Abstract
Serum lipid levels are closely related to the structure and function of blood vessels. Chronic hyperlipidemia may lead to damage in both the cardio- and the cerebrovascular systems. Vascular dysfunctions, including impairments of the blood-brain barrier, are known to be associated with neurodegenerative diseases. A growing number of evidence suggests that cardiovascular risk factors, such as hyperlipidemia, may increase the likelihood of developing dementia. Due to differences in lipoprotein metabolism, wild-type mice are protected against diet-induced hypercholesterolemia, and their serum lipid profile is different from that observed in humans. Therefore, several transgenic mouse models have been established to study the role of different apolipoproteins and their receptors in lipid metabolism, as well as the complications related to pathological lipoprotein levels. This mini-review focused on a transgenic mouse model overexpressing an apolipoprotein, the human ApoB-100. We discussed literature data and current advancements on the understanding of ApoB-100 induced cardio- and cerebrovascular lesions in order to demonstrate the involvement of this type of apolipoprotein in a wide range of pathologies, and a link between hyperlipidemia and neurodegeneration.
Keywords: ApoB-100 lipoprotein, hyperlipidemia, atherosclerosis, blood-brain barrier (BBB), endothelial dysfunction, cerebrovascular disease, neurodegeneration, dementia, transgenic mice
1. introduction
Lipids, as being water-insoluble molecules, are transported in the blood as protein-bound structures, called lipoprotein particles. These are composed of a hydrophobic core consisting of triglycerides and cholesterol esters, encompassed by a hydrophilic phospholipid monolayer containing embedded apolipoproteins. Lipoprotein particles are classified into different groups according to their density, size, lipid and apolipoprotein contents. Apolipoproteins are essential structural molecules of lipoprotein particles, and play an important role in lipid metabolism by regulating numerous metabolic enzymes, as well as mediating molecular interactions with lipoprotein receptors [1, 2].
Following intestinal absorption, dietary fats are packaged into chylomicron (CM) particles within the enterocytes, and are delivered to the circulation. The core protein component of CMs is apolipoprotein B (ApoB)-48, which is the truncated variant of ApoB-100, produced by the ApoB mRNA editing enzyme, APOBEC1 [1, 3]. In the capillaries, triglycerides carried in CMs are hydrolyzed by lipoprotein lipase. Released free fatty acids are taken up by muscle cells and adipocytes, while triglyceride-depleted chylomicron remnants (smaller particles) are removed from the circulation by the liver [1-3]. In the liver, triglycerides and cholesterol esters (from chylomicron remnants, as well as those newly synthesized by hepatocytes) are incorporated into triglyceride-rich, very-low-density lipoprotein (VLDL) particles, and are secreted into the blood. In humans, the core structural protein of VLDL is ApoB-100, while in mice VLDL contains both apoB-100 and apoB-48 due to the hepatic expression of APOBEC1 in mice and rats [3-5]. Fatty acids are released from VLDL particles by lipoprotein lipase in peripheral tissues, leading to the formation of cholesterol-enriched intermediate-density lipoprotein (IDL) particles. IDL particles are partially cleared from circulation via hepatocytes LDL receptors (LDLRs) and LDL receptor-related proteins (LRPs) which recognize the ApoE protein present in IDL particles. The rest of the IDL undergoes further triglyceride hydrolyzation by hepatic lipase, leading to the formation of low-density lipoproteins (LDLs). Therefore, in humans, LDL particles contain mainly cholesterol esters and ApoB-100 [2, 3]. Hepatic LDLRs play a crucial role in the control of serum LDL levels, as the clearance of LDL from the blood is mediated mainly by these receptors. A smaller fraction of LDL is taken up by peripheral tissues [2]. Data on the clearance of ApoB-48 and ApoB-100 indicate that ApoB-48 is removed faster from circulation than ApoB-100, which may partially explain why mice have lower serum LDL and VLDL levels as compared to humans [3, 6]. High-density lipoprotein (HDL) particles, containing ApoA1, are responsible for reverse cholesterol transport. Cholesterol esters of HDL particles are transferred into ApoB containing particles by the cholesterol ester transfer protein (CETP) in humans. In contrast, mice lack CETP activity, therefore the predominant lipoproteins carrying cholesterol are HDL in mice, and they are typically characterized by quite low LDL levels [2, 3]. The differences in lipoprotein metabolism result in a different serum lipid profile between humans and wild-type mice. Due to the low LDL to HDL ratio, wild-type mice are protected against hypercholesterolemia, and are resistant to atherosclerosis [7]. Therefore, several transgenic mouse models have been established for the animal studies of hyperlipidemia and hypercholesterolemia [3]. Although the lipid profile of these animals is still not completely equal to that of humans, they are useful tools to study the role of different apolipoproteins and receptors involved in lipid metabolism, and the consequences of pathological lipoprotein levels. For example, the LDLR -/- mouse strain, which expresses exclusively ApoB-100 in the liver, is an accepted rodent model of familial hypercholesterolemia [8]. Another transgenic mouse model of dyslipidemia, the ApoE -/- strain, develops atherosclerotic lesions showing even more severe symptoms on high-fat or high-cholesterol diet [7]. Moreover, many different genetically modified mouse lines have been generated to study the role of ApoB proteins in lipid metabolism and atherogenesis [5].
2. The ApoB-100 transgenic mouse strain: a model of hyperlipidemia and atherosclerosis
One of the first ApoB-100 transgenic mouse strains was generated in Hobbs’s laboratory in the early 1990s [9]. They used a minigene construct containing exons 1-26 of the human ApoB-100 cDNA, and the last three exons and introns from the human ApoB gene, including 2.3 kb of the 3’-untranslated region driven from the mouse transthyretin promoter/enhancer sequences. Transgenic human ApoB was detected in the liver, kidney and brain of these mice. The researchers found that human ApoB-100 mRNA was edited in the mouse liver with a slightly decreased efficiency as compared to the endogenous mouse transcript. Soon after that, the same group generated transgenic mice using a 79.5-kb genomic DNA fragment spanning the entire human ApoB gene, inserted into P1 phagemid. In these transgenics, the human ApoB transgene was expressed at high levels in the liver, but not in the intestine [10, 11].
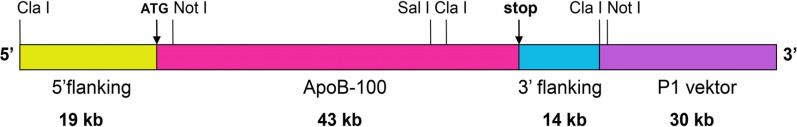
A transgenic mouse strain overexpressing the human ApoB-100 protein was established in our laboratory in 2005 [13], using the transgene presented here. The gene construct was produced in Rubin’s laboratory in 1993 [12]. The construct contained the complete human ApoB-100 transcription unit, including the long 5’ promoter, as well as intragenic and 3’specific enhancer/silencer sequences, cloned into a P1 phagemid vector. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Almost simultaneously, another ApoB-100 transgenic strain was developed in Rubin’s laboratory in 1993 [12]. They cloned the complete human ApoB-100 transcription unit into a P1 phagemid vector. This construct expressed the human ApoB-100 similarly to the endogenous mouse protein, due to the long 5’ promoter, intragenic and 3’specific enhancer/silencer sequences.
Our group produced transgenic mice overexpressing the human ApoB-100 protein in 2005, using the same P1 phagemid transgene (Fig. 1) for microinjection as the one constructed in Rubin’s laboratory (kindly provided by Prof. Rubin) [13]. We have been maintaining this transgenic strain on the C57B6N genetic background, being stable for 14 years without any modification in the transgene expression.
Fig. (1).
Physical map of human ApoB-100 transgene. Based on Callow et al. 1994. PNAS, 91. 2130-34.
ApoB-100 deficient mice were produced in Jan Breslow’s lab in 1995 [14]. Homozygote knockout animals were not viable; embryos died at embryonic day 9 (E9). Heterozygote animals were viable, but showed increased intrauterine lethality due to incomplete neural tube closure. Several newborn heterozygotes developed hydrocephalus. Heterozygote male mice were mostly sterile. Plasma triglyceride levels were normal, but total, LDL, and HDL cholesterol levels were substantially reduced. Independently, another ApoB-100 deficient mouse line was established by Farese and coworkers, who found that heterozygote animals were protected against hypercholesterolemia induced by a diet enriched in fat and cholesterol [15].
ApoB-100 is the major constituent of lipoprotein particles that are considered to be atherogenic [5]. The majority of plasma ApoB-100 molecules are associated with LDL, although they are also found in VLDL and IDL particles. As each LDL particle contains a single molecule of ApoB-100, increasing concentrations of ApoB-100 induce the elevation of LDL particle number in the blood [2, 16]. Therefore, the plasma lipid profile of mouse strains overexpressing ApoB-100 is similar to that of humans.
Experiments with the first ApoB-100 transgenic mice model revealed that transgenics have elevated serum levels of ApoB, total and LDL cholesterol, as well as triglyceride [10-12]. Transgenic mice on a high-fat or high-cholesterol diet had significantly increased serum levels of total and LDL cholesterol as compared to non-transgenic animals fed with the same diet [11, 12]. Our results also demonstrated that ApoB-100 transgenics receiving a regular chow diet had permanently elevated plasma triglyceride levels as compared to wild-type controls at the age of 3, 7, 9 and 12 months [17, 18]. ApoB-100 transgenic mice on a high-cholesterol diet showed increased levels of serum total and LDL cholesterol, while serum HDL levels did not change [19]. Interestingly, serum triglyceride levels were normalized by the cholesterol-enriched diet in our transgenic mice [19]. This coincides with the results of Young and coworkers, who found that lipoproteins containing the ApoB-100 protein were enriched in triglycerides in transgenic mice receiving normal chow, while in those fed with high-fat diet LDL particles contained very low levels of triglycerides [5, 10, 11]. Therefore, two different types of hyperlipidemia can be modelled by ApoB-100 overexpressing mice depending on the diet: hypertriglyceridemia on regular chow, and hypercholesterolemia induced by cholesterol supplementation. The severity of hyperlipidemia induced by ApoB-100 overexpression can be increased by combining this transgene with other mutations. For example, LDLR deficient ApoB-100 transgenic mice show a markedly increased plasma LDL level, and develop atherosclerotic lesions by the age of 6 months on a regular diet [20]. Transgenic mice expressing the human CETP protein along with ApoB-100 and fed with a normal diet also show an increased ratio of LDL to HDL fraction, which resembles the plasma cholesterol distribution of normolipidemic humans [21].
Several studies confirm that hyperlipidemia and increased serum levels of LDL cholesterol are important risk factors for ischemic heart disease [22]. Accumulation of LDL in the arterial wall is a key event in early atherogenesis, as it promotes the aggregation and oxidation of LDL particles, leading to inflammation and the formation of atherosclerotic lesions [23]. As ApoB-100 overexpressing mice are not protected against diet-induced hypercholesterolemia, a lipid-enriched diet induces the formation of atherosclerotic plaques in these animals. Oil-Red O stained sections of the proximal aorta of ApoB-100 transgenic mice fed with an atherogenic, fat- and cholesterol-rich diet showed significantly increased plaque numbers as compared to non-transgenics [11, 24]. The aorta in these transgenic mice was characterized by fatty streak lesions along its entire length. The features of these lesions, including the presence of oxidized lipoproteins, macrophages, and immunoglobulins, were identical to those commonly seen in human lesions [24]. Electron microscopic studies revealed the presence of foam cells and cholesterol crystal deposits in the wall of the thoracic aorta [11]. ApoB-100 transgenic mice fed with a cholesterol-rich (2%) diet for 18 weeks demonstrated significantly increased levels of myocardial superoxide, NADPH oxidase and nitrotyrosine [19]. In addition, serum malondialdehyde level, a marker of systemic lipid oxidation and oxidative stress, was considerably increased as well. These biochemical alterations in myocardial tissues led to decreased aortic flow and reduced cardiac work in cholesterol-fed transgenic mice, as measured in isolated working hearts [19].
Chronic hyperlipidemia affects not only the cardiovascular, but also the cerebrovascular system. An early cross-sectional study revealed that hallmarks of atherosclerosis, such as an increased thickness of vessel walls or accumulation of plaques in the carotid arteries, are related to vascular dementia and Alzheimer’s disease (AD) [25]. A growing number of evidence suggests that established risk factors of cardiovascular disease, including mid-life obesity, high systolic blood pressure, diabetes or hyperlipidemia, may also increase the probability of developing dementia, and may accelerate the cognitive decline in AD patients [26-30]. A follow-up study showed that the cognitive performance of elderly persons was negatively affected by a history of vascular disease, and this effect was most pronounced in AD patients. Moreover, the development of cardiovascular diseases was more prevalent in the AD group [28].
3. Blood-brain barrier
Atherosclerotic changes may occur throughout the entire vasculature, including blood vessels supplying the brain. The structure and function of brain capillaries are tailored to the requirements of maintaining a strictly controlled microenvironment essential for proper neural functioning. The interface between the circulating blood and the neural tissue is the blood-brain barrier (BBB), which is formed by endothelial cells closely interacting with astrocytic endfeet and pericytes surrounding brain capillaries. The brain receives its nutrient supply through the BBB, while at the same time, the BBB protects the brain from toxic compounds and pathogens. Gases (i.e., oxygen and carbon dioxide) transported in the blood, as well as lipid-soluble compounds, diffuse passively through the BBB. In contrast, the exchange of water-soluble molecules between the blood and the brain is dynamically and actively regulated by the BBB [31].
The basic cellular components of the BBB are capillary endothelial cells. They are characterized by a lack of fenestrae, low rate of transcytosis and expression of tight junctions (TJs), extrusion pumps and selective transporters. TJs represent the most characteristic feature of the BBB. They are contact points between endothelial cells where transmembrane proteins, mainly occludin, claudin-5 and junctional adhesion molecules, span the intercellular cleft. TJ transmembrane proteins are anchored to scaffolding proteins (e.g., zonula occludens-1 (ZO-1)) regulating TJ assembly [32]. TJs block the paracellular transport of macromolecules and restrict the diffusion of ions and small water-soluble molecules through the intercellular cleft between endothelial cells. Another function of TJs is the maintenance of polarized distribution of endothelial membrane proteins, including extrusion pumps and selective transporters. Water-soluble nutrients, such as glucose, amino acids, neurotransmitter amino acids GABA and glycine, monocarboxylates and vitamins, as well as organic anions and cations pass through endothelial cells using their specific transporters [33]. Lipids, which are transported in the blood in the form of lipoprotein complexes, also use specific transporters for their uptake by endothelial cells [34, 35]. Brain capillary endothelial cells express not only transporters that mediate the entry of nutrients into the brain, but also efflux transporters that are involved in removing harmful, potentially toxic substances, like amyloid-β (Aβ) peptides, from the brain into the blood. The major efflux transporters, such as P-glycoprotein (Pgp), multidrug resistance associated proteins and breast cancer resistance protein belong to the B and G subfamilies of ATP binding cassette transporters [36].
The induction and maintenance of brain endothelial characteristics are largely dependent on astroglial cells [37]. Endothelial cells, in turn, also influence astrocyte function [38]. The anatomical basis of astroglia-endothelial cross-talk is a network of fine lamellae made of astrocytic endfeet opposed to the outer surface of brain capillary endothelial cells [39]. Direct contact between endothelial and astroglial cells is required for the proper expression of TJ proteins, including occludin, claudin-5 and ZO-1, in endothelial cells [40]. On the other hand, endothelial-astroglial interaction plays a role in the polarized expression of the water-channel protein aquaporin-4 in glial endfeet [41, 42]. Aquaporin-4 is essential for the maintenance of volume and osmotic balances of the neuronal microenvironment [43].
Pericytes are embedded in the basement membrane of small blood vessels, such as pre-capillary arterioles, capillaries and post-capillary venules. They regulate the thickness of basal lamina by producing and depositing its components or synthesizing proteases [44, 45]. Pericytes are contractile cells with processes surrounding the vessel wall. They actively communicate with endothelial cells, astrocytes and neurons [46, 47], regulate cerebral blood flow [48], as well as the expression of TJ and adhesion junction proteins [49], neuroinflammation [50], angiogenesis [51], clearance [52], and stem cell activity [53]. During development, features, specific for brain capillary endothelial cells are induced by pericytes, and later by astrocytes [54].
4. Dyslipidemia-related changes in BBB function
The cellular components of the BBB communicate with each other through cytokines. Each cell type has a specific pattern of cytokine secretion, and all of them are capable of producing pro- and anti-inflammatory cytokines [55]. Upon a shift towards proinflammatory cytokine secretion (e.g., tumor necrosis factor-α or interleukin-6), an increase in BBB permeability can be observed [56]. BBB functional damage may include microbleeds, leukocyte infiltration and entry of blood-derived macromolecules (fibrinogen, thrombin, albumin, IgG, haemosiderin) into the brain, as well as impaired glucose transport and Pgp function. BBB dysfunction is usually accompanied by morphological alterations, such as disrupted TJs, basement membrane changes and pericyte loss. Most signs suggesting a compromised BBB are observed in neurodegenerative diseases, including AD, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis and HIV-associated dementia [57].
Inflammatory processes may be induced by a series of factors, including injuries, infections, ischemia and metabolic disorders, such as hyperglycemic and hyperlipidemic conditions [58-60]. Dyslipidemia is a known risk factor for intracranial atherosclerosis [61, 62], and is prevalent in 75% of AD patients with BBB impairment [63]. Brain capillary endothelial cell functions may be affected by an increased production of arachidonic acid metabolites, which occurs in hyperlipidemic conditions. These metabolites are reported to modulate serine/threonine phosphorylation of TJ proteins, leading to TJ disruption and barrier dysfunction [64]. Lipolysis products of triglyceride-rich lipoproteins may also increase BBB permeability via the disruption of intercellular junctions, which in turn induces apoptosis and influences lipid raft morphology and composition [65, 66]. Reactive oxygen species might also be involved in endothelial cell injury induced by triglyceride-rich lipoproteins [66, 67]. Indeed, serum triglyceride levels in humans are positively correlated with the level of protein oxidation, irrespective of total cholesterol levels [68]. An in vivo study performed in mice confirmed that lipolysis products of triglyceride-rich lipoproteins transiently increased the BBB transfer coefficient. It suggests that serum lipids may induce neurovascular injury, which may be involved in the development of cognitive dysfunction [69].
Dyslipidemia-related permeability changes of the BBB may also result from alterations in endothelial transport systems. Glucose transporter-1 (Glut-1) expression in brain endothelial cells was transiently downregulated in mice fed with a high-fat diet, which was followed by a compensatory Glut-1 upregulation leading to cognitive disturbances through inflammatory pathways [70]. Inflammatory conditions also modulate the expression and function of BBB efflux transporters, including Pgp and LRP1 [71]. A reduction in either LRP1 or Pgp levels or both leads to impaired Aβ clearance from the brain and to cognitive deficits in mice [72, 73]. This finding is in accordance with verapamil-PET studies showing a decrease in Pgp function in the brain of patients with AD or Parkinson’s disease [57].
Besides endothelial cells, pericytes may also participate in dyslipidemia-induced permeability changes of the BBB. Hyperlipidemia was reported to prevent the effects of vascular endothelial growth factor on pericytes in mice, provoking a decrease in pericyte coverage of brain capillaries and an increase in BBB permeability [74].
5. Role of dyslipidemia in neurodegenerative diseases
Long-term high dietary intake of cholesterol reduces the density of vessel branches in the brain [75]. Pathological changes linked to hypercholesterolemia can be observed despite the fact that the BBB blocks cholesterol entry into the brain. Oxidized cholesterol metabolites, such as 24S-hydroxycholesterol and 27-hydroxy-cholesterol, however, are able to cross the BBB, and recent data suggest that they contribute to the development of AD [76]. Moreover, significantly increased serum levels of LDL and decreased levels of HDL cholesterol were measured in AD patients compared to healthy controls, while only a slight, non-significant difference regarding total cholesterol levels was observed between the groups [77]. Higher serum LDL levels were associated with early-onset AD [78], and with compromised integrity of white matter tissue in different brain regions, even in healthy individuals [79].
Dysregulation of circulatory apolipoprotein composition may be an early sign of cognitive decline. Patients with mild cognitive impairment were found to have an increased ApoB/ApoA1 ratio, which was negatively correlated with the volume of the hippocampus, and positively correlated with the incidence of later-onset cognitive decline [80]. Other studies also indicated that plasma ApoB-100 levels were 20-25% higher in AD patients as compared to controls, and a positive correlation was observed between plasma ApoB-100 and brain Aβ42 concentrations [16, 77]. On the other hand, the reduced ApoA1 level was strongly correlated with cognitive decline in AD patients [81, 82].
Interestingly, the accumulation of ApoB-100 protein in senile plaques and neurofibrillary tangles was found in the brain of AD patients [83], and this observation was experimentally confirmed in APP/PS1 transgenic mice using a highly sensitive 3-dimensional immuno-fluorescence microscopy technique [84]. ApoB-100 is known to be synthesized mainly in the liver and small intestine, and serum ApoB-100 is generally unable to cross the BBB. However, pathological alterations of the BBB found in AD may allow different serum-derived proteins to penetrate the brain. ApoB-100 was identified in the cholesterol-enriched membrane microdomains of human primary cerebral endothelial cells in vitro [85], and our studies confirmed the presence of the ApoB-100 protein in brain capillary endothelial cells in mice as well [86].
Increased serum triglyceride levels may also be involved in the development of neurodegenerative diseases. Hypertriglyceridemia has been found to significantly increase the risk of vascular dementia in elderly subjects [87]. Increased serum triglyceride levels in midlife can predict the later development of Aβ and tau pathology in cognitively normal individuals [88]. Moreover, transgenic mouse models of AD with high levels of circulating Aβ show an increase in plasma triglyceride levels prior to amyloid plaque formation [89]. Elevated levels of triglyceride-rich lipoproteins and their derivatives produced by lipolysis may contribute to neurodegeneration via inducing oxidative stress, inflammation and dysfunction of the BBB.
Certain mutations in the genes of apolipoproteins or their receptors, frequently leading to dyslipidemia, are also associated with neurodegeneration. For example, the presence of the E4 allele of ApoE is a known risk factor for developing atherosclerosis, and is involved in AD pathology as well. Two copies of the E4 allele are established to lead increased serum cholesterol levels and accelerate the development of both diseases [29, 90]. Indeed, the frequency of the E4 allele is significantly increased in patients with AD as compared to controls or unaffected siblings [91]. These findings are experimentally confirmed in transgenic mice as well, demonstrating that ApoE4 knock-in diminishes adult hippocampal neurogenesis [92]. A recent study has also shown that ApoE4 can negatively influence microvascular functions of the brain, thereby contributing to white matter degradation and cognitive impairment [93]. Furthermore, ApoE4 expression in a mouse model of AD is reported to exacerbate the accumulation of Aβ plaques in the hippocampus [94-96]. Interestingly, polymorphism of the ApoE gene may influence serum levels of other lipoproteins as well, for example, E4 carriers show significantly increased serum levels of ApoB [80].
Although ApoE is probably the most studied factor related to lipid metabolism and AD, there are other potential candidates that may play a role in linking serum lipid regulation to cognitive functions. LDLR mutations are known to be involved in familial hypercholesterolemia [97], which in turn may increase the risk of mild cognitive impairment [98]. In transgenic animals, brain LDLR deficiency can induce processes similar to those occurring in AD, such as cognitive decline or increased levels of oxidative stress [99-102].
More interestingly, it was found that rare variants of the ApoB gene are more frequent in patients with early-onset AD. These polymorphisms in the ApoB gene probably lead to an altered level, structure or function of the protein, suggesting a direct link between ApoB and dementia [78].
6. BBB characteristics and neurodegeneration in ApoB-100 transgenic mice
6.1. Expression of the Human ApoB-100 Protein in Transgenic Mice
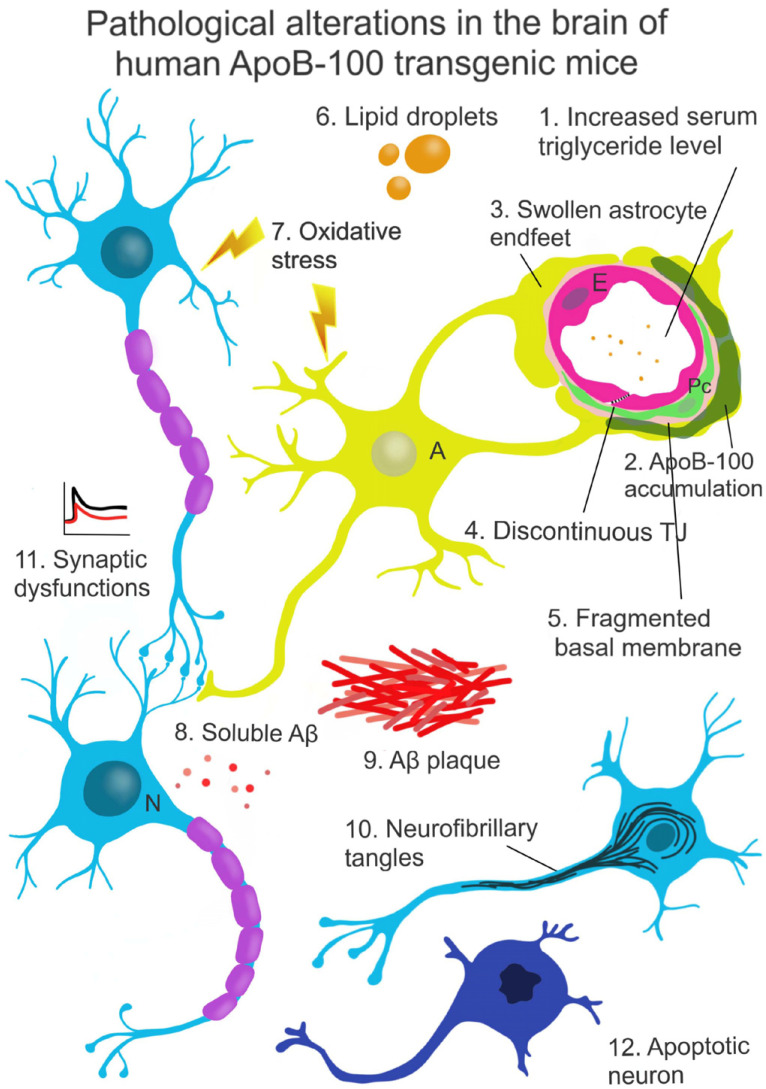
Data from our laboratory, as well as other studies, suggest that dyslipidemia and/or altered levels of certain apolipoproteins, including ApoB-100, might be involved in neurodegenerative processes [18, 103]. We showed that serum triglyceride levels in ApoB-100 transgenic mice were two-fold higher as compared to those seen in wild-types when fed with a normal diet. Moreover, fluorescent immunohistochemical staining revealed that the human ApoB-100 protein was accumulated in the capillaries of the thalamic region in our transgenic mice [104]. The presence of transgenic ApoB-100 protein in brain vessels may result either from extravasation or from in situ production. Our unpublished RT-PCR studies demonstrated the presence of mRNA encoding the human ApoB-100 protein in the microvessel fraction isolated from the brains of transgenic mice. It can be assumed that ApoB-100 overexpression, together with abnormal serum lipid levels, may disturb cerebrovascular functions (Fig. 2).
Fig. (2).
Neurodegenerative changes in the brain of ApoB-100 transgenic mice.
6.2. Morphological Characteristics of the BBB in ApoB-100 Transgenic Mice
These transgenic mice are characterized by a decrease in density and an increase in lumen diameter of cortical microvessels [105]. An increase in permeability for sodium fluorescein (376 Da) was observed in the hippocampus of ApoB-100 overexpressing mice, suggesting TJ impairment. In accordance with this finding, a decreased expression of genes encoding TJ proteins occludin and ZO-1 was detected in microvessels isolated from ApoB-100 transgenic mice, as compared to wild-type controls. In contrast, the mRNA level of claudin-5, another key TJ protein, did not change significantly. Other BBB-associated genes, including the mesenchyme homeobox 2 (Meox2) gene regulating BBB functions [106] also showed a decreased expression in the ApoB-100 transgenic group as compared to wild-type mice. Reduced mRNA levels were also detected for genes encoding selected key BBB transporters, such as Glut-1, docosahexaenoic acid transporter (encoded by the Major Facilitator Superfamily Domain Containing 2A (Mfsd2a) gene), and Pgp, one of the main efflux transporters involved in Aβ clearance (encoded by the ATP-binding cassette transporter B1a (Abcb1a) gene) [18]. These findings coincide with earlier observations showing a decreased expression and function of these BBB transporters in AD-related conditions [103, 107]. A difference in the morphology of brain capillaries was also evident upon comparing ApoB-100 transgenic mice and wild-type controls. Using immunohistochemical labeling, a reduced immunofluorescence intensity for Pgp and vimentin, a cytoskeletal protein, and for the astroglia marker glial fibrillary acidic protein (GFAP) was demonstrated in the cortex of ApoB-100 transgenic animals. In contrast, TJ protein staining patterns were unchanged as compared to wild-type controls. At the ultrastructural level, several discontinuous TJs, edematic astroglial endfeet, a thickened basal membrane, and a reduction of pericyte coverage were detected in ApoB-100 transgenic mice [18]. The observed structural changes may lead to increased BBB permeability and are in line with pathological alterations occurring in AD [103, 108].
The morphology and immunostaining patterns of endothelial cells, astroglia and pericytes of ApoB-100 transgenic and wild-type mice were also compared in cell cultures. Claudin-5 immunolabeling of endothelial cells, GFAP immunostaining of astroglial cells and α-smooth muscle actin labeling of pericytes were similar in both experimental groups, while the immunofluorescence intensities of cytoplasmic linker proteins ZO-1 and β-catenin were higher in cells isolated from the transgenic group. Increased basal production of reactive oxygen species was detected in ApoB-100 transgenic endothelial cells, which was further elevated following exposure to oxidized LDL. Endothelial cells from the transgenic group showed higher membrane rigidity as compared to wild-type cells, which remained unchanged after treatment with oxidized LDL. In contrast, oxidized LDL treatment resulted in increased membrane rigidity in wild-type cells [86].
6.3. Biochemical Characteristics of ApoB-100 Transgenic Mice
The lipoprotein profile of ApoB-100 transgenic mice was significantly different from that of wild-type controls: a decrease in ApoA and an increase in ApoE and LDLR levels were observed in 6-month-old transgenic animals, which might predict later-onset cognitive decline. Using Nile red staining, triglyceride droplets were detected in the cortex and hippocampus of ApoB-100 transgenic mice, suggesting an altered brain lipid homeostasis [17]. In accordance with previous data showing a link between serum triglyceride levels and oxidative stress [68], the level of malonaldehyde, an indicator of lipid peroxidation, was significantly increased in the hippocampus and cortex of hypertriglyceridemic ApoB-100 transgenic mice [104].
Overexpression of the human ApoB-100 protein in transgenic mice was found to affect the level of amyloid precursor protein (APP) in a diet-dependent manner. An increase in the level of membrane-bound APP was detected in transgenic mice fed with a high-cholesterol diet, while a decrease in membrane-bound APP levels was observed in those on a normal diet, compared to wild-type controls [13]. Abnormal lipid metabolism was linked to Aβ plaque formation in the brain of homozygous ApoB transgenic mice, but not in hemizygous animals, as detected by immunohistochemical labeling [109, 17]. However, Löffler et al. demonstrated elevated Aβ levels even in the brain of hemizygous ApoB-100 transgenic animals with an Aβ Triplex assay. The concentration of Aβ in hemizygous ApoB-100 transgenic mice may not be sufficient for plaque formation or may be below the detection limit of immunohistochemical stainings [104]. In contrast to Aβ plaque formation, increased levels of Tau phosphorylation, another major pathological hallmark of AD, were observed in hemizygous ApoB-100 overexpressing mice using immunohistochemical labeling and western blot analysis [17].
In ApoB-100 transgenic mice, characterized by high serum levels of triglyceride (1), extensive accumulation of the ApoB-100 protein in cerebral blood vessels and brain parenchyma was observed (2). Overexpression of the human ApoB-100 protein resulted in morphological alterations of the BBB, including swollen astrocyte endfeet (3), discontinuous intercellular junctions (4) and a thick, fragmented basal membrane, suggesting neurovascular dysfunction (5). Further pathological changes indicating perturbed brain homeostasis include large lipid droplets (6) found in the cortex of aged transgenic animals. This abnormal brain metabolism induced oxidative stress (7). Elevated level of Aβ in the brain of hemizygous mice was detected (8), and in homozygous animals, this same alteration reached the concentration level required for plaque formation (9). The level of Tau phosphorylation was also increased in the brain of ApoB-100 transgenic mice, leading to a disorganized neural microtubular network and the formation of neurofibrillary tangles (10), which were reported to associate with neurodegenerative processes. Smaller PPF ratios and reduced LTP were measured in the brain of ApoB-100 transgenic mice as compared to wild-type controls, indicating synaptic dysfunction (11). The observed molecular alterations might contribute to a high rate of neuronal apoptosis (12) detected in several brain areas of ApoB-100 transgenic mice.
Abbreviations: N - Neuron, A - Astroglia, Pc - Pericyte, E - Endothelial cell. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6.4. Neuropathological Changes in ApoB-100 Transgenic Mice
Our group reported a high rate of neuronal cell death mainly in the hippocampal, cerebrocortical and hypothalamic brain areas of ApoB-100 transgenic animals, detected in a TUNEL assay. This finding was confirmed with Fluoro Jade C staining. Western blot analysis of signaling proteins indicating ongoing apoptosis, such as the proline-rich tyrosine kinase 2 and the mitogen-activated protein kinase, revealed increased expression in the brain of ApoB-100 transgenic animals as compared to wild-type controls. In addition, homozygous ApoB-100 transgenic mice also showed elevated production of proteins associated with neuronal damage, such as S100B, glutamine synthetase, heat shock protein 70, syntaxin, and neuronal nitric oxide synthase. The observed neuronal cell death was associated with an enlargement of the third and lateral ventricles, according to MRI studies [109].
The neuronal function was assessed using electrophysiological methods, including paired-pulse facilitation (PPF) and long-term potentiation (LTP) measurements. Transgenic mice overexpressing the human ApoB-100 protein showed smaller PPF ratios at each interstimulus interval at the age of 3 and 6 months as compared to wild-type littermates, suggesting impaired presynaptic function. Long-term synaptic plasticity, another aspect of synaptic activity, was analyzed in LTP induction measurements. Reduced LTP was detected in ApoB-100 transgenic mice as compared to the wild-type control group [17]. In line with these findings, at the age of 12 months, ApoB-100 transgenic mice showed disturbed spatial and emotional memory and learning deficits as evaluated with Morris Water Maze and Contextual Fear Conditioning tests, respectively [104].
CONCLUSION
A growing number of evidence suggests that the transgenic mouse strain overexpressing the human ApoB-100 protein is not only a model of atherosclerosis and cardiovascular diseases, but it may serve as an animal model to study neurodegenerative alterations as well. An excess of the ApoB-100 protein leads to cerebrovascular dysfunctions, which in turn, result in synaptic abnormalities, cognitive impairment and neuronal cell death. Further studies are needed to shed more light on the specific molecular and cellular mechanisms linking hyperlipidemia to cerebrovascular and neuronal dysfunctions.
Acknowledgements
The authors thank Dora Bokor, PharmD, for proofreading the manuscript.
list of Abbreviations
- Abcb1a
ATP-binding cassette transporter B1a
- AD
Alzheimer’s disease
- Apo
Apolipoprotein
- APP
Amyloid precursor protein
- Aβ
Amyloid-β
- BBB
Blood-brain barrier
- CETP
Cholesterol ester transfer protein
- CM
Chylomicron
- GFAP
Glial fibrillary acidic protein
- GLUT-1
Glucose transporter-1
- HDL
High-density lipoprotein
- IDL
Intermediate-density lipoprotein
- LDL
Low-density lipoprotein
- LDLR
LDL receptor
- LRP
LDL receptor-related protein
- LTP
Long-term potentiation
- Meox2
Mesenchyme homeobox 2
- Mfsd2a
Major Facilitator Superfamily Domain Containing 2A
- Pgp
P-glycoprotein
- PPF
Paired-pulse facilitation
- TJ
Tight junction
- VLDL
Very-low-density lipoprotein
- ZO-1
Zonula occludens-1
Consent for Publication
Not applicable.
Funding
The study was funded by the National Research, Development and Innovation Office, (GINOP 2.3.2.15.2016-00060, GINOP 2.3.2.-15.2016-00040) and by the ÚNKP-19-3-SZTE-67 New National Excellence Program of the Ministry for Innovation and Technology, Hungary.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014;52(12):1695–1727. doi: 10.1515/cclm-2013-0358. [DOI] [PubMed] [Google Scholar]
- 2.Feingold K.R., Grunfeld C. Introduction to lipids and lipoproteins. http://www.ncbi.nlm.nih.gov/pubm ed/26247089
- 3.Shiomi M., Koike T., Ishi T. 2012. Genetically modified animal models for lipoprotein research. [Google Scholar]
- 4.Greeve J., Altkemper I., Dieterich J.H., Greten H., Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J. Lipid Res. 1993;34(8):1367–1383. [PubMed] [Google Scholar]
- 5.Kim E., Young S.G. Genetically modified mice for the study of apolipoprotein B. J. Lipid Res. 1998;39(4):703–723. [PubMed] [Google Scholar]
- 6.Li X., Catalina F., Grundy S.M., Patel S. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J. Lipid Res. 1996;37(1):210–220. [PubMed] [Google Scholar]
- 7.Breslow J.L. Mouse models of atherosclerosis. Science. 1996;272(5262):685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 8.Powell-Braxton L., Véniant M., Latvala R.D., et al. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat. Med. 1998;4(8):934–938. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa G., Johnson D.F., Yao Z., et al. Expression of human apolipoprotein B100 in transgenic mice. Editing of human apolipoprotein B100 mRNA. J. Biol. Chem. 1993;268(32):23747–23750. [PubMed] [Google Scholar]
- 10.Linton M.F., Farese R.V., Jr, Chiesa G., et al. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J. Clin. Invest. 1993;92(6):3029–3037. doi: 10.1172/JCI116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purcell-Huynh D.A., Farese R.V., Jr, Johnson D.F., et al. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J. Clin. Invest. 1995;95(5):2246–2257. doi: 10.1172/JCI117915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callow M.J., Stoltzfus L.J., Lawn R.M., Rubin E.M. Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc. Natl. Acad. Sci. USA. 1994;91(6):2130–2134. doi: 10.1073/pnas.91.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjelik A., Bereczki E., Gonda S., et al. Human apoB overexpression and a high-cholesterol diet differently modify the brain APP metabolism in the transgenic mouse model of atherosclerosis. Neurochem. Int. 2006;49(4):393–400. doi: 10.1016/j.neuint.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Huang L.S., Voyiaziakis E., Markenson D.F., Sokol K.A., Hayek T., Breslow J.L. apo B gene knockout in mice results in embryonic lethality in homozygotes and neural tube defects, male infertility, and reduced HDL cholesterol ester and apo A-I transport rates in heterozygotes. J. Clin. Invest. 1995;96(5):2152–2161. doi: 10.1172/JCI118269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farese R.V., Jr, Ruland S.L., Flynn L.M., Stokowski R.P., Young S.G. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 1995;92(5):1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caramelli P., Nitrini R., Maranhão R., et al. Increased apolipoprotein B serum concentration in Alzheimer’s disease. Acta Neurol. Scand. 1999;100(1):61–63. doi: 10.1111/j.1600-0404.1999.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 17.Lénárt N., Szegedi V., Juhász G., et al. Increased tau phosphorylation and impaired presynaptic function in hypertriglyceridemic ApoB-100 transgenic mice. PLoS One. 2012;7(9):e46007. doi: 10.1371/journal.pone.0046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyk Z., Tóth M.E., Lénárt N., et al. Cerebrovascular pathology in hypertriglyceridemic APOB-100 transgenic mice. Front. Cell. Neurosci. 2018;12:380. doi: 10.3389/fncel.2018.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csont T., Bereczki E., Bencsik P., et al. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc. Res. 2007;76(1):100–109. doi: 10.1016/j.cardiores.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Sanan D.A., Newland D.L., Tao R., et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc. Natl. Acad. Sci. USA. 1998;95(8):4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grass D.S., Saini U., Felkner R.H., et al. Transgenic mice expressing both human apolipoprotein B and human CETP have a lipoprotein cholesterol distribution similar to that of normolipidemic humans. J. Lipid Res. 1995;36(5):1082–1091. [PubMed] [Google Scholar]
- 22.Collaboration P.S. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 23.Williams K.J., Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995;15(5):551–561. doi: 10.1161/01.ATV.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callow M.J., Verstuyft J., Tangirala R., Palinski W., Rubin E.M. Atherogenesis in transgenic mice with human apolipoprotein B and lipoprotein (a). J. Clin. Invest. 1995;96(3):1639–1646. doi: 10.1172/JCI118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofman A., Ott A., Breteler M.M., et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 26.Kivipelto M., Ngandu T., Fratiglioni L., et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 27.Xu W., Qiu C., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58(1):71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laukka E.J., Fratiglioni L., Bäckman L. The influence of vascular disease on cognitive performance in the preclinical and early phases of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2010;29(6):498–503. doi: 10.1159/000313978. [DOI] [PubMed] [Google Scholar]
- 29.Lathe R., Sapronova A., Kotelevtsev Y. Atherosclerosis and Alzheimer-diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014;14:36. doi: 10.1186/1471-2318-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos C.Y., Snyder P.J., Wu W-C., Zhang M., Echeverria A., Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement. (Amst.) 2017;7:69–87. doi: 10.1016/j.dadm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Fanning A.S., Anderson J.M. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos-Bedolla P., Walter F.R., Veszelka S., Deli M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014;45(8):610–638. doi: 10.1016/j.arcmed.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Blasiole D.A., Davis R.A., Attie A.D. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 2007;3(9):608–619. doi: 10.1039/b700706j. [DOI] [PubMed] [Google Scholar]
- 35.Brown M.S., Kovanen P.T., Goldstein J.L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- 36.Begley D.J. ABC transporters and the blood-brain barrier. Curr. Pharm. Des. 2004;10(12):1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 37.Janzer R.C., Raff M.C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 38.Estrada C., Bready J.V., Berliner J.A., Pardridge W.M., Cancilla P.A. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J. Neuropathol. Exp. Neurol. 1990;49(6):539–549. doi: 10.1097/00005072-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Kacem K., Lacombe P., Seylaz J., Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23(1):1–10. doi: 10.1002/(SICI)1098-1136(199805)23:1<1:AID-GLIA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 40.Willis C.L., Leach L., Clarke G.J., Nolan C.C., Ray D.E. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48(1):1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- 41.Rash J.E., Yasumura T., Hudson C.S., Agre P., Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA. 1998;95(20):11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palazzo C., Buccoliero C., Mola M.G., et al. AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathol. Commun. 2019;7(1):51. doi: 10.1186/s40478-019-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen S., Nagelhus E.A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O.P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nehls V., Schuchardt E., Drenckhahn D. The effect of fibroblasts, vascular smooth muscle cells, and pericytes on sprout formation of endothelial cells in a fibrin gel angiogenesis system. Microvasc. Res. 1994;48(3):349–363. doi: 10.1006/mvre.1994.1061. [DOI] [PubMed] [Google Scholar]
- 45.Arihiro S., Ohtani H., Hiwatashi N., Torii A., Sorsa T., Nagura H. Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology. 2001;39(1):50–59. doi: 10.1046/j.1365-2559.2001.01142.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakaoke R., Verma S., Niwa M., et al. Glucose-regulated blood-brain barrier transport of insulin: pericyte-astrocyte-endothelial cell cross talk. Int. J. Neuroprot. Neuroregener. 2007;3:195–200. [Google Scholar]
- 47.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 2008;14(16):1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- 48.Kutcher M.E., Herman I.M. The pericyte: cellular regulator of microvascular blood flow. Microvasc. Res. 2009;77(3):235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armulik A., Genové G., Mäe M., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 50.Wang S., Voisin M-B., Larbi K.Y., et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 2006;203(6):1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armulik A., Genové G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Sagare A.P., Bell R.D., Zhao Z., et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Nakagomi T., Kubo S., Nakano-Doi A., et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33(6):1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 54.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banks W.A., Kovac A., Morofuji Y. Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow Metab. 2018;38(6):1104–1118. doi: 10.1177/0271678X17740793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rochfort K.D., Cummins P.M. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015;43(4):702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 57.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muñoz-Fernández M.A., Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998;56(3):307–340. doi: 10.1016/S0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 59.Incalza M.A., D’Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Dias H.K.I., Brown C.L.R., Polidori M.C., Lip G.Y., Griffiths H.R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin. Sci. (Lond.) 2015;129(12):1195–1206. doi: 10.1042/CS20150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J.H., Hong K.S., Lee E.J., Lee J., Kim D.E. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke. 2011;42(11):3040–3046. doi: 10.1161/STROKEAHA.111.620104. [DOI] [PubMed] [Google Scholar]
- 62.Turan T.N., Makki A.A., Tsappidi S., et al. WASID Investigators Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41(8):1636–1640. doi: 10.1161/STROKEAHA.110.584672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowman G.L., Kaye J.A., Quinn J.F. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr. Gerontol. Geriatr. Res. 2012;2012:184042. doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chattopadhyay R., Dyukova E., Singh N.K., Ohba M., Mobley J.A., Rao G.N. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C ε-mediated zona occludens-1 phosphorylation at threonine 770/772. J. Biol. Chem. 2014;289(6):3148–3163. doi: 10.1074/jbc.M113.528190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eiselein L., Wilson D.W., Lamé M.W., Rutledge J.C. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2007;292(6):H2745–H2753. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- 66.Wang L., Sapuri-Butti A.R., Aung H.H., Parikh A.N., Rutledge J.C. Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2008;295(1):H237–H244. doi: 10.1152/ajpheart.01366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonios N., Angiolillo D.J., Silliman S. Hypertriglyceridemia and ischemic stroke. Eur. Neurol. 2008;60(6):269–278. doi: 10.1159/000157880. [DOI] [PubMed] [Google Scholar]
- 68.Klafke J.Z., Porto F.G., Batista R., et al. Association between hypertriglyceridemia and protein oxidation and proinflammatory markers in normocholesterolemic and hypercholesterolemic individuals. Clin. Chim. Acta. 2015;448:50–57. doi: 10.1016/j.cca.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Lee L.L., Aung H.H., Wilson D.W., Anderson S.E., Rutledge J.C., Rutkowsky J.M. Triglyceride-rich lipoprotein lipolysis products increase blood-brain barrier transfer coefficient and induce astrocyte lipid droplets and cell stress. Am. J. Physiol. Cell Physiol. 2017;312(4):C500–C516. doi: 10.1152/ajpcell.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jais A., Solas M., Backes H., et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;165(4):882–895. doi: 10.1016/j.cell.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 71.Erickson M.A., Dohi K., Banks W.A. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121–130. doi: 10.1159/000330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storck S.E., Hartz A.M.S., Bernard J., et al. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav. Immun. 2018;73:21–33. doi: 10.1016/j.bbi.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cirrito J.R., Deane R., Fagan A.M., et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 2005;115(11):3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zechariah A., ElAli A., Hagemann N., et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013;33(7):1561–1567. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 75.Hohsfield L.A., Daschil N., Orädd G., Strömberg I., Humpel C. Vascular pathology of 20-month-old hypercholesterolemia mice in comparison to triple-transgenic and APPSwDI Alzheimer’s disease mouse models. Mol. Cell. Neurosci. 2014;63:83–95. doi: 10.1016/j.mcn.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Kuo Y.M., Emmerling M.R., Bisgaier C.L., et al. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain abeta 1-42 levels. Biochem. Biophys. Res. Commun. 1998;252(3):711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 78.Wingo T.S., Cutler D.J., Wingo A.P., et al. Association of early-onset alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB. JAMA Neurol. 2019;76(7):809–817. doi: 10.1001/jamaneurol.2019.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams V.J., Leritz E.C., Shepel J., et al. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum. Brain Mapp. 2013;34(8):1826–1841. doi: 10.1002/hbm.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song F., Poljak A., Crawford J., et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One. 2012;7(6):e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawano M., Kawakami M., Otsuka M., Yashima H., Yaginuma T., Ueki A. Marked decrease of plasma apolipoprotein AI and AII in Japanese patients with late-onset non-familial Alzheimer’s disease. Clin. Chim. Acta. 1995;239(2):209–211. doi: 10.1016/0009-8981(95)06115-T. [DOI] [PubMed] [Google Scholar]
- 82.Merched A., Xia Y., Visvikis S., Serot J.M., Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging. 2000;21(1):27–30. doi: 10.1016/S0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 83.Namba Y., Tsuchiya H., Ikeda K. Apolipoprotein B immunoreactivity in senile plaque and vascular amyloids and neurofibrillary tangles in the brains of patients with Alzheimer’s disease. Neurosci. Lett. 1992;134(2):264–266. doi: 10.1016/0304-3940(92)90531-B. [DOI] [PubMed] [Google Scholar]
- 84.Takechi R., Galloway S., Pallebage-Gamarallage M., Wellington C., Johnsen R., Mamo J.C. Three-dimensional colocalization analysis of plasma-derived apolipoprotein B with amyloid plaques in APP/PS1 transgenic mice. Histochem. Cell Biol. 2009;131(5):661–666. doi: 10.1007/s00418-009-0567-3. [DOI] [PubMed] [Google Scholar]
- 85.Dodelet-Devillers A., Cayrol R., van Horssen J., et al. Functions of lipid raft membrane microdomains at the blood-brain barrier. J. Mol. Med. (Berl.) 2009;87(8):765–774. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- 86.Lénárt N., Walter F.R., Bocsik A., et al. Cultured cells of the blood-brain barrier from apolipoprotein B-100 transgenic mice: effects of oxidized low-density lipoprotein treatment. Fluids Barriers CNS. 2015;12:17. doi: 10.1186/s12987-015-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raffaitin C., Gin H., Empana J-P., et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the three-city study. Diabetes Care. 2009;32(1):169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nägga K., Gustavsson A-M., Stomrud E., et al. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology. 2018;90(1):e73–e81. doi: 10.1212/WNL.0000000000004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burgess B.L., McIsaac S.A., Naus K.E., et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A β in plasma. Neurobiol. Dis. 2006;24(1):114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Houlden H., Crook R., Hardy J., Roques P., Collinge J., Rossor M. Confirmation that familial clustering and age of onset in late onset Alzheimer’s disease are determined at the apolipoprotein E locus. Neurosci. Lett. 1994;174(2):222–224. doi: 10.1016/0304-3940(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 91.Houlden H., Crook R., Duff K., et al. Apolipoprotein E alleles but neither apolipoprotein B nor apolipoprotein AI/CIII alleles are associated with late onset, familial Alzheimer’s disease. Neurosci. Lett. 1995;188(3):202–204. doi: 10.1016/0304-3940(95)11422-S. [DOI] [PubMed] [Google Scholar]
- 92.Li G., Bien-Ly N., Andrews-Zwilling Y., et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5(6):634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koizumi K., Hattori Y., Ahn S.J., et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 2018;9(1):3816. doi: 10.1038/s41467-018-06301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tachibana M., Holm M-L., Liu C-C., et al. APOE4-mediated amyloid-β pathology depends on its neuronal receptor LRP1. J. Clin. Invest. 2019;129(3):1272–1277. doi: 10.1172/JCI124853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng K.Y., Pérez-González R., Alldred M.J., et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019;142(1):163–175. doi: 10.1093/brain/awy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nuriel T., Peng K.Y., Ashok A., et al. The endosomal-lysosomal pathway is dysregulated by APOE4 expression in vivo. Front. Neurosci. 2017;11:702. doi: 10.3389/fnins.2017.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pullinger C.R., Kane J.P., Malloy M.J. Primary hypercholesterolemia: genetic causes and treatment of five monogenic disorders. Expert Rev. Cardiovasc. Ther. 2003;1(1):107–119. doi: 10.1586/14779072.1.1.107. [DOI] [PubMed] [Google Scholar]
- 98.Zambón D., Quintana M., Mata P., et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am. J. Med. 2010;123(3):267–274. doi: 10.1016/j.amjmed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elder G.A., Ragnauth A., Dorr N., et al. Increased locomotor activity in mice lacking the low-density lipoprotein receptor. Behav. Brain Res. 2008;191(2):256–265. doi: 10.1016/j.bbr.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreira E.L.G., de Oliveira J., Nunes J.C., et al. Age-related cognitive decline in hypercholesterolemic LDL receptor knockout mice (LDLr-/-): evidence of antioxidant imbalance and increased acetylcholinesterase activity in the prefrontal cortex. J. Alzheimers Dis. 2012;32(2):495–511. doi: 10.3233/JAD-2012-120541. [DOI] [PubMed] [Google Scholar]
- 101.Wang S.H., Huang Y., Yuan Y., Xia W.Q., Wang P., Huang R. LDL receptor knock-out mice show impaired spatial cognition with hippocampal vulnerability to apoptosis and deficits in synapses. Lipids Health Dis. 2014;13:175. doi: 10.1186/1476-511X-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Oliveira J., Hort M.A., Moreira E.L.G., et al. Positive correlation between elevated plasma cholesterol levels and cognitive impairments in LDL receptor knockout mice: relevance of cortico-cerebral mitochondrial dysfunction and oxidative stress. Neuroscience. 2011;197:99–106. doi: 10.1016/j.neuroscience.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Löffler T., Flunkert S., Havas D., et al. Impact of ApoB-100 expression on cognition and brain pathology in wild-type and hAPPsl mice. Neurobiol. Aging. 2013;34(10):2379–2388. doi: 10.1016/j.neurobiolaging.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 105.Süle Z., Mracskó E., Bereczki E., et al. Capillary injury in the ischemic brain of hyperlipidemic, apolipoprotein B-100 transgenic mice. Life Sci. 2009;84(25-26):935–939. doi: 10.1016/j.lfs.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 106.Wu Z., Guo H., Chow N., et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 2005;11(9):959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 107.Zhao Z., Zlokovic B.V. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82(4):728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomimoto H., Akiguchi I., Wakita H., Suenaga T., Nakamura S., Kimura J. Regressive changes of astroglia in white matter lesions in cerebrovascular disease and Alzheimer’s disease patients. Acta Neuropathol. 1997;94(2):146–152. doi: 10.1007/s004010050686. [DOI] [PubMed] [Google Scholar]
- 109.Bereczki E., Bernát G., Csont T., Ferdinandy P., Scheich H., Sántha M. Overexpression of human apolipoprotein B-100 induces severe neurodegeneration in transgenic mice. J. Proteome Res. 2008;7(6):2246–2252. doi: 10.1021/pr7006329. [DOI] [PubMed] [Google Scholar]