Abstract
Consumers’ demand for healthier foods with functional properties has had a clear influence on the food industry and in this sense, they have been attaching natural sources of bioactive ingredients into food products. Vaccinium myrtillus L. (bilberry) is known to be a functional food, presenting its fruits in the form of a small dark blueberry. This coloration is due to its high content in anthocyanin, being also associated with bilberries’ beneficial health effects. In the bilberry industry, there is a very high annual loss of this fruit due to the less aesthetic shape or appearance, in which they cannot be considered suitable for sale and are therefore disposed of as biological waste. Therefore, it is of great importance to valorize this fruit and this review aimed to completely characterize the fruits of V. myrtillus in order to comprehend the relationship between their consumption and the beneficial effects regarding consumer’s health. Thus, this review provides a description of the nutritional and bioactive compounds present in bilberry fruits, followed by their beneficial health effects. An overview of the natural pigments present in these fruits was also explored, focusing particularly in the anthocyanins composition, which represents the most widely studied class of bioactive compounds of V. myrtillus fruits. Finally, industrial applications of these fruits and by-products, as an efficient approach to the production of value-added products with economical and environmental impact, were also discussed.
In general, V. myrtillus is a rich source of micronutrients and phytochemical compounds, such as organic acids, sugars, vitamins, fibers and phenolic compounds (anthocyanin and non-anthocyanin compounds), with nutritional and functional properties, that justify the growing interest in these berries, not only for food applications, but also in the pharmaceutical industry.
Keywords: Vaccinium myrtillus L., phenolic compounds, natural pigments, health benefits, anthocyanin, vitamins
1. Introduction
Bilberry (Vaccinium myrtillus L.) is a dark blue fruit that belongs to the genus Vaccinium, family Ericaceae, which comprises around 450 species of trees, shrubs, sub-shrubs and hemiphytes distributed all over the world [1]. These fruits are usually consumed in the fresh form, however, due to their short shelf life, they are also frozen, dried or processed in the form of jams, juices and wines or liqueurs [2]. Bilberries have been conventionally consumed and used in traditional medicine since ancient times, being harvested from wild bushes, although currently the cultivation of these fruits is commonly performed in northern and eastern Europe, and also in northern Africa [3].
These fruits are described as being an important source of phenolic compounds and carotenoids, also containing moderate levels of other micronutrients such as vitamins. Nevertheless, it is due to their high levels of anthocyanins that these fruits are recognized for their bioactive properties [4]. Anthocyanins, besides being responsible for the blue color of bilberries, are the major group of flavonoids in these berries and have been associated to many beneficial health effects, such as prevention or treatment of cancers, cardiovascular diseases, obesity, diabetes, aging diseases, urinary tract infections and periodontal diseases [5, 6]. The high content of these flavonoids has also highlighted these fruits as interesting sources of coloring compounds for food and pharmaceutical applications [7]. According to the literature, the anthocyanin profile in bilberry consists of fifteen main compounds, derived from five aglycones (delphinidin, cyanidin, petunidin, peonidin, and malvidin) linked to different sugar moieties (galactose, glucose and arabinose) [8, 9]. Consumers are increasingly concerned about choosing foods labeled as healthier and more natural. In this sense, the food industry has been exploring various natural sources in order to enrich different products [10]. Currently, there are many products in the market with the incorporation of berries, namely bilberry, highlighting their beneficial effects, usually their antioxidant potential [11].
In this review, the nutritional characterization and phenolic composition of V. myrtillus L. fruits, and also their beneficial health effects, are discussed. Natural pigment exploitation and industrial application are also the main focus of this review.
2. Nutritional characterization
Due to their reduced environmental adaptability, it is extremely difficult to cultivate bilberries. Moreover, there are still few producers of these fruits because of their low productivity, justified by the small size of the fruits when compared to similar blueberries (V. corymbosum L.), which have a larger fruit size. Most of the available bilberries (V. myrtillus L.) are mainly obtained from wild plants that grow in northern and southern Europe [12], while highbush blueberries (V. corymbosum) are originated from North America [13]. Sometimes bilberry is also called blueberry because both have a similar appearance and are close relatives, but the true blueberry is native to the United States [14]. According to Coudun & Gégout [15], there are several environmental factors, such as climatic conditions, soil type or cultivation conditions, among others, which may affect the plant growth and consequently, fruit productivity [15]. In the case of bilberries plants, some authors report that light availability influences plant development and consequently, fruit yield [16]. Additionally, it is found that weather conditions can also influence the nutritional and chemical composition of the fruits. This was proven through studies performed from several authors, which demonstrated that bilberries fruits growing in northern latitudes present higher phenolic contents than those from southern latitudes [17, 18]. There are more than 70 registered ethnomedical and food uses of 36 Vaccinium species, being V. myrtillus, the species with the highest number of described uses [5]. The American Herbal Products Association classified V. myrtillus as a Class 1 product category assigned when considering it as safe to consume [19]. The fruits of this plant are usually consumed as food, while the leaves or aerial parts are commonly associated with their medicinal use [5, 20]. V. myrtillus berries can be consumed fresh, frozen and dried, as well as in their processed forms, such as juices, jams or food supplements [14].
The interesting nutritional and functional properties described for bilberries justify the growing interest in these berries. The fruits of V. myrtillus are rich sources of micronutrients and phytochemical compounds with health benefits, such as organic acids [21-23], sugars [21, 22], vitamins [24], fibres [25], and phenolic compounds [20, 26]. The organoleptic properties of these fruits are clearly influenced by the different content of the mentioned compounds, namely sugars and organic acids [27]. Since sugars and organic acids are considered the main soluble constituents of berries, some authors describe their content as a direct effect on fruit taste and ripeness and consequently, on consumer’s acceptability [26, 28]. According to Michalska & Łysiak [29], besides the taste of the bilberry fruits, the vitamin C content is important in the consumption of these fruits because 100 g of the fruit provides, on average, 10 mg of ascorbic acid, which is equal to 1/3 of the daily recommended intake [29]. However, Uleberg et al. [21] indicated that cool temperatures and genetic factors influence the taste of bilberry fruits, which explained the sweeter taste of fruits cultivated in Northern areas in comparison to those from Southern areas [21].
Bilberries have different organic acids in their composition (malic, citric, gallic, chlorogenic, ascorbic and quinic acids), being citric, malic and quinic acids the main ones. Fructose and glucose are described as the highest group of free sugars in bilberry, although the presence of sucrose has also been reported as a relevant constituent [21, 22, 26].
The mineral content in fruits and vegetables is very important, contributing to their nutritional value. However, it is necessary to balance these mineral elements in the human diet, because trace elements can be toxic when consumed at a higher amount than the recommended intake. Some wild fruits, including bilberry, have been explored and identified as interesting sources of minerals [30, 31]. Regarding mineral composition, these fruits have three main macroelements (Ca, P, and Mg) and seven microelements (Fe, Ba, Na, Mn, Cu, Sr, and Zn) [16]. Comparative studies between wild and commercial fruits have shown that wild bilberry has higher concentrations of minerals than commercial fruits [16]. As mentioned above, these differences may be justified by the cultivation conditions, namely the soil composition [32].
Regarding the proximate composition, the fresh fruits contain around 84% of water, 9.7% of carbohydrates, 0.6% of proteins, 0.4% of fats, and 3-3.5% of fibres, with an estimated energetic value of 192 kJ [29].
3. Bioactive compounds
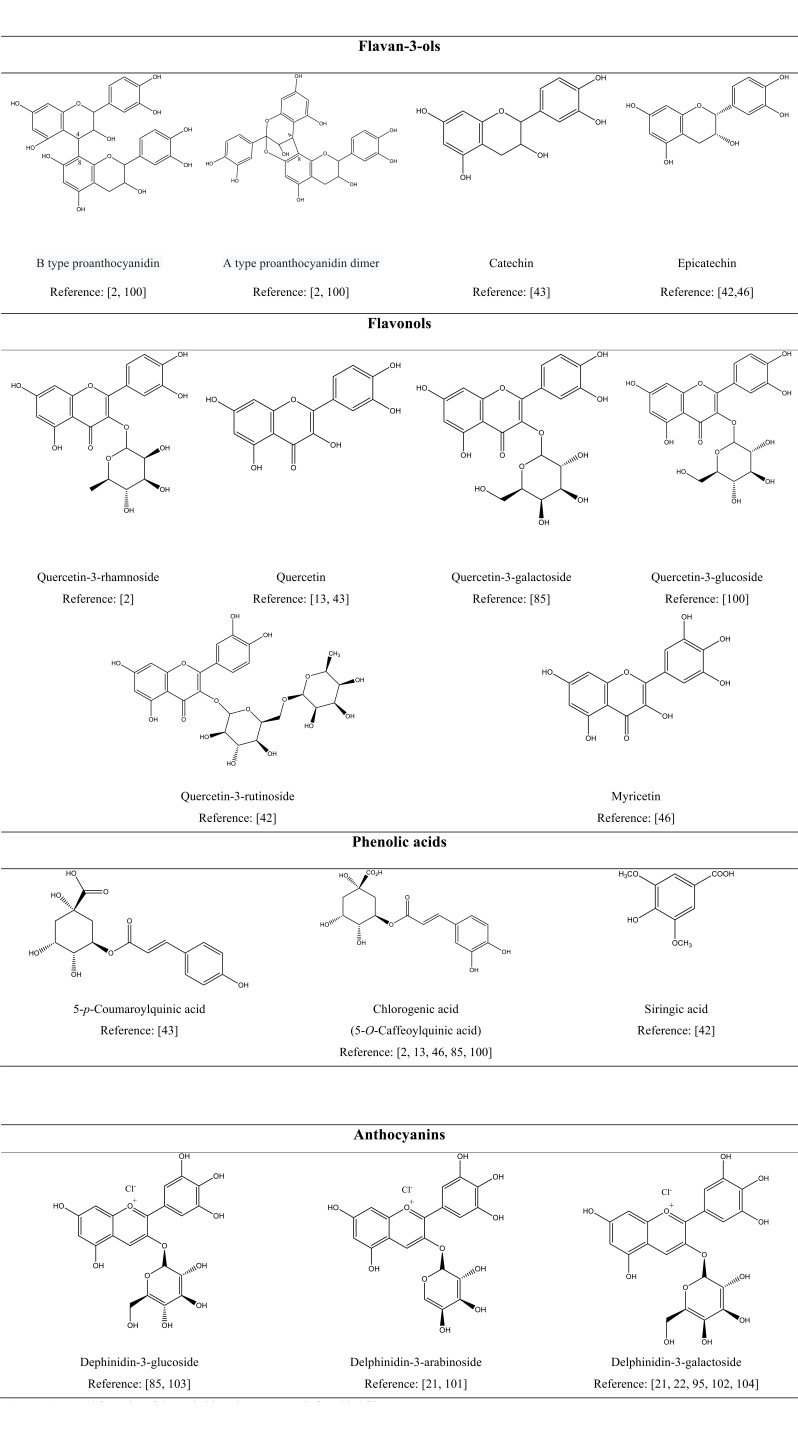
Phenolic compounds are a group of secondary plant metabolites recognized for their health-protective action, namely as an antioxidant [2, 33], anti-inflammatory [34] and antihypertensive [35], antimicrobial [36] and anticancer agents [37]. The fact that V. myrtillus has a high content of phenolic compounds may account for the growing demand for this fruit. Vaccinium myrtillus L. is rich in polyphenols, with anthocyanins flavan-3-ols, flavonols and phenolic acids as the main group of compounds identified by several authors [2, 13, 42, 43, 46, 91, 106] (Fig. 1). Flavonoids, such as flavan-3-ols (catechins and proanthocyanidins) and flavonols (i.e., kaempferol, quercetin, myricetin), phenolic acids (mainly hydroxycinnamic and hydroxybenzoic acids) and derivatives of stilbenes, are the major non-anthocyanin polyphenols present in V. myrtillus fruits [20, 29, 38].
Fig. (1).
Structural formulas of the main bioactive compounds found in bilberry.
The flavan-3-ols are usually found with varying concentrations in commonly consumed foods such as fruits, legumes, vegetables and nuts. They are natural antioxidants that may contribute to prevent rancidity due to the oxidation of unsaturated fats and stabilize food colours, as well as being involved in chemoprevention against a variety of diseases [39]. The most common flavan-3-ols are procyanidins, consisting of (epi)catechin oligomers [39, 40] and can be classified into A-type and B-type, depending on the stereo configuration and linkage between monomers. B-type procyanidins are the most abundant, with procyanidins B1, B2, B3 and B4 occurring most frequently [39]. Table 1 shows the contents of the main phenolic compounds determined in bilberries in recent studies by several authors. Data are expressed in different units and as a dry or fresh weight basis, as reported by the authors. Regarding flavan-3-ols, the concentrations of catechin, epicatechin, procyanidin dimers and trimers, and gallocatechin are collected, as well as total flavan-3-ol contents. As it can be seen, only catechin was identified and quantified in all the represented studies.
Table 1.
Content levels of flavan-3-ols, flavonols and phenolic acids in fruits of Vaccinium myrtillus L.
| Phenolic Compounds | [2] | [43] | [85] | [100] | [13] | [42] | [46] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechin | 29.67 mg/100 g (dw) | 15.04 μg/100 g (dw) | 91.64 mg/g (dw) | 96 mg/100 g (dw) | 0.2 mg/100g (fw) | 2.72 mg/kg (fw) | 13.9 mg/kg (dw) | |||||||
| Epicatechin | 59.14 mg/100 g (dw) | nd | nd | 7.9 mg/100 g (dw) | 2 mg/100g (fw) | 28.54 mg/kg (fw) | 255 mg/kg (dw) | |||||||
| Procyanidin dimers | 72.10 mg/100 g (dw) | nd | nd | 117 mg/100 g (dw) | nd | nd | nd | |||||||
| Procyanidin trimers | 59.33 mg/100 g (dw) | nd | nd | 109 mg/100 g (dw) | nd | nd | nd | |||||||
| Gallocatechin | 35.72 mg/100 g (dw) | nd | nd | nd | nd | 6.66 mg/kg (fw) | nd | |||||||
| Total flavan-3-ols | 244.09 mg/100 g (dw) | 15.04 μg/100 g (dw) | 91.64 mg/g (dw) | 329.9 mg/100 g (dw) | 2.2 mg/100g (fw) | 37.92 mg/kg (fw) | 268.9 mg/kg (dw) | |||||||
| Myricetin hexoside | 29.61 mg/100 g (dw) | nd | 28.60 mg/g (dw) | nd | nd | nd | nd | |||||||
| Myricetin pentoside | 3.34 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Myricetin-3-glucuronide | 2.23 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Myricetin-3-rhamnoside | 2.12 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Laricitrin-3-galactoside | 0.10 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Laricitrin-3-glucoside | 4.33 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Laricitrin-3-glucuronide | 0.34 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Myricetin | 6.20 mg/100 g (dw) | 40.66 μg/100 g (dw) | nd | nd | 0.4 mg/100g (fw) | nd | 36.9 mg/kg (dw) | |||||||
| Syringetin-3-galactoside | 8.69 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Syringetin-3-glucoside | 1.01 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Isorhamnetin-3-arabinoside | nd | nd | nd | 6 mg/100 g (dw) | nd | nd | nd | |||||||
| Isorhamnetin-3-galactoside | 5.27 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Isorhamnetin-3-glucoside | 36.60 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Quercetin-3-arabinoside | nd | nd | 38.14 mg/g (dw) | nd | nd | nd | nd | |||||||
| Quercetin-3-rhamnoside | 67.36 mg/100 g (dw) | 51.80 μg/100 g (dw) | nd | 6 mg/100 g (dw) | nd | nd | nd | |||||||
| Phenolic Compounds | [2] | [43] | [85] | [100] | [13] | [42] | [46] | |||||||
| Quercetin-7-rhamnoside | nd | nd | nd | 48 mg/100 g (dw) | nd | nd | nd | |||||||
| Quercetin-3-galactoside | 4.03 mg/100 g (dw) | nd | 45.78 mg/g (dw) | nd | nd | nd | nd | |||||||
| Quercetin-3-glucuronide | 2.82 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Quercetin-3-glucoside | 4.03 mg/100 g (dw) | nd | nd | 236 mg/100 g (dw) | nd | nd | nd | |||||||
| Quercetin-3-(6”-acetyl) glucoside | nd | nd | nd | 97 mg/100 g (dw) | nd | nd | nd | |||||||
| Quercetin-3-pentoside | nd | nd | 29.29 mg/g (dw) | 64 mg/100 g (dw) | nd | nd | nd | |||||||
| Quercetin-3-rutinoside | nd | 51.80 μg/100 g (dw) | 27.03 mg/g (dw) | 196 mg/100 g (dw) | 0.2 mg/100g (fw) | 3.84 mg/kg (fw) | nd | |||||||
| Quercetin | nd | 243.30 μg/100 g (dw) | 4.40 mg/g (dw) | 146 mg/100 g (dw) | 0.8 mg/100g (fw) | 1.62 mg/kg (fw) | 2.2 mg/kg (dw) | |||||||
| Kaempferol | nd | 15.64 μg/100 g (dw) | nd | 30 mg/100 g (dw) | nd | 1.72 | nd | |||||||
| Kaempferol-3-glucoside | nd | nd | nd | nd | nd | nd | nd | |||||||
| Kaempferol-3-glucuronide | 2.82 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Kaempferol-3-(6”-acetylglucoside) | nd | nd | nd | 8 mg/100 g (dw) | nd | nd | nd | |||||||
| Kaempferol-3-rhamnoside | nd | nd | nd | 10.3 mg/100 g (dw) | nd | nd | nd | |||||||
| Total flavonols | 173.03 mg/100 g (dw) | 403.2 μg/100 g (dw) | 173.24 mg/g (dw) | 847.3 mg/100g (dw) | 1.4 mg/100g (fw) | 57.82 mg/kg (fw) | 39.1 mg/kg (dw) | |||||||
| Dicaffeoylquinic acid | nd | nd | nd | 31.6 mg/100g (dw) | nd | nd | nd | |||||||
| 4-O-Caffeoylquinic acid | nd | nd | nd | 37 mg/100g (dw) | nd | nd | nd | |||||||
| trans 5-O-Caffeoylquinic acid | 818.39 mg/100 g (dw) | nd | nd | 649 mg/100g (dw) | nd | nd | nd | |||||||
| cis 5-O-Caffeoylquinic acid | 31.84 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Caffeic acid derivatives | 66.83 mg/100 g (dw) | nd | nd | nd | nd | nd | nd | |||||||
| Caffeic acid | 4.24 mg/100 g (dw) | 15.33 mg/100 g (dw) | 6.26 mg/g (dw) | nd | 0.3 mg/100g (fw) | 5.46 mg/kg (fw) | 3.1 mg/kg (dw) | |||||||
| Chlorogenic acid | nd | 21.0 mg/100 g (dw) | 6.40 mg/g (dw) | nd | 23.1 mg/100g (fw) | 1.25 mg/kg (fw) | 1320 mg/kg (dw) | |||||||
| 3-p-Coumaroylquinic acid | nd | nd | nd | 7.3 mg/100g (dw) | nd | nd | nd | |||||||
| 4-p-Coumaroylquinic acid | nd | nd | nd | 3.5 mg/100g (dw) | nd | nd | nd | |||||||
| 5-p-Coumaroylquinic acid | 3.04 mg/100 g (dw) | 57.87 mg/100 g (dw) | nd | 29.2 mg/100g (dw) | nd | nd | nd | |||||||
| p-Coumaric acid hexoside | 3.99 mg/100 g (dw) | nd | nd | nd | 0.3 mg/100g (fw) | 7.63 mg/kg (fw) | 1.20 mg/kg (dw) | |||||||
| Coumaroyl iridoid isomers | 31.68 mg/100 g (dw) | nd | nd | 82.5 mg/100g (dw) | nd | nd | nd | |||||||
| Coumaric acid derivatives | 3.18 mg/100 g (dw) | nd | nd | 243.7 mg/100g (dw) | nd | nd | nd | |||||||
| Ellagic acid | nd | 9.99 mg/100 g (dw) | nd | nd | 1.2 mg/100g (fw) | nd | nd | |||||||
| Ferulic acid | 4.84 mg/100 g (dw) | 22.76 mg/100 g (dw) | nd | 7.3 mg/100g (dw) | 0.4 mg/100g (fw) | 10.60 mg/kg (fw) | 0.44 mg/kg (dw) | |||||||
| Gallic acid | nd | 7.24 mg/100 g (dw) | nd | nd | 6.2 mg/100g (fw) | 19.19 mg/kg (fw) | 33.3 mg/kg (dw) | |||||||
| Gallic acid derivatives | nd | nd | nd | 36 mg/100g (dw) | nd | nd | nd | |||||||
| Protocatechuic acid | nd | 19.41 mg/100 g (dw) | 7.66 mg/g (dw) | nd | nd | nd | nd | |||||||
| Syringic acid | nd | 27.43 mg/100 g (dw) | nd | nd | nd | 637.43 mg/kg (fw) | nd | |||||||
| Vanillic acid | nd | nd | nd | nd | nd | 532.97 mg/kg (fw) | nd | |||||||
| Total phenolic acids | 968.02 mg/100 g (dw) | 181.03 mg/100 g (dw) | 20.32 mg/g (dw) | 1127.1 mg/100 g (dw) | 31.5 mg/100 g (fw) | 1214.53 mg/kg (fw) | 1358.04 mg/kg (dw) | |||||||
Abbreviations used: nd- Not detected, fw- Fresh weight, dw- Dry weight
The individual and total contents of flavonols in fruits of V. myrtillus are also presented in Table 1. All the reported studies identified kaempferol, quercetin and myricetin glycosides as the main compounds, although Zorenc et al. [2] also reported laricitrin, syringetin and isorhamnetin glycosides [2].
Phenolic acids are phenols that possess one carboxylic acid function and include two main groups: the hydroxycinnamic and hydroxybenzoic acids [41]. Caffeic, p-coumaric, vanillic, ferulic, and protocatechuic acids are widely present in many plants, whereas gentisic and syringic acids have a more restrictive distribution, being reported in V. myrtillus by Deǧirmencioǧlu et al. [42] and Tumbas et al. [43] (Table 1).
Regarding stilbenes, there are few studies that reveal its presence in bilberry fruits, however, Ehala, Vaher, & Kaljurand [44] and Može et al. [13] identified and quantified trans-resveratrol in concentrations ranging 97.8% and 0.3 mg/100 g fw, relating their presence to the antioxidant activity of the fruit [13, 44].
Anthocyanins are pigments commonly present in plants, where they are responsible for the characteristic blue, red or purple colour. Actually, anthocyanins are considered the most important water-soluble pigments in plants, being particularly relevant in flowers and berries, namely bilberry, cherry or blackcurrant [45]. Many studies describe anthocyanins as the most abundant polyphenol class in V. myrtillus fruits, being responsible for the characteristic dark blue colour of bilberry. Glucosides, galactosides and arabinosides of delphinidin, cyanidin, petunidin, peonidin and malvidin characterize the anthocyanin profile of bilberry [46]. Owing to their interest and relevance in the sensory and health properties of fruits, a more profound discussion regarding this group of compounds is made in the following section.
4. Natural pigments
Textile, food, painting, cosmetic, and pharmaceutical industries are some of the sectors where colour plays a very important role. Research on natural pigments is of enormous importance as it has a direct impact on environmental, economic and human health safety [47]. Natural pigments generally extracted from different parts of plants, namely seeds, fruits, vegetables or roots, have played an important role since ancient times and particularly, in textile dyeing [48]. For its part, the food industry routinely applies pigments to foods to restore colour losses or make them more attractive to consumers. Food colorants can be classified as artificial or natural. It is estimated that around 31% of the colorants used by the food industry are obtained from natural sources, such as plants, animals and microorganisms [49].
Currently, due to several published scientific studies that associate the consumption of certain artificial additives with potential adverse health effects, an aversion has been created to this type of colorants. This has put pressure on the industry that is looking for natural alternatives capable of performing the same functions as artificial colorants and additionally offering bioactive properties [50].
Several studies have shown that extracts rich in anthocyanins could be used not only as colorants but also as potential functional food ingredients and/or dietary supplements due to their biological properties [21]. Anthocyanins are classified in the USA as natural food colourings in the fruit (21 CFR 73,250) and vegetable (21 CFR 73,260) category, and in the EU, they are included as additives under code E163 [51].
Anthocyanins are the most abundant and widely studied class of bioactive compounds in the fruits of V. myrtillus. The anthocyanin profile in bilberry is characterized by fifteen main compounds, among which delphinidin glycosides constitute the best represented ones, as it is shown in Table 2, where the concentrations determined by different authors are collected.
Table 2.
Contents of individual anthocyanins in Vaccinium myrtillus fruits.
| Anthocyanins | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | Cy-3-arab | Cy-3-gal | Cy-3-glu | Dp-3-arab | Dp-3-gal | Dp-3-glu | Mv-3-arab | Mv-3-gal | Mv-3-glu | Pn-3-arab | Pn-3-gal | Pn-3-glu | Pt-3-arab | Pt-3- gal | Pt-3-glu | References |
| Finland Southern (mg/100 g fw) | 37.0 | 34.0 | 41.0 | 57.5 | 45.7 | 54.6 | 2.6 | 13.3 | 16.6 | nd | 2.1 | 9.3 | nd | 10.0 | 25.9 | [21] |
| Finland Northern (mg/100 g fw) | 44.0 | 59.5 | 50.9 | 87.8 | 98.9 | 76.4 | 9.6 | 34.2 | 46.8 | nd | 4.8 | 17.7 | nd | 26.3 | 45.3 | [21] |
|
Slovenia (mg/kg fw) |
161.92 | 542.45 | 194.47 | 640.31 | 700.46 | 381.11 | 42.45 | 109.97 | 149.63 | 9.66 | 19.42 | 108.09 | 78.31 | 213.63 | 416.06 | [22] |
|
Poland (mg/g dw) |
936 | 375 | 396 | 741 | 1060 | 1247 | 295 | 127 | 506 | 15 | 107 | 198 | 340 | 581 | 541 | [104] |
| Italy Northern (mg/100 g fw) | 37.1 | 56.3 | 55.0 | 97.4 | 127.1 | 119.0 | nd | 19.7 | 56.1 | nd | nd | 24.8 | 20.1 | 33.1 | 65.9 | [95] |
| Slovenia (mg/100g fw) | 110.6 | 122.6 | 13.04 | 152.3 | 167.1 | 169.1 | 28.24 | 21.66 | 24.89 | 22.99 | 17.82 | 20.75 | 19.98 | 14.76 | 16.72 | [13] |
|
Finland (mg/kg fw) |
450 | 493 | 488 | 632 | 629 | 562 | 91 | 124 | 350 | 20 | 34 | 187 | 137 | 167 | 359 | [101] |
|
Mustasaari, Finland (mg/kg fw) |
220 | 300 | 220 | 340 | 360 | 300 | 53 | 100 | 150 | 18 | 36 | 75 | 84 | 120 | 170 | [102] |
|
Milan, Italy (mg/g extract) |
32.18 | 40.03 | 41.81 | 49.06 | 49.07 | 54.21 | 8.75 | 13.23 | 40.15 | 1.75 | 4.34 | 15.90 | 13.44 | 17.92 | 38.49 | [79] |
| Bulgaria (mg/100 g dw) | 78.13 | 244.81 | 563.38 | 101.57 | 91.54 | 733.01 | 27.65 | 17.80 | 46.65 | 15.42 | 14.39 | 57.61 | 38.67 | 27.74 | 17.53 | [103] |
Abbreviations used: dp- delphinidin; cy- cyanidin; pt- petunidin; pn- peonidin; mv- malvidin; gal- galactoside; glc- glucoside; arab- arabinoside; nd- not detected; fw- fresh weight; dw- dry weight.
The type and quantity of anthocyanins are affected by several internal and external factors, such as genetic factors (varietal and regional), environmental variables (light intensity, temperature, humidity, the use of fertilisers), fruit size, ripening stage, pre-harvest environmental conditions and storage. The influence of regional factors on the anthocyanin content in bilberry fruits has been shown by Uleberg et al. [21], which demonstrated differences between anthocyanin content in samples collected in northern and southern Finland, with higher anthocyanin and total polyphenol contents in the northern region.
The use of extracts obtained from natural sources rich in anthocyanins has gained prominence, in view of their use by the food and pharmaceutical industries, with different purposes. The low stability of these compounds requires the utmost care. In most cases, water and alcoholic solutions are used as extraction solvents under acidic conditions, so as to maintain the stability of flavylium ion and increase the intensity of the red hue of the anthocyanin [52]. Anthocyanins, in addition to making products more attractive, play a key role as bioactive compounds with putative healthy effects. Studies developed by Prior et al. [53] and Fernandes et al. [54] suggest the potential of anthocyanins to be used as nutraceuticals and functional food ingredients to fight obesity and type 2 diabetes. Fernandes et al. [54], in a study carried out with mice, showed the hypoglycemic effects of cyanidin-3-glucoside, reducing blood glucose levels and enhancing insulin sensitivity by the downregulation of the retinol binding protein-4 expression. Prior et al. [53] found that purified anthocyanins from V. myrtillus, as well as blueberry juice, could prevent dyslipidemia and obesity in the same animal model. Moreover, Yamaura et al. [55] showed that cyanidin-3-glucoside and a quercetin fraction from bilberry have beneficial dermatological effects and inhibit inflammation of injured skin, correcting the Th1/Th2 balance and reducing IL-17.
Not only individual anthocyanins from V. myrtillus present beneficial effects, but Cooke et al. [56] demonstrated that one extract with 40% anthocyanins, containing the main fifteen bilberry compounds, possessed chemopreventive effects for colorectal cancer in rats.
The use of V. myrtillus fruits as a source of natural pigments has also been evaluated by some authors. Thus Camire et al. [57] compared breakfast cereals coloured with an extract rich in anthocyanin from V. myrtillus and an extract obtained from grape juice. The results concluded that bilberry concentrate possessed much higher anthocyanin content (125.4 mg/100 g, fresh weight) than the grape juice extract (28.3 mg/100 g, fresh weight) and storage over a period of three months did not change the anthocyanin content in bilberry extrudates.
Although the external appearance of food is the organoleptic characteristic that gains the first attention of consumers, it is necessary to assess acceptability. Pasqualone, Bianco, & Paradiso [58] evaluated the acceptability of consumers to biscuits added with anthocyanin extracts obtained from bilberry fruits. This study revealed that most consumers, while detecting differences in colour when compared to biscuits without anthocyanin incorporation, accepted the anthocyanin-enriched product satisfactorily. Another study revealed that the incorporation of V. myrtillus in ice-cream increased the levels of some major and minor essential elements, such as K, Se, Mn and Zn, and presented a good score by the panellists. Therefore, the addition of V. myrtillus extracts to ice cream was recommended as a natural source to increase the nutritional value and improve physicochemical properties, thus increasing the added value of the product [59].
5. Health benefits
The berries of V. myrtillus are popular worldwide and consumed since ancient times, constituting an important part of the usual diet, as well as used in several popular medicines [9]. These fruits are recognized as rich natural sources of polyphenols and other bioactive compounds with health benefits. Among others, antioxidant, anti-obesity, anti-proliferative, cardioprotective, anti-inflammatory, ocular, hypoglycemic and antibacterial effects have been associated with consuming bilberry fruits [60-62]. The numerous scientific studies that support the beneficial effects associated with these fruits explain the increase in the production and consumption of novel products and dietary supplements containing bilberry [63]. However, some studies have warned that high dose consumption of this berry may cause some unwanted effects, including possible interactions with concurrently and subsequently administered medicinal products [64]. It is important to consider that there is still a long way to go in order to confirm the relationship between the consumption of this type of berries and the bioactive properties, since most of the scientific research carried out in this field has been carried out in vitro. In this section, the principal health benefits associated with the consumption of berries or product development from V. myrtillus are reviewed.
5.1. Antioxidant Properties
Antioxidants are thought to prevent chronic complications in part through their interactions with reactive oxygen species (ROS) and their ability to scavenge free radicals [38]. The antioxidant properties of polyphenols have been strongly related to their ability to act as reducing agents [65, 66].
Dróżdż, Šėžienė, & Pyrzynska [67] demonstrated that fruits of V. myrtillus exhibited high antioxidant activity as good electron donors, and their extracts were able to reduce copper (II)-neocu-proine chelate, as well as to quench 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH•). Veljković et al. [68], in studies in rats, found relevant EC50 values for the DPPH radical scavenging activity and inhibition of lipid peroxidation in liposomes (0.202 ± 0.008 mg/mL and 0.33 ± 0.01 mg/mL respectively) for bilberry fruits . The same authors, in assays with Wistar albino rats, showed significantly decreased lipid peroxidation (MDA) and protein oxidation (AOPP) levels (p < 0.001) in the groups in which V. myrtillus was supplemented than in the control group [68].
5.2. Anti-obesity Effects
The anti-obesity mechanisms for berries may include a reduction in lipid absorption, a decrease in differentiation and proliferation of preadipocytes, a decrease in lipogenesis, an increase in lipolysis, and the inhibition of pro-inflammatory adipokine secretion [69, 70]. Studies carried by Kowalska et al. [69], demonstrated the capability of V. myrtillus to diminish lipid accumulation with a concomitant down-regulation of peroxisome proliferator-activated receptor gamma (PPARγ), enhancer-binding protein alpha (C/EBPα) and sterol regulatory element binding transcription factor 1 (SREBP1c) in mouse embryo 3T3-L1 adipocytes, as well as to suppress the expression of adipocyte fatty acid-binding protein (aP2) and resistin [71].
5.3. Anti-proliferative Properties
Polyphenols, which are plentiful in V. myrtillus, are among the most promising anti-carcinogenic agents in plants. Demirel Sezer et al. [72] found that quercetin and kaempferol, the most abundant non-anthocyanin polyphenols present in V. myrtillus extracts, showed strong cytotoxic, antioxidant and apoptotic effects. V. myrtillus anthocyanins have also demonstrated the ability to upregulate tumor suppressor genes, induce apoptosis in cancer cells, repair and protect genomic DNA integrity and improve neuronal and cognitive brain function [73]. Nguyen et al. [74] concluded that extracts from V. myrtillus were able to inhibit proliferation and induce apoptosis in breast cancer cells through a mechanism that did not involve action in the microtubules or mitosis, although when the concentration of the extracts increases, the organization of the microtubules was affected, leading to the accumulation of cells at mitosis by a direct action on microtubules.
5.4. Cardioprotective Effects
Cardiovascular diseases remain one of the leading causes of death and they are, therefore, a primary focus of research and treatment [75]. Several studies have shown that the intake of berry fruits was associated with a reduced risk of cardiovascular diseases. Ashour et al. [76] demonstrated the ability of V. myrtillus to protect against DOX-induced cardiotoxicity in rats, which could be attributed, at least in part, to its antioxidant activity. V. myrtillus significantly inhibited DOX-induced elevations of LDH, CPK and CK-MB activity in serum, as well as troponin I levels; furthermore, in the histopathological examination, the severity of the histological changes was much lower in sections from rats pre-treated with V. myrtillus [76].
Erlund et al. [77] studied the effects of V. myrtillus berries consumption on platelet function, blood pressure and HDL-cholesterol, and concluded that daily consumption of moderate amounts (100 mg) during two months could contribute to explain the cardiovascular disease protective role of a diet rich in fruits and vegetables. Žiberna et al. [78] observed that the administration of a V. myrtillus extract (0.01-5 mg/L) to rats increased coronary flow up to 2.5-fold, and decreased lactate dehydrogenase (LDH) release rate during reperfusion by 3.7-fold at 0.1 mg/L and by 6.7-fold at 1 mg/L, compared to controls. It was also effective in the prevention of arrhythmias, whose duration was maximally shortened at 0.1 mg/L to 3.2 ± 0.2%, and at 1 mg/L to 4.4 ± 0.3%, in relation to the untreated group.
5.5. Anti-inflammatory Effects
The progression and development of several diseases, such as autoimmune diseases, organ fibrosis, diabetes, obesity, allergies and dysfunction, are influenced by acute and chronic inflammation. Schink et al. [62], in a screening of 99 ethanolic plants extracts, found that V. myrtillus displayed strong anti-inflammatory activity combined with high cell viability in THP-1, HeLa-TLR4, and HEK-TLR2/HEK-TLR4 cell lines. Anthocyanin-rich extracts from bilberry also showed anti-inflammatory effects against liver inflammation in mice, leading to the suppression of LPS-induced inducible nitric oxide synthase (iNOS), TNF-α, IL-1β and IL-6 transcripts, and iNOS, TNF-α and NF-κB protein levels [79]. Furthermore, bilberry also demonstrated the ability to reduce serum C-reactive protein (CRP), IL-6, IL-12 and LPS levels, and downregulate genes associated with the TLR pathway in individuals with metabolic syndrome [62, 80]. All in all, these studies reveal not only the anti-inflammatory potential of V. myrtillus, but also its oral effectiveness in humans.
5.6. Hypoglycemic Effects
Postprandial hyperglycemia is a condition that can be improved through lifestyle and diet, preventing the development of cardiovascular diseases and diabetes type 2 [81, 82]. Xu et al. [83] showed that the consumption of fruits, like V. myrtillus, rich in phenolic compounds, can attenuate postprandial glycemic and insulin responses in young adults, with bilberry fruits having the most insulin lowering effect at 30 min postprandial; this effect was maintained throughout the early postprandial period and was related with the consumed amount of phenolic compounds. Different authors also reported inhibitory effects on α-glucosidase and amyloglucosidase activities of polyphenol-rich extracts from V. myrtillus fruits; in particular, phenolic acid-enriched fractions were able to inhibit α-glucosidase in vitro, which is considered one of the most effective ways to control type 2 diabetes [60, 84-86].
5.7. Ocular Effects
Eye fatigue, pain, dry eye sensation, excess of tears, blurry vision, glaucoma and cataracts are the most common changes that can impair the quality of vision, especially in individuals with daily work that requires more eye strain [87]. Thus, the development of products which could improve eye health has gained prominence from researchers. Riva et al. [88] studied the bioavailability of a standardized V. myrtillus extract and its ability to alleviate dry eye symptoms and concluded that it could improve tear secretion in subjects suffering from dry eye symptoms. Other studies described beneficial ocular effects of V. myrtillus extracts, namely night vision improvement [89], cataract and glaucoma prevention [90].
5.8. Antimicrobial Effects
Some studies suggest that bilberry may protect against human pathogenic bacteria, due to its composition in phenolic compounds and organic acids [91]. Toivanen et al. [92] demonstrated that juices produced from V. myrtillus showed potential against pneumococcal infections caused by Neisseria meningitidis with a 63% growth inhibition at a concentration of 10 mg/mL. Huttunen et al. [93] studied the inhibitory activity of wild berry juice fractions composed mostly of sugars and some amounts of small size phenolics against Streptococcus pneumoniae binding to human bronchial cells and concluded that the highest concentration used in their antimicrobial tests (~86 mg/g) was extremely effective and the growth of S. pneumonia was totally inhibited by V. myrtillus extract.
Overall, bilberry has potential to be used in vision improvement, and in the treatment or prevention of conditions associated with dyslipidemia, inflammation, hyperglycemia, increased oxidative stress, cardiovascular disease (CVD), cancer, diabetes, and other age-related diseases, besides their antimicrobial activity.
6. Industrial applications
Appearance is a recurrent concern of the food and pharmaceutical industries where colour plays a key role. The demand for the use of natural pigments has increased not only because of the concern with the use of artificial colorants, which is increasingly evident, but also for their nutraceutical properties [10]. The discovery of compounds able to meet the colouring and healthy requirements, with minimal toxicity, has led many researchers to focus on the use of extracts rich in anthocyanins obtained from fruits, including the fruits of V. myrtillus, in the development of innovative products.
Daily anthocyanin consumption can range from milligrams to hundreds of milligrams per person, depending on the diet and the sources they are ingested from. The use of anthocyanins as food colourings, especially in more acidic foods, which may favour their stability, has exponentially raised. The exploitation of V. myrtillus fruits for medicine and human diet purposes has gained significant attention, being the economically most important wild berries of Northern Europe, widely used by the food industry [94]. This fruit is consumed not only in a fresh manner, but also in processed products (press cake) and derivatives (juice, jam and liqueur) [95, 96]. Fruits drying and its transformation into powder represent a suitable alternative widely used by consumers and the food industry that allow having them available throughout the year for subsequent use as an ingredient in based foods (extruded products, bakeries, sauces, beverages, ice creams, yogurts, and confectionary) [11, 97]. The by-products resulting from the production of bilberries, as well as the fruits that, due to their exterior appearance or size do not meet commercialization standards, may also be used for the preparation of polyphenols extracts and the production of novel foods, conferring them added value and reducing the environmental impact. Some authors showed that the press cake of V. myrtillus, a by-product of juice production, can be a suitable and green approach to be used in the preparation of value-added products by the food industry [96, 98, 99]. For instance, Fidaleo et al. [33] studied the suitability of phenolic extracts obtained from V. myrtillus residues as an ingredient in drinking yogurt and condensed milk with a high antioxidant capacity [33]. There are, already in the market, several companies that have incorporated bilberry in products from several sectors claiming benefits to consumer health. Lusoberry® (http://lusoberry.com/), a Portuguese company based in Oliveira do Hospital, is a bilberry producer that began to add value to the fruits using them in the production of oil and wine. The company expounds that bilberry oil does not have as strong taste or acidity as olive oil and that has a higher concentration of magnesium and potassium. For its part, the bilberry wine, not being a novelty in the world, emerges for the first time in the Iberian Peninsula.
Yogurts are an important element of the human diet and often consumed by a large part of the population of all age groups. This may justify the diversity of dairy companies interested in developing bilberry-enriched yogurt. PIÁ®, a Brazilian company, has developed a yogurt with bilberry preparations, containing 25% less sugar content than usual (http://www.pia.com.br/produto/tipo/iogurtes/). Furthermore, Biedermann® company from Switzerland has developed BioSkyr®, protein-rich fermented milk containing 9.2% bilberry fruit (https://biomolkerei.ch). Both companies have promoted marketing campaigns around these products, highlighting the health benefits. A Brazilian company dedicated to the preparation of more natural ice cream has launched a claimed healthy product based on bilberry fruit (http://www.santofruto.eco.br/).
Another sector that is of great importance in the human diet and which has also been influenced by consumer demand for healthier foods is the bakery industry. Mirtiflor® (https://www.mirtiflor.pt/), a family Portuguese company producer of wild fruits, has invested in the development of several products aiming in the full use of these fruits. In the particular case of bilberry, the company, in addition to preparing traditional jams and liqueurs, has launched in the market bilberry cookies. Moreover, the well-known BioGerminal® (https://www.germinalbio.it/), which is dedicated to the production of healthy food, has developed an integral spelled pie with bilberry. Moreover, Little Bellies® (https://bellies.com.au/) is a well-known children's food brand that has been focusing on launching products labeled as healthier and more natural. In its diversity of choices, there is a snack for children from 9 months of age based on puffed corn with organic bilberry. In addition to being advertised as a natural snack with antioxidant properties, this snack is also gluten-free.
Similarly, the big chocolate companies have also been testing new recipes using powerful fruits to somehow make unhealthy products more attractive to consumers. Thus, Schogetten® (https://www.schogetten.com) features chocolate with dehydrated bilberry and muesli and the well-known Guylian® Belgian chocolate (https://www.guylian.com/) features a variety of fruit berries topped with its famous chocolate, among them bilberries.
In addition to the various sectors of the food industry, the cosmetic industry has also been focusing on enriching different products with bilberry. Panvel Vert® (https://www.panvel.com) has extensive experience in cosmetic products and has developed a body spray with soothing and relaxing properties with bilberry extract enriching the product with vitamins, minerals and other antioxidants. Furthermore, the hair cosmetics industry has been betting on innovation, and brand Loweel® (http://www.lowell.com.br/) has developed a hair care kit for repair and nutrition incorporating bilberry antioxidant active agents, claiming the protection from hair aging.
In conclusion, V. myrtillus is a widely consumed fruit, with interesting nutritional and therapeutic properties, rich in phytochemical compounds, which can be used in various industrial sectors, not only as such or as derived products, but also taking advantage of the resulting by-products, without losing its claimed beneficial effects.
Concluding remarks
The fruits of V. myrtillus are appreciated for their nutritional characteristics and their rich content in micronutrients and phytochemicals proven health benefits, including organic acids, sugars, minerals and vitamins, fiber and phenolic compounds, which have been associated to health benefits. Anthocyanins are one of the main compounds present in bilberry fruits composition, responsible for the characteristic colouration of these berries and supposed healthy effects. Thus, they are promising natural colourant molecules, capable of replacing artificial additives. The industry has been exploring the use of bilberries, which has had a huge environmental and economic impact as it makes use of all the fruit, including waste products. Several industrial sectors have been focusing on the production of new products enriched with bioactive compounds obtained from bilberry in order to obtain novel products with the associated beneficial properties. However, at the level of application as a colouring ingredient, there are some further stability studies to be carried out in order to ensure its stability in the final products so as to replace artificial colours in food and textiles.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support to CIMO (UIDB/00690/2020) and T.C.S.P. Pires grant (SFRH/BD/129551/2017). L. Barros would like to thank the national funding by FCT, P.I., through the institutional scientific employment program-contract. To the project AllNat for the contract of C. Caleja (Project AllNat POCI-01-0145-FEDER-030463). The authors are also grateful to FEDER-Interreg España-Portugal programme for financial support through the project 0377_Iberphenol_6_E. The GIP-USAL is financially supported by the Spanish Government through the project AGL2015-64522-C2-2-R.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Nagulsamy P., Ponnusamy R., Thangaraj P. Evaluation of antioxidant, anti-inflammatory, and antiulcer properties of Vaccinium leschenaultii Wight: A therapeutic supplement. Yao Wu Shi Pin Fen Xi. 2015;23:376–386. doi: 10.1016/j.jfda.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zorenc Z., Veberic R., Mikulic-Petkovsek M. Are processed bilberry products a good source of phenolics. J. Food Sci. 2018;83(7):1856–1861. doi: 10.1111/1750-3841.14209. [DOI] [PubMed] [Google Scholar]
- 3.Zoratti L., Klemettilä H., Jaakola L. Bilberry (Vaccinium myrtillus L.). Ecotypes Nutr Compos Fruit Cultiv; 2016. pp. 83–99. [Google Scholar]
- 4.Colak N., Primetta A.K., Riihinen K.R., et al. Phenolic compounds and antioxidant capacity in different-colored and non-pigmented berries of bilberry (Vaccinium myrtillus L.). Food Biosci. 2017;20:67–78. [Google Scholar]
- 5.Abreu O.A., Barreto G., Prieto S. Vaccinium (ericaceae): Ethnobotany and pharmacological potentials. Emir. J. Food Agric. 2014;26:577–591. [Google Scholar]
- 6.Crespo M.C., Visioli F. A brief review of blue- and bilberries’ potential to curb cardio-metabolic perturbations: focus on diabetes. Curr. Pharm. Des. 2017;23(7):983–988. doi: 10.2174/1381612822666161010120523. [DOI] [PubMed] [Google Scholar]
- 7.Li R., Wang P. Guo Q qi, Wang Z yu. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011;125:116–120. [Google Scholar]
- 8.Primetta A.K., Jaakola L., Ayaz F.A., Inceer H., Riihinen K.R. Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control. 2013;30:662–667. [Google Scholar]
- 9.Colak N., Torun H., Gruz J., Strnad M., Hermosín-Gutiérrez I., Hayirlioglu-Ayaz S., et al. Bog bilberry phenolics, antioxidant capacity and nutrient profile. J Food Chem. 2016;201:339–349. doi: 10.1016/j.foodchem.2016.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Murley T., Chambers E. The influence of colorants, flavorants and product identity on perceptions of naturalness. Foods. 2019;8(8) doi: 10.3390/foods8080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karam M.C., Petit J., Zimmer D., Baudelaire Djantou E., Scher J. Effects of drying and grinding in production of fruit and vegetable powders. A review. J. Food Eng. 2016;188:32–49. [Google Scholar]
- 12.Nin S., Petrucci W.A., Del Bubba M., Ancillotti C., Giordani E. Effects of environmental factors on seed germination and seedling establishment in bilberry (Vaccinium myrtillus L.). Sci. Hortic. (Amsterdam) 2017;226:241–249. [Google Scholar]
- 13.Može Š., Polak T., Gašperlin L., et al. Phenolics in slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011;59:6998–7004. doi: 10.1021/jf200765n. [DOI] [PubMed] [Google Scholar]
- 14.Chu W.K., Cheung S.C., Lau R.A., Benzie I.F. Herbal medicine -biomolecular and clinical aspects.Herb Med -biomolecular Clin Asp second. Group T& F.; 2011. pp. 55–67. [Google Scholar]
- 15.Coudun C., Gégout J. Quantitative prediction of the distribution and abundance of Vaccinium myrtillus with climatic and edaphic factors. J. Veg. Sci. 2007;18(4):517–524. [Google Scholar]
- 16.Barizza E., Guzzo F., Fanton P., et al. Nutritional profile and productivity of bilberry (Vaccinium myrtillus L.) in different habitats of a protected area of the Eastern Italian Alps. J. Food Sci. 2013;78(5):C673–C678. doi: 10.1111/1750-3841.12120. [DOI] [PubMed] [Google Scholar]
- 17.Åkerström A., Jaakola L., Bång U., Jäderlund A. Effects of latitude-related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (Bilberries). J. Agric. Food Chem. 2010;58(22):11939–11945. doi: 10.1021/jf102407n. [DOI] [PubMed] [Google Scholar]
- 18.Lätti A.K., Jaakola L., Riihinen K.R., Kainulainen P.S. Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland. Agric Food Chem. 2010;58(1):427–433. doi: 10.1021/jf903033m. [DOI] [PubMed] [Google Scholar]
- 19.Upton R. Bilberry Fruit Vaccinium myrtillus. L. Standards of Analysis, Quality Control, and Therapeutics; 2001. [Google Scholar]
- 20.Ferlemi A.V., Lamari F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants. 2016;•••:5. doi: 10.3390/antiox5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uleberg E., Rohloff J., Jaakola L., et al. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012;60:10406–10414. doi: 10.1021/jf302924m. [DOI] [PubMed] [Google Scholar]
- 22.Zorenc Z., Veberic R., Stampar F., Koron D., Mikulic-Petkovsek M. White versus blue: Does the wild “albino” bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem. 2016;211:876–882. doi: 10.1016/j.foodchem.2016.05.142. [DOI] [PubMed] [Google Scholar]
- 23.Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2015;95:776–785. doi: 10.1002/jsfa.6897. [DOI] [PubMed] [Google Scholar]
- 24.Cocetta G., Karppinen K., Suokas M., et al. Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. J. Plant Physiol. 2012;169:1059–1065. doi: 10.1016/j.jplph.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Aura A.M., Holopainen-Mantila U., Sibakov J., Kössö T., Mokkila M., Kaisa P. Bilberry and bilberry press cake as sources of dietary fibre. Food Nutr. Res. 2015;59 doi: 10.3402/fnr.v59.28367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012;77:1064–1071. doi: 10.1111/j.1750-3841.2012.02896.x. [DOI] [PubMed] [Google Scholar]
- 27.Silva B.M., Andrade P.B., Mendes G.C., Seabra R.M., Ferreira M.A. Study of the organic acids composition of quince (Cydonia oblonga Miller) fruit and jam. J. Agric. Food Chem. 2002;50:2313–2317. doi: 10.1021/jf011286+. [DOI] [PubMed] [Google Scholar]
- 28.Giné Bordonaba J., Terry L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria× ananassa) from different cultivars using deficit irrigation. Food Chem. 2010;122(4):1020–1026. [Google Scholar]
- 29.Michalska A., Łysiak G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015;16:18642–18663. doi: 10.3390/ijms160818642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damascos M.A., Arribere M., Svriz M., Bran D. Fruit mineral contents of six wild species of the north andean patagonia, Argentina. Biol. Trace Elem. Res. 2008;125(1):72–80. doi: 10.1007/s12011-008-8159-y. [DOI] [PubMed] [Google Scholar]
- 31.Desideri D., Meli M.A., Roselli C. Determination of essential and non-essential elements in some medicinal plants by polarised X ray fluorescence spectrometer (EDPXRF). Microchem. J. 2010;95(2):174–180. [Google Scholar]
- 32.Kabata-Pendias A. Trace elements in soils and plants. 4th ed. 2010. [Google Scholar]
- 33.Fidaleo M., Lavecchia R., Maffei G., Zuorro A. Phenolic extracts from bilberry (Vaccinium myrtillus L.) residues as new functional food ingredients. Int J App Eng Research. 2015;10(16):37125–37128. [Google Scholar]
- 34.Ambriz-Pérez D.L., Leyva-López N., Gutierrez-Grijalva E.P., Heredia J.B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Congesnt Food Agric; 2016. p. 2. [Google Scholar]
- 35.Rodrigo R., Gil D., Miranda-Merchak A., Kalantzidis G. Antihypertensive role of polyphenols. Adv. Clin. Chem. 2012;58:225–254. doi: 10.1016/B978-0-12-394383-5.00014-X. [DOI] [PubMed] [Google Scholar]
- 36.Bouarab-Chibane L., Forquet V., Lantéri P., et al. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019;10:1–23. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anantharaju P.G., Gowda P.C., Vimalambike M.G., Madhunapantula S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016;15:1–16. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Y., Liimatainen J., Alanne A-L., et al. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017;220:266–281. doi: 10.1016/j.foodchem.2016.09.145. [DOI] [PubMed] [Google Scholar]
- 39.Rue E.A., Rush M.D., van Breemen R.B. Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018;17(1):1–16. doi: 10.1007/s11101-017-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge Y.W., Zhu S., Kazuma K., Wei S.L., Yoshimatsu K., Komatsu K. Molecular ion index assisted comprehensive profiling of B-type oligomeric proanthocyanidins in rhubarb by high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016;408(13):3555–3570. doi: 10.1007/s00216-016-9433-z. [DOI] [PubMed] [Google Scholar]
- 41.Abo K a Phenolic acids in foods. An overview of analytical methodology phenolic acids in foods : an overview of analytical methodology. J. Agric. Food Chem. 2013;115:137–189. [Google Scholar]
- 42.Deǧirmencioǧlu N., Gürbüz O., Karatepe G.E., Irkin R. Influence of hot air drying on phenolic compounds and antioxidant capacity of blueberry (Vaccinium myrtillus) fruit and leaf. J. Appl. Bot. Food Qual. 2017;90:115–125. [Google Scholar]
- 43.Tumbas Šaponjac V., Čanadanović-Brunet J., Ćetković G., Djilas S., Četojević-Simin D. Dried bilberry (Vaccinium myrtillus L.) extract fractions as antioxidants and cancer cell growth inhibitors. LWT Food Sci Tech. 2015;61:615–621. [Google Scholar]
- 44.Ehala S., Vaher M., Kaljurand M. Characterization of phenolic profiles of Northern European berries by capillary electrophoresis and determination of their antioxidant activity. J. Agric. Food Chem. 2005;53(16):6484–6490. doi: 10.1021/jf050397w. [DOI] [PubMed] [Google Scholar]
- 45.Silva S., Costa E.M., Calhau C., Morais R.M., Pintado M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017;57(14):3072–3083. doi: 10.1080/10408398.2015.1087963. [DOI] [PubMed] [Google Scholar]
- 46.Ancillotti C., Ciofi L., Pucci D., et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow). S.B. Young. Food Chem. 2016;204:176–184. doi: 10.1016/j.foodchem.2016.02.106. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y., Wang L., Xue Y., et al. Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China. Biochem. Eng. J. 2009;43:135–141. [Google Scholar]
- 48.Parmar R.S., Singh C. A comprehensive study of eco-friendly natural pigment and its applications. Biochem. Biophys. Rep. 2018;13:22–26. doi: 10.1016/j.bbrep.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mapari S.A.S., Thrane U., Meyer A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010;28(6):300–307. doi: 10.1016/j.tibtech.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Carocho M., Barreiro M.F., Morales P., Ferreira I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014;13:377–399. doi: 10.1111/1541-4337.12065. [DOI] [PubMed] [Google Scholar]
- 51.Loypimai P., Moongngarm A., Chottanom P., Moontree T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colourant. Innov. Food Sci. Emerg. Technol. 2015;27:102–110. doi: 10.1016/j.ifset.2014.12.009. [DOI] [Google Scholar]
- 52.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prior R.L.E., Wilkes S.R., Rogers T., Khanal R.C., Wu X., Howard L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010;58:3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes I., Marques C., Évora A., Faria A., Mateus N., De Freitas V. Anthocyanins : nutrition and health. bioact mol foods. Nature Switzerland. Springer; 2019. pp. 1097–1133. [Google Scholar]
- 55.Yamaura K., Ishiwatari M., Yamamoto M., Shimada M., Bi Y., Ueno K. Anthocyanins, but not anthocyanidins, from bilberry (Vaccinium myrtillus L.) alleviate pruritus via inhibition of mast cell degranulation. J. Food Sci. 2012;77(12):H262–H267. doi: 10.1111/j.1750-3841.2012.02974.x. [DOI] [PubMed] [Google Scholar]
- 56.Cooke D., Schwarz M., Boocock D., et al. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis - Relationship with tissue anthocyanin levels. Int. J. Cancer. 2006;119:2213–2220. doi: 10.1002/ijc.22090. [DOI] [PubMed] [Google Scholar]
- 57.Camire M.E., Chaovanalikit A., Dougherty M.P., Briggs J. Blueberry and grape anthocyanins as breakfast cereal colorants. J. Food Sci. 2002;67(1):438–441. doi: 10.1111/j.1365-2621.2002.tb11425.x. [DOI] [Google Scholar]
- 58.Pasqualone A., Bianco A.M., Paradiso V.M. Production trials to improve the nutritional quality of biscuits and to enrich them with natural anthocyanins. CYTA J. Food. 2013;11:301–308. doi: 10.1080/19476337.2012.753113. [DOI] [Google Scholar]
- 59.Eekaya Kotan T. Mineral composition and some quality characteristics of ice creams manufactured with the addition of Blueberry. J Food. 2018;43:635–643. doi: 10.15237/gida.GD18042. [DOI] [Google Scholar]
- 60.de Mello V.D.F., Lankinen M.A., Lindström J., et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 2017;61(9) doi: 10.1002/mnfr.201700019. [DOI] [PubMed] [Google Scholar]
- 61.Karcheva-Bahchevanska D.P., Lukova P.K., Nikolova M.M., Mladenov R.D., Iliev I.N. Effect of extracts of bilberries (Vaccinium myrtillus L.) on amyloglucosidase and α-glucosidase activity. Folia Med. (Plovdiv) 2017;59(2):197–202. doi: 10.1515/folmed-2017-0028. [DOI] [PubMed] [Google Scholar]
- 62.Schink A., Neumann J., Leifke A.L., et al. Screening of herbal extracts for TLR2-and TLR4-dependent anti-inflammatory effects. PLoS One. 2018;13(10):e0203907. doi: 10.1371/journal.pone.0203907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prokop J., Lněničková K., Cibiček N., et al. Effect of bilberry extract (Vaccinium myrtillus L.)on drug-metabolizing enzymes in rats. Food Chem. Toxicol. 2019;129:382–390. doi: 10.1016/j.fct.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 64.Prakash C., Zuniga B., Song C.S., et al. Nuclear receptors in drug metabolism, drug response and drug interactions. Nucl. Receptor Res. 2015;2:1–20. doi: 10.11131/2015/101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima G.P.P., Vianello F., Corrêa C.R. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014;5:1065–1082. [Google Scholar]
- 66.Tumbas V., Čanadanović-Brunet J., Gille L., Dilas S., Ćetković G. Superoxide anion radical scavenging activity of bilberry (Vaccinium myrtillus L.). J. Berry Res. 2010;1(1):13–23. [Google Scholar]
- 67.Dróżdż P., Šėžienė V., Pyrzynska K. phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods Hum. Nutr. 2017;72(4):360–364. doi: 10.1007/s11130-017-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veljković M., Pavlović D.R., Stojiljković N., et al. Bilberry: chemical profiling, in vitro and in vivo antioxidant activity and nephroprotective effect against gentamicin toxicity in rats. Phytother. Res. 2017;31(1):115–123. doi: 10.1002/ptr.5738. [DOI] [PubMed] [Google Scholar]
- 69.Kowalska K., Olejnik A., Szwajgier D., Olkowicz M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kowalska K, Olejnik A, Rychlik J, Grajek W. 2015.
- 71.Suzuki R, Tanaka M, Takanashi M, Hussain A, Yuan B, Toyoda H, et al. 2011. [DOI] [PMC free article] [PubMed]
- 72.Demirel Sezer E., Oktay L.M., Karadadaş E., Memmedov H., Selvi Gunel N., Sözmen E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food. 2019;22(11):1118–16. doi: 10.1089/jmf.2019.0098. [DOI] [PubMed] [Google Scholar]
- 73.Thibado S., Thornthwaite J., Ballard T., Goodman B. Anticancer effects of Bilberry anthocyanins compared with NutraNanoSphere encapsulated Bilberry anthocyanins. Mol. Clin. Oncol. 2017;8(2):330–335. doi: 10.3892/mco.2017.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen V., Tang J., Oroudjev E., et al. Cytotoxic effects of bilberry extract on MCF7-GFP-tubulin breast cancer cells. J. Med. Food. 2010;13(2):278–285. doi: 10.1089/jmf.2009.0053. [DOI] [PubMed] [Google Scholar]
- 75.Brader L., Overgaard A., Christensen L.P., Jeppesen P.B., Hermansen K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013;10(4):270–282. doi: 10.1900/RDS.2013.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashour O.M., Elberry A.A., Alahdal A.M., et al. Protective effect of bilberry (Vaccinium myrtillus) against doxorubicin-induced oxidative cardiotoxicity in rats. Med Sci Montii. 2011;17(4):110–115. doi: 10.12659/MSM.881711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erlund I., Koli R., Alfthan G., et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008;87(2):323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 78.Žiberna L., Lunder M., Može Š., Vanzo A., Drevenšek G. Cardioprotective effects of bilberry extract on ischemia-reperfusion-induced injury in isolated rat heart. BMC Pharmacol. 2009;9 doi: 10.1186/1471-2210-9-S2-A55. [DOI] [Google Scholar]
- 79.Luo H., Lv X.D., Wang G.E., Li Y.F., Kurihara H., He R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. 2014;65(5):594–601. doi: 10.3109/09637486.2014.886184. [DOI] [PubMed] [Google Scholar]
- 80.Kolehmainen M., Mykkänen O., Kirjavainen P.V., et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012;56(10):1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 81.Granfeldt Y.E., Björck I.M.E. A bilberry drink with fermented oatmeal decreases postprandial insulin demand in young healthy adults. Nutr. J. 2011;10(57):333–353. doi: 10.1186/1475-2891-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ceriello A., Genovese S. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Rev. Endocr. Metab. Disord. 2016;17:111–116. doi: 10.1007/s11154-016-9341-8. [DOI] [PubMed] [Google Scholar]
- 83.Xu J., Jönsson T., Plaza M., et al. Probiotic fruit beverages with different polyphenol profiles attenuated early insulin response. Nutr. J. 2018;17(1):34. doi: 10.1186/s12937-018-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDougall G.J., Stewart D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors. 2005;23(4):189–195. doi: 10.1002/biof.5520230403. [DOI] [PubMed] [Google Scholar]
- 85.Xiao T., Guo Z., Sun B., Zhao Y. Identification of anthocyanins from four kinds of berries and their inhibition activity to α-glycosidase and protein tyrosine phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017;65(30):6211–6221. doi: 10.1021/acs.jafc.7b02550. [DOI] [PubMed] [Google Scholar]
- 86.de Sales P.M., de Souza P.M., Simeoni L.A., Magalhães P de O., Silveira D. α-amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012;15(1):141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- 87.Ozawa Y., Kawashima M., Inoue S., et al. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging. 2015;19(5):548–554. doi: 10.1007/s12603-014-0573-6. [DOI] [PubMed] [Google Scholar]
- 88.Riva A., Togni S., Franceschi F., et al. The effect of a natural, standardized bilberry extract (Mirtoselect®) in dry eye: a randomized, double blinded, placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017;21(10):2518–2525. [PubMed] [Google Scholar]
- 89.Canter P.H., Ernst E. Anthocyanosides of Vaccinium myrtillus (bilberry) for night vision a systematic review of placebo-controlled trials. Surv. Ophthalmol. 2004;49(1):38–50. doi: 10.1016/j.survophthal.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Head K.A. Natural therapies for ocular disorders part two: Cataracts and glaucoma. Rev. 6th ed. Altern. Med; 2001. pp. 141–166. [PubMed] [Google Scholar]
- 91.Puupponen-Pimiä R., Nohynek L., Ammann S., Oksman-Caldentey K.M., Buchert J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J. Agric. Food Chem. 2008;56(3):681–688. doi: 10.1021/jf072001h. [DOI] [PubMed] [Google Scholar]
- 92.Toivanen M., Huttunen S., Lapinjoki S., Tikkanen-Kaukanen C. Inhibition of adhesion of Neisseria meningitidis to human epithelial cells by berry juice polyphenolic fractions. Phytother. Res. 2011;25(6):828–832. doi: 10.1002/ptr.3349. [DOI] [PubMed] [Google Scholar]
- 93.Huttunen S., Toivanen M., Arkko S., Ruponen M., Tikkanen-Kaukanen C. Inhibition activity of wild berry juice fractions against streptococcus pneumoniae binding to human bronchial cells. Phytother. Res. 2011;25(1):122–127. doi: 10.1002/ptr.3240. [DOI] [PubMed] [Google Scholar]
- 94.Trivedi P., Karppinen K., Klavins L., et al. Compositional and morphological analyses of wax in northern wild berry species. Food Chem. 2019;295:441–448. doi: 10.1016/j.foodchem.2019.05.134. [DOI] [PubMed] [Google Scholar]
- 95.Benvenuti S., Brighenti V., Pellati F. High-performance liquid chromatography for the analytical characterization of anthocyanins in Vaccinium myrtillus L. (bilberry) fruit and food products. Anal. Bioanal. Chem. 2018;410:3559–3571. doi: 10.1007/s00216-018-0915-z. [DOI] [PubMed] [Google Scholar]
- 96.Zhou L., Lie Y., Briers H., Fan J., et al. natural product recovery from bilberry (Vaccinium myrtillus l.) presscake via microwave hydrolysis. ACS Sustain. Chem.& Eng. 2018;6(3):3676–3685. [Google Scholar]
- 97.Oliveira G., Eliasson L., Ehrnell M., Höglund E., Andlid T., Alminger M. Tailoring bilberry powder functionality through processing: Effects of drying and fractionation on the stability of total polyphenols and anthocyanins. Food Sci. Nutr. 2019;7(3) doi: 10.1002/fsn3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bobinaitė R., Pataro G., Lamanauskas N., Šatkauskas S., Viškelis P., Ferrari G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015;52(9):5898–5905. doi: 10.1007/s13197-014-1668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pataro G., Bobinaitė R., Bobinas Č., et al. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017;10(9):1595–1605. [Google Scholar]
- 100.Stanoeva J.P., Stefova M., Andonovska K.B., Vankova A., Stafilov T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017;20:S863–S883. doi: 10.1080/10942912.2017.1315592. [DOI] [Google Scholar]
- 101.Kähkönen M.P., Heinämäki J., Ollilainen V., Heinonen M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003;83:1403–1411. [Google Scholar]
- 102.Laaksonen O., Sandell M., Kallio H. Chemical factors contributing to orosensory profiles of bilberry (Vaccinium myrtillus) fractions. Eur. Food Res. Technol. 2010;231(2):271–285. [Google Scholar]
- 103.Ivayla D., Ilian B. Assesment of the anthocyanin variation in bulgarian bilberry (Vaccinium Myrtillus L.) and lingonberry (Vaccinium Vitis-Idaea L.). Int J Med Pharm Sci. 2016;6:39–49. [Google Scholar]
- 104.Müller D., Schantz M., Richling E. High Performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012;77(4):C340–C345. doi: 10.1111/j.1750-3841.2011.02605.x. [DOI] [PubMed] [Google Scholar]