Abstract
Background
Symptomatic treatments of Alzheimer’s Disease (AD) with cholinesterase inhibitors and/or memantine are relatively ineffective and there is a need for new treatments targeting the underlying pathology of AD. In most of the failed disease-modifying trials, patients have been allowed to continue taking symptomatic treatments at stable doses, under the assumption that they do not impair efficacy. In recently completed Phase 3 trials testing the tau aggregation inhibitor leuco-methylthioninium bis (hydromethane-sulfonate) (LMTM), we found significant differences in treatment response according to whether patients were taking LMTM either as monotherapy or as an add-on to symptomatic treatments.
Methods
We have examined the effect of either LMTM alone or chronic rivastigmine prior to LMTM treatment of tau transgenic mice expressing the short tau fragment that constitutes the tangle filaments of AD. We have measured acetylcholine levels, synaptosomal glutamate release, synaptic proteins, mitochondrial complex IV activity, tau pathology and Choline Acetyltransferase (ChAT) immunoreactivity.
Results
LMTM given alone increased hippocampal Acetylcholine (ACh) levels, glutamate release from synaptosomal preparations, synaptophysin levels in multiple brain regions and mitochondrial complex IV activity, reduced tau pathology, partially restored ChAT immunoreactivity in the basal forebrain and reversed deficits in spatial learning. Chronic pretreatment with rivastigmine was found to reduce or eliminate almost all these effects, apart from a reduction in tau aggregation pathology. LMTM effects on hippocampal ACh and synaptophysin levels were also reduced in wild-type mice.
Conclusion
The interference with the pharmacological activity of LMTM by a cholinesterase inhibitor can be reproduced in a tau transgenic mouse model and, to a lesser extent, in wild-type mice. Long-term pretreatment with a symptomatic drug alters a broad range of brain responses to LMTM across different transmitter systems and cellular compartments at multiple levels of brain function. There is, therefore, no single locus for the negative interaction. Rather, the chronic neuronal activation induced by reducing cholinesterase function produces compensatory homeostatic downregulation in multiple neuronal systems. This reduces a broad range of treatment responses to LMTM associated with a reduction in tau aggregation pathology. Since the interference is dictated by homeostatic responses to prior symptomatic treatment, it is likely that there would be similar interference with other drugs tested as add-on to the existing symptomatic treatment, regardless of the intended therapeutic target or mode of action. The present findings outline key results that now provide a working model to explain interference by symptomatic treatment.
Keywords: Tau aggregation inhibitor, hydromethylthionine, mouse model, Alzheimer’s disease, tauopathy, acetylcholinesterase inhibitor (AChEI), drug interaction, synaptic proteins
1. INTRODUCTION
The only treatments currently available for Alzheimer’s Disease (AD) are symptomatic. The most widely used of these are the Acetylcholinesterase Inhibitors (AChEIs), which work by chronically increasing the levels of Acetylcholine (ACh) in the synaptic cleft. In experimental models, the cholinergic function is associated primarily with selective attention [1-3], and is not particularly sensitive to more broadly based measures of functional impairment/improvement [4, 5]. Similar considerations apply to memantine, that also modulates brain function in a non-specific manner [6, 7]. The therapeutic benefits of these treatments are relatively short-lasting [8], with fewer than 30% of patients continuing on AChEIs 12 months after initiation [9-11]. A substantial proportion of AD patients are in any case not treated, ~44% in the US [12] and ~77% in the UK [13]. In France, reimbursement for these drugs has been withdrawn because of “insufficient medical benefit and dangerousness because of side effects” [14]. The low medical use and patient adherence are due to low perceived efficacy and side effects, with the rate of decline after temporary symptomatic improvement, no different from that occurring in the absence of treatment [8]. Hence it is agreed generally that a major unmet medical need exists to develop a treatment able to slow the progression of AD. A Lancet Neurology Commission report [15] noted that “no treatment is yet available to halt or reverse the underlying pathology of established AD. Indeed, an effective therapy for AD is perhaps the greatest unmet need facing modern medicine”. The last new treatment to have been approved for AD was in 2003 for memantine [16]. From 2002 to 2012, there have been 289 clinical trials at Phase 2 or Phase 3, with an overall failure rate of 99.6% [17]. There have been 19 further trial failures since 2012 targeting various aspects of pathological processing of β-amyloid [18].
Despite the limitations of available symptomatic treatments, almost all late-stage clinical trials currently ongoing or recently completed aiming to test new therapeutic approaches have been conducted in patient populations where the majority of subjects continue taking symptomatic treatments [18]. This is determined, in part, by ethical concerns that patients participating in potentially lengthy clinical trials would deny access to any treatment if randomised to the placebo arm. A further consideration is an unproven assumption that symptomatic treatments do not interfere with treatments targeting the underlying pathology because their modes of action are different. We show in the present report that this assumption is incorrect.
Leuco-methylthioninium bis(hydromethanesulfonate) (LMTM; USAN name hydromethylthionine mesylate) is being developed as a treatment targeting pathological aggregation of tau protein in AD [19]. The methylthioninium (MT) moiety can exist in oxidised (MT+) and reduced (LMT) forms. LMTM is a stabilised salt of LMT which has much better pharmaceutical properties than the oxidised MT+ form [20, 21]. We have recently reported that LMT rather than MT+ is the active species blocking tau aggregation in vitro [22]. LMT blocks tau aggregation in vitro in cell-free and cell-based assays [20, 22] and reduces tau aggregation pathology and associated behavioural deficits in tau transgenic mouse models in vivo at clinically relevant doses [23]. LMT also disaggregates the Paired Helical Filaments (PHFs) isolated from AD brain tissues converting the tau protein into a form that becomes susceptible to proteases [20, 24].
The MT moiety also has a range of other potentially beneficial properties. It has been known for some time that at low concentrations (10 - 100 nM), it enhances mitochondrial activity by acting as a supplementary electron carrier in the electron transfer chain. The MT moiety undergoes redox cycling catalysed by complex I using NADH as a co-factor whereby it accepts electrons that are subsequently transferred to complexes III and IV [25-27]. It is able also to induce mitochondrial biogenesis and to activate Nrf2-mediated oxidative stress response elements in vivo [28]. Other activities include neuroprotective effects in the brain by inhibiting microglial activation and increasing autophagy [29]. The MT moiety has been shown to increase the clearance of pathological tau in vivo via enhancement of autophagy at the 10 - 20nM concentration range [30]. Therefore, in addition to the dissolution of AD tau aggregates, LMTM has numerous complementary actions that address many of the pathways currently advocated as having potential for the treatment of AD [31, 32].
Although LMTM given orally produces brain levels sufficient for activity in vitro and in vivo [21], it had minimal apparent efficacy if taken as an add-on to symptomatic treatments in two large Phase 3 clinical trials [33, 34]. In subjects receiving LMTM as monotherapy, however, the treatment produced marked reduced cognitive and functional decline, reduction in the rate of progression of brain atrophy measured by MRI and reduction in the loss of glucose uptake measured by FDG-PET [33, 34]. In recent analyses that include steady-state plasma concentration data, consistent concentration-dependent treatment effects have been found for LMTM as monotherapy and as add-on therapy, but reduced by about half as an add-on [35].
In this report, we provide a preliminary summary of results from a large body of ongoing work addressing the unexpected differences in treatment effects for monotherapy and add-on therapy that were seen in two large clinical trials of LMTM. We have back-translated the clinical experimental design into a preclinical context and report that similar differences in neuropharmacological activity can be reproduced in a well-characterised tau transgenic mouse model (Line 1, “L1”; [36]). The purpose of the present report is to provide an initial overview of results to date, which shows that chronic pretreatment with a cholinesterase inhibitor reduces the pharmacological effects of LMTM at multiple brain levels and systems, including networks, transmitters, cellular compartments and intracellular pathways. This report will be followed in due course by more detailed reports for each of the experimental paradigms we have examined. However, the results already available provide support for a general working hypothesis to explain the mechanisms responsible for anticholinesterase interference with tau aggregation inhibitor activity in the brain.
Our findings suggest that homeostatic mechanisms downregulate multiple neuronal systems at different levels of brain function to compensate for the chronic pharmacological activation induced by symptomatic treatments. Compared with LMTM given alone, the effect of this downregulation is to reduce neurotransmitter release, levels of synaptic proteins, mitochondrial function and behavioural benefits if LMTM is given against a background of chronic exposure prior to acetylcholinesterase inhibitor. Therefore, the interference in the pharmacological activity of LMTM first seen clinically has a clear neuropharmacological basis that can be reproduced in a tau transgenic mouse model. The homeostatic effects we have identified are likely to have more general relevance for the conduct of disease-modifying trials in AD that need not be restricted to tau aggregation inhibitors.
2. METHODS
2.1. Features of the Tau Transgenic Mouse Model Used for Interference Studies
In the L1 mouse model, which forms the basis of the present studies, there is an over-expression of a three-repeat tau fragment encompassing residues 296-390 of the 2N4R tau isoform under the control of the Thy 1 promotor in an NMRI mouse strain [36]. This fragment corresponds to the segment of tau first identified within the proteolytically stable core of the PHF [37, 38] and recently confirmed by cryo-electron microscopy [39]. The site of action of LMT is located within the core tau unit of the PHF [20, 22]. In the L1 mouse, the core tau unit is fused with a signal sequence that directs the protein to the Endoplasmic Reticulum (ER) where it initiates aggregation. Tau aggregation remains at the oligomer stage and spreads in an age-dependent fashion from medial temporal lobe structures into isocortex, mimicking the spread of tau pathology in the human brain [36]. Cognitive impairment appears from 3 months onwards and is reversed by treatment with LMT salts at a dose of 15 mg/kg/day given by oral gavage. This cognitive improvement is accompanied by a decrease in tau pathology [23].
2.2. Experimental Paradigms
In what follows, we summarise some of the key results obtained so far for the AChEI, rivastigmine. Similar experiments with memantine are still ongoing. Although donepezil is more widely used clinically than rivastigmine (in the UK, for example, see https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis), initial ex-periments showed that donepezil is too toxic at therapeutic doses in mice to permit chronic administration.
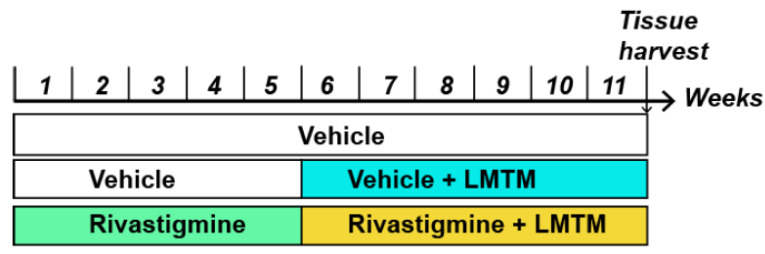
The treatment schedule used to study the negative interaction between symptomatic treatments and LMTM was designed to model the clinical situation in which subjects are first treated chronically with a cholinesterase inhibitor or memantine before receiving LMTM Fig. (1). After five weeks of daily gavaging with vehicle or rivastigmine, combination treatment proceeded in some groups while others received only LMTM monotherapy.
Fig. (1).
Treatment regime outline used in this study to mimic previous clinical trials. While there has been no inclusion of placebo control in the clinical trials, we have included a vehicle group gavaged with saline throughout. In addition, a 5-week monotherapy was typically followed by a 6-week treatment of either vehicle +LMTM or rivastigmine +LMTM followed by tissue harvest. Drugs were administered as a daily oral cocktail. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Wild-type and L1 female mice (n = 7-16 for each group) were pre-treated with rivastigmine (0.1 or 0.5 mg/kg/day) or vehicle for 5 weeks by gavage. For the following 6 weeks, LMTM (5 and 15 mg/kg) was added to this daily treatment regime, also administered by gavage Fig. (1). Animals were then sacrificed for immunohistochemical and other tissue analyses, as described in a study [36]. Although 5 mg/kg/day in mice corresponds approximately to 8 mg/day in humans in terms of Cmax levels of parent MT in plasma, this dose is at the threshold for effects on pathology and behaviour. The higher dose of 15 mg/kg/day is generally required for LMTM to be fully effective in the L1 mouse model [23]. This may relate to the much shorter half-life of MT in mice (4 hours) compared to humans (37 hours in elderly humans).
2.3. Immunohistochemistry of Presynaptic Proteins
The tissue was harvested from intracardiac perfused mice, and right hemispheres were used for paraffin sectioning at different levels, according to stereotaxic coordinates [40], for specific staining of synaptic proteins in regions of interest: Medial septum, vertical or horizontal limb of the diagonal band of Broca, nucleus basalis of Meynert, nucleus accumbens, striatum, primary motor cortex, visual cortex and hippocampal CA1. Primary antibodies to synaptic proteins were purchased from Cell Signaling (α-synuclein), Biolegend (SNAP25), abcam (syntaxin) and Synaptic Systems (VAMP2, and synaptophysin 1). For immunohistochemistry, the previously described protocols were used [41, 42]. Sections were analysed using a Zeiss Axiovert 135 microscope by an investigator blinded to genotype and treatment details. Images were saved in jpg format and labelling was measured as integrated density in 8-bit images using the ImageJ tool (version 1.48v, NIH, USA). Data are presented as Z-score transformations without units.
2.4. Tau and ChAT Immunohistochemistry
The brains of five animals from each treatment group were cut coronally through the forebrain for anti-tau (paraffin sections of 6 μm thickness) and anti-ChAT (frozen sections of 40 μm thickness) immunohistochemistry. Tau mAb, raised against and recognizing truncated tau (2N4R)297-391 (TauRx Therapeutics Ltd.), was used as the primary antibody (1:100) with goat anti-mouse IgG-horseradish peroxidase (HRP) (AP124P; Merck) as the secondary antibody (1:100). Staining was visualised using 3,3’-diaminobenzidine (DAB; Sigma; 0.025%) as a chromogen. Images of anti-tau stained sections were taken at 100× magnification by using a Nikon Eclipse Ni-E microscope equipped with a DSRi2 camera and NIS Elements BR4.30.00 image capture software (Nikon, Japan). Two representative sections of each mouse brain were imaged and the areas and densities of the setting regions of interest in the vertical limb of the diagonal band of Broca for anti-tau were measured using the software. The staining intensity was defined from 0 for white to 255 for black and the intensity of specific staining was expressed as the mean intensity of pixels in the region of interest. The measured values were expressed as Relative Optical Intensity (ROI).
For the immunohistochemical determination of ChAT, mAb AP144P (Merck; 1:200) was used followed by a rabbit anti-goat-HRP secondary antibody (AP106P; Merck; 1:100) and visualized using DAB. For the analysis of cholinergic neurons, packing density sections were imaged using a Nikon Eclipse Ni-E microscope and NIS-Elements software. ChAT-immunoreactive (ChAT-ir) neurons were counted in the vertical limb of the diagonal band of Broca (VDB) identified in accordance with the Mouse Brain Stereotaxic Atlas [40]. Regions of interest were outlined using the X-Y plotting system of the software that measures the square area (mm2) of the marked frame, and ChAT-ir neurons were counted at 400x magnification. Cell counts per section were then corrected using Abercrombie’s formula [43], and the packing density of cholinergic neurons was calculated as a function of the rostrocaudal level and location within the VDB by using the cell counts divided by area of the marked frame in each analyzed section. The number of ChAT-ir neurons was counted in two sections per mouse in VDB of five mice from each treatment group.
2.5. Brain Mitochondrial Complex IV Activity
Total brain homogenates from 12-17 animals of 13 treatment groups Fig. (1) were used to extract proteins and specific cytochrome-c oxidase (complex IV) activity was measured using a commercially available kit (abcam, #ab109911) and normalized to the activity of citrate synthase, determined using a commercially available kit (BioVision, #K318-100) as an index of mitochondrial enrichment in the preparations.
2.6. Acetylcholine Measurement in Hippocampus
Animals were treated with LMTM (5 mg/kg/day for 2 weeks, gavage) after prior treatment for 2 weeks with or without rivastigmine (0.5 mg/kg/day subcutaneous Alzet minipump). Levels of ACh were measured in the hippocampus via indwelling microdialysis probes and HPLC analysis of the extracellular fluid. After the experiment, brains were harvested and histologically assessed for correct cannula placement [44].
2.7. Statistical Analyses
Data are presented as group averages and standard errors of mean and were analysed using parametric statistics, with alpha set to 0.05.
3. RESULTS
3.1. Features of the Tau Transgenic Mouse Model
Features of the L1 mouse model that have emerged in the course of the present studies include a prominent loss of neuronal immunoreactivity for ChAT in the basal forebrain region, and a corresponding reduction in AChE activity in the cortex and hippocampus. There is also an approximate 50% reduction in glutamate release from brain synaptosomal preparations in L1 mice compared with wild-type mice. In these respects, therefore, L1 mice also model the neurochemical impairments in cholinergic [45, 46] and glutamatergic [47] function that are characteristic of AD.
Underlying these impairments in neurotransmitter function, the L1 mouse model shows a disturbance in the integration of synaptic proteins. Quantitative immunohistochemistry for multiple synaptic proteins in the basal forebrain (vertical diagonal band of Broca selected as a representative example) shows that there is normally a high degree of correlation in levels of proteins of the SNARE complex (SNAP-25, syntaxin, VAMP2) and the vesicular glycoprotein synaptophysin and α-synuclein in wild-type mice. All these correlations are lost in L1 mice (Table 1). New correlations appeared between synaptophysin and VAMP2, and syntaxin and synapsin. Therefore, synaptic vesicular protein levels are no longer linked quantitatively to the proteins of the SNARE complex or α-synuclein. This suggests that the tau oligomer pathology of the L1 mice interferes with the functional integration between vesicular and membrane-docking proteins in the synapse.
Table 1.
Positive correlations between levels of a range of presynaptic proteins in the basal forebrain (vertical diagonal band of Broca as a representative example) measured immunohistochemically in (A) wild-type mice or (B) tau transgenic L1 mice.
| A Wild-Type Mice | α-Synuclein | SNAP25 | Syntaxin | VAMP2 | Synaptophysin |
|---|---|---|---|---|---|
| α-Synuclein | |||||
| SNAP25 | * | ||||
| Syntaxin | ─ | ** | |||
| VAMP2 | ─ | * | * | ||
| Synaptophysin | ─ | ** | * | ─ | |
| Synapsin | ─ | ─ | ─ | ─ | ─ |
| B L1 Mice | α-Synuclein | SNAP25 | Syntaxin | VAMP2 | Synaptophysin |
| α-Synuclein | |||||
| SNAP25 | ─ | ||||
| Syntaxin | ─ | ─ | |||
| VAMP2 | ─ | ─ | ─ | ||
| Synaptophysin | ─ | ─ | ─ | * | |
| Synapsin | ─ | ─ | * | ─ | ─ |
Note: n = 9 per genotype. Significance of correlations, by linear regression analysis, are denoted as: *, p < 0.05; **, p < 0.01; ─, no significance at p = 0.05.
3.2. Effects of Treatment with LMTM and Rivastigmine in Wild-Type Mice
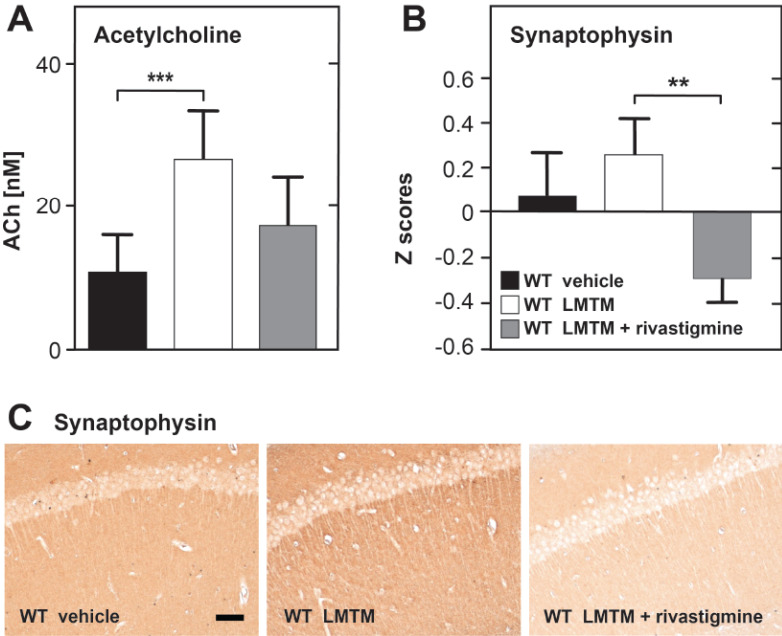
In wild-type mice, there was a significant, 2-fold increase in basal ACh levels in the hippocampus following LMTM treatment and a 30% reduction when mice received LMTM after chronic treatment with rivastigmine Fig. (2A). There was also an increase in mean synaptophysin levels measured in the hippocampus, visual cortex, diagonal band and septum following LMTM treatment alone and a statistically significant reduction of the same magnitude when LMTM was given against a background of prior treatment with rivastigmine Fig. (2B).
Fig. (2).
Treatment effects of LMTM alone or following chronic pretreatment with rivastigmine in wild-type mice. Hippocampal levels of ACh (A), measured by high performance liquid chromatography as described previously [44], or synaptophysin levels for the hippocampus, visual cortex, diagonal band of Broca and septum (B) are shown (total n = 25). Data expressed as mean values + SE (**, p< 0.01; ***, p< 0.001). Photomicrographs (C) show representative images of synaptophysin labelling in hippocampal CA1 in respective groups. Scale bar, 100 µm. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.3. Effects of Treatment with LMTM and Rivastigmine in Tau Transgenic L1 Mice
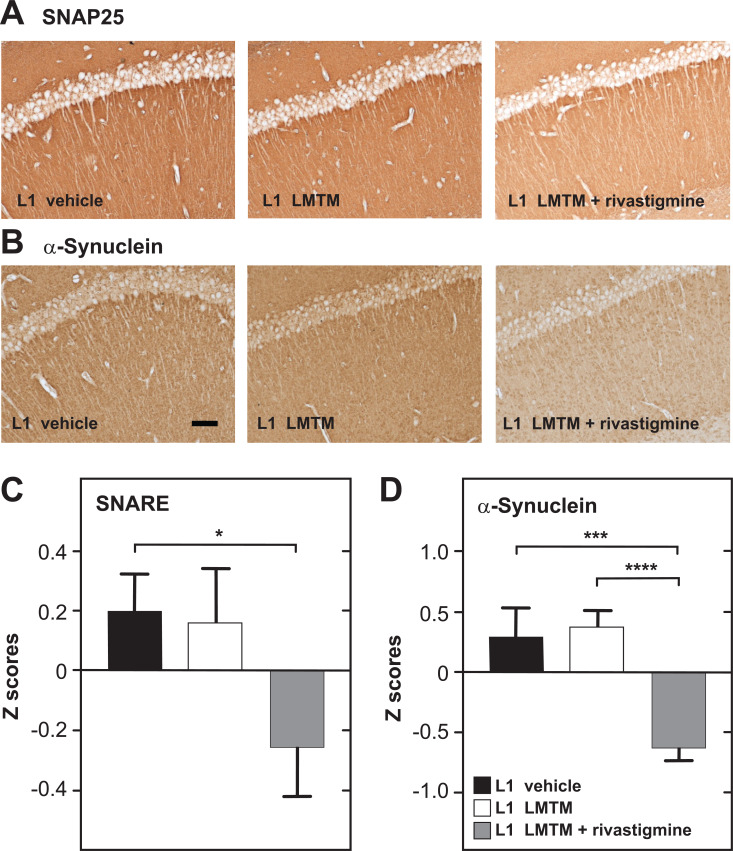
In tau transgenic L1 mice, LMTM alone produced significant increases in ACh release in the hippocampus, in glutamate release from synaptosomal brain preparations (not shown here), in synaptophysin levels, in mitochondrial complex IV activity and in spatial cognition (water maze, data not shown). None of these effects were seen when LMTM was preceded by chronic rivastigmine. Indeed, in the case of SNARE complex proteins Fig. (3A) and α-synuclein Fig. (3B), the reduction produced by the combination was to levels below the Z scores of the control group.
Fig. (3).
Treatment effects of LMTM alone or following chronic pretreatment with rivastigmine in tau transgenic L1 mice. Photomicrographs of hippocampal CA1 sections for labelled for SNAP25 as one of the SNARE proteins (A) and for α-synuclein (B) in respective treatment groups. Scale bar, 100 µm. Quantitative data for (C) SNARE complex proteins (SNAP25, syntaxin and VAMP2) and (D) α-synuclein expressed as the mean + SE for the hippocampus, visual cortex, diagonal band of Broca and septum. (*, p< 0.05; ***, p< 0.001; ****, p< 0.0001). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
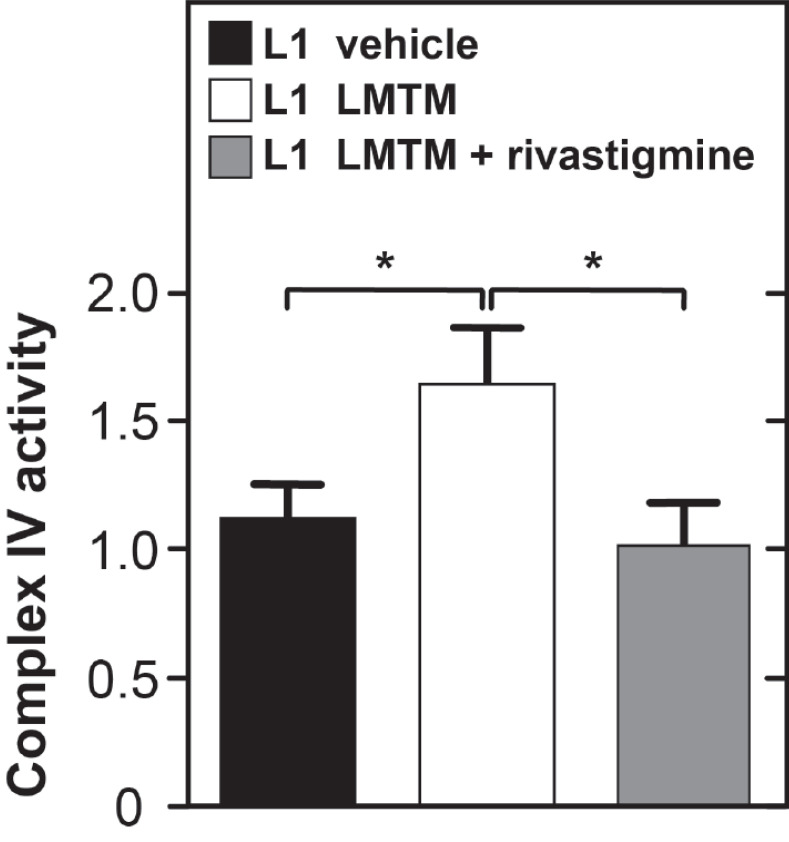
LMTM given alone produced a significant enhancement of complex IV activity in brain mitochondria from tau transgenic L1 mice. Chronic pretreatment with rivastigmine eliminated this effect Fig. (4).
Fig. (4).
Treatment effects of LMTM alone or following chronic pretreatment with rivastigmine in tau transgenic L1 mice on complex IV activity. Data normalized to citrate synthase activity and expressed as mean + SE. (*, p< 0.05). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
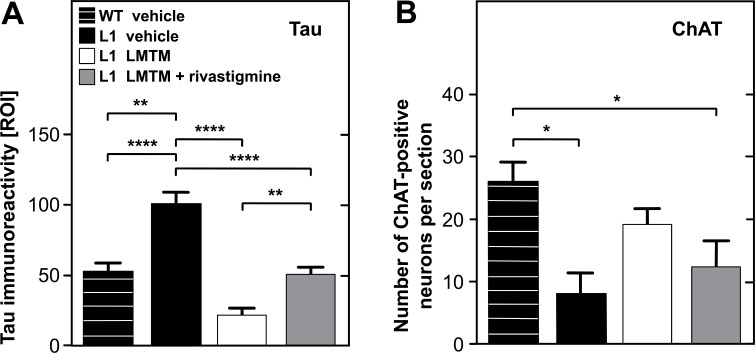
In contrast to the effects on neurotransmitter release, synaptic protein levels and mitochondrial complex IV activity, chronic pretreatment with rivastigmine showed no effect on the primary action of LMTM as a tau aggregation inhibitor. As expected, immunoreactivity against the core tau unit of the PHF measured by optical density was elevated in tau transgenic L1 mice, and this was decreased following treatment with LMTM Fig. (5A). Conversely, counts of ChAT-positive neurons were reduced in L1 mice, but these were only partially restored by treatment with LMTM Fig. (5B). Although the effect on tau immunoreactivity persisted in L1 mice when LMTM was given following prior chronic treatment with rivastigmine, interference in ChAT-reactivity was still evident.
Fig. (5).
Treatment effects of LMTM alone or following chronic pretreatment with rivastigmine in tau transgenic L1 mice. Values are compared with vehicle-treated wild-type mice for levels of tau immunoreactivity (relative optical intensity, ROI) (A) and number of neurons immunoreactive for choline acetyltransferase (B) in vertical diagonal band of Broca. Data are expressed as mean + SE. (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
The results presented here demonstrate that the reduction in pharmacological activity of LMTM when given as an add-on to symptomatic treatment in humans can be reproduced both in wild-type mice and in a tau transgenic mouse model. Therefore, it is based on neuropharmacological mechanisms that have an effect of altering how the brain responds to LMTM. The results imply that the differences in clinical response to LMTM as monotherapy or add-on therapy are likely to be explained by differences in the underlying neuropharmacology of LMTM in these two contexts [33, 34]. Alternative explanations based on the presumption that patients who are prescribed symptomatic treatments are somehow different from untreated patients fail for a number of reasons. The minor and variable differences in baseline severity between these two patient groups have been shown not to account for differences in treatment response [33, 34]. Apparent differences in the rate of decline in treated and untreated MCI patients in the ADNI program [48] disappear when severity at baseline is accounted for in the analysis [33]. The presumption that untreated patients do not really have AD, or have a different form of AD, is also inconsistent with baseline neuroimaging data from subjects participating in the Phase 3 trials [33].
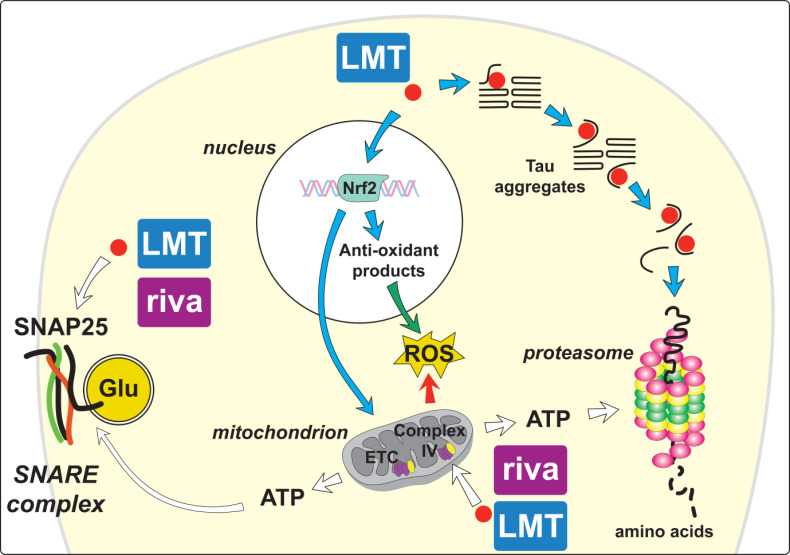
The results we now report demonstrate that there are two classes of the effect produced by LMTM treatment in wild-type and tau transgenic mice (Table 2): Those that are subject to dynamic modulation by prior exposure to cholinesterase inhibitor and those that are not. In tau transgenic mice, the treatment effects that can be modulated include an increase in ACh release in the hippocampus, changes in synaptic proteins, an increase in mitochondrial complex IV activity and reversal of behavioural impairment (not shown). The only treatment effect that is not subject to pharmacological modulation is the primary effect on tau aggregation pathology. The two classes of LMTM treatment effects are summarised in Fig. (6).
Table 2.
Treatment effects of LMTM (5 or 15 mg/kg/day) given alone or following chronic pretreatment with rivastigmine (0.1 or 0.5 mg/kg/day) in wild-type and L1 mice.
| - | Effects in Wild-Type Mice | Effects in Line 1 Mice | ||
|---|---|---|---|---|
| LMTM Alone | Rivastigmine + LMTM | LMTM Alone | Rivastigmine + LMTM | |
| ACh release | ↑ | ↓ | ↑ | ↓ |
| Glutamate release | = | n/a | ↑ | n/a |
| SNARE complex | = | = | = | ↓ |
| Synaptophysin | ↑ | ↓ | ↑ | ↓ |
| α-Synuclein | = | = | = | ↓ |
| Mitochondrial complex IV | = | = | ↑ | ↓ |
| Spatial cognition | = | = | ↑ | ↓ |
| ChAT-positive neurons | = | = | ↑ | ↓ |
| Tau labelling | n/a | n/a | ↓ | ↓ |
Note: Arrows indicate scale and direction of change (red arrows signify treatment effects which reached statistical significance, those in black were directional); =, indicates no effect; and n/a signifies that results are not available yet.
Fig. (6).
Summary schema of likely treatment effects of LMT that are subject to dynamic modulation by chronic pretreatment with rivastigmine (riva). Particular attention is afforded to changes in mitochondrial metabolism, presynaptic proteins and to tau aggregation inhibitor activity. Combined treatment with AChEI does not impair LMT effects on tau aggregation pathology (blue arrows). By contrast, the combination prevents the increases in synaptic proteins, glutamate (Glu) release and increased complex IV activity within the mitochondrial electron transport chain (ETC) that are observed following treatment with LMTM alone (white arrows). LMT also induces mitochondrial biogenesis and activates Nrf2-mediated oxidative stress response elements that can protect against damaging reactive oxygen species (ROS) (green and red arrows). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Effects that are subject to pharmacological modulation are of two types: those which are augmented by the effect on tau aggregation pathology and those which are also seen in wild-type mice. Of the outcomes we have measured, the positive treatment effects of LMTM given alone in wild-type mice are restricted to an increase in ACh levels in the hippocampus, and an increase in synaptophysin levels in multiple brain regions. Therefore, LMTM treatment is able to activate neuronal function at therapeutically relevant doses in wild-type mice lacking tau aggregation pathology (Table 2). Although it has been reported that MT is a weak cholinesterase inhibitor [49, 50], this is unlikely to be the mechanism responsible for the increase in ACh levels because rivastigmine did not potentiate but decreased the effect of LMTM on ACh levels. The increase in ACh and synaptophysin levels might theoretically be explained by an increase in presynaptic mitochondrial activity, since MT is known to enhance mitochondrial complex IV activity [25], and mitochondria have an important role in the homeostatic regulation of presynaptic function [51]. Direct measurement of complex IV activity in wild-type mice, however, did not show any increase following LMTM treatment. The activating effects of LMTM on ACh levels were also not associated with improvement in spatial recognition memory in wild-type mice (not shown).
Chronic pretreatment with rivastigmine suppressed the cholinergic activation in the hippocampus and reduced synaptophysin levels more generally in the brain of wild-type mice. This effect is clearly not dependent on the effects of LMTM on tau aggregation pathology, since there is no pathology in wild-type mice. Rather, they point to a generalised homeostatic downregulation that counteracts the effect of combining two drugs which each have activating effects on neuronal function. Presumably, the primary mechanism that would normally protect against excessive levels of ACh in the synaptic cleft would be an increase in AChE activity. Since rivastigmine produces chronic impairment of this control system, alternative pathways are downregulated in order to preserve homeostasis in cholinergic and other neuronal systems. The LMTM-induced effects are subject to this dynamic downregulation in the context of chronic stimulation by a cholinesterase inhibitor.
Although qualitatively similar, the effects of LMTM given alone are much more prominent and more broad-ranging in tau transgenic L1 mice, the most likely explanation for this is that LMTM combines an inhibitory effect on tau oligomers with inherent neuronal activating effects. The reduction in tau oligomer levels following LMTM treatment facilitates a more pronounced activation of synaptic function and release of neurotransmitters such as ACh and glutamate. Likewise, LMTM reverses the spatial memory deficit seen in tau transgenic L1 mice [23]. The negative effects seen when LMTM is introduced on a chronic rivastigmine background appear simply to reflect the reversal of the activation seen with LMTM alone.
A deleterious effect of tau oligomers on the function of synaptic proteins is readily understandable as being the result of direct interference with the docking of synaptic vesicles, membrane fusion and release of neurotransmitters. In tau transgenic L1 mice, synaptic vesicular protein levels are no longer linked quantitatively either to the proteins of the SNARE complex or to α-synuclein, implying a loss of functional integration between vesicular and membrane-docking proteins at the synapse. The consequence of this was seen directly as an impairment in glutamate release from synaptosomal preparations from tau transgenic mice, and a restoration of normal glutamate release following treatment with LMTM.
The mechanisms responsible for the mitochondrial effects of LMTM are more complex. The MT moiety is thought to enhance oxidative phosphorylation by acting as an electron shuttle between complex I and complex IV [25]. The MT moiety has a redox potential of approximately 10 mV, midway between the redox potential of complex I (-0.4 V) and complex IV (+0.4 V). However, LMTM has no effect on complex IV activity in brain mitochondria isolated from wild-type mice. By contrast, a strong effect was seen in tau transgenic L1 mice. This suggests that tau oligomers interfere with mitochondrial function. It has been shown recently that C-terminally truncated tau protein is bound both to the mitochondrial outer membrane and also enters the intermembrane space of mitochondria [52]. Truncated PHF-tau protein isolated from brain tissues of AD patients forms SDS-resistant complexes with the voltage-dependent anion-selective channel protein (VDAC; formerly porin) in the mitochondrial outer membrane, and also with ATP synthase subunit 9 and core protein 2 of complex III in the intermembrane space [53]. These binding interactions are likely to be deleterious on the function of the electron transport chain in mitochondria and the effect of LMTM in reducing tau oligomer accumulation in and around mitochondria may contribute to the activation of complex IV seen in L1 mice. It is not known how homeostatic downregulation resulting from rivastigmine treatment might affect mitochondrial function. Mitochondria are known to be important homeostatic regulators of synaptic function via buffering of Ca2+ levels and ATP generation [51].
It is striking that the positive effects of LMTM and their reversal or suppression by pretreatment with anticholinesterase can be seen across different transmitter systems and cellular compartments at multiple levels of brain function. This implies that there is no single locus responsible for the interference in the LMTM treatment response. Rather, the negative interaction appears to be part of a generalised homeostatic downregulation in multiple neuronal systems that compensate for the chronic pharmacological activation resulting from blockade of acetylcholinesterase. Likewise, preliminary data available for memantine pretreatment show similar elimination of effects of LMTM on spatial learning deficits (neurobiological studies are still ongoing). This is not unexpected, since the interference with the effects of LMTM treatment seen clinically is very similar for the two drug classes.
More generally, it appears unlikely that the interference affecting LMTM treatment is specific to LMTM. Any treatment that has an activating effect on synaptic function, whether by reducing primary pathology or by another mechanism, is likely to be subject to similar interference, since it is driven primarily by the pre-existing symptomatic treatment. Thus if a reduction in amyloid load results in synaptic activation, as has been proposed [54], then it is likely that symptomatic treatments will also interfere with the ability to demonstrate this effect clinically. A further consideration is whether the homeostatic downregulation that we have demonstrated would operate in the same way if LMTM treatment were primary and symptomatic treatment were added at a later date. The experiments we have conducted to date were designed to mimic the clinical situation in which LMTM is added in patients already receiving symptomatic treatments. If homeostatic downregulation is determined by the treatment that comes first, as we suspect, it is quite possible that the treatment effects of LMTM would dominate, and that the response to add-on symptomatic treatment could be reduced to some extent. This remains to be tested experimentally in the tau transgenic L1 mouse model and, potentially, within the context of a clinical trial of LMTM monotherapy.
CONCLUSION
Homeostatic control systems play an important role in the brain. Such systems are well understood and well documented in many neurophysiological contexts. It is therefore not surprising that treatment interventions designed to boost neuronal function induce homeostatic controls that limit the extent of neuronal over-activation. In the case of cholinergic function, excessive activity is highly deleterious and results clinically in tremors and convulsions. It should also not be surprising that chronic stimulation of the brain by symptomatic treatments alters the way in which it responds to other interventions. Indeed, it would be surprising if this were not the case, and the widespread presumption that symptomatic treatments do not have the potential to interfere with novel treatment approaches is itself counterintuitive. We have demonstrated in two large Phase 3 clinical trials, supported now by a substantial body of preclinical research, that symptomatic treatments do indeed interfere with the treatment effects of a drug that would have been expected a priori to act via an entirely unrelated mechanism. Our experience to date with LMTM has led us to the view that it is necessary to conduct disease-modifying trials as placebo-controlled monotherapy interventions. For such trials, the treatment effects need to be high enough to be demonstrable over a period of at least 12 months, as is the case for LMTM. Alternative approaches that include rescue therapy introduce confounding effects in the analysis and reduce power. Long trials seeking to demonstrate small effects against a background of ongoing symptomatic treatment are at the risk of simply contributing to the very high failure rates of AD trials to date.
Acknowledgements
Declared none.
AUTHORS' CONTRIBUTIONS
All authors have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Ethics Approval and Consent to Participate
Animal studies were approved by University of Aberdeen (No. PPL 7008396) and First Local Ethics Committee for Animal Experiments in Warsaw, Poland (Approval No. 345/2017).
Human and Animal Rights
No humans were used in this study. Experiments on animals were carried out in accordance with the European Communities Council Directive (63/2010/EU) with local ethical approval, a project licensed under the UK Scientific Procedures Act (1986), and in accordance with the Polish Law on the Protection of Animals.
Consent for Publication
Not applicable.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author [CMW], upon reasonable request.
Funding
This study was sponsored entirely by WisTa Laboratories Ltd. under the following grants: PAR1395, PAR1561, PAR1562, PAR1577 and PAR1763. The sponsor was involved in the design of the study; in the collection, analysis and interpretation of data; and in the writing of the report. The corresponding author had full access to all the data and had final responsibility for submission of the report for publication.
conflict of interest
C.R. Harrington and C.M. Wischik are employees and officers of TauRx Therapeutics Ltd. and are inventors on patents relating to LMTM and tau-aggregation inhibitors that are owned by WisTa Laboratories Ltd., an affiliate of TauRx Therapeutics Ltd.
REFERENCES
- 1.Sarter M., Lustig C., Blakely R.D., Koshy Cherian A. Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. J. Physiol. Paris. 2016;110(1-2):10–18. doi: 10.1016/j.jphysparis.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botly L.C., De Rosa E. Cholinergic influences on feature binding. Behav. Neurosci. 2007;121(2):264–276. doi: 10.1037/0735-7044.121.2.264. [DOI] [PubMed] [Google Scholar]
- 3.Botly L.C., De Rosa E. A cross-species investigation of acetylcholine, attention, and feature binding. Psychol. Sci. 2008;19(11):1185–1193. doi: 10.1111/j.1467-9280.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson L., Platt B., Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011;221(2):443–465. doi: 10.1016/j.bbr.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Klinkenberg I., Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010;34(8):1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Ding Z., Brown J.W., Rueter L.E., Mohler E.G. Profiling attention and cognition enhancing drugs in a rat touchscreen-based continuous performance test. Psychopharmacology (Berl.) 2018;235(4):1093–1105. doi: 10.1007/s00213-017-4827-y. [DOI] [PubMed] [Google Scholar]
- 7.Gastambide F., Cotel M.C., Gilmour G., O’Neill M.J., Robbins T.W., Tricklebank M.D. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology. 2012;37(4):1057–1066. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney C., Farrell D., Gray R., et al. AD2000 Collaborative Group. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet. 2004;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 9.Singh G., Thomas S.K., Arcona S., Lingala V., Mithal A. Treatment persistency with rivastigmine and donepezil in a large state medicaid program. J. Am. Geriatr. Soc. 2005;53(7):1269–1270. doi: 10.1111/j.1532-5415.2005.53384_9.x. [DOI] [PubMed] [Google Scholar]
- 10.Raina P., Santaguida P., Ismaila A., et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann. Intern. Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 11.Mauskopf J.A., Paramore C., Lee W.C., Snyder E.H. Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings. J. Manag. Care Pharm. 2005;11(3):231–251. doi: 10.18553/jmcp.2005.11.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koller D., Hua T., Bynum J.P.W. Treatment patterns with antidementia drugs in the United States Medicare Cohort Study. J. Am. Geriatr. Soc. 2016;64(8):1540–1548. doi: 10.1111/jgs.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez C., Jones R.W., Rietbrock S. Trends in the prevalence of antipsychotic drug use among patients with Alzheimer’s disease and other dementias including those treated with antidementia drugs in the community in the UK: A cohort study. BMJ Open. 2013;3(1):e002080. doi: 10.1136/bmjopen-2012-002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krolak-Salmon P., Dubois B., Sellal F., et al. France will no more reimburse available symptomatic drugs against Alzheimer’s disease. J. Alzheimers Dis. 2018;66(2):425–427. doi: 10.3233/JAD-180843. [DOI] [PubMed] [Google Scholar]
- 15.Winblad B., Amouyel P., Andrieu S., et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 16.Lanctôt K.L., Rajaram R.D., Herrmann N. Therapy for Alzheimer’s disease: how effective are current treatments? Ther. Adv. Neurol. Disorder. 2009;2(3):163–180. doi: 10.1177/1756285609102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings J.L., Morstorf T., Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014;6(4):37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panza F., Lozupone M., Logroscino G., Imbimbo B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019;15(2):73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 19.Wischik C.M., Schelter B.O., Wischik D.J., Storey J.M.D., Harrington C.R. Modeling prion-like processing of tau protein in Alzheimer’s disease for pharmaceutical development. J. Alzheimers Dis. 2018;62(3):1287–1303. doi: 10.3233/JAD-170727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington C.R., Storey J.M.D., Clunas S., et al. Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer’s disease. J. Biol. Chem. 2015;290(17):10862–10875. doi: 10.1074/jbc.M114.616029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baddeley T.C., McCaffrey J., Storey J.M.D., et al. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2015;352(1):110–118. doi: 10.1124/jpet.114.219352. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hilaly Y.K., Pollack S.J., Rickard J.E., et al. Cysteine-independent inhibition of Alzheimer’s disease-like paired helical filament assembly by leuco-methylthioninium (LMT). J. Mol. Biol. 2018;430(21):4119–4131. doi: 10.1016/j.jmb.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Melis V., Magbagbeolu M., Rickard J.E., et al. Effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models. Behav. Pharmacol. 2015;26(4):353–368. doi: 10.1097/FBP.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wischik C.M., Edwards P.C., Lai R.Y.K., Roth M., Harrington C.R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA. 1996;93(20):11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atamna H., Mackey J., Dhahbi J.M. Mitochondrial pharmacology: Electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors. 2012;38(2):158–166. doi: 10.1002/biof.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atamna H., Nguyen A., Schultz C., et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22(3):703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 27.Gureev A.P., Shaforostova E.A., Popov V.N., Starkov A.A. Methylene blue does not bypass Complex III antimycin block in mouse brain mitochondria. FEBS Lett. 2019;593(5):499–503. doi: 10.1002/1873-3468.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stack C., Jainuddin S., Elipenahli C., et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 2014;23(14):3716–3732. doi: 10.1093/hmg/ddu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao M., Liang F., Xu H., Yan W., Zhang J. Methylene blue exerts a neuroprotective effect against traumatic brain injury by promoting autophagy and inhibiting microglial activation. Mol. Med. Rep. 2016;13(1):13–20. doi: 10.3892/mmr.2015.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Congdon E.E., Wu J.W., Myeku N., et al. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8(4):609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schirmer R.H., Adler H., Pickhardt M., Mandelkow E. Lest we forget you -- methylene blue .... Neurobiol. Aging. 2011;32(2325):e7–e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Oz M., Lorke D.E., Petroianu G.A. Methylene blue and Alzheimer’s disease. Biochem. Pharmacol. 2009;78(8):927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Wilcock G.K., Gauthier S., Frisoni G.B., et al. Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: cohort analysis as modified primary outcome in a phase 3 clinical trial. J. Alzheimers Dis. 2018;61(1):435–457. doi: 10.3233/JAD-170560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauthier S., Feldman H.H., Schneider L.S., et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388(10062):2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schelter B.O., Shiells H., Baddeley T.C., et al. Concentration-dependent activity of hydromethylthionine on cognitive decline and brain atrophy in mild to moderate Alzheimer’s disease. J. Alzheimers Dis. 2019;72(3):931–946. doi: 10.3233/JAD-190772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melis V., Zabke C., Stamer K., et al. Different pathways of molecular pathophysiology underlie cognitive and motor tauopathy phenotypes in transgenic models for Alzheimer’s disease and frontotemporal lobar degeneration. Cell. Mol. Life Sci. 2015;72(11):2199–2222. doi: 10.1007/s00018-014-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wischik C.M., Novak M., Thøgersen H.C., et al. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1988;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wischik C.M., Novak M., Edwards P.C., Klug A., Tichelaar W., Crowther R.A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1988;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzpatrick A.W.P., Falcon B., He S., et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547(7662):185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G., Franklin K.B.J. The mouse brain in stereotaxic coordinates. New York: Academic Press; 2012. [Google Scholar]
- 41.Niewiadomska G., Komorowski S., Baksalerska-Pazera M. Amelioration of cholinergic neurons dysfunction in aged rats depends on the continuous supply of NGF. Neurobiol. Aging. 2002;23(4):601–613. doi: 10.1016/S0197-4580(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 42.Schwab K., Frahm S., Horsley D., et al. A protein aggregation inhibitor, leuco-methylthioninium bis(hydromethanesulfonate), decreases α-synuclein inclusions in a transgenic mouse model of synucleinopathy. Front. Mol. Neurosci. 2018;10:447. doi: 10.3389/fnmol.2017.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 44.König M., Berlin B., Schwab K., et al. Increased cholinergic response in α-synuclein transgenic mice (h-α-synL62). ACS Chem. Neurosci. 2019;10(4):1915–1922. doi: 10.1021/acschemneuro.8b00274. [DOI] [PubMed] [Google Scholar]
- 45.Mesulam M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013;521(18):4124–4144. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pepeu G., Grazia Giovannini M. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 2017;1670:173–184. doi: 10.1016/j.brainres.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Revett T.J., Baker G.B., Jhamandas J., Kar S. Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013;38(1):6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider L.S., Insel P.S., Weiner M.W. Alzheimer’s Disease Neuroimaging Initiative. Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch. Neurol. 2011;68(1):58–66. doi: 10.1001/archneurol.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deiana S., Harrington C.R., Wischik C.M., Riedel G. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: Comparison with rivastigmine. Psychopharmacology (Berl.) 2009;202(1-3):53–65. doi: 10.1007/s00213-008-1394-2. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffendorf M., Bruning T.A., Batnik H.D., van Zwieten P.A. The interaction between methylene blue and the cholinergic system. Br. J. Pharmacol. 1997;122(1):95–98. doi: 10.1038/sj.bjp.0701355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devine M.J., Kittler J.T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 2018;19(2):63–80. doi: 10.1038/nrn.2017.170. [DOI] [PubMed] [Google Scholar]
- 52.Cieri D., Vicario M., Vallese F., et al. Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca2+ handling. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864(10):3247–3256. doi: 10.1016/j.bbadis.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Wischik C.M., Lai R.Y.K., Harrington C.R. Modelling prion-like processing of tau protein in Alzheimer’s disease for pharmaceutical development. Brain Microtubule Associated Proteins: Modifications in Disease. Amsterdam: Harwood Academic Publishers; 1997. pp. 185–241. [Google Scholar]
- 54.Marsh J., Alifragis P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018;13(4):616–623. doi: 10.4103/1673-5374.230276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [CMW], upon reasonable request.