Abstract
Introduction
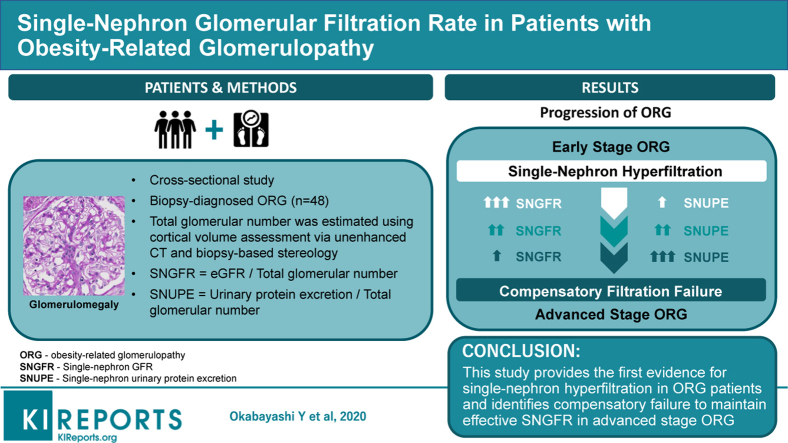
Obesity-related glomerulopathy (ORG) is a slowly progressive kidney disease occurring in association with obesity. It is characterized histopathologically by glomerulomegaly, likely caused by single-nephron hyperfiltration that has not been demonstrated in humans because of technical difficulty in measuring single-nephron glomerular filtration rate (SNGFR) in the clinical setting.
Methods
Total glomerular number per kidney, with or without global glomerulosclerosis, was estimated by the combination of cortical volume assessment via unenhanced computed tomography and biopsy-based stereology. Mean glomerular volume was calculated from the measured area of glomerular tufts. Both SNGFR and single-nephron urinary protein excretion (SNUPE) were estimated by dividing values for estimated glomerular filtration rate and urinary protein excretion by the number of nonsclerotic glomeruli. Living kidney donors were used as healthy controls.
Results
A total of 48 ORG patients with average nonsclerotic glomerular numbers of 456,000 ± 235,000 per kidney were included. The values for SNGFR in ORG patients with chronic kidney disease (CKD) stages 1 and 2 were higher than for nonobese and obese controls (97 ± 43 vs. 59 ± 21 vs. 64 ± 21 nl/min, respectively, P = 0.001). Nonsclerotic glomerular number decreased with advancing stages of renal functional impairment. The presence of ORG with more advanced CKD stages was associated with lower SNGFR and marked elevation in SNUPE levels, with no difference in the mean glomerular volume between the stages.
Conclusions
These results provide functional evidence for single-nephron hyperfiltration in patients with ORG, and identify compensatory failure to maintain effective SNGFR as a feature of advanced-stage ORG.
Keywords: hyperfiltration, nephron number, obesity, proteinuria, renal biopsy
Graphical abstract
See Commentary on Page 1126
Obesity-related complications have emerged as increasing socioeconomic problems alongside the worldwide epidemic of obesity.1, 2, 3 The kidney is 1 of the target organs for obesity-related health disorders, and obesity is an independent risk factor for the development of kidney disease.4,5 A well-characterized renal complication of obesity is obesity-related glomerulopathy (ORG).6, 7, 8, 9, 10 Since the first autopsy cases were reported in 1974, much knowledge has been accumulated about the clinicopathological characteristics of ORG. The condition ORG is defined as a proteinuric glomerulopathy with glomerulomegaly in obese patients, in the absence of clinical and histopathological evidence of other renal diseases.6, 7, 8, 9, 10 Previous interventional studies examining renal physiological parameters before and after bariatric surgery have shown that obesity is closely associated with increased total kidney glomerular filtration rate (GFR).11,12 Histopathologically, glomerulomegaly and focal segmental glomerulosclerosis (FSGS) found in ORG are very likely caused by single-nephron hyperfiltration following abnormally increased renal plasma flow and filtration fraction.7,11,12 However, abnormalities in single-nephron GFR (SNGFR) in ORG have not been demonstrated because of technical difficulties in measuring SNGFR in the clinical setting.
Recent studies have developed a method to estimate total glomerular number per kidney in living kidney donors by the combination of computed tomographic angiography and biopsy-based stereology.13,14 By dividing the total nephron GFR by the total number of nonsclerotic (functioning) glomeruli in both kidneys, the method showed that the mean values for SNGFR were stable during age-related loss of functioning nephrons, and identified several plausible factors influencing SNGFR in healthy subjects, such as height of more than 190 cm, obesity, and a family history of end-stage renal disease.15 Recently, we have further modified the method and established a regression equation model to estimate kidney cortical volume by measuring kidney parenchymal volume using unenhanced CT images in humans.16 Evaluation of cortical volume by this method allows estimation of total glomerular number per kidney in patients with renal diseases who are often not suitable candidates for image analyses using contrast media. This study applied the combined methods of unenhanced computed tomography (CT) and biopsy-based stereology to estimate total glomerular number and SNGFR in patients with ORG.
Methods
Patients
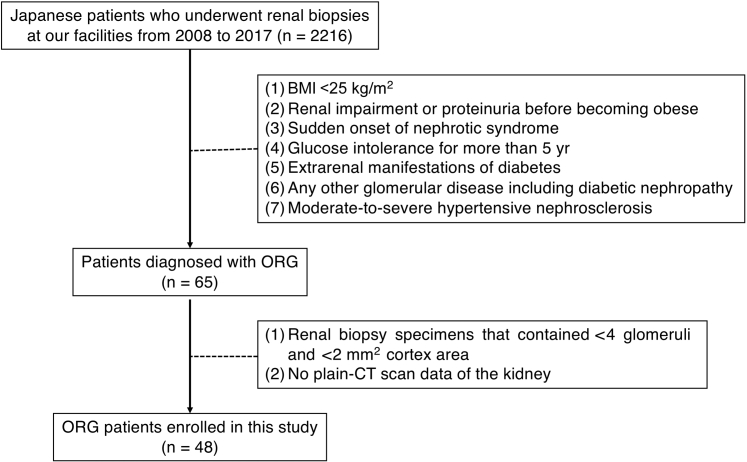
The study cohort included obese adult Japanese patients with native kidney biopsy diagnosis of ORG at Jikei Hospital, Tokyo, and Jikei Kashiwa Hospital, Chiba, from 2008 to 2017. Indications for biopsy were renal impairment (eGFR <60 ml/min) and/or overt proteinuria (>0.5 g/d). Patients were recruited according to exclusion criteria as previously described.17 Briefly, patients with renal dysfunction or urinalysis abnormalities prior to becoming obese and those who exhibited acute-onset nephrotic syndrome, which is an atypical presentation for ORG, were excluded. Patients with impaired glucose tolerance alone, as defined as an HbA1c of 6.5% or greater (National Glycohemoglobin Standardization Program [NGSP]) at the time of biopsy diagnosis, were not excluded. However, patients were excluded if they had a history of impaired glucose tolerance for more than 5 years or any extra-renal complications of diabetes. Renal histological features of ORG were defined as glomerulomegaly with or without FSGS lesions.18 Patients were excluded if they showed typical clinical and histological findings of other primary or secondary kidney diseases including diabetic nephropathy. This study was approved by the ethics review board of Jikei University School of Medicine (30-385 9406) and conducted according to the Declaration of Helsinki.
Definitions
Obesity in Japanese subjects was defined as body mass index (BMI) greater than 25 kg/m2.19 Body surface area (BSA) was determined by the equation as follows: BSA (m2) = weight0.425 (kg) × height0.725 (cm) × 71.84 × 10–4.20 Patients were recruited retrospectively from renal biopsy archives during the study period (Figure 1). Renal biopsy specimens of kidney transplant donors with or without obesity and showing no evidence of kidney disease were used as control groups. Urinary protein excretion (UPE) was determined as total amount of UPE per day by 24-hour urine collection. The 24-hour creatinine clearance rate (CCr) was calculated using measured serum creatinine concentration and urine creatinine concentration in the urine collection. The estimated GFR (eGFR) was calculated using a modified 3-variable equation for the GFR of Japanese individuals21: eGFR = 194 × age−0.287 × sCr−1.094 (×0.739 if female), where sCr is the serum creatinine level. Values for CCr and eGFR were calculated without adjustment for BSA. Chronic kidney disease (CKD) stages were defined as follows: G1, G2, G3a, G3b, G4, and G5 for eGFR ≥90, 60–89, 45–59, 30–44, 15–29 and <15 ml/min per 1.73 m2, respectively. Hypertension was defined as a systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg, or use of antihypertensive drugs. Subjects using antihypertensive medications, such as renin−angiotensin−aldosterone system (RAAS) inhibitors, for the purpose of renoprotection despite a normal blood pressure, were considered to be normotensive. The SNGFR and single-nephron UPE (SNUPE) were calculated by dividing per day values for eGFR and UPE by total number of glomeruli excluding globally sclerotic glomeruli in both kidneys.
Figure 1.
Patient selection. A total of 2216 patients underwent renal biopsy at our facility from 2008 to 2017. Of these patients, 65 were diagnosed with obesity-related glomerulopathy (ORG), and 17 were excluded from this study due to lack of computed tomographic data or inadequate glomerular number or cortical area in biopsy specimens. BMI, body mass index.
Pathological Analysis
All kidney specimens included in this study were obtained by 18-gauge needle biopsy. Implantation biopsies of 1 hour after recirculation were used for the analyses of kidney transplant donors. Kidney tissues were embedded in paraffin, cut into 3-μm sections, and stained with hematoxylin–eosin, periodic acid−Schiff, Masson’s trichrome, and periodic acid–methenamine silver. All biopsy specimens were subjected to immunohistochemical staining with the routine panel of antibodies, including IgG, IgA, IgM, C3, and C1q, and electron microscopy studies. The area of interstitial fibrosis/tubular atrophy was semiquantitatively evaluated and scored as the percentage of total cortex involved, with 1% to 5% rounded to 5%, and other values rounded to the closest 10%. The average values for interstitial fibrosis/tubular atrophy were estimated across the entirety of each biopsy specimen. The percentages of all glomeruli in the biopsy specimen with segmental glomerulosclerosis and with global glomerulosclerosis were calculated. The histologic subtype of FSGS was determined for each glomerulus including tip, collapsing, cellular, perihilar, and not otherwise specified (NOS) lesions.18
Morphological Measurements
Computed tomographic images of kidneys were acquired using the Aquilion Prime (Toshiba Medical Systems, Otawara, Japan), the Definition AS+ (Siemens, Munich, Germany) or the Definition flash (Siemens). The thickness of the obtained image was in the range of 3.0 to 5.0 mm. The parenchymal volume of kidneys was measured by the unenhanced CT images as previously described using software (ITK-SNAP version 1.1; University of Pennsylvania, Philadelphia, PA; www.itksnap.org).14 The cortical volume of kidneys was created by the estimation equation models as follows: estimated cortical volume (cm3) = −1.3 (intercept) + 0.71 × parenchymal volume (cm3).22
The nonsclerotic nephron number was estimated as the number of non−globally sclerotic glomeruli, including glomeruli with segmentally sclerotic lesions, according to the method established by Denic et al.13 The number of nonsclerotic glomeruli per kidney was calculated by multiplying the total cortical volume (mm3) by the numerical density of nonsclerotic glomeruli (number per cubic millimeter [mm3] of cortex). These values were divided by 2 (per kidney), by 1.43 to correct for tissue volume shrinkage due to paraffin embedding, and by 1.268 to correct for volume shrinkage due to loss of tissue perfusion pressure.23,24
Computerized image analyzer (Aperio Image Scope 12.4, Leica Microsystems, Wetzlar, Germany) was used to measure the areas of all glomerular capillary tufts and the area of obtained renal cortex. The area of the outer capillary loops including podocytes was defined as glomerular tuft area. (Supplementary Figure S1A). Glomeruli with global glomerulosclerosis were excluded from the measurements of tuft area, whereas glomeruli with segmental glomerulosclerosis were included (Supplementary Figure S1B). Mean glomerular capillary area (GA) was calculated by averaging all the measured areas of glomerular capillary loops.22,25 The mean glomerular volume (GV) was calculated from the measured GA: GV = (GA)3/2 × β/d, where β is a dimensionless shape coefficient (β = 1.38 for spheres) and d is a size distribution coefficient (d = 1.01) used to adjust for variation in glomerular size.23,26 The nonsclerotic glomerular density (per cubic millimeter of cortex) was calculated using the stereological method of Weibel−Gomez as follows26:NSG density = , where β is a dimensionless shape coefficient (β = 1.382 for spheres). The sclerotic glomerular density (per cubic millimeter of cortex) was identically calculated: sclerotic glomerular density = . The total number of nephrons was estimated based on glomerular density (per cubic millimeter of cortex) as the sum of non−globally sclerotic glomerular density and globally sclerotic glomerular density.
Statistical Analysis
Continuous variables were expressed as mean ± SD. Continuous variables were compared by the Mann–Whitney U test to compare variables between 2 groups. Categorical variables were expressed as percentages and compared by the χ2 test. The Kruskal–Wallis test and the Jonckheere–Terpstra test were used to compare variables among 3 or more groups, as appropriate. The Dunn–Bonferroni test was used as a post hoc analysis. Values of P < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software package version 25.0 (IBM Corp., Armonk, NY).
Results
Clinical Characteristics at the Time of Biopsy Diagnosis
The clinicopathological characteristics of the patients at the time of renal biopsy are shown in Table 1. All ORG patients included in this cohort were Japanese, with mean age of 48.6 ± 11.5 years, and 61% were male. Of the 48 ORG patients, 37 (77%) were diagnosed as hypertensive at the time of biopsy. By histopathological analysis, 20 ORG patients (42%) had FSGS lesions, and 31 of 931 glomeruli from 48 patients showed FSGS lesions, 13 (42%) of which were perihilar and 18 (58%) of which were NOS lesions. No cellular, collapsing, or tip lesions of FSGS were identified in this cohort. The values for mean glomerular volume that included the glomeruli with segmental sclerosis in the calculation were not significantly different from those that excluded the glomeruli with segmental sclerosis (P = 0.204). Among these 48 Japanese ORG patients, nonsclerotic nephron number ranged from 149,000 to 1,055,000 per kidney. The mean nonsclerotic glomerular number and mean glomerular number including globally sclerotic glomeruli in ORG patients were 456,000 ± 235,000 and 676,000 ± 272,000 per kidney, respectively.
Table 1.
Clinical characteristics at the time of biopsy diagnosis in ORG patients
| Variable | All patients (n = 48) |
|---|---|
| Clinical findings | |
| Age, yr | 48.6 ± 11.5 |
| Sex, male, % (n) | 60.4 (29) |
| BMI, kg/m2 | 29.5 ± 3.4 |
| BSA, m2 | 1.88 ± 0.19 |
| Hypertension, % (n) | 77.1 (37) |
| Serum creatinine, mg/dl | 1.16 ± 0.70 |
| eGFR, ml/min | 68 ± 32 |
| 24-h CCr, ml/min | 102 ± 50 |
| UPE, mg/d | 1347 ± 1498 |
| Serum albumin, g/dl | 4.0 ± 0.4 |
| Serum uric acid, mg/dl | 7.0 ± 1.4 |
| Triglycerides, mg/dl | 280 ± 253 |
| RAAS inhibitors, % (n) | 62.5 (30) |
| Histopathological findings | |
| Total number of glomeruli per biopsy specimen | 19.1 ± 10.4 |
| Number of nonsclerotic glomeruli per biopsy specimen | 14.6 ± 9.7 |
| Glomeruli affected by global glomerulosclerosis, % | 23.8 ± 19.4 |
| Patients with segmental glomerulosclerosis, % (n) | 41.7 (20) |
| Glomeruli affected by segmental glomerulosclerosis, % | 4.0 ± 7.2 |
| FSGS variant, % | |
| Glomeruli with perihilar lesion | 1.7 ± 4.0 |
| Glomeruli with NOS lesion | 2.3 ± 5.7 |
| Glomeruli with cellular lesion | 0 |
| Glomeruli with collapsing lesion | 0 |
| Glomeruli with tip lesion | 0 |
| Interstitial fibrosis/ tubular atrophy, % | 17.1 ± 19.9 |
| Arteriosclerotic lesions, %, grade 0/1/2a | 28/40/32 |
| Arteriolar hyalinosis, %, grade 0/1/2b | 25/46/29 |
| Renal morphological parameters | |
| Renal parenchymal volume, cm3/kidney | 154 ± 44 |
| Renal cortical volume, cm3/kidney | 110 ± 32 |
| Mean glomerular volume, ×106 μm3 | |
| Including glomeruli with segmental glomerulosclerosis | 5.01 ± 2.47 |
| Excluding glomeruli with segmental glomerulosclerosis | 5.05 ± 2.50 |
| Mean areal glomerular density, /mm2 | 1.54 ± 0.65 |
| Mean volumetric glomerular density, /mm3 | 7.73 ± 3.81 |
| Total nonsclerotic glomerular number, ×106/kidney | 0.456 ± 0.235 |
| Total globally sclerotic glomerular number, ×106/kidney | 0.220 ± 0.198 |
| Single-nephron eGFR, nl/min | 85 ± 42 |
| Single-nephron UPE, μg/day | 2.36 ± 3.60 |
BMI, body mass index; BSA, body surface area; CCr, creatinine clearance rate; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; NOS, not otherwise specified; ORG, obesity-related glomerulopathy; RAAS, renin−angiotensin−aldosterone system; UPE, urinary protein excretion.
Severity of arterial lesions with no intimal thickening was defined as grade 0, those with ≤50% intimal thickening as grade 1, and those with >50% as grade 2.
Grade of arteriolar lesions was semiquantitatively evaluated based on the percentage of arterioles per biopsy specimen exhibiting hyalinosis, as follows: grade 0, <5% lesions; grade 1, 5% to 25% lesions; and grade 2, >25% lesions.
Comparison of Clinicopathological Findings Between ORG Patients With Preserved Renal Function and Kidney Transplant Donors With or Without Obesity
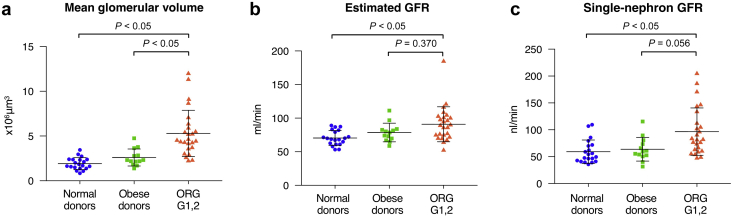
Clinicopathological characteristics were evaluated in the ORG subgroups with preserved renal function of CKD stage 1 or 2 and compared with kidney transplantation donors as healthy controls (Table 2). The measured parenchymal volume and the estimated cortical volume of the kidneys were significantly higher in patients with ORG than in obese and nonobese controls. By contrast, mean areal and volumetric glomerular densities were significantly lower in the ORG patients than in obese and nonobese controls. The degrees of global and segmental glomerulosclerosis were higher in ORG patients with preserved renal function than in obese and nonobese donor controls. The nonsclerotic glomerular number was not statistically different among these 3 study groups. In comparison with those of obese and nonobese donor controls, the globally sclerotic glomerular number was significantly larger in ORG patients with preserved renal function. Total and single-nephron parameters were evaluated among the ORG patients with preserved renal function and compared with living kidney donors (Figure 2). The ORG patients with CKD stage 1 or 2 had significantly higher mean GV than the obese and nonobese donor controls. The ORG patients with CKD stage 1 or 2 had higher eGFR and SNGFR than the nonobese donor controls and showed a trend toward higher eGFR and SNGFR than the obese donor controls.
Table 2.
Comparison of clinicopathological findings between ORG patients with a preserved renal function and kidney transplant donors
| Variable | Nonobese donors (n = 20) | Obese donors (n = 13) | ORG CKD stage 1, 2 (n = 25) |
P valuea |
|---|---|---|---|---|
| Clinical findings | ||||
| Age, yr | 52.8 ± 6.4 | 52.1 ± 8.3 | 45.0 ± 10.7b | 0.010 |
| Sex, male % (n) | 40.0 (8) | 38.5 (5) | 64.0 (16) | 0.178 |
| BMI, kg/m2 | 21.4 ± 1.9 | 27.0 ± 1.8b | 29.4 ± 3.2b | <0.001 |
| BSA, m2 | 1.58 ± 0.15 | 1.74 ± 0.13 | 1.90 ± 0.22b | <0.001 |
| Hypertension, % (n) | 5.0 (1) | 15.4 (2) | 68.0 (17) | <0.001 |
| Serum creatinine, mg/dl | 0.71 ± 0.13 | 0.71 ± 0.09 | 0.77 ± 0.17 | 0.347 |
| eGFR, ml/min | 70 ± 11 | 79 ± 13 | 91 ± 26b | 0.003 |
| 24-h CCr, ml/min | 108 ± 27 | 109 ± 23 | 136 ± 39b | 0.016 |
| Urinary protein excretion, mg/d | 25 ± 22 | 29 ± 41 | 874 ± 770b,c | <0.001 |
| Serum albumin, g/dl | 4.1 ± 0.3 | 4.4 ± 0.3b | 4.2 ± 0.4 | 0.015 |
| Serum uric acid, mg/dl | 4.6 ± 0.9 | 5.2 ± 0.7 | 6.5 ± 1.5b,c | <0.001 |
| Triglyceride, mg/dl | 206 ± 24 | 144 ± 51b | 291 ± 284 | 0.023 |
| RAAS inhibitors, % (n) | 0 (0) | 7.7 (1) | 60.0 (15) | 0.002 |
| Histopathological findings | ||||
| Total number of glomeruli per biopsy | 6.8 ± 2.4 | 8.2 ± 3.5 | 19.1 ± 10.7b,c | <0.001 |
| Number of nonsclerotic glomeruli per biopsy | 6.6 ± 2.3 | 8.0 ± 3.1 | 16.3 ± 9.9b,c | <0.001 |
| Total cortical area, mm2 | 2.7 ± 0.8 | 3.3 ± 0.7 | 9.5 ± 4.7 | <0.001 |
| Global glomerulosclerosis, % | 3.3 ± 6.3 | 1.6 ± 4.3 | 15.6 ± 14.9b,c | <0.001 |
| Segmental glomerulosclerosis, % | 0 | 0 | 1.8 ± 3.6b,c | 0.006 |
| Interstitial fibrosis/ tubular atrophy, % | 4.8 ± 4.8 | 6.1 ± 4.8 | 7.4 ± 6.8 | 0.459 |
| Renal morphological parameters | ||||
| Renal parenchymal volume, cm3/kidney | 119 ± 23 | 138 ± 22 | 173 ± 48b,c | <0.001 |
| Renal cortical volume, cm3/kidney | 85 ± 17 | 98 ± 17 | 123 ± 34b,c | <0.001 |
| Mean areal glomerular density, /mm2 | 2.50 ± 0.87 | 2.34 ± 0.59 | 1.71 ± 0.63b,c | 0.002 |
| Mean volumetric glomerular density, /mm3 | 14.6 ± 5.4 | 12.4 ± 3.5 | 8.42 ± 3.89b,c | <0.001 |
| Total nonsclerotic glomerular number, ×106/kidney | 0.652 ± 0.211 | 0.673 ± 0.217 | 0.542 ± 0.227 | 0.103 |
| Total globally sclerotic glomerular number, ×106/kidney | 0.034 ± 0.066 | 0.031 ± 0.079 | 0.161 ± 0.168b,c | <0.001 |
| Single-nephron eGFR, nl/min | 59 ± 21 | 64 ± 21 | 97 ± 43b | 0.001 |
BMI, body mass index; BSA, body surface area; eGFR, estimated glomerular filtration rate; ORG, obesity-related glomerulopathy; RAAS, renin–angiotensin–aldosterone system.
Kruskal–Wallis test with Dunn–Bonferroni test.
P < 0.05 vs. nonobese donors.
P < 0.05 vs. obese donors.
Figure 2.
Single-nephron parameters in obesity-related glomerulopathy (ORG) patients with preserved renal function. Total and single-nephron parameters, including (a) mean glomerular volume (GV), (b) estimated glomerular filtration rate (eGFR), and (c) single-nephron glomerular filtration rate (SNGFR) were evaluated among the ORG patients with preserved renal function of chronic kidney disease (CKD) stage G 1, 2 and compared with kidney transplantation donors with or without obesity. Values represent the mean ± SD of evaluations from each group. Differences among groups were analyzed by the Kruskal−Wallis test with the Dunn−Bonferroni test. GFR, glomerular filtration rate.
Comparison of Clinicopathological Findings in ORG Patients With Different Renal Function Stages
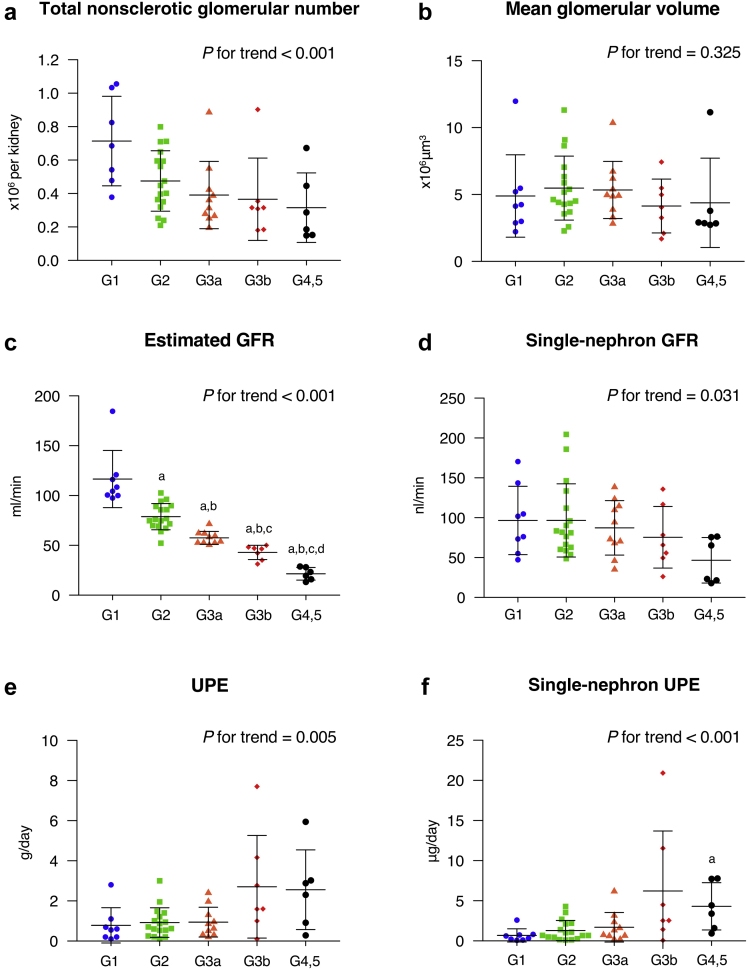
Clinicopathological characteristics were compared among the ORG subgroups defined by CKD stage (Table 3). Estimated cortical volume of the kidneys and mean volumetric glomerular density tended to decrease in accordance with the progression of renal function stages, with no statistically significant differences among the ORG subgroups. Mean areal glomerular density showed a statistically significant tendency to decrease in accordance with the progression of CKD stages. Total and single-nephron parameters were compared among the ORG subgroups categorized by CKD stage (Figure 3). The total nonsclerotic glomerular number decreased as renal function declined. Mean GV did not show statistically significant differences among the ORG subgroups with different stages of CKD. Both eGFR and SNGFR decreased in accordance with the advancement of CKD stages. Values for UPE increased and the values for SNUPE were further increased with the advancement of CKD stages. Similar results for single-nephron parameters were confirmed in the subgroups treated with antihypertensive medications including RAAS inhibitors at the time of biopsy diagnosis (Supplementary Figure S2).
Table 3.
Comparison of clinicopathological findings in ORG patients with different renal function stages
| Variable | ORG CKD stage 1 (n = 8) |
ORG CKD stage 2 (n = 17) |
ORG CKD stage 3a (n = 10) |
ORG CKD stage 3b (n = 7) |
ORG CKD stage 4, 5 (n = 6) |
P value for trenda |
|---|---|---|---|---|---|---|
| Clinical findings | ||||||
| Age, yr | 41.1 ± 15.2 | 46.9 ± 7.7 | 49.2 ± 8.6 | 55.9 ± 10.6 | 50.7 ± 14.8 | 0.019 |
| Sex, male % (n) | 50.0 (4) | 70.6 (12) | 60.0 (6) | 71.4 (5) | 50.0 (3) | 0.974 |
| BMI, kg/m2 | 30.3 ± 3.7 | 29.1 ± 3.0 | 30.6 ± 4.3 | 28.6 ± 3.5 | 29.1 ± 2.7 | 0.665 |
| BSA, m2 | 1.89 ± 0.24 | 1.91 ± 0.22 | 1.85 ± 0.16 | 1.90 ± 0.20 | 1.83 ± 0.13 | 0.479 |
| Hypertension, % (n) | 50.0 (4) | 76.5 (13) | 70.0 (7) | 71.4 (5) | 100 (6) | 0.046 |
| Serum creatinine, mg/dl | 0.59 ± 0.08 | 0.86 ± 0.14b | 1.09 ± 0.15b,c | 1.42 ± 0.18b,c,d | 2.67 ± 0.92b,c,d,e | <0.001 |
| eGFR, ml/min per 1.73 m2 | 106 ± 14 | 71 ± 8b | 54 ± 3b,c | 39 ± 5b,c,d | 20 ± 5b,c,d,e | <0.001 |
| 24-h CCr, ml/min | 145 ± 42 | 132 ± 39 | 83 ± 18b,c | 61 ± 19b,c | 33 ± 15b,c,d | <0.001 |
| Urinary protein excretion, mg/d | 780 ± 879 | 918 ± 737 | 940 ± 743 | 2703 ± 2557 | 2555 ± 1986 | 0.005 |
| Serum albumin, g/dl | 4.1 ± 0.1 | 4.2 ± 0.4 | 4.1 ± 0.4 | 3.7 ± 0.5 | 3.7 ± 0.4 | 0.003 |
| Serum uric acid, mg/dl | 6.6 ± 2.2 | 6.5 ± 1.1 | 7.2 ± 1.3 | 7.0 ± 0.9 | 8.3 ± 0.8c | 0.014 |
| Triglyceride, mg/dl | 362 ± 459 | 258 ± 158 | 254 ± 109 | 365 ± 344 | 172 ± 113 | 0.761 |
| RAAS inhibitors, % (n) | 25.0 (2) | 70.6 (12) | 60.0 (6) | 57.1 (4) | 83.3 (5) | 0.281 |
| Histopathological findings | ||||||
| Total number of glomeruli per biopsy specimen | 22.3 ± 14.0 | 17.6 ± 8.9 | 19.0 ± 11.9 | 19.9 ± 10.7 | 18.3 ± 8.5 | 0.825 |
| Number of non-sclerotic glomeruli per biopsy specimen | 20.3 ± 13.1 | 14.4 ± 7.8 | 14.4 ± 11.5 | 12.7 ± 9.8 | 10.1 ± 4.4 | 0.075 |
| Global glomerulosclerosis, % | 10.0 ± 9.3 | 18.2 ± 16.5 | 25.0 ± 19.6 | 34.9 ± 23.6 | 43.3 ± 12.0b,c | <0.001 |
| Segmental glomeruloscrelosis, % | 3.0 ± 3.7 | 1.2 ± 3.5 | 1.7 ± 2.7 | 8.5 ± 8.2c | 11.8 ± 13.7c | 0.014 |
| Interstitial fibrosis/ tubular atrophy, % | 5.0 ± 2.7 | 8.5 ± 7.9 | 12.5 ± 19.2 | 28.6 ± 16.5b,c | 51.7 ± 19.7b,c,d | <0.001 |
| Renal morphological parameters | ||||||
| Renal parenchymal volume, cm3/kidney | 184 ± 65 | 168 ± 40 | 140 ± 20 | 135 ± 31 | 122 ± 39 | 0.003 |
| Renal cortical volume, cm3/kidney | 131 ± 46 | 119 ± 28 | 100 ± 14 | 96 ± 22 | 87 ± 28 | 0.057 |
| Mean areal glomerular density, /mm2 | 2.06 ± 0.82 | 1.54 ± 0.45 | 1.51 ± 0.70 | 1.31 ± 0.72 | 1.17 ± 0.36 | 0.005 |
| Mean volumetric glomerular density, /mm3 | 10.70 ± 5.35 | 7.34 ± 2.51 | 7.37 ± 4.11 | 7.02 ± 4.25 | 6.27 ± 2.48 | 0.050 |
| Total nonsclerotic glomerular number, ×106/kidney | 0.686 ± 0.260 | 0.475 ± 0.181 | 0.391 ± 0.201 | 0.365 ± 0.246 | 0.315 ± 0.208c | <0.001 |
| Total globally sclerotic glomerular number, ×106/kidney | 0.103 ± 0.080 | 0.188 ± 0.185 | 0.229 ± 0.191 | 0.289 ± 0.213 | 0.368 ± 0.200 | 0.007 |
BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ORG, obesity-related glomerulopathy; RAAS, renin−angiotensin−aldosterone system.
Jonckheere−Terpstra test with Dunn−Bonferroni test.
P < 0.05 vs. CKD stage 1.
P < 0.05 vs. CKD stage 2.
P < 0.05 vs. CKD stage 3a.
P < 0.05 vs. CKD stage 3b.
Figure 3.
Comparison of single-nephron parameters in obesity-related glomerulopathy (ORG) patients with different renal function stages. Total and single-nephron parameters, including (a) otal nonsclerotic glomerular number, (b) mean glomerular volume (GV), (c) estimated glomerular filtration rate (eGFR), (d) single-nephron glomerular filtration rate (SNGFR), (e) urinary protein excretion (UPE), and (f) single-nephron urinary protein excretion (SNUPE) were compared among the ORG subgroups categorized based on different renal function stages of chronic kidney disease (CKD) G1, G2, G3a, G3b, and G4, 5. Values represent the mean ± SD of evaluations from each group. Differences among groups were analyzed by the Jonckheere−Terpstra test with the Dunn−Bonferroni test. GFR, glomerular filtration rate. aP < 0.05 versus CKD stage 1. bP < 0.05 versus CKD stage 2. cP < 0.05 versus CKD stage 3a. dP < 0.05 versus CKD stage 3b.
Discussion
Using unenhanced CT and biopsy-based stereology, we demonstrate for the first time in humans the development of elevated SNGFR levels in ORG patients compared to those in healthy subjects. Importantly, an increase in SNGFR was not observed in transplant donors with comparable levels of obesity, suggesting that obesity alone is not sufficient to induce these changes in all obese subjects. These results are consistent with the typical histopathological features of marked glomerulomegaly and maladaptive FSGS lesions, indicating abnormal intraglomerular hemodynamics in ORG patients.6,27,28 Our results further show that SNGFR is decreased in accordance with the progression of CKD stages, with no difference in the mean GV between the subgroups. This may be caused in part by a tendency toward more afferent arteriolar hyalinosis with advancing CKD.29 Mesangial cell contraction caused by RAAS activation leading to a decreased capillary surface area also could contribute to decreased SNGFR without reducing mean GV.30 Of note, SNUPE was much higher in the ORG subgroups with more advanced CKD stages. These results suggest that the initial glomerular hyperfiltration with compensatory glomerular enlargement is an early response that is followed by vulnerability of the glomerular filtration barrier to injury, representing a process of decompensated hyperfiltration at the single-nephron level during ORG progression. Experimental studies in obese rats suggest that glomerular hypertrophy exerts mechanical strain on maximally hypertrophied podocytes, leading to podocyte failure and depletion, which in turn could mediate increasing proteinuria.31
Although the origin of glomerulomegaly in patients with ORG is incompletely understood, several plausible mechanisms have been proposed.28 These include glomerular adaptive changes due to a mismatch between body size and nephron mass and an increase in the glomerular capillary number. Our previous studies identified low areal glomerular density as a typical renal histopathological finding in ORG biopsy specimens.17,32 In our current study cohort, we again confirmed that mean areal glomerular density in ORG patients was significantly lower than in obese or nonobese control subjects. Based on this simple metric, we had assumed that the nephron endowment in ORG patients was low.28,32, 33, 34 Unexpectedly, our current results fail to support this hypothesis. As a whole, ORG patients did not show an apparently low total glomerular number per kidney as compared to donor control subjects when globally sclerotic glomeruli were included in the calculation.
An overriding question in the pathogenesis of ORG is why only a small subset of obese individuals develop ORG. Our current results show that ORG closely associates with a decrease in nonsclerotic glomerular number. Thus, our results support the possibility that a low number of functioning nephrons, either inherent or acquired, is one of the risk factors sensitizing obese individuals to ORG progression. However, the similar numbers of nephrons (with or without globally sclerotic glomeruli) in ORG patients with preserved renal function and in healthy donor controls suggest that low nephron number is not likely to be the sole predisposing factor to induce ORG in combination with obesity. Similar to variability in nephron number, recent studies have revealed broad variability in podocyte number per glomerulus among normal individuals.35,36 Mismatch between abnormally enlarged glomeruli and individual podocyte endowment could constitute another predisposing mechanism to glomerular injury in ORG patients.37 Finally, our findings of low areal glomerular density together with enlarged parenchymal volume and cortical volume support tubular hypertrophy occurring as part of a co-ordinated hypertrophic nephron response in ORG.
A recent study reported that CCr overestimates measured GFR due to tubular secretion of creatinine.38 In fact, CCr was approximately 30% higher than eGFR in this study cohort. This study therefore used eGFR-based SNGFR as a substitute for measured GFR-based SNGFR. In our donor controls, the eGFR-based SNGFR was 20% lower and CCr-based SNGFR was 10% higher as compared to the SNGFR value (80 ± 40 nl/min) estimated by an iothalamate-based measurement of the GFR in the US donor cohort.15 Given the potential racial differences in nephron numbers and SNGFR, validation studies using measured GFR are needed among different races.
This study has several important limitations. First, the small number of patients recruited in this study may have influenced the statistical power. Second, our study’s cross-sectional design does not prove causal relationships between glomerular number and renal outcomes. Third, a large proportion of our patients were receiving treatment with antihypertensive medications including RAAS inhibitors, which could modify the SNGFR and SNUPE. Fourth, information on birth weight, a surrogate for nephron number at birth, was unavailable in this study. Finally, the small glomerular sampling by needle biopsy may have significantly influenced the results, as the distribution of individual glomerular adaptive responses is not uniform across the kidney. Furthermore, the glomeruli and cortical area obtained in the biopsy specimen of donors were substantially smaller than those of ORG patients. These discrepancies in the number of glomeruli and/or cortical area may have affected the measurement of glomerular density and total glomerular number.
In conclusion, our results provide morphometric and functional correlates for single-nephron parameters in ORG patients. Our data suggest, for the first time, that single-nephron glomerular hyperfiltration indeed occurs in human kidneys with ORG and is a feature of early-stage ORG with preserved renal function. Despite persistent elevations in mean GV during ORG progression, SNGFR tended to decline with more advanced stages of CKD, in parallel with worsening proteinuria. Together, our findings suggest that impairment in individual glomerular barrier function, as a result of decompensated hyperfiltration responses, underlie ORG progression.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by JSPS KAKENHI grant numbers JP25461236 and JP16K0936 (to NT). Parts of this study were presented at the American Society of Nephrology Kidney Week 2018, San Diego, California (November 26, 2018).
Author Contributions
YO and NT designed the study. YO performed the data collection and measurement analysis. YO and NT drafted the manuscript. VDD provided intellectual input to the project and helped draft and edit the manuscript. All authors contributed to data interpretation and approved the final version of the manuscript.
Footnotes
Figure S1. Measurement of glomeruli. Representative light microscopic measurement for non-sclerotic glomeruli (A) and glomeruli with FSGS lesions (B) (PAS, ×200).
Figure S2. Comparison of single-nephron parameters in ORG patients treated with RAAS inhibitors at the time of biopsy diagnosis. Total and single-nephron parameters, including total nonsclerotic glomerular number (A), mean glomerular volume (GV) (B), estimated GFR (eGFR) (C), single-nephron GFR (SNGFR) (D), urinary protein excretion (UPE) (E), and single-nephron urinary protein excretion (SNUPE) (F) were compared among the ORG subgroups categorized based on different renal function stages of CKD G1, G2, G3a, G3b, and G4, 5 (n = 4, 11, 6, 4, and 5, respectively.). Values represent the mean ± SD of evaluations from each group. Differences among groups were analyzed by the Jonckheere−Terpstra test with the Dunn−Bonferroni test. aP < 0.05 versus CKD stage 1, bP < 0.05 versus CKD stage 2, cP < 0.05 versus CKD stage 3a, dP < 0.05 versus CKD stage 3b.
Supplementary Material
References
- 1.Ogden C.L., Carroll M.D., Kit B.K. Prevalence of childhood and adult obesity in the United States, 2011−2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock G., Lewington S., Sherliker P. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y.1, Beydoun M.A., Liang L. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.1, Chen X., Song Y. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 5.Fox C.S., Larson M.G., Leip E.P. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 6.Weisinger J.R., Kempson R.L., Eldridge F.L. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
- 7.Kambham N., Markowitz G.S., Valeri A.M. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 8.Praga M., Hernandez E., Morales E. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.M., Li S.J., Chen H.P. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi N., Koike K., Hirano K. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol. 2013;17:379–385. doi: 10.1007/s10157-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 11.Chagnac A., Weinstein T., Korzets A. Glomerular hemodynamics in severe obesity. Am J Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 12.Chagnac A., Weinstein T., Herman M. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 13.Denic A., Lieske J.C., Chakkera H.A. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2017;28:313–320. doi: 10.1681/ASN.2016020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki T., Tsuboi N., Kanzaki G. Biopsy-based estimation of total nephron number in Japanese living kidney donors. Clin Exp Nephrol. 2019;23:629–637. doi: 10.1007/s10157-018-01686-2. [DOI] [PubMed] [Google Scholar]
- 15.Denic A., Mathew J., Lerman L.O. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376:2349–2357. doi: 10.1056/NEJMoa1614329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki T., Tsuboi N., Okabayashi Y. Estimation of nephron number in living humans by combining unenhanced computed tomography with biopsy-based stereology. Sci Rep. 2019;9:14400. doi: 10.1038/s41598-019-50529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okabayashi Y., Tsuboi N., Sasaki T. Glomerulopathy associated with moderate obesity. Kidney Int Rep. 2016;1:250–255. doi: 10.1016/j.ekir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Agati V.D. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 19.Japan Society for the Study of Obesity New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 20.Du Bois D., Du Bois E.F. Tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Internal Med. 1916;17:863–871. [PubMed] [Google Scholar]
- 21.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T., Tsuboi N., Haruhara K. Bowman’s capsule volume and related factors in adults with normal renal function. Kidney Int Rep. 2017;3:314–320. doi: 10.1016/j.ekir.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulladosa X., Moreso F., Narváez J.A. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003;14:2662–2668. doi: 10.1097/01.asn.0000088025.33462.b0. [DOI] [PubMed] [Google Scholar]
- 24.Lerman L.O., Bentley M.D., Bell M.R. Quantitation of the in vivo kidney volume with cine computed tomography. Invest Radiol. 1990;25:1206–1211. doi: 10.1097/00004424-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Haruhara K., Tsuboi N., Sasaki T. Volume ratio of glomerular tufts to Bowman capsules and renal outcomes in nephrosclerosis. Am J Hypertens. 2019;32:45–53. doi: 10.1093/ajh/hpy147. [DOI] [PubMed] [Google Scholar]
- 26.Weibel E.R. 44–45. Academic Press; London, UK: 1979. pp. 131–134. (Stereological Method: Practical Methods of Biological Morphometry). [Google Scholar]
- 27.D’Agati V.D., Chagnac A., de Vries V.D. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi N., Okabayashi Y., Shimizu A. The renal pathology of obesity. Kidney Int Rep. 2017;2:251–260. doi: 10.1016/j.ekir.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamami R., Kohagura K., Miyagi T. Modification of the impact of hypertension on proteinuria by renal arteriolar hyalinosis in nonnephrotic chronic kidney disease. J Hypertens. 2016;34:2274–2279. doi: 10.1097/HJH.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 30.Pfeilschifter J. Cross-talk between transmembrane signaling systems: a prerequisite for the delicate regulation of glomerular hemodynamics by mesangial cells. Eur J Clin Invest. 1989;19:347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda A., Chowdhury M.A., Venkatareddy M.P. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23:1351–1363. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuboi N., Utsunomiya Y., Kanzaki G. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol. 2012;7:735–741. doi: 10.2215/CJN.07270711. [DOI] [PubMed] [Google Scholar]
- 33.Tsuboi N., Utsunomiya Y., Hosoya T. Obesity-related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28(suppl 4):iv108–iv113. doi: 10.1093/ndt/gft258. [DOI] [PubMed] [Google Scholar]
- 34.Praga M. Synergy of low nephron number and obesity: new focus on hyperfiltration nephropathy. Nephrol Dial Transplant. 2005;20:2594–2597. doi: 10.1093/ndt/gfi201. [DOI] [PubMed] [Google Scholar]
- 35.Venkatareddy M., Wang S., Yang Y. Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol. 2014;25:1118–1129. doi: 10.1681/ASN.2013080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puelles V.G., Douglas-Denton R.N., Cullen-McEwen L.A. Podocyte number in children and adults: associations with glomerular size and numbers of other glomerular resident cells. J Am Soc Nephrol. 2015;26:2277–2288. doi: 10.1681/ASN.2014070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H.M., Liu Z.H., Zeng C.H. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48:772–779. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Rule A.D., McCulloch C.E., Lieske J.C., Ku E., Hsu C.Y. Tubular secretion of creatinine and kidney function: an observational study. BMC Nephrol. 2020;21:108. doi: 10.1186/s12882-020-01736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.