin therapy in this age group?
In patients with severe hypercholesterolemia, what are the efficacy and net benefit of PCSK9 inhibitors as add-on treatment to maximal statin therapy?
What is the efficacy of moderate-intensity and high-intensity statin therapy in patients with risk-enhancing factors (eg, chronic inflammatory disease, CKD, metabolic syndrome)?
8.2. Risk Assessment
In primary prevention, the appropriate selection of patients for cholesterol-lowering drug therapy is highly dependent on risk assessment. Previous guidelines made use of risk-assessment algorithms (eg, Framingham risk scoring or PCE) to estimate risk. Although these equations are useful, they may overestimate or underestimate risk for individual patients. For this reason, the 2013 ACC/AHA guidelinesS8.2-1 introduced the clinician–patient risk discussion to facilitate clinical decisions about appropriate therapy. In the present guidelines, the clinician–patient risk discussion has been amplified and made an integral part of the clinical decision. In addition, in cases in which uncertainty exists, the measurement of CAC has been proposed as a third step in making a treatment decision. Each of these steps could be improved for future guidelines.
8.2.1. . Continuing Refinement of PCE
Because the population baseline risk may be continually declining in the US population, ongoing epidemiological study is needed to assess and update population risk. An example is the development of QRISK in the U.K. population, which is continually expanding its scope.
8.2.2. . Improvement in Lifetime Risk Estimate
The present guidelines include a lifetime ASCVD risk algorithm for those 20 to 59 years of age, but it is based on an insufficient database. Along with a risk algorithm for short-term risk of ASCVD (eg, 10 years), a more robust lifetime risk algorithm would facilitate the clinician–patient risk discussion for treatment decisions.
8.2.3. Refinement of Clinician–Patient Risk Discussion
An ongoing study of how a clinician can best interact with a patient to arrive at an informed decision must be done, taking multiple factors into consideration. This is particularly important because cholesterol-lowering therapy is meant to be a lifetime therapy.
8.2.4. Monitoring and Adjustment of Treatment
The clinician–patient risk discussion will likely prove inadequate unless an ongoing interaction between the patient and clinician occurs. This involves monitoring the effectiveness of therapy and adherence to therapy. Thus, the clinician–patient risk discussion should include more than the initial treatment decision. Ongoing research on how to improve the entire process of initial decision-making and long-term follow-up is necessary.
8.2.5. Prognostic Significance of CAC
The present guideline makes use of the available data to predict the risk associated with CAC. These data need to be amplified by new and ongoing studies to guide treatment decisions. Particular uncertainty exists about the predictive value of intermediate CAC scores. In addition, the predictive significance of a CAC score of zero must be further verified in follow-up studies. For patients with a CAC score of zero, it is currently uncertain when and if follow-up CAC measurements should be done to reassess risk status.
Supplementary Material
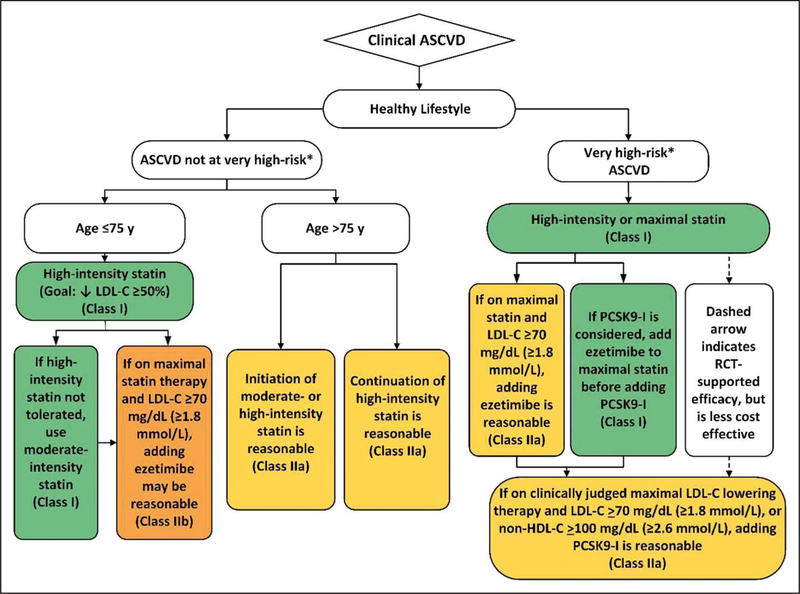
Figure 1. Secondary prevention in patients with clinical ASCVD.
Colors correspond to Class of Recommendation in Table 2. Clinical ASCVD consists of acute coronary syndrome (ACS), those with history of myocardial infarction (MI), stable or unstable angina or coronary other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral artery disease (PAD) including aortic aneurysm, all of atherosclerotic origin. Very high-risk includes a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions (Table 4). ACS indicates acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MI, myocardial infarction; and PCSK9i, PCSK9 inhibitor.
Table 4.
Very High-Risk* of Future ASCVD Events
| Major ASCVD Events |
| Recent ACS (within the past 12 mo) |
| History of MI (other than recent ACS event listed above) |
| History of ischemic stroke |
| Symptomatic peripheral arterial disease (history of claudication with ABI <0.85, or previous revascularization or amputationS4.1-39) |
| High-Risk Conditions |
| Age ≥65 y |
| Heterozygous familial hypercholesterolemia |
| History of prior coronary artery bypass surgery or percutaneous coronary intervention outside of the major ASCVD event(s) |
| Diabetes mellitus |
| Flypertension |
| CKD (eGFR 15–59 MI/min/1.73 m2)S4.1-15,S4.1-17 |
| Current smoking |
| Persistently elevated LDL-C (LDL-C ≥100 mg/dL [≥2.6 mmol/L]) despite maximally tolerated statin therapy and ezetimibe |
| History of congestive HF |
Very high-risk includes a history of multiple major ASCVD events or one major ASCVD event and multiple high-risk conditions.
ABI indicates ankle-brachial index; ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; eGFR,estimated glomerular filtration rate; HF, heart failure; LDL, low-density lipoprotein cholesterol; and MI, myocardial infarction.
Table 8.
Selected Examples of Candidates for CAC Measurement Who Might Benefit From Knowing Their CAC Score Is Zero
| CAC Measurement Candidates Who Might Benefit From Knowing Their CAC Score Is Zero |
| Patients reluctant to initiate statin therapy who wish to understand their risk and potential for benefit more precisely |
| Patients concerned about need to reinstitute statin therapy after discontinuation for statin-associated symptoms |
| Older patients (men, 55–80 y of age; women, 60–80 y of age) with low burden of risk factorsS4.4.2-25 who question whether they would benefit from statin therapy |
| Middle-aged adults (40–55 y of age) with PCE-calculated 10-year risk of ASCVD 5% to <7.5% with factors that increase their ASCVD risk, although they are in a borderline risk group |
Caveats: If patient is intermediate risk and if a risk decision is uncertain and a CAC score is performed, it is reasonable to with hold statin therapy unless higher risk conditions such as cigarette smoking, family history of premature ASCVD, or diabetes mellitus are present, and to reassess CAC score in 5–10 years. Moreover, if CAC is recommended, it should be performed in facilities that have current technology that delivers the lowest radiation possible.
ASCVD indicates atherosclerotic cardiovascular disease; CAC, coronary artery calcium; LDL-C, low-density lipoprotein cholesterol; and PCE, pooledcohort equations.
1.6. Abbreviations
- Abbreviation
Meaning/Phrase
- ABI
ankle-brachial index
- ACS
acute coronary syndrome
- AIDS
acquired immunodeficiency syndrome
- apoB
apolipoprotein B
- ARR
absolute risk reduction
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CK
creatine kinase
- CKD
chronic kidney disease
- COR
Class of Recommendation
- CTT
Cholesterol Treatment Trialists
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- FH
familial hypercholesterolemia
- HDL
high-density lipoprotein
- HF
heart failure
- HIV
human immunodeficiency virus
- LDL-C
low-density lipoprotein cholesterol
- LOE
Level of Evidence
- Lp(a)
lipoprotein (a)
- MI
myocardial infarction
- PCE
pooled cohort equations
- QALY
quality-adjusted life-year
- RA
rheumatoid arthritis
- RCT
randomized controlled trials
- RRR
relative risk reduction
- RWI
relationships with industry and other entities
- SAMS
statin-associated muscle symptoms
- SR
systematic review
- TC
total cholesterol
- VLDL
very low-density lipoprotein
- VLDL-C
very low-density lipoprotein cholesterol
Appendix
Appendix 1.
Author Relationships With Industry and Other Entities (Relevant)–2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol* (August 2018)
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/ Partnership/ Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Scott M. Grundy (Chair) | VA North Texas Health Care System and University of Texas Southwestern Medical Center at Dallas–Professor of Internal Medicine | None | None | None | None | None | None |
| Neil J. Stone (Vice Chair) | Northwestern Medicine/Northwestern University–Bo now Professor of Medicine, Cardiology | None | None | None | None | None | None |
| Alison L. Bailey | Erlanger Health System/University of Tennessee College of Medicine– Program Director, Cardiovascular Diseases Fellowship; Director, Preventive cardiology and Cardiac Rehabilitation | None | None | None | None | None | None |
| Craig Beam | CBRE–Managing Director; National Cultivation/Strategic Investments Leader | None | None | None | None | None | None |
| Kim K. Birtcher | University of Houston College of Pharmacy–Clinical Professor | None | None | None | None | None | None |
| Roger S. Blumenthal | Johns Hopkins University, Ciccarone Center for the Prevention of Heart Disease–Professor of Medicine | None | None | None | None | None | None |
| Lynne T. Braun | Rush University Medical Center– Professor of Nursing and Medicine | None | None | None | None | None | None |
| Sarah De Ferranti | Boston Children's Hospital–Assistant Professor of Pediatrics | None | None | None | None | None | None |
| Joseph Faiella- Tommasino | Touro College, School of Health Sciences–Chairman and Assistant Dean of Physician Assistant Programs | None | None | None | None | None | None |
| Daniel E. Forman | University of Pittsburgh–Chair, Geriatric Cardiology | None | None | None | None | None | None |
| Ronald Goldberg | University of Miami, Diabetes Research Institute–Professor of Medicine, Division of Endocrinology, Metabolism and Diabetes | None | None | None | None | None | None |
| Paul A. Fleidenreich | Stanford University, Department of Medicine–Professor, Vice Chair for Quality | None | None | None | None | None | None |
| Mark A. Hlatky | Stanford University, School of Medicine– Professor of Health Research Policy, Professor of Cardiovascular Medicine | None | None | None | None | None | None |
| Daniel W. Jones | University of Mississippi Medical Center–Professor of Medicine and Physiology; Director, Clinical and Population Science | None | None | None | None | None | None |
| Donald Lloyd-Jones | Northwestern University–Eileen M. Foe 11 Professor; Chair, Department of Preventive Medicine | None | None | None | None | None | None |
| Nuria Lopez-Pajares | Temple University–Physician | None | None | None | None | None | None |
| Chiadi Ndumele | Johns Hopkins University School of Medicine–Robert E. Meyerhoff Assistant Professor of Medicine | None | None | None | None | None | None |
| Carl E. Orringer | University of Miami, Soffer Clinical Research Center–Associate Professor | None | None | None | None | None | None |
| Carmen Peralta | University of California, San Francisco– Associate Professor of Medicine; Kidney Health Research Collaborative–Executive Director | None | None | None | None | None | None |
| Joseph Saseen | University of Colorado, Anschutz Medical Campus–Professor and Vice Chair, Department of Clinical Pharmacy; Professor, Department of Family Medicine | None | None | None | None | None | None |
| Sidney C. Smith, Jr | University of North Carolina, Chapel Hill–Professor of Medicine | None | None | None | None | None | None |
| Laurence S. Sperling | Emory University, Rollins School of Public Health–Professor of Medicine, Cardiology; Professor of Global Health | None | None | None | None | None | None |
| Salim S. Virani | Baylor College of Medicine–Professor, Section of Cardiovascular Research and Director, Cardiology Fellowship Training Program; Michael E. DeBakey VA Medical Center–Staff Cardiologist and Investigator, Health Policy, Quality & Informatics Program, Center for Innovations in Quality, Effectiveness and Safety | None | None | None | None | None | None |
| Joseph Yeboah | Wake Forest Baptist Health– Assistant Professor, Internal Medicine, Cardiovascular | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5000 of the fair market value of the businessentity; or if funds received by the person from the business entity exceed 5% of the person's gross income for the previous year. Relationships that exist withno financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. According to the ACC/
AHA, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, orissue addressed in the document; or b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document ormakes a competing drug or device addressed in the document, or c) the person ora member of the person's household, has a reasonable potential for financial,professional or other personal gain or loss as a result of the issues/content addressed in the document.
The Cholesterol Guideline began in September 2016. Over the initial years of the CMS Open Payment System, understandably, there have been manyissues related to the accurate reporting of food and beverage payments. For this reason, the ACC and AHA have not considered these minor charges relevant relationships with industry.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAPA, American Academy of Physician Assistants; ABC, Association ofBlack Cardiologists; ACC, American College of Cardiology; ACPM, American College of Preventive Medicine; ADA, American Diabetes Association; AGS, American Geriatrics Society; AHA, American Heart Association; APhA, American Pharmacists Association; ASPC, American Society for Preventive Cardiology; PCNA, Preventive Cardiovascular Nurses Association; and VA, Veterans Affairs.
Appendix 2.
Reviewer Relationships With Industry and Other Entities (Comprehensive)–2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA GuideManagement of Blood Cholesterol (August 2018)
| Peer Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness | Salary |
|---|---|---|---|---|---|---|---|---|---|
| Philip A. Ades | Official Reviewer– AACVPR | University of Vermont Medical Center– Professor of Medicine | None | None | None | None | None | None | None |
| Karen P. Alexander | Official Reviewer– ACC Science and Quality Committee | Duke University Medical Center–Professor of Medicine/Cardiology | None | None | None | • GSK • NIH |
None | None | None |
| Theresa M. Beckie | Official Reviewer– AACVPR | University of South Florida–Professor and Associate Dean of the PhD Program | None | None | None | None | None | None | None |
| Kathy Berra | Official Reviewer– PCNA | Stanford University | • Omada Health | None | None | None | • Council on Aspirin for Health and Prevention - a committee of the Alta rum Institute • Preventive Cardiovascular Nurses Association | None | None |
| William T. Cefalu | Official Reviewer– ADA | American Diabetes Assoc i at i o n–C h i ef Scientific, Medical and Mission Officer | None | None | None | None | None | None | None |
| Mary Ann Champagne | Official Peer Reviewer–PCNA | Stanford Hospital and Clinics–Clinical Nurse Specialist and Coordinator | None | None | None | None | None | None | None |
| Joaquin Cigarroa | Official Reviewer– ACC/AHATask Force on Clinical Practice Guidelines | Oregon Health and Science University– Clinical Professor of Medicine | None | None | None | None | None | None | None |
| Stephen R. Daniels | Official Reviewer– AAP | University of Colorado School of Medicine– Professor and Chair, Department of Pediatrics; Children's Hospital Colorado–Pediatrician- in-Chief and L. Joseph Butterfield Chair in Pediatrics | • Sanofi-Aventis | None | None | None | • Novo Nordisk Inc. | None | None |
| Dave Dixon | Official Reviewer– NLA | Virginia Commonwealth University School of Pharmacy–Associate Professor and Vice-Chair for Clinical Services | None | None | None | None | None | None | None |
| Earl W. Ferguson | Official Reviewer– AC PM | Ridgecrest Regional Hospital–Independent Consultant | None | None | • Bakersfield Heart Hospitalf | None | • Growth Creators Inc./Radekal/ Pertexa • California Health Information Partnership and Services Organizationt |
None | None |
| Edward A. Gill, Jr | Official Reviewer– NLA | University of Colorado Cardiology Division– Professor of Clinic Practice, Medicine- Cardiology | None | None | None | None | None | None | • Acute Coronary Syndrome-2007† |
| Tyler J. Gluckman | Official Reviewer– ACC Board of Governors | Providence St. Vincent Heart Clinic–Medical Director | • Boehringer Ingelheim Pharmaceuticals | None | None | None | None | None | None |
| Rita Kalyani | Official Reviewer– ADA | Johns Hopkins School of Medicine–Associate Professor of Medicine | None | None | None | None | None | None | None |
| Norma M. Keller | Official Reviewer– ACC Board of Governors | New York University Medical Center–Chief of Cardiology | None | None | None | None | None | None | None |
| Amit Khera | Official Reviewer– AS PC | University of Texas Southwestern Medical Center–Assistant Professor of Medicine | None | None | None | None | None | None | None |
| Carol Kirkpatrick | Official Reviewer– NLA | Idaho State University– Wellness Center Director/ Clinical Associate Professor Kasiska Division of Health Sciences | • National Lipid Association | None | None | None | None | None | None |
| G. B. John Mancini | Official Reviewer– ACC Board of Governors | Vancouver Hospital Research Pavilion– Professor of Medicine | • Amgen • Bayer • Boehringer Ingelheim Pharmaceuticals, Inc • Eli Lilly and Company • Esperion • Merck • Pfizer • Regeneran • Sanofi-aventis/ Regeneron • Servier |
None | None | None | None | None | None |
| Laxmi S. Mehta | Official Reviewer– ACC Science and Quality Committee | Ohio State University– Professor of Medicine; Section Director of Preventative Cardiology and Women's Cardiovascular Health | None | None | None | None | • AHAt | None | None |
| David Montgomery | Official Reviewer– ABC | Piedmont Heart Institute–Cardiologist | None | None | None | None | None | None | None |
| Michelle Odden | Official Reviewer– AGS | Oregon State University–Associate Professor | None | None | None | None | None | None | None |
| Daniel J. Rader | Official Reviewer– AHA | C oo per-M cC 1 u re– Professor of Medicine; University of Pennsylvania School of Medicine– Director, Preventive Cardiovascular Medicine | • Alnylam*
• Novartis* • Pfizer* • DalCor • Medlmmune, Inc |
None | • Staten Bio*
• VascularStrategies* |
None | None | None | None |
| Michael W. Rich | Official Reviewer– AGS | Washington University School of Medicine– Professor of Medicine | None | None | None | None | None | None | None |
| Mirvat A. Alasnag | Content Reviewer– ACC Early Career Member Section | King Fahd Armed Forces Hospital, Jeddah- KSA–1 nterve nt i o n a 1 Cardiologist | None | None | None | None | None | None | None |
| Kim K. Birtcher | Content Reviewer– ACC/AHATask Force on Clinical Practice Guidelines | University of Houston College of Pharmacy– Clinical Professor | • Jones & Bartlett Learning | None | None | None | • Accreditation Council for Clinical Lipidologyt | None | |
| Conrad B. Blum | Content Reviewer– ACC/AHA | Medicine at Columbia University Medical Center–Professor | None | None | None | None | • ACC-AHAt | None | None |
| Bernard Dennis | Content Reviewer– ACC/AHA Lay Reviewer | Dennis Associates, LLC | None | None | None | None | None | None | None |
| Henry Ginsberg | Content Reviewer– AHA | Columbia University, Irving–Professor of Medicine | • Merck • Resverlogix • Sanofi- Regeneron • Amgen • Akcea • Kowa • Janssen • Esperion |
None | None | None | None | None | None |
| Ira Goldberg | Content Reviewer– AHA | NYU Division of Endocrinology, Diabetes, and Metabolism– Director | • Akcea*
• Amgen • Arrowhead • Intarcia • Merck • Regeneran |
None | None | None | None | None | None |
| José A. Jog la r | Content Reviewer– ACC/AHATask Force on Clinical Practice Guidelines | UT Southwestern Medical Center University–Professor of Medicine | None | None | None | None | None | None | None |
| Peer Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/ Partnership/ Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness | Salary |
| Glenn N. Levine | Content Reviewer– ACC/AHA Task Force on Clinical Practice Guidelines | Baylor College of Medicine–Professor of Medicine; Michael E. DeBakey Medical Center–Director, Cardiac Care Unit | None | None | None | None | None |
• Defendant, Out-of-hospital cardiopulmonary arrest, 2017* |
None |
| Daniel Levy | Content Reviewer– ACC/AHA | Center for Population Studies–Director; Journal of the American Society of Hypertension–E ditor- in-Chief | None | None | None | None | None | None | None |
| Theodore Mazzone | Content Reviewer– ACC/AHA | Northshore University Health System– Chairman, Department of Medicine | None | None | None | None | None | None | None |
| Patrick E. McBride | Content Reviewer– ACC/AHA | University of Wisconsin School of Medicine and Public Health–Professor Emeritus, Departments of Medicine (Cardiovascular Medicine) and Family Medicine | None | None | • Health Decisions, Inct | None | None | None | None |
| Karen J. McConnell | Content Reviewer– APhA | Catholic Health Initiatives–System Director of Clinical Pharmacy Services | None | None | None | None | None | None | None |
| Pamela B. Morris | Content Reviewer– ACC Prevention of Cardiovascular Disease Member Section | The Medical University of South Carolina– Professor of Medicine, Director of Preventative Cardiology | • Amgen • Esperion • Sanofi Regeneran |
None | None | None | None | None | None |
| Nathalie Pamir | Content Reviewer– AHA Scientific Council | Oregon Health and Science University– Assistant Professor | None | None | None | None | None | None | None |
| Janelle F. Ruisinger | Content Reviewer– APhA | The University of Kansas School of Pharmacy, Department of Pharmacy Practice–Clinical Pharmacist; KUMC Atherosclerosis and LDL-Apheresis Center– Clinical Associate Professor | None | None | None | • Amgen†
• Regeneran† • Sanofi-Aventis† |
• American Society of Health System Pharmacists | None | None |
| Joshua Schulman- Marcus | Content Reviewer– ACC Early Career Member Section | Albany Medical Center– Assistant Professor of Medicine | None | None | None | None | None | None | None |
| Michael D. Shapiro | Content Reviewer– ACC Prevention of Cardiovascular Disease Member Section | Oregon Health & Science University–Associate Professor of Medicine and Radiology | • Akcea • Amgen • Kastle* • Novartis Corporation • Regeneran |
None | None | • Akcea† • Amarin† • Amgen† |
None | None | None |
| Susan Shero | Content Reviewer– ACC/AHA | NIHNHLBI–Public Health Advisor | None | None | None | None | None | None | None |
| James L. Young II | Content Reviewer– AHA | Beaumont Health– Patient/Family Liaison | None | None | None | None | None | None | None |
This table represents all relationships of reviewers with industry and other entities that were reported at the time of peer review, including those not deemed to be relevant to this document, at the time thisdocument was under review The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest representsownership of >5% of the voting stock or share of the business entity, or ownership of >$5000 of the fair market value of the business entity; or if funds received by the person from the business entityexceed 5% of the person's gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unlessotherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional informationabout the ACC/AHA Disclosure Policy for Writing Committees.
Significant relationship.
No financial benefit.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAPA, American Academy of Physician Assistants; ABC, Association of Black Cardiologists; ACC, AmericanCollege of Cardiology; AC PM, American College of Preventive Medicine; ADA, American Diabetes Association; AGS, American Geriatrics Society; AHA, American Heart Association; APhA, AmericanPharmacists Association; ASPC, American Society for Preventive Cardiology; GSK, GlaskoSmithKIine; KSA, Kingdom of Saudi Arabia; KUMC, University of Kansas Medical Center; LDL, low-density lipoprotein;NHLBI, National Heart, Lung, and Blood Institute; NIH, National Institutes of Health; NLA, National Lipid Association; NYU, New York University; PCNA, Preventive Cardiovascular Nurses Association; and UT,University of Texas.
Footnotes
Former Task Force member; current member during the writing effort.
ACC/AHA TASK FORCE MEMBERS Glenn N. Levine, MD, FACC, FAHA, Chair; Patrick T. O’Gara, MD, MACC, FAHA, Chair-Elect; Jonathan L. Halperin, MD, FACC, FAHA, Immediate Past Chair*; Sana M. Al-Khatib, MD, MHS, FACC, FAHA; Joshua A. Beckman, MD, MS, FAHA; Kim K. Birtcher, PharmD, MS, AACC; Biykem Bozkurt, MD, PhD, FACC, FAHA*; Ralph G. Brindis, MD, MPH, MACC*; Joaquin E. Cigarroa, MD, FACC; Lesley H. Curtis, PhD, FAHA*; Anita Deswal, MD, MPH, FACC, FAHA; Lee A. Fleisher, MD, FACC, FAHA; Federico Gentile, MD, FACC; Samuel Gidding, MD, FAHA*; Zachary D. Goldberger, MD, MSc, FACC, FAHA; Mark A. Hlatky, MD, FACC, FAHA; John Ikonomidis, MD, PhD, FAHA*; José A. Joglar, MD, FACC, FAHA; Laura Mauri, MD, MSc, FAHA*; Mariann R. Piano, RN, PhD, FAHA; Susan J. Pressler, PhD, RN, FAHA*; Barbara Riegel, PhD, RN, FAHA*; Duminda N. Wijeysundera, MD, PhD
PRESIDENTS AND STAFF
American College of Cardiology
C. Michael Valentine, MD, FACC, President Timothy W. Attebery, MBA, FACHE, Chief Executive Officer
William J. Oetgen, MD, MBA, FACC, Executive Vice President, Science, Education, Quality, and Publishing
MaryAnne Elma, MPH, Senior Director, Science, Education, Quality, and Publishing
Amelia Scholtz, PhD, Publications Manager, Science, Education, Quality, and Publishing
American College of Cardiology/ American Heart Association
Katherine A. Sheehan, PhD, Director, Guideline Strategy and Operations
Abdul R. Abdullah, MD, Senior Manager, Guideline Science
Thomas S.D. Getchius, Manager, Guideline Operations
American Heart Association
Ivor Benjamin, MD, FAHA, President
Nancy Brown, Chief Executive Officer
Rose Marie Robertson, MD, FAHA, Chief Science and Medicine Officer
Gayle R. Whitman, PhD, RN, FAHA, FAAN, Senior Vice President, Office of Science Operations
Prashant Nedungadi, PhD, Science and Medicine Advisor, Office of Science Operations
Jody Hundley, Production and Operations Manager, Scientific Publications, Office of Science Operations
ARTICLE INFORMATION
This document was approved by the American College of Cardiology Clinical Policy Approval Committee, the American Heart Association Science Advisory and Coordinating Committee, American Association of Cardiovascular and Pulmonary Rehabilitation, American Academy of Physician Assistants, Association of Black Cardiologists, American College of Preventive Medicine, American Diabetes Association, American Geriatrics Society, American Pharmacists Association, American Society for Preventive Cardiology, National Lipid Association, and Preventive Cardiovascular Nurses Association in October 2018, and the American Heart Association Executive Committee in October 2018.
Supplemental materials are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIR.0000000000000624.
This article has been copublished in the Journal of the American College of Cardiology.
Copies: This document is available on the websites of the American College of Cardiology (www.acc.org) and the American Heart Association (professional.heart.org). A copy of the document is also available at https://professional.heart.org/statements by selecting the “Guidelines & Statements” button. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu near the top of the web page, then click “Publication Development.”
REFERENCES
PREAMBLE
- P-1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/ AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–1143. DOI: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
1. INTRODUCTION
1.1. Methodology and Evidence Review
- S1.1-1.Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1144–61. DOI: 10.1161/CIR.0000000000000626. [DOI] [PubMed] [Google Scholar]
- S1.1-2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/ AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–1143. DOI: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
1.4. Scope of the Guideline
- S1.4-1.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170?000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S1.4-2.Chou R, Dana T, Blazina I, et al. Statin use for the prevention of cardiovascular disease in adults: a systematic review for the US Preventive Services Task Force. 2016. US Agency for Healthcare Research and Quality; Rockville, MD: Report No.: 14-05206-EF-2. [PubMed] [Google Scholar]
- S1.4-3.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S1.4-4.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
1.5. Class of Recommendation and Level of Evidence
- S1.5-1.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2015;133:1426–8. [DOI] [PubMed] [Google Scholar]
2. HIGH BLOOD CHOLESTEROL AND ASCVD
2.1. Measurements of LDL-C and Non–HDL-C
- S2.1-1.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–56. [DOI] [PubMed] [Google Scholar]
- S2.1-2.Langsted A, Nordestgaard BG. Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen General Population Study. Clin Chem. 2011;57:482–9. [DOI] [PubMed] [Google Scholar]
- S2.1-3.Mora S, Rifai N, Buring JE, et al. Comparison of LDL cholesterol concentrations by Friedewald calculation and direct measurement in relation to cardiovascular events in 27?331 women. Clin Chem. 2009;55:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-4.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172:1707–10. [DOI] [PubMed] [Google Scholar]
- S2.1-5.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-6.Doran B, Guo Y, Xu J, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. 2014;130:546–53. [DOI] [PubMed] [Google Scholar]
- S2.1-7.Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013;62:732–9. [DOI] [PubMed] [Google Scholar]
- S2.1-8.Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-9.Sathiyakumar V, Park J, Golozar A, et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation. 2018;137:10–9. [DOI] [PubMed] [Google Scholar]
3. THERAPEUTIC MODALITIES
3.1. Lipid-Lowering Drugs
- S3.1-1.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. [DOI] [PubMed] [Google Scholar]
3.1.1. Statin Therapy
- S3.1.1-1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- S3.1.1-2.Karlson BW, Wiklund O, Palmer MK, et al. Variability of low-density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: results from VOYAGER. Eur Heart J Cardiovasc Pharmacother. 2016;2:212–7. [DOI] [PubMed] [Google Scholar]
- S3.1.1-3.Naito R, Miyauchi K, Daida H. Racial differences in the cholesterol-lowering effect of statin. J Atheroscler Thromb. 2017;24:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1.1-4.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. [DOI] [PubMed] [Google Scholar]
- S3.1.1-5.American College of Cardiology. American College of Cardiology LDL-C Manager. Available at: http://tools.acc.org/ldl Accessed: January 8, 2018.
- S3.1.1-6.US Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/ Accessed January 8, 2018.
- S3.1.1-7.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S3.1.1-8.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- S3.1.1-9.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. [DOI] [PubMed] [Google Scholar]
- S3.1.1-10.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. [DOI] [PubMed] [Google Scholar]
- S3.1.1-11.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. [DOI] [PubMed] [Google Scholar]
- S3.1.1-12.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. [DOI] [PubMed] [Google Scholar]
- S3.1.1-13.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [DOI] [PubMed] [Google Scholar]
- S3.1.1-14.Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. [DOI] [PubMed] [Google Scholar]
- S3.1.1-15.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]
- S3.1.1-16.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. [DOI] [PubMed] [Google Scholar]
- S3.1.1-17.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–63. [DOI] [PubMed] [Google Scholar]
- S3.1.1-18.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45. [DOI] [PubMed] [Google Scholar]
- S3.1.1-19.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- S3.1.1-20.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
4. PATIENT MANAGEMENT GROUPS
4.1. Secondary ASCVD Prevention
- S4.1-1.Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. [DOI] [PubMed] [Google Scholar]
- S4.1-2.Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–8. [DOI] [PubMed] [Google Scholar]
- S4.1-3.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-4.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. [DOI] [PubMed] [Google Scholar]
- S4.1-5.Group HPSC. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–54; discussion 53–4. [DOI] [PubMed] [Google Scholar]
- S4.1-6.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- S4.1-7.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. [DOI] [PubMed] [Google Scholar]
- S4.1-8.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. [DOI] [PubMed] [Google Scholar]
- S4.1-9.Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–62. [DOI] [PubMed] [Google Scholar]
- S4.1-10.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [DOI] [PubMed] [Google Scholar]
- S4.1-11.Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–85. [DOI] [PubMed] [Google Scholar]
- S4.1-12.Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44:1772–9. [DOI] [PubMed] [Google Scholar]
- S4.1-13.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–22. [DOI] [PubMed] [Google Scholar]
- S4.1-14.Bohula EA, Morrow DA, Giugliano RP, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–21. [DOI] [PubMed] [Google Scholar]
- S4.1-15.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- S4.1-16.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- S4.1-17.Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633–43. [DOI] [PubMed] [Google Scholar]
- S4.1-18.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- S4.1-19.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018. In press. [DOI] [PubMed] [Google Scholar]
- S4.1-20.Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1144–61. DOI: 10.1161/CIR.0000000000000626. [DOI] [PubMed] [Google Scholar]
- S4.1-21.Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–87. [DOI] [PubMed] [Google Scholar]
- S4.1-22.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–53. [DOI] [PubMed] [Google Scholar]
- S4.1-23.Gandra SR, Villa G, Fonarow GC, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-24.Bellosta S, Corsini A. Statin drug interactions and related adverse reactions. Expert Opin Drug Saf. 2012;11:933–46. [DOI] [PubMed] [Google Scholar]
- S4.1-25.Gnjidic D, Le Couteur DG, Blyth FM, et al. Statin use and clinical outcomes in older men: a prospective population-based study. BMJ Open. 2013;3:e002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-26.Gray SL, Boudreau RM, Newman AB, et al. Angiotensin-converting enzyme inhibitor and statin use and incident mobility limitation in community-dwelling older adults: the Health, Aging and Body Composition study. J Am Geriatr Soc. 2011;59:2226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-27.Ho CK, Walker SW. Statins and their interactions with other lipid-modifying medications: safety issues in the elderly. Ther Adv Drug Saf. 2012;3:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-28.LaCroix AZ, Gray SL, Aragaki A, et al. Statin use and incident frailty in women aged 65 years or older: prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci. 2008;63:369–75. [DOI] [PubMed] [Google Scholar]
- S4.1-29.Pilotto A, Panza F, Copetti M, et al. Statin treatment and mortality in community-dwelling frail older patients with diabetes mellitus: a retrospective observational study. PLoS One. 2015;10:e0130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-30.Qi K, Reeve E, Hilmer SN, et al. Older peoples’ attitudes regarding polypharmacy, statin use and willingness to have statins deprescribed in Australia. Int J Clin Pharm. 2015;37:949–57. [DOI] [PubMed] [Google Scholar]
- S4.1-31.Scott D, Blizzard L, Fell J, et al. Statin therapy, muscle function and falls risk in community-dwelling older adults. Qjm. 2009;102:625–33. [DOI] [PubMed] [Google Scholar]
- S4.1-32.Thai M, Reeve E, Hilmer SN, et al. Prevalence of statin-drug interactions in older people: a systematic review. Eur J Clin Pharmacol. 2016;72:513–21. [DOI] [PubMed] [Google Scholar]
- S4.1-33.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–64. [DOI] [PubMed] [Google Scholar]
- S4.1-34.Houx PJ, Shepherd J, Blauw GJ, et al. Testing cognitive function in elderly populations: the PROSPER study. PROspective Study of Pravastatin in the Elderly at Risk. J Neurol Neurosurg Psychiatry. 2002;73:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-35.Rodriguez F, Maron DJ, Knowles JW, et al. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- S4.1-36.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-37.Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. [DOI] [PubMed] [Google Scholar]
- S4.1-38.Feinstein MJ, Jhund P, Kang J, et al. Do statins reduce the risk of myocardial infarction in patients with heart failure? A pooled individual-level reanalysis of CORONA and GISSI-HF. Eur J Heart Fail. 2015;17:434–41. [DOI] [PubMed] [Google Scholar]
- S4.1-39.Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–50. [DOI] [PubMed] [Google Scholar]
4.2. Severe Hypercholesterolemia (LDL-C ≥190 mg/dL [≥4.9 mmol/L])
- S4.2-1.Besseling J, Hovingh GK, Huijgen R, et al. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol. 2016;68:252–60. [DOI] [PubMed] [Google Scholar]
- S4.2-2.Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-3.Nanchen D, Gencer B, Muller O, et al. Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation. 2016;134:698–709. [DOI] [PubMed] [Google Scholar]
- S4.2-4.Perak AM, Ning H, de Ferranti SD, et al. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia ohenotype. Circulation. 2016;134:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-5.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. [DOI] [PubMed] [Google Scholar]
- S4.2-6.Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-7.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170?000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-8.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- S4.2-9.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- S4.2-10.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. [DOI] [PubMed] [Google Scholar]
- S4.2-11.Huijgen R, Abbink EJ, Bruckert E, et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin Ther. 2010;32:615–25. [DOI] [PubMed] [Google Scholar]
- S4.2-12.Ross S, D’Mello M, Anand SS, et al. Effect of bile acid sequestrants on the risk of cardiovascular events: a Mendelian randomization analysis. Circ Cardiovasc Genet. 2015;8:618–27. [DOI] [PubMed] [Google Scholar]
- S4.2-13.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-14.Perez de Isla L, Alonso R, Mata N, et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation. 2017;135:2133–44. [DOI] [PubMed] [Google Scholar]
- S4.2-15.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40. [DOI] [PubMed] [Google Scholar]
- S4.2-16.Nicholls SJ, Brandrup-Wognsen G, Palmer M, et al. Meta-analysis of comparative efficacy of increasing dose of Atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol. 2010;105:69–76. [DOI] [PubMed] [Google Scholar]
- S4.2-17.Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. 2016;176:1834–42. [DOI] [PubMed] [Google Scholar]
4.3. Diabetes Mellitus in Adults
- S4.3-1.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. [DOI] [PubMed] [Google Scholar]
- S4.3-2.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–16. [DOI] [PubMed] [Google Scholar]
- S4.3-3.de Vries FM, Denig P, Pouwels KB, et al. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: a meta-analysis. Drugs. 2012;72:2365–73. [DOI] [PubMed] [Google Scholar]
- S4.3-4.Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–85. [DOI] [PubMed] [Google Scholar]
- S4.3-5.Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of myocardial infarction in men and women with type 2 diabetes in the UK: a cohort study using the General Practice Research Database. Diabetologia. 2008;51:1639–45. [DOI] [PubMed] [Google Scholar]
- S4.3-6.Rana JS, Liu JY, Moffet HH, et al. Diabetes and prior coronary heart disease are not necessarily risk equivalent for future coronary heart disease events. J Gen Intern Med. 2016;31:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-7.Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151–7. [DOI] [PubMed] [Google Scholar]
- S4.3-8.Soedamah-Muthu SS, Fuller JH, Mulnier HE, et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804. [DOI] [PubMed] [Google Scholar]
- S4.3-9.Wong ND, Glovaci D, Wong K, et al. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9:146–52. [DOI] [PubMed] [Google Scholar]
- S4.3-10.Karmali KN, Goff DC Jr, Ning H, et al. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–68. [DOI] [PubMed] [Google Scholar]
- S4.3-11.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-12.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-13.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S4.3-14.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- S4.3-15.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-16.Brownrigg JR, de Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart. 2014;100:1837–43. [DOI] [PubMed] [Google Scholar]
- S4.3-17.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-18.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-19.Guo VY, Cao B, Wu X, et al. Prospective association between diabetic retinopathy and cardiovascular disease–a systematic review and meta-analysis of cohort studies. J Stroke Cerebrovasc Dis. 2016;25:1688–95. [DOI] [PubMed] [Google Scholar]
- S4.3-20.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4:115–24. [DOI] [PubMed] [Google Scholar]
- S4.3-21.Nezarat N, Budoff MJ, Luo Y, et al. Presence, characteristics, and volumes of coronary plaque determined by computed tomography angiography in young type 2 diabetes mellitus. Am J Cardiol. 2017;119:1566–71. [DOI] [PubMed] [Google Scholar]
- S4.3-22.Ogren M, Hedblad B, Engstrom G, et al. Prevalence and prognostic significance of asymptomatic peripheral arterial disease in 68-year-old men with diabetes. Results from the population study ‘Men born in 1914’ from Malmo, Sweden. Eur J Vasc Endovasc Surg. 2005;29:182–9. [DOI] [PubMed] [Google Scholar]
- S4.3-23.Pambianco G, Costacou T, Ellis D, et al. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–9. [DOI] [PubMed] [Google Scholar]
- S4.3-24.Pang XH, Han J, Ye WL, et al. Lower extremity peripheral arterial disease is an independent predictor of coronary heart disease and stroke risks in patients with type 2 diabetes mellitus in China. Int J Endocrinol. 2017;2017:9620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-25.Svensson MK, Cederholm J, Eliasson B, et al. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. Diab Vasc Dis Res. 2013;10:520–9. [DOI] [PubMed] [Google Scholar]
4.4. Primary Prevention
- S4.4-1.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-2.Avorn J. The psychology of clinical decision making - implications for medication use. N Engl J Med. 2018;378:689–91. [DOI] [PubMed] [Google Scholar]
- S4.4-3.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- S4.4-4.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785–822. [DOI] [PubMed] [Google Scholar]
- S4.4-5.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-6.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- S4.4-7.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-8.US Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans. Available at: https://health.gov/paguidelines/pdf/paguide.pdf Accessed August 24, 2018.
- S4.4-9.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- S4.4-10.US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. December 2015. Available at: http://health.gov/dietaryguide-lines/2015/guidelines/ Accessed August 24, 2018.
- S4.4-11.Lichtenstein AH, Appel LJ, Brands M, et al. Summary of American Heart Association diet and lifestyle recommendations revision 2006 Arterioscler Thromb Vasc Biol. 2006;26:2186–91. [DOI] [PubMed] [Google Scholar]
- S4.4-12.Ginsberg HN, Kris-Etherton P, Dennis B, et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1 Arterioscler Thromb Vasc Biol. 1998;18:441–9. [DOI] [PubMed] [Google Scholar]
- S4.4-13.Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390:2050–62. [DOI] [PubMed] [Google Scholar]
- S4.4-14.Chen M, Li Y, Sun Q, et al. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr. 2016;104:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-15.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–20. [DOI] [PubMed] [Google Scholar]
- S4.4-16.Carr JJ, Jacobs DR Jr, Terry JG, et al. Association of cronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-17.Choosing interventions that are cost effective (WHO-CHOICE): cost-effectiveness thresholds. 2009. World Health Organization; Geneva. Switzerland. [Google Scholar]
- S4.4-18.Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67:114–20. [DOI] [PubMed] [Google Scholar]
- S4.4-19.Warburton DE, Charlesworth S, Ivey A, et al. A systematic review of the evidence for Canada’s physical activity guidelines for adults. Int J Behav Nutr Phys Act. 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-20.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–52. [DOI] [PubMed] [Google Scholar]
- S4.4-21.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.4.1. Evaluation and Risk Assessment
4.4.1.1. Risk-Enhancing Factors
- S4.4.1.1-1.American College of Cardiology. ASCVD Risk Predictor Plus. Available at: http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/ Accessed September 1, 2018.
- S4.4.1.1-2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- S4.4.1.1-3.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
4.4.2. Primary Prevention Adults 40 to 75 Years of Age With LDL-C levels 70 to 189 mg/dL (1.7 to 4.8 mmol/L)
- 4.4.2-1.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170?000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-2.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-3.Cholesterol Treatment Trialists C, Herrington WG, Emberson J, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–39. [DOI] [PubMed] [Google Scholar]
- S4.4.2-4.Chou R, Dana T, Blazina I, et al. Statin use for the prevention of cardiovascular disease in adults: a systematic review for the US Preventive Services Task Force Rockville, MD: U.S. Agency for Health care Research and Quality, 2016. Report No.: 14-05206-EF-2. [PubMed] [Google Scholar]
- S4.4.2-5.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. [DOI] [PubMed] [Google Scholar]
- S4.4.2-6.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S4.4.2-7.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-8.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- S4.4.2-9.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- S4.4.2-10.Karmali KN, Goff DC Jr, Ning H, et al. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–68. [DOI] [PubMed] [Google Scholar]
- S4.4.2-11.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-12.Krumholz HM. Treatment of cholesterol in 2017 JAMA. 2017;318:417–8. [DOI] [PubMed] [Google Scholar]
- S4.4.2-13.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-14.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-15.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage study. J Am Coll Cardiol. 2016;68:881–91. [DOI] [PubMed] [Google Scholar]
- S4.4.2-16.Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64:851–60. [DOI] [PubMed] [Google Scholar]
- S4.4.2-17.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–68. [DOI] [PubMed] [Google Scholar]
- S4.4.2-18.Ridker PM, Mora S, Rose L. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-19.Yano Y, O’Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-20.Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2017;2:1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-21.Sniderman AD, Tsimikas S, Fazio S. The severe hypercholesterolemia phenotype: clinical diagnosis, management, and emerging therapies. J Am Coll Cardiol. 2014;63:1935–47. [DOI] [PubMed] [Google Scholar]
- S4.4.2-22.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–45. [DOI] [PubMed] [Google Scholar]
- S4.4.2-23.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.2-24.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.4.3. Monitoring in Response to LDL-C–Lowering Therapy
- S4.4.3-1.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. [DOI] [PubMed] [Google Scholar]
- S4.4.3-2.Benner JS, Tierce JC, Ballantyne CM, et al. Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy. Pharmacoeconomics. 2004;22 suppl 3:13–23. [DOI] [PubMed] [Google Scholar]
- S4.4.3-3.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. [DOI] [PubMed] [Google Scholar]
- S4.4.3-2.Benner JS, Tierce JC, Ballantyne CM, et al. Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy. Pharmacoeconomics. 2004;22 suppl 3:13–23. [DOI] [PubMed] [Google Scholar]
- S4.4.3-3.Chiavaroli L, Nishi SK, Khan TA, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61:43–53. [DOI] [PubMed] [Google Scholar]
4.4.4. Primary Prevention in Other Age Groups
4.4.4.1. Older Adults
- S4.4.4.1-1.Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152:488–96. w174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.1-2.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S4.4.4.1-3.Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the Contemporary JUPITER and HOPE-3 Randomized Trials. Circulation. 2017;135:1979–81. [DOI] [PubMed] [Google Scholar]
- S4.4.4.1-4.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- S4.4.4.1-5.Orkaby AR, Gaziano JM, Djousse L, et al. Statins for primary prevention of cardiovascular events and mortality in older men. J Am Geriatr Soc. 2017;65:2362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.1-6.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.1-7.Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62:2090–9. [DOI] [PubMed] [Google Scholar]
- S4.4.4.1-8.Teng M, Lin L, Zhao YJ, et al. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta-analysis. Drugs Aging. 2015:32:649–61. [DOI] [PubMed] [Google Scholar]
- S4.4.4.1-9.Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med. 2015:175:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.1-10.Mahabadi AA, Mohlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. J Am Coll Cardiol Img. 2017:10:143–53. [Google Scholar]
- S4.4.4.1-11.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the Biolmage study. J Am Coll Cardiol. 2016:68:881–91. [DOI] [PubMed] [Google Scholar]
4.4.4.2. Children and Adolescents
- S4.4.4.2-1.Iannuzzi A, Licenziati MR, Vacca M, et al. Comparison of two diets of varying glycemic index on carotid subclinical atherosclerosis in obese children. Heart Vessels. 2009;24:419–24. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-2.Murphy EC, Carson L, Neal W, et al. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int J Pediatr Obes. 2009;4:205–14. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-3.Pratt RE, Kavey RE, Quinzi D. Combined dyslipidemia in obese children: response to a focused lifestyle approach. J Clin Lipidol. 2014;8:181–6. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-4.de Ferranti SD, Milliren CE, Denhoff ER, et al. Providing food to treat adolescents at risk for cardiovascular disease. Obesity (Silver Spring). 2015;23:2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-5.Niinikoski H, Lagstrom H, Jokinen E, et al. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation. 2007; 116:1032–40. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-6.Obarzanek E, Kimm SY, Barton BA, et al. Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the Dietary Intervention Study in Children (DISC). Pediatrics. 2001;107:256–64. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-7.Dorgan JF, Liu L, Barton BA, et al. Adolescent diet and metabolic syndrome in young women: results of the Dietary Intervention Study in Children (DISC) follow-up study. J Clin Endocrinol Metab. 2011;96:E 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-8.Wong H, Chahal N, Manlhiot C, et al. Flaxseed in pediatric hyperlipidemia: a placebo-controlled, blinded, randomized clinical trial of dietary flaxseed supplementation for children and adolescents with hypercholesterolemia. JAMA Pediatr. 2013;167:708–13. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-9.Zachariah JP, Chan J, Mendelson MM, et al. Adolescent dyslipidemia and standardized lifestyle modification: benchmarking real-world practice. J Am Coll Cardiol. 2016;68:2122–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-10.Torvik K, Narverud I, Ottestad I, et al. Dietary counseling is associated with an improved lipid profile in children with familial hypercholesterolemia. Atherosclerosis. 2016;252:21–7. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-11.Koletzko B, Kupke I, Wendel U. Treatment of hypercholesterolemia in children and adolescents. Acta Paediatr. 1992;81:682–5. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-12.Tershakovec AM, Shannon BM, Achterberg CL, et al. One-year follow-up of nutrition education for hypercholesterolemic children. Am J Public Health. 1998;88:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-13.Kusters DM, Caceres M, Coll M, et al. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J Pediatr. 2015;166:1377–84. e1–3. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-14.Yeste D, Chacon P, Clemente M, et al. Ezetimibe as monotherapy in the treatment of hypercholesterolemia in children and adolescents. J Pediatr Endocrinol Metab. 2009;22:487–92. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-15.Clauss S, Wai KM, Kavey RE, et al. Ezetimibe treatment of pediatric patients with hypercholesterolemia. J Pediatr. 2009; 1 54:869–72. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-16.Sonnett T, Robinson J, Milani P, et al. Role of colesevelam in managing heterozygous familial hypercholesterolemia in adolescents and children. Adolesc Health Med Ther. 2010; 1:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-17.Wald DS, Kasturiratne A, Godoy A, et al. Child-parent screening for familial hypercholesterolemia. J Pediatr. 2011; 159:865–7. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-18.Wald DS, Bestwick JP, Morris JK, et al. Child-parent familial hypercholesterolemia screening in primary care. N Engl J Med. 2016;375:1628–37. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-19.Ritchie SK, Murphy EC, Ice C, et al. Universal versus targeted blood cholesterol screening among youth: the CARDIAC project. Pediatrics. 2010;126:260–5. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-20.Skovby F, Micic S, Jepsen B, et al. Screening for familial hypercholesterolaemia by measurement of apolipoproteins in capillary blood. Arch Dis Child. 1991;66:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-21.Garcia RE, Moodie DS. Routine cholesterol surveillance in childhood. Pediatrics. 1989;84:751–5. [PubMed] [Google Scholar]
- S4.4.4.2-22.Ned RM, Sijbrands EJ. Cascade screening for familial hypercholesterolemia (FH). PLoS Curr. 2011;3:RRN1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-23.Bender R, Bell DA, Elooper AJ, et al. Screening for familial hypercholesterolaemia. Pathology. 2012;44:122–8. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-24.National Institute for Health and Care Excellence (NICE) (UK). Familial hypercholesterolaemia: identification and management. Available at: http://www.nice.org.uk/guidance/CG71 Accessed AugUst 24, 2018. [PubMed]
- S4.4.4.2-25.Kit BK, Kuklina E, Carroll MD, et al. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4.4.2-26.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–41. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-27.Lozano P, Elenrikson NB, Morrison CC, et al. Lipid screening in childhood and adolescence for detection of multifactorial dyslipidemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316: 634–14. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-28.Gidding SS, Champagne MA, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–92. [DOI] [PubMed] [Google Scholar]
- S4.4.4.2-29.Lozano P, Henrikson NB, Dunn J, et al. Lipid screening in childhood and adolescence for detection of familial hypercholesterolemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:645–55. [DOI] [PubMed] [Google Scholar]
4.5. Other Populations at Risk
4.5.1. Ethnicity
- S4.5.1-1.Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- S4.5.1-2.Gujral UP, Vittinghoff E, Mongraw-Chaffin M, et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med. 2017;166:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-3.Rao G, Powell-Wiley TM, Ancheta I, et al. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation. 2015;132:457–72. [DOI] [PubMed] [Google Scholar]
- S4.5.1-4.Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77:1923–32. [DOI] [PubMed] [Google Scholar]
- S4.5.1-5.Group H-TC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-6.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-7.Daviglus ML, Pirzada A, Talavera GA. Cardiovascular disease risk factors in the Hispanic/Latino population: lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis. 2014;57:230–6. [DOI] [PubMed] [Google Scholar]
- S4.5.1-8.Daviglus ML, Pirzada A, Durazo-Arvizu R, et al. Prevalence of low cardiovascular risk profile among diverse Hispanic/Latino adults in the United States by age, sex, and level of acculturation: the Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc. 2016;5:e003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-9.Gonzalez HM, Tarraf W, Rodriguez CJ, et al. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J. 2016;176:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-10.Qureshi WF, Kaplan RC, Swett K, et al. American College of Cardiology/American Heart Association (ACC/AHA) Class I guidelines for the treatment of cholesterol to reduce atherosclerotic cardiovascular risk: implications for US Hispanics/Latinos based on findings from the Hispanic Community Health Study/Study of Latios (HCHS/SOL). J Am Heart Assoc. 2017; 6: e005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-11.Schneiderman N, Chirinos DA, Aviles-Santa ML, et al. Challenges in preventing heart disease in hispanics: early lessons learned from the Hispanic Community Health Study/Study of Latinos (HCHS/ SOL). Prog Cardiovasc Dis. 2014;57:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-12.Hutchinson RN, Shin S. Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska Native populations. PLoS One. 2014;9: e80973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-13.Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018; 138:e1–34. [DOI] [PubMed] [Google Scholar]
- S4.5.1-14.Conomos MP, Laurie CA, Stilp AM, et al. Genetic diversity and association studies in US Hispanic/Latino populations: applications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet. 2016;98:165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-15.Frank AT, Zhao B, Jose PO, et al. Racial/ethnic differences in dyslipidemia patterns. Circulation. 2014;129:570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-16.Pu J, Romanelli R, Zhao B, et al. Dyslipidemia in special ethnic populations. Cardiol Clin. 2015;33:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-17.Basu S, Hong A, Siddiqi A. Using decomposition analysis to identify modifiable racial disparities in the distribution of blood pressure in the United States. Am J Epidemiol. 2015;182:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-18.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the U 9. J Diabetes. 2010;2:180–9S4.5.1–19. 3. [DOI] [PubMed] [Google Scholar]
- S4.5.1-19.Gujral UP, Pradeepa R, Weber MB, et al. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013; 1281:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-20.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-21.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- S4.5.1-22.DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J. 2017;38:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-23.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- S4.5.1-24.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-25.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67: 2118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-26.Kandula NR, Kanaya AM, Liu K, et al. Association of 10-year and lifetime predicted cardiovascular disease risk with subclinical atherosclerosis in South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. J Am Heart Assoc. 2014;3:e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-27.Alluri K, McEvoy JW, Dardari ZA, et al. Distribution and burden of newly detected coronary artery calcium: results from the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Com put Tomogr. 2015:9337–344 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-28.Cho YK, Jung CH, Kang YM, et al. 2013 ACC/AHA cholesterol guideline versus 2004 NCEP ATP III guideline in the prediction of coronary artery calcification progression in a Korean population. J Am Heart Assoc. 2016;5:e003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-29.Manolio TA, Arnold AM, Post W, et al. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;197:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-30.Osawa K, Nakanishi R, Budoff M. Coronary artery calcification. Glob Heart. 2016;11:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-31.Kanaya AM, Kandula NR, Ewing SK, et al. Comparing coronary artery calcium among US South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis. 2014;234:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-32.Greenland P, Blaha MJ, Budoff MJ, et al. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-33.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–63. [DOI] [PubMed] [Google Scholar]
- S4.5.1-34.Kimura T, Inoue T, Taguchi I, et al. Does high-intensity pitavastatin therapy further improve clinical outcomes? The REAL-CAD study in 13054 patients with stable coronary artery disease. Circulation. 2017;136:e450. [Google Scholar]
- S4.5.1-35.Birmingham BK, Bujac SR, Elsby R, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71:329–40. [DOI] [PubMed] [Google Scholar]
- S4.5.1-36.Lee E, Ryan S, Birmingham B, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330–41. [DOI] [PubMed] [Google Scholar]
- S4.5.1-37.Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.1-38.Lloret R, Yeas J, Stein M, et al. Comparison of rosuvastatin versus atorvastatin in Hispanic-Americans with hypercholesterolemia (from the STARSHIP trial). Am J Cardiol. 2006;98:768–73. [DOI] [PubMed] [Google Scholar]
- S4.5.1-39.George MD, McGill NK, Baker JF. Creatine kinase in the US population: Impact of demographics, comorbidities, and body composition on the normal range. Medicine (Baltimore). 2016;95:e4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.5.2. Hypertriglyceridemia
- S4.5.2-1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- S4.5.2-2.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9. [PubMed] [Google Scholar]
- S4.5.2-3.Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- S4.5.2-4.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–52. [DOI] [PubMed] [Google Scholar]
- S4.5.2-5.Karlson BW, Palmer MK, Nicholls SJ, et al. A VOYAGER meta-analysis of the impact of statin therapy on low-density lipoprotein cholesterol and triglyceride levels in patients with hypertriglyceridemia. Am J Cardiol. 2016;117:1444–8. [DOI] [PubMed] [Google Scholar]
- S4.5.2-6.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.2-7.Christian JB, Arondekar B, Buysman EK, et al. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6:450–61. [DOI] [PubMed] [Google Scholar]
- S4.5.2-8.Duane WC, Hunninghake DB, Freeman ML, et al. Simvastatin, a competitive inhibitor of HMG-CoA reductase, lowers cholesterol saturation index of gallbladder bile. Hepatology. 1988;8:1147–50. [DOI] [PubMed] [Google Scholar]
- S4.5.2-9.Rhodes KS, Weintraub M, Marchlewicz EH, et al. Medical nutrition therapy is the essential cornerstone for effective treatment of ‘refractory’ severe hypertriglyceridemia regardless of pharmaceutical treatment: Evidence from a Lipid Management Program. J Clin Lipidol. 2015;9:559–67. [DOI] [PubMed] [Google Scholar]
4.5.3. Issues Specific to Women
- S4.5.3-1.Ouyang P, Wenger NK, Taylor D, et al. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.3-2.Grandi SM, Vallee-Pouliot K, Reynier P, et al. Hypertensive disorders in pregnancy and the risk of subsequent cardiovascular disease. Paediatr Perinat Epidemiol. 2017;31:412–21. [DOI] [PubMed] [Google Scholar]
- S4.5.3-3.Shostrom DCV, Sun Y, Oleson JJ, et al. History of gestational diabetes mellitus in relation to cardiovascular disease and cardiovascular risk factors in US women. Front Endocrinol (Lausanne). 2017;8:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.3-4.Catov JM, Newman AB, Roberts JM, et al. Preterm delivery and later maternal cardiovascular disease risk. Epidemiology. 2007;18:733–9. [DOI] [PubMed] [Google Scholar]
- S4.5.3-5.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–76. [DOI] [PubMed] [Google Scholar]
- S4.5.3-6.Roeters van Lennep JE, Heida KY, Bots ML, et al. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:178–86. [DOI] [PubMed] [Google Scholar]
- S4.5.3-7.Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med. 2004;350:1579–82. [DOI] [PubMed] [Google Scholar]
- S4.5.3-8.Ofori B, Rey E, Berard A. Risk of congenital anomalies in pregnant users of statin drugs. Br J Clin Pharmacol. 2007;64:496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.3-9.Taguchi N, Rubin ET, Hosokawa A, et al. Prenatal exposure to HMG-CoA reductase inhibitors: effects on fetal and neonatal outcomes. Reprod Toxicol. 2008;26:175–7. [DOI] [PubMed] [Google Scholar]
- S4.5.3-10.Winterfeld U, Allignol A, Panchaud A, et al. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG. 2013;120:463–71. [DOI] [PubMed] [Google Scholar]
- S4.5.3-11.Zarek J, Koren G. The fetal safety of statins: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2014;36:506–9. [DOI] [PubMed] [Google Scholar]
- S4.5.3-12.McGrogan A, Snowball J, Charlton RA. Statins during pregnancy: a cohort study using the General Practice Research Database to investigate pregnancy loss. Pharmacoepidemiol Drug Saf. 2017;26:843–52. [DOI] [PubMed] [Google Scholar]
4.5.4 Adults With CKD
- S4.5.4-1.Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington W, Emberson J, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–39. [DOI] [PubMed] [Google Scholar]
- S4.5.4-2.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.4-3.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407. [DOI] [PubMed] [Google Scholar]
- S4.5.4-4.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. [DOI] [PubMed] [Google Scholar]
4.5.5. Adults With Chronic Inflammatory Disorders and HIV
- S4.5.5-1.Mantel A, Holmqvist M, Jernberg T, et al. Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur Heart J. 2015;36:3413–22. [DOI] [PubMed] [Google Scholar]
- S4.5.5-2.Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-3.Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. 2011;70: 929–34. [DOI] [PubMed] [Google Scholar]
- S4.5.5-4.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- S4.5.5-5.Wajed J, Ahmad Y, Durrington PN, et al. Prevention of cardiovascular disease in systemic lupus erythematosus–proposed guidelines for risk factor management. Rheumatology (Oxford). 2004;43:7–12. [DOI] [PubMed] [Google Scholar]
- S4.5.5-6.Westerweel PE, Luyten RK, Koomans HA, et al. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1384–96. [DOI] [PubMed] [Google Scholar]
- S4.5.5-7.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-8.Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012 Clin Infect Dis. 2016;63:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-9.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-10.Kearns A, Gordon J, Burdo TH, et al. HIV-1-associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol. 2017;69:3084–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-11.Dregan A, Chowienczyk P, Molokhia M. Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders. Heart. 2017;103:1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-12.Fernandez-Montero JV, Barreiro P, de Mendoza C, et al. Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat. 2016;23:47–52. [DOI] [PubMed] [Google Scholar]
- S4.5.5-13.Bartels CM, Kind AJ, Everett C, et al. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-14.Toms TE, Panoulas VF, Douglas KM, et al. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann Rheum Dis. 2010;69:683–8. [DOI] [PubMed] [Google Scholar]
- S4.5.5-15.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
- S4.5.5-16.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-17.Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74: 668–74. [DOI] [PubMed] [Google Scholar]
- S4.5.5-18.Mulligan K, Grunfeld C, Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. [DOI] [PubMed] [Google Scholar]
- S4.5.5-19.Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45:111–19. [DOI] [PubMed] [Google Scholar]
- S4.5.5-20.Davis JM 3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56:820–30. [DOI] [PubMed] [Google Scholar]
- S4.5.5-21.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5.5-22.Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–15. [DOI] [PubMed] [Google Scholar]
- S4.5.5-23.Navarro-Millan I, Charles-Schoeman C, Yang S, et al. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum. 2013;65:1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
5. STATIN SAFETY AND STATIN-ASSOCIATED SIDE EFFECTS
- S5-1.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-2.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-3.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-4.Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473–81. [DOI] [PubMed] [Google Scholar]
- S5-5.Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315:1580–90. [DOI] [PubMed] [Google Scholar]
- S5-6.Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–69. [DOI] [PubMed] [Google Scholar]
- S5-7.Joy TR, Monjed A, Zou GY, et al. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med. 2014;160:301–10. [DOI] [PubMed] [Google Scholar]
- S5-8.Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-9.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. [DOI] [PubMed] [Google Scholar]
- S5-10.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. [DOI] [PubMed] [Google Scholar]
- S5-11.Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–30. [DOI] [PubMed] [Google Scholar]
- S5-12.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-13.Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-14.Thompson PD, Panza G, Zaleski A, et al. Statin-associated side effects. J Am Coll Cardiol. 2016;67:2395–410. [DOI] [PubMed] [Google Scholar]
- S5-15.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- S5-16.Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–22. [DOI] [PubMed] [Google Scholar]
- S5-17.Foster T, Budoff MJ, Saab S, et al. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–7. [DOI] [PubMed] [Google Scholar]
- S5-18.Tikkanen MJ, Fayyad R, Faergeman O, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168:3846–52. [DOI] [PubMed] [Google Scholar]
- S5-19.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- S5-20.Taylor BA, Lorson L, White CM, et al. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015;238:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-21.Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24–34. [DOI] [PubMed] [Google Scholar]
- S5-22.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170?000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-23.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. [DOI] [PubMed] [Google Scholar]
- S5-24.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- S5-25.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. [DOI] [PubMed] [Google Scholar]
- S5-26.Cohen JD, Brinton EA, Ito MK, et al. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10?138 current and former statin users. J Clin Lipidol. 2012;6:208–15. [DOI] [PubMed] [Google Scholar]
- S5-27.Serban MC, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–95. [DOI] [PubMed] [Google Scholar]
- S5-28.Zhang H, Plutzky J, Shubina M, et al. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med. 2017;167:221–7. [DOI] [PubMed] [Google Scholar]
- S5-29.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
6. IMPLEMENTATION
- S6-1.Brown BG, Bardsley J, Poulin D, et al. Moderate dose, three-drug therapy with niacin, lovastatin, and colestipol to reduce low-density lipoprotein cholesterol <100 mg/dl in patients with hyperlipidemia and coronary artery disease. Am J Cardiol. 1997;80:111–5. [DOI] [PubMed] [Google Scholar]
- S6-2.Tamblyn R, Reidel K, Huang A, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Med Decis Making. 2010;30:176–88. [DOI] [PubMed] [Google Scholar]
- S6-3.Thom S, Poulter N, Field J, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918–29. [DOI] [PubMed] [Google Scholar]
- S6-4.van Driel ML, Morledge MD, Ulep R, et al. Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev. 2016;12:CD004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S6-5.Chan WV, Pearson TA, Bennett GC, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI Implementation Science Work Group: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e122–37 [DOI] [PubMed] [Google Scholar]
- S6-6.Fischer F, Lange K, Klose K, et al. Barriers and strategies in guideline implementation-a scoping review. Healthcare (Basel). 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S6-7.Stacey D, Hill S, McCaffery K, et al. Shared decision making interventions: theoretical and empirical evidence with implications for health literacy. Stud Health Technol Inform. 2017;240:263–83. [PubMed] [Google Scholar]
- S6-8.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
7. COST AND VALUE CONSIDERATIONS
7.1. Economic Value Considerations: PCSK9 Inhibitors
- S7.1-1.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–45. [DOI] [PubMed] [Google Scholar]
- S7.1-2.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- S7.1-3.Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–87. [DOI] [PubMed] [Google Scholar]
- S7.1-4.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90?056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- S7.1-5.Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S7.1-6.Robinson JG, Huijgen R, Ray K, et al. Determining when to add nonstatin therapy: a quantitative approach. J Am Coll Cardiol. 2016;68:2412–21. [DOI] [PubMed] [Google Scholar]
- S7.1-7.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–53. [DOI] [PubMed] [Google Scholar]
- S7.1-8.Gandra SR, Villa G, Fonarow GC, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S7.1-9.Choosing interventions that are cost effective (WHO-CHOICE): cost-effectiveness thresholds. Geneva, Switzerland: World Health Organization, 2009. [Google Scholar]
8. LIMITATIONS AND KNOWLEDGE GAPS
8.2. Risk Assessment
- S8.2-1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.