Abstract
tRNA modifications at the anti‐codon loop are critical for accurate decoding. FTSJ1 was hypothesized to be a human tRNA 2′‐O‐methyltransferase. tRNAPhe(GAA) from intellectual disability patients with mutations in ftsj1 lacks 2′‐O‐methylation at C32 and G34 (Cm32 and Gm34). However, the catalytic activity, RNA substrates, and pathogenic mechanism of FTSJ1 remain unknown, owing, in part, to the difficulty in reconstituting enzymatic activity in vitro. Here, we identify an interacting protein of FTSJ1, WDR6. For the first time, we reconstitute the 2′‐O‐methylation activity of the FTSJ1‐WDR6 complex in vitro, which occurs at position 34 of specific tRNAs with m1G37 as a prerequisite. We find that modifications at positions 32, 34, and 37 are interdependent and occur in a hierarchical order in vivo. We also show that the translation efficiency of the UUU codon, but not the UUC codon decoded by tRNAPhe(GAA), is reduced in ftsj1 knockout cells. Bioinformatics analysis reveals that almost 40% of the high TTT‐biased genes are related to brain/nervous functions. Our data potentially enhance our understanding of the relationship between FTSJ1 and nervous system development.
Keywords: FTSJ1, RNA modification, translational regulation, tRNA, WDR6
Subject Categories: Molecular Biology of Disease, Neuroscience, RNA Biology
FTSJ1, an intellectual disability associated protein, is the human cytoplasmic tRNA 32 and 34 2′‐O‐methyltransfearse that interacts with auxiliary protein WDR6 to catalyze Nm34 formation. Knockout of ftsj1 affects the translation efficiency of UUU codon usage biased genes.
Introduction
More than 100 types of RNA modifications are identified and functionally characterized in coding and non‐coding RNAs, which termed as the field of “RNA epigenetics” or “epitranscriptomics” (He, 2010; Frye et al, 2016; Garber, 2019). As one of the heaviest modified cellular RNAs, transfer RNA (tRNA) serves as the star and prominent molecule for studying RNA epigenetics (El Yacoubi et al, 2012; Pan, 2018). Modifications on anti‐codon stem‐loop (ASL) of tRNA usually contribute to efficiency and fidelity of protein synthesis. Modifications at the first anti‐codon position, base 34 (wobble), typically impact the codon–anti‐codon interaction and could expand the decoding capability of a tRNA to read the fourfold degenerate codons (Agris et al, 2007; Nedialkova & Leidel, 2015). Another hotspot modification at position 37, adjacent to the anti‐codon 3′ end, modulates codon–anti‐codon interaction by strengthening base stacking and maintaining the reading frame (Maraia & Arimbasseri, 2017).
Recently, regulation between distinct modifications shows emerging importance in RNA epigenetics. The influence between two distinct modifications, such as the first modification is prerequisite to or acts as another recognition element for the second modification, has been found through individual studies on mRNA and tRNA (Xiang et al, 2018; Dixit et al, 2019). Intriguingly, the hierarchical order of two modifications is mainly detected at the ASL region on tRNA (Han & Phizicky, 2018; Dixit et al, 2019). In T. brucei tRNAThr, C32‐to‐U32 editing stimulates adenosine‐to‐inosine editing at position 34 (I34) (Rubio et al, 2006). 5‐methylcytidine (m5C) at position 38 depends on prior queuosine at position 34 (Q34) formation in Schizosaccharomyces pombe and D. discoideum tRNAAsp (Muller et al, 2015). In Escherichia coli tRNALeu, N6‐isopentenyladenosine (i6A) at position 37 is the prerequisite for 2′‐O‐methylation at C34 and U34 (Cm34 and Um34) (Zhou et al, 2015).
Although most of the tRNA modifications were identified over 50 years ago (Hurwitz et al, 1964), the enzymes for tRNA modifications in humans remain largely unknown, likely resulting from the difficulty in reconstituting the enzyme activity in vitro (Rubio et al, 2017). In particular, the interdependence of modifications at the ASL region hinders the identification of modifying enzymes, which may require complicated recognition elements for catalytic processes (Han & Phizicky, 2018; Dixit et al, 2019). Therefore, reconstituting the enzyme activity in vitro is of vital importance for accurate identification of tRNA‐modifying enzymes and revealing the interdependence of tRNA modifications.
Aberrant tRNA modifications were closely linked to human diseases and neurological diseases in particular (Abedini et al, 2018). Up to now, the defects of eleven genes which are probably responsible for tRNA modifications have been reported to be closely related to neurological disorders (Leschziner et al, 2011; Abedini et al, 2018). The underlying mechanisms connecting the defects of tRNA modifications and neurological diseases remain largely unknown. One of the prominent examples is human ftsj1, a non‐syndromic X‐linked intellectual disability (NSXLID)‐associated gene (Freude et al, 2004; Ramser et al, 2004). Human FTSJ1 (UniProt: Q9UET6; 49% identity and 63% similarity with Saccharomyces cerevisiae Trm7; Fig EV1), which contains 327 AA residues, is a homolog of yeast tRNA 2′‐O‐methyltransferase Trm7 at C32 and N34 (N=A, G, C, U) (Pintard et al, 2002; Guy et al, 2012; Guy & Phizicky, 2015). The tRNAPhe(GAA) from NSXLID patients with loss‐of‐function mutations of ftsj1 lacks Cm32 and Gm34 (Guy et al, 2015), hinting that FTSJ1 is a human tRNA 32 and 34 2′‐O‐methyltransferase. However, the catalytic activity and RNA substrates of FTSJ1 have not yet been elucidated, as well as the pathogenic mechanism. Additionally, tRNAPhe(GAA) from an NSXLID patient with the FTSJ1 p.A26P missense allele lacks Gm34 but contains Cm32, suggesting that Gm34 is an indispensable modification for neurodevelopment (Guy et al, 2015).
Figure EV1. Sequence alignments of human FTSJ1, eukaryotic Trm7, and E. coli FTSJ/RrmJ.
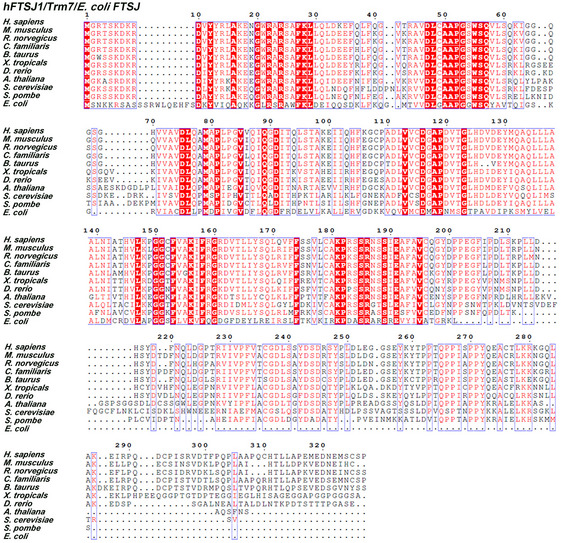
H. sapiens, Homo sapiens; M. musculus, Mus musculus; R. norvegicus, Rattus norvegicus; C. familiaris, Canis familiaris; B. taurus, Bos taurus; X. tropicalis, Xenopus tropicalis; D. rerio, Danio rerio; A. thaliana, Arabidopsis thaliana; S. cerevisiae, Saccharomyces cerevisiae; S. pombe, Schizosaccharomyces pombe; E. coli, Escherichia coli. Source data are available online for this figure.
In S. cerevisiae and S. pombe, Trm7 interacts separately with Trm732 and Trm734 to 2′‐O‐methylate C32 and N34 of tRNATrp(CCA), tRNAPhe(GAA), and tRNALeu(UAA) (Guy et al, 2012; Guy & Phizicky, 2015). In yeast, ▵Trm7 and ▵Trm732▵Trm734 cause growth defects due to loss of functional tRNAPhe(GAA) and reduced available charged tRNAPhe(GAA), which activates a robust general AA control (GAAC) response (Guy et al, 2012; Guy & Phizicky, 2015; Han et al, 2018). Intriguingly, it was found from yeast tRNAPhe(GAA) that Cm32 and Gm34 influenced the efficient conversion from 1‐methylguanosine at position 37 (m1G37) to wybutosine (yW) (Guy et al, 2012; Guy & Phizicky, 2015). These previous reports demonstrated that tRNAPhe(GAA) is the crucial substrate and may serve as the main functional executor of Trm7.
Human THADA (UniProt: Q6YHU6) and WDR6 (UniProt: Q9NNW5) are proposed to be the homologs of Trm732 and Trm734, respectively (Guy & Phizicky, 2015). THADA contains 1,953 AA residues, but only its 988–1,470 peptide shares 28% identity and 46% sequence similarity with the 596–1,028 peptide of S. cerevisiae Trm732. However, ftsj1 and thada separately complement the growth defects of S. cerevisiae ▵Trm7 and ▵Trm7▵Trm732 (Guy & Phizicky, 2015), indicating that FTSJ1 is a putative tRNA 32 and 34 2′‐O‐methyltransferase and THADA may have the similar function as Trm732. In contrast to the study of thada, co‐expression of wdr6 and ftsj1 did not complement the growth defects of S. cerevisiae ▵Trm7▵Trm734 (Guy & Phizicky, 2015). WDR6 consists of 1,121 AA residues, and S. cerevisiae Trm734 contains 1,013 AA residues; the identity and sequence similarity of the two proteins are only 20% and 37% (Fig EV2), raising the question that whether WDR6 is the human functional equivalent of Trm734.
Figure EV2. Sequence alignments of WDR6 or Trm734 from different species.
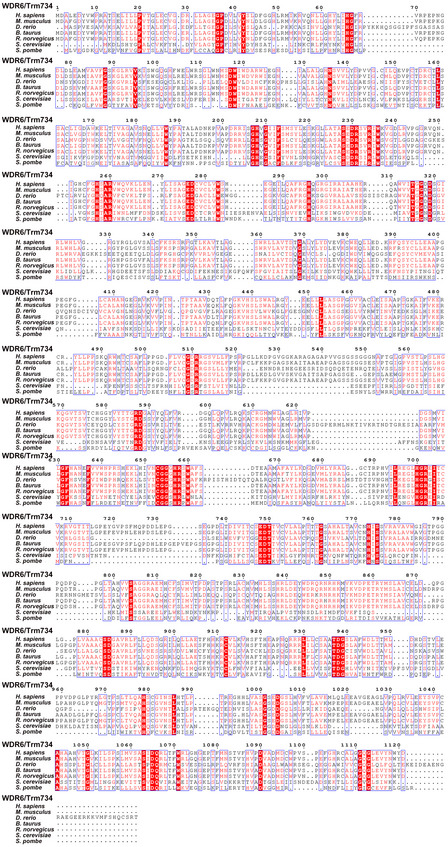
S. cerevisiae and S. pombe contain Trm734, the auxiliary protein for Trm7. Some higher eukaryotes, such as H. sapiens, M. musculus, D. rerio, B. taurus, and R. norvegicus, contain the Trm734 homolog, WDR6. Source data are available online for this figure.
In this study, we showed that FTSJ1 localizes mainly in cytoplasm and directly binds with WDR6. By using FTSJ1 and WDR6 that were purified using the baculovirus/insect cell system, we found that the binary complex of FTSJ1 and WDR6 (FTSJ1‐WDR6) produced Nm34 in vitro by using some specific tRNAs that carrying pre‐existing modifications as its substrates. In the complex, FTSJ1 could bind with S‐adenosyl‐L‐methionine (SAM), the methyl donor, and may support the catalytic role, while WDR6 mainly serves as a tRNA‐binding component. Additionally, FTSJ1 could catalyze Nm formation on different tRNA substrates at positions 32 and 34. Critically, we found that m1G37 is a prerequisite for Nm34 formation by FTSJ1‐WDR6, and modifications are interdependent among positions 32, 34, and 37 of tRNAPhe(GAA). Moreover, we found that the translation efficiency of the UUU codon but not the UUC codon decoded by tRNAPhe(GAA) was decreased in ftsj1 knockout HEK293T cells, suggesting that loss of intricate modifications has an effect on TTT‐biased genes. Intriguingly, our bioinformatics study showed that there are many TTT‐biased genes in the human genome; in the top 488 high TTT‐biased genes, 189 of them are associated with the nervous system and brain function. Taken together, our findings are the first to demonstrate the catalytic activity of FTSJ1 in vitro, shed light on the interdependence of tRNA modifications in regulating codon translation efficiency, and provided a new approach for investigating the correlation between FTSJ1 and NSXLID.
Results
Subcellular localization and determination of the interacting protein of FTSJ1
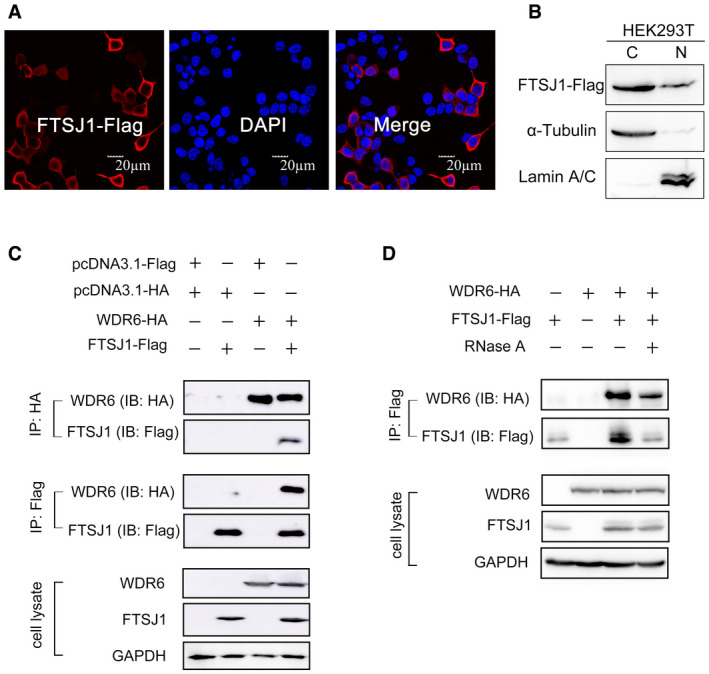
To determine the subcellular localization of FTSJ1, we expressed the gene encoding C‐terminal Flag‐tagged FTSJ1 (FTSJ1‐Flag) in HEK293T cells and detected the protein by immunolabeling. The FTSJ1 was predominantly located in the cytoplasm with a small amount in the nucleus (Fig 1A). The subcellular localization of FTSJ1 was further confirmed by nuclear/cytosol fractionation assays, and the data by Western blotting analysis showed that FTSJ1 was mainly in the cytosolic fraction of HEK293T cells (Fig 1B). We performed immunoprecipitation combined with mass spectrometry (IP‐MS) to identify proteins that potentially interact with FTSJ1. The FTSJ1‐Flag in HEK293T cells was immunoprecipitated by the anti‐Flag antibody (Appendix Fig S1), and more than 100 proteins were detected as potential FTSJ1‐interacting partners by IP‐MS analysis (Appendix Table S1). Surprisingly, WDR6, but not THADA, showed up in the list (Appendix Table S1). WDR6, which is the homolog of S. cerevisiae Trm734, belongs to the WD‐repeat protein family, which contains a beta WD propeller and forms a platform without any catalytic activity on which multiple protein complexes assemble reversibly (Smith, 2008). The interaction between FTSJ1 and WDR6 was further confirmed by co‐immunoprecipitation (co‐IP). WDR6 with an HA‐tag (WDR6‐HA) could be pulled down by FTSJ1‐Flag, and vice versa (Fig 1C).
Figure 1. Subcellular localization and protein–protein interactions of FTSJ1.
-
AImmunofluorescence labeling of FTSJ1‐Flag (red) in HEK293T cells. The nucleus was stained by DAPI (blue). Scale bar, 20 μm.
-
BSubcellular localization of FTSJ1. Cytoplasmic (C) and nuclear (N) fractions were separated from HEK293T cells expressing FTSJ1‐Flag. α‐Tubulin and lamin A/C were used as indicators of the cytoplasmic and nuclear fractions, respectively.
-
CCo‐IP assays of the interaction between FTSJ1 and WDR6 in co‐transfected HEK293T cells. Anti‐HA IP (top) and anti‐Flag IP (middle) were immunoblotted by anti‐HA and anti‐Flag antibodies, respectively. Bottom: cell lysates immunoblotted by anti‐HA or anti‐Flag antibodies, with GAPDH serving as a loading control.
-
DCo‐IP assays of the interaction between FTSJ1 and WDR6 with RNase A addition.
To investigate whether the interaction between FTSJ1 and WDR6 depends on intracellular RNAs, we added RNase A to the HEK293T cell lysates to digest intracellular RNAs. The results of co‐IP showed that intracellular RNAs were not involved in the interaction between FTSJ1 and WDR6 (Fig 1D). The above results indicate that FTSJ1 interacts with WDR6, and this interaction does not require intracellular RNAs.
FTSJ1 directly binds to WDR6
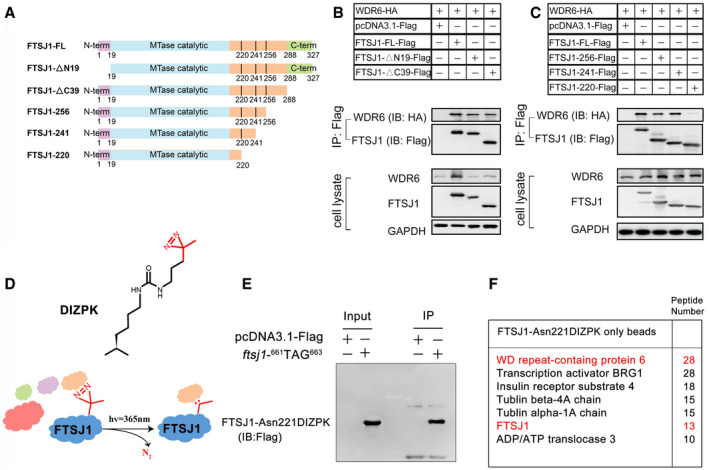
Based on the sequence alignment of FTSJ1 and E. coli FTSJ (PDB code: 1EIZ) (Bugl et al, 2000), 19–220 AA residues of FTSJ1 are presumed to be the methyltransferase (MTase) catalytic domain (Figs EV1 and 2A). To further dissect the domain(s) in FTSJ1 that interacts with WDR6, we tested the interaction between full‐length WDR6 and a series of truncated mutants of FTSJ1 (Fig 2A). We identified the 220FNQLDGPTRIIVPFVTCGDLSS241 peptide in FTSJ1 is an essential motif for WDR6 binding (Fig 2B and C).
Figure 2. FTSJ1 directly binds to WDR6.
-
ASchema showing a series of truncated FTSJ1 mutants used to map FTSJ1 domain(s) responsible for WDR6 binding.
-
B, CCo‐IP assays of the interaction between FTSJ1‐Flag or truncated FTSJ1 mutants and WDR6‐HA in co‐transfected HEK293T cells. Anti‐Flag IP (top) was, respectively, immunoblotted by anti‐HA and anti‐Flag antibodies. Bottom: cell lysates immunoblotted by anti‐HA or anti‐Flag antibodies, with GAPDH serving as a loading control.
-
DThe structure of DIZPK and scheme showing the capture of FTSJ1‐interacting proteins using DIZPK.
-
EIP assays of FTSJ1‐Asn221DIZPK. The empty vector was transfected as a negative control.
-
FA list of top FTSJ1‐Asn221DIZPK putative interacting proteins identified by IP‐MS in HEK293T cells.
Recently, protein photocrosslinking, via unnatural AAs, has been developed as an effective method for capturing proximal protein–protein interacting partners in vivo (Tanaka et al, 2008; Ai et al, 2011). DIZPK is an unnatural AA probe, and under UV light (365 nm), its high‐activity carbene molecules are rapidly and efficiently inserted into the adjacent protein within a 10 Å distance (Zhang et al, 2011). Based on the identified region of FTSJ1 that interacts with WDR6, we introduced DIZPK to Asn221 in FTSJ1 (Fig 2D), via the change of 661AAC663 in ftsj1 to TAG to obtain FTSJ1‐Asn221DIZPK. We expressed and immunoprecipitated FTSJ1‐Asn221DIZPK (Fig 2E). Critically, WDR6 had the highest abundance in the FTSJ1‐associated proteins by proximity labeling IP‐MS analysis (Fig 2F; Appendix Table S2), indicating that WDR6 directly binds to FTSJ1.
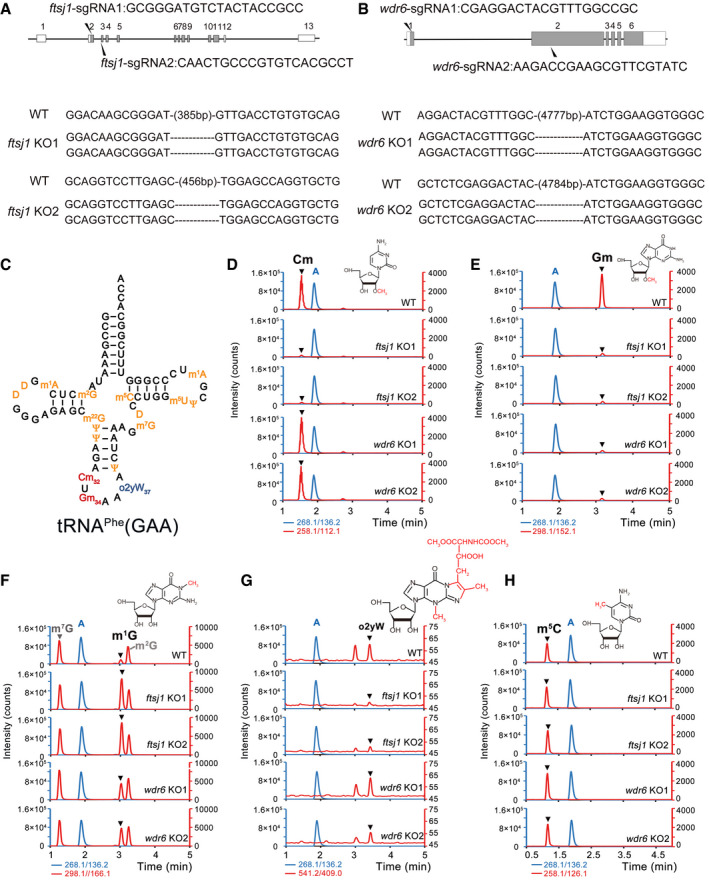
Knockout of ftsj1 and/or wdr6 affects Cm, Gm, m1G, and o2yW levels of tRNAPhe(GAA)
In order to identify the function of FTSJ1 and WDR6, we knocked out ftsj1 in HEK293T cells using the CRISPR‐Cas9 system and obtained two knockout (KO) cell lines in which both alleles contained frameshift mutations (Fig 3A); Similarly, we generated two wdr6 KO HEK293T cell lines (Fig 3B). The knockout efficiency of ftsj1 and wdr6 is shown in Appendix Fig S2. Cm32 and Gm34 are conserved in yeast and human cytoplasmic tRNAPhe(GAA) (Appendix Fig S3; Fig 3C) (Boccaletto et al, 2018). Thus, by using a biotinylated single‐stranded DNA probe, we isolated cytoplasmic tRNAPhe(GAA) from wild‐type (WT), two ftsj1 KO cell lines, and two wdr6 KO cell lines and subjected them to UPLC‐MS/MS to analyze the Cm, Gm, m1G, o2yW, and m5C levels. Fig 3D–H are the representative images of Cm, Gm, m1G, o2yW, and m5C levels of tRNAPhe(GAA) from WT, ftsj1 KO, and wdr6 KO cells, and the corresponding bar charts together with statistics are shown in Fig EV3. In WT cells, Cm and Gm were abundantly detected in tRNAPhe(GAA) (Figs 3D and E, and EV3). However, compared to that of WT cells, the level of Cm and Gm both decreased significantly in ftsj1 KO cells (Figs 3D and E, and EV3), while in wdr6 KO cells, only the level of Gm obviously decreased, and the level of Cm showed no significant difference (Figs 3D and E, and EV3). As a control, the quantity of m5C in tRNAPhe(GAA) had no apparent change in WT cells, ftsj1 KO cells, and wdr6 KO cells (Figs 3H and EV3). According to the tRNA database (Boccaletto et al, 2018), human cytoplasmic tRNAPhe(GAA) only possesses Cm at position 32 and Gm at position 34, and our results showed that FTSJ1 is responsible for the Cm32 and Gm34 modification, while WDR6 is only involved in Gm34 formation in vivo.
Figure 3. Knockout of ftsj1 and/or wdr6 affects Cm, Gm, m1G, and o2yW levels of tRNAPhe(GAA).
-
A, BSchematic depiction of ftsj1 and wdr6, and target sites of mutations introduced by the CRISPR‐Cas9 system in HEK293T cells. Shaded and open boxes indicate coding regions and untranslated regions of exons, respectively. Lines indicate introns. For ftsj1, two sgRNA sequences for targeting exons 2 and 3 are noted. For wdr6, two sgRNA sequences for targeting exons 1 and 2 are noted. Sequences of both alleles of ftsj1 or wdr6 in KO1 and KO2 cell lines are aligned. Deleted nucleotides are indicated as dashed lines.
-
CSecondary structures of human tRNAPhe(GAA) with modified nucleosides.
-
D–HMass chromatograms of the nucleosides, Cm (Q1/Q3 = 258.1/112.1), Gm (Q1/Q3 = 298.1/152.1), m1G (Q1/Q3 = 298.1/166.1), m7G (Q1/Q3 = 298.1/166.1), m2G (Q1/Q3 = 298.1/166.1), o2yW (Q1/Q3 = 541.2/409.0), m5C (Q1/Q3 = 258.1/126.1), and A (Q1/Q3 = 268.1/136.2) of tRNAPhe(GAA) isolated from WT, ftsj1 KO, and wdr6 KO cells. Target peaks are indicated by black triangles. m2G, 2‐methylguanosine; D, dihydrouridine; m22G, N2,N2‐dimethylguanosine; Cm, 2′‐O‐methylcytidine; Gm, 2′‐O‐methylguanosine; m5C, 5‐methylcytidine; m7G, 7‐methylguanosine; m5U, 5‐methyluridine; m1A, 1‐methyladenosine; ψ, pseudouridine; o2yW, peroxywybutosine. Q1/Q3: the mass of the precursor ion and the mass of the product ion. Combined the retention time of standard product, we marked the o2yW in Fig 3G. Considering the change of this peak area was consistent with that of o2yW, we speculated the other peak was generated by the intermediate product of o2yW with nature isotope labeled.
Figure EV3. Knockout of ftsj1 and/or wdr6 affects Cm32, Gm34, m1G37, and o2yW37 levels of tRNAPhe(GAA).
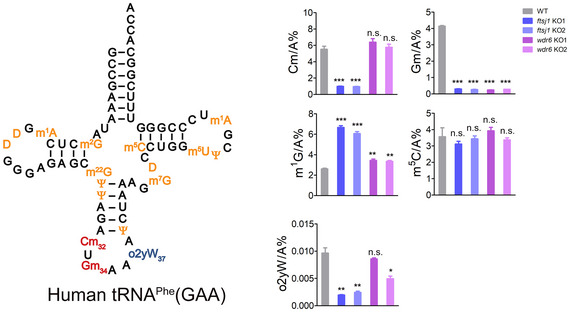
Quantification of the Cm/A, Gm/A, m1G/A, m5C/A, and o2yW/A ratios in endogenous tRNAPhe(GAA) isolated from WT, ftsj1 KO, and wdr6 KO cell lines by UPLC‐MS/MS analysis. Error bars represent the standard deviation of three independent experiments. P values were determined using two‐tailed Student's t‐test for paired samples. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., no significance.
The hypermodification wybutosine (yW) or its derivatives specifically occurred to archaeal and eukaryotic tRNAPhe(GAA) at position 37 from m1G (Perche‐Letuvee et al, 2014). Interestingly, the corresponding m1G level significantly increased in ftsj1 KO cells and in wdr6 KO cells (Figs 3F and EV3); the peroxywybutosine (o2yW) level obviously decreased in ftsj1 KO cells compared with that of WT cells (Figs 3G and EV3). These results suggested that there is a balance at G37, which can be modified to m1G37 or further modified to o2yW37. In WT cells, m1G37 is undetectable, suggesting that all the m1G37 are hypermodified to o2yW37; in ftsj1 KO cells, the formation of o2yW37 is hindered, and G37 is mainly modified to m1G37. Indeed, the o2yW level decreased in NSXLID patients with ftsj1 mutation (Guy et al, 2015). In yeast, the loss of Cm32 and Gm34 modifications from Trm7 deletion hindered the yW37 modification (Guy et al, 2012; Guy & Phizicky, 2015). Our results suggested a hierarchical order that the hypomodification of Cm32 and Gm34 impeded the formation of yW37 or o2yW37 modifications from m1G37 is evolutionarily conserved between yeast and human.
FTSJ1‐WDR6 catalyzes Gm34 on tRNAPhe(GAA) with m1G37 as a prerequisite
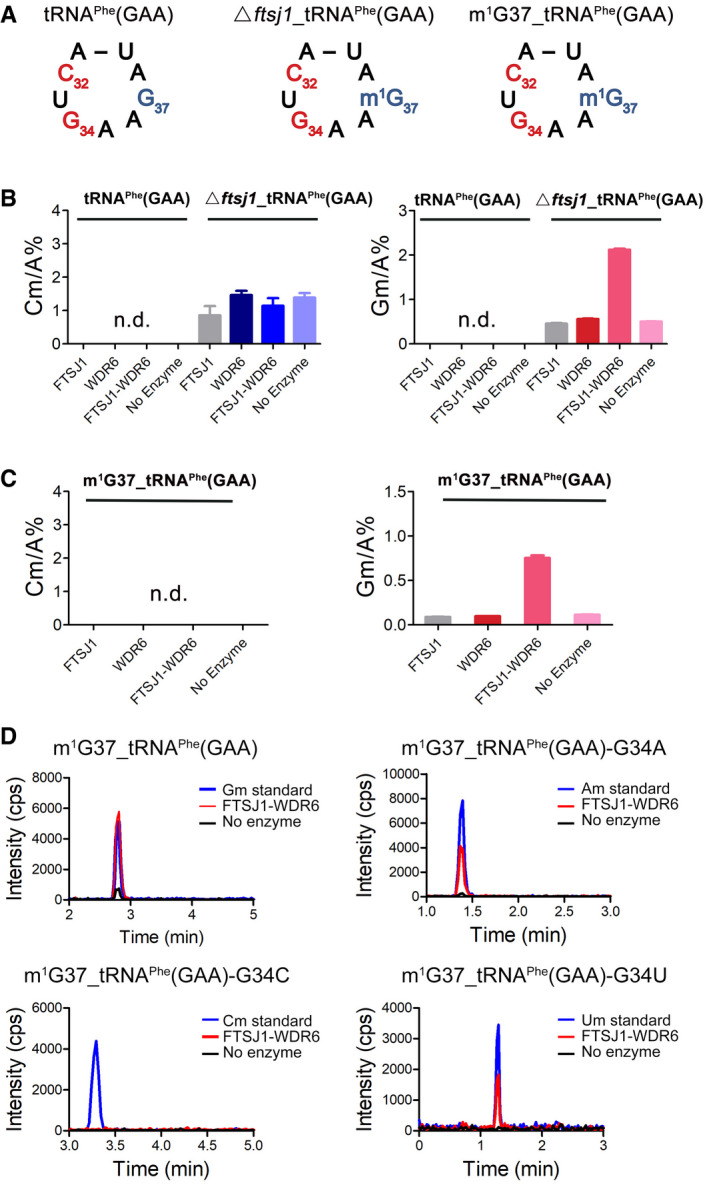
To reconstruct the catalytic activity of FTSJ1 in vitro, we used the baculovirus insect cell expression system to obtain FTSJ1 protein and used transcript tRNAPhe(GAA) without any modification or tRNAPhe(GAA) with pre‐existing modified nucleosides that were obtained by a biotinylated DNA probe from ftsj1 KO cells (▵ftsj1_tRNAPhe(GAA)) as substrate, but no methylation activity could be detected (Fig 4A and B), suggesting that standalone FTSJ1 could not catalyze 2′‐O‐methylation on tRNAPhe(GAA). We further purified GST‐WDR6 from baculovirus‐infected insect cells to test the catalytic activity of the mixture of FTSJ1 and WDR6 (FTSJ1‐WDR6) in vitro. Surprisingly, formation of Cm or Gm was undetectable when using unmodified T7‐transcribed tRNAPhe(GAA) as substrate (Fig 4B). When using ▵ftsj1_tRNAPhe(GAA) as substrate, robust Gm formation was detected after incubation with FTSJ1‐WDR6, but not with either FTSJ1 or WDR6 alone (Fig 4B), suggesting that FTSJ1‐WDR6 catalyzes the Gm34 modification in vitro, and this reaction requires the presence of some other pre‐existing tRNA modifications.
Figure 4. FTSJ1‐WDR6 catalyzes Gm34 formation on tRNAPhe(GAA) with m1G37 in vitro .
-
ASecondary structures of the anti‐codon loop of three types of tRNAPhe(GAA)s: tRNAPhe(GAA), ▵ftsj1_tRNAPhe(GAA), and m1G37_tRNAPhe(GAA).
-
B, CQuantification of the Cm/A and Gm/A of tRNAPhe(GAA), ▵ftsj1_tRNAPhe(GAA), and m1G37_tRNAPhe(GAA) after incubation with FTSJ1, WDR6, and FTSJ1‐WDR6 by UPLC‐MS/MS analysis, respectively. Error bars represent the standard deviation of three independent experiments. n.d., not detected.
-
DUPLC‐MS/MS analysis of 2′‐O‐methylation of m1G37_tRNAPhe(GAA), m1G37_tRNAPhe(GAA)‐G34A, m1G37_tRNAPhe(GAA)‐G34C, and m1G37_tRNAPhe(GAA)‐G34U after incubation with FTSJ1‐WDR6. 2 μl of Gm (1 ng/ml), Am (1 ng/ml), Cm (1 ng/ml), and Um (5 ng/ml) standards was injected to UPLC‐MS/MS as control. cps, counts per second.
One of our former studies showed that Cm/Um34 formation in E. coli tRNALeu requires i6A37 as the prerequisite (Zhou et al, 2015). Position 37 of tRNA is semi‐conserved that they can be either A or G (Chan & Lowe, 2016; Boccaletto et al, 2018). For tRNAPhe(GAA), position 37 is G. Considering that m1G37 is a conserved modification and occurs in an earlier step during tRNA maturation (Hou et al, 2018), we tried to test whether the m1G37 modification has an impact on the formation of Gm34. Herein, we introduced m1G37 to tRNAPhe(GAA) via E. coli TrmD (UniProt: P0A873) to generate tRNAPhe(GAA) carrying m1G37 (m1G37_tRNAPhe(GAA) (Fig 4A). Remarkably, Gm modification was detected in the m1G37_tRNAPhe(GAA) after incubation with FTSJ1‐WDR6 (Fig 4C), suggesting m1G37 is a prerequisite for Gm34 formation that is catalyzed by FTSJ1‐WDR6. Intriguingly, the ratio of Gm/A in m1G37_tRNAPhe(GAA) after the incubation with FTSJ1‐WDR6 was lower than that in ▵ftsj1_tRNAPhe(GAA) after the incubation with FTSJ1‐WDR6 (Fig 4B and C), indicating that there may be other modifications that could also enhance the formation of Gm34. Notably, Cm32 cannot be generated by FTSJ1 or FTSJ1‐WDR6 when using ▵ftsj1_tRNAPhe(GAA) or m1G37_tRNAPhe(GAA) as substrate (Fig 4B and C), suggesting that other molecules may involve in this process.
In order to confirm that the Gm modification occurred at position 34 by FTSJ1‐WDR6, we constructed tRNAPhe(GAA) transcript mutants with G34 mutated to A, C, or U (tRNAPhe(GAA)‐G34A, tRNAPhe(GAA)‐G34C, or tRNAPhe(GAA)‐G34U) and added m1G37 to all the tRNAs by TrmD. The results showed that m1G37_tRNAPhe(GAA)‐G34A or m1G37_tRNAPhe(GAA)‐G34U contained only Am or Um, but not Gm after incubation with FTSJ1‐WDR6 (Fig 4D), indicating that 2′‐O‐methylation catalyzed by FTSJ1‐WDR6 is indeed happened at position 34 of tRNA. Surprisingly, we could not detect Cm in m1G37_tRNAPhe(GAA)‐G34C after the incubation with FTSJ1‐WDR6 (Fig 4D), indicating that C at position 34 is not the optimum base for catalysis. We also replaced A35 with three other bases to obtain the tRNAPhe(GAA)‐A35C, tRNAPhe(GAA)‐A35G, and tRNAPhe(GAA)‐A35U mutants and added m1G37 to all the three mutants by TrmD. Intriguingly, after incubation with FTSJ1‐WDR6, Gm could be detected at m1G37_tRNAPhe(GAA)‐A35G or m1G37_tRNAPhe(GAA)‐A35U, but not at m1G37_tRNAPhe(GAA)‐A35C (Fig EV4). These results showed that C35 of tRNA probably serves as an anti‐recognition element for FTSJ1‐WDR6.
Figure EV4. UPLC‐MS/MS analysis of Gm34 of m1G37_tRNAPhe(GAA), m1G37_tRNAPhe(GAA)‐A35C, m1G37_tRNAPhe(GAA)‐A35G and m1G37_tRNAPhe(GAA)‐A35U after incubation with or without FTSJ1‐WDR6.
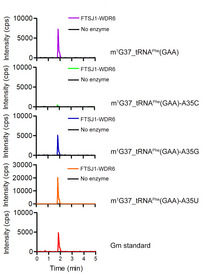
2 μl of Gm standard (1 ng/ml) was loaded as control. cps, counts per second.
In a word, our results showed that FTSJ1‐WDR6 could catalyze tRNA:Nm34 modification in vitro, and m1G37 is one of the prerequisites for Gm34 formation. Importantly, Cm32 and Gm34 will promote m1G37 to be hypermodified into o2yW. These observations suggested that tRNA modifications at positions 32, 34, and 37 occur in a highly ordered way.
Functional role division in the FTSJ1‐WDR6 complex
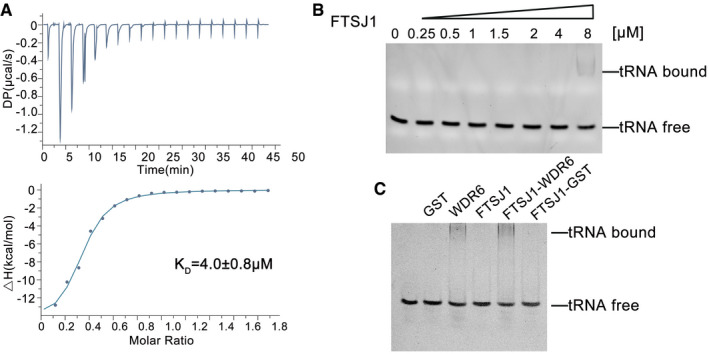
Generally, two‐subunit enzymes for tRNA modifications consist of a catalytic subunit and a partner RNA‐binding protein (Guy & Phizicky, 2014; Maraia & Arimbasseri, 2017). Therefore, we carried out a series of experiments to validate the functional role division of FTSJ1‐WDR6. Structural‐based sequence alignment (Fig EV1) indicates that FTSJ1 contains the MTase catalytic domain. Therefore, we performed ITC to measure the SAM‐binding capability of FTSJ1. The dissociation constant (KD) of SAM with FTSJ1 was about 4 μM (Fig 5A), suggesting that FTSJ1 could bind to SAM efficiently and is the potential catalytic component. Next, we analyzed the binding affinity of FTSJ1 and WDR6 for tRNAPhe(GAA) by a gel mobility shift assay. These results showed that standalone FTSJ1 had very weak binding affinity for the tRNA (Fig 5B). The binding affinity of FTSJ1‐WDR6 to tRNA was significantly higher (Fig 5C). WDR6 was able to bind tRNA alone, but the binding affinity was lower than that of FTSJ1‐WDR6 (Fig 5C).
Figure 5. The substrate‐binding affinity of FTSJ1.
-
AThe SAM‐binding affinity of FTSJ1 as measured by ITC.
-
B, CThe binding affinity of FTSJ1 alone or FTSJ1‐WDR6 for tRNA analyzed by the gel mobility shift assay. For the reaction in (C), 0.2 μM GST, 0.2 μM GST‐WDR6, 1 μM FTSJ1, the mixture of 1 μM FTSJ1 with 0.2 μM GST‐WDR6 or 0.2 μM GST, 0.5 mM SAM, and 250 nM tRNAPhe(GAA) transcript were incubated.
Our results showed that in this binary complex, FTSJ1 is the SAM‐binding catalytic subunit and has weaker tRNA‐binding affinity, while WDR6 mainly plays a role in binding to tRNA substrates.
tRNA substrates of FTSJ1 at position 34
The above results showed that m1G37 is one of the prerequisites for Gm34 formation by FTSJ1‐WDR6. There are a total of 49 human cytoplasmic tRNA molecular species (Boccaletto et al, 2018), and 17 of which are tRNAs containing G37 (G37‐tRNAs) (Appendix Table S3). To test whether there are more tRNA substrates of FTSJ1‐WDR6, we tested all human cytoplasmic G37‐tRNAs, except for tRNALeu(CAA) with f5Cm34 which was already reported as the substrate of FTSJ1 (Kawarada et al, 2017). We screened the other 16 tRNAs: 3 tRNAArg isoacceptors, 4 tRNALeu isoacceptors, 3 tRNAPro isoacceptors, 2 tRNATyr isoacceptors, tRNATrp(CCA), tRNACys(GCA), tRNAHis(GUG), and tRNAPhe(GAA). Interestingly, in the tRNA database (Boccaletto et al, 2018), the Cm34 modification is present in one of the tRNAs containing A37 (A37‐tRNA), elongator tRNAMet(CAU). Thus, we also isolated this tRNA and four other A37‐tRNAs to test whether they are substrates of FTSJ1‐WDR6. The purities of the isolated products by correlated biotinylated DNA probes were further detected using denatured electrophoresis (Fig EV5). Among them, tRNACys(GCA), tRNATyr(GUA), or tRNATyr(AUA) could not be enriched or purified, while the other tRNAs, including 13 G37‐tRNAs and 5 A37‐tRNAs, could all be isolated from cells with high purity (Fig EV5).
Figure EV5. Analysis of the purity of the fished tRNAs by biotinylated DNA probes using urea PAGE.
Among them, tRNACys(GCA), tRNATyr(GUA), or tRNATyr(AUA) could not be purified (in red box).
The Nm quantities of each tRNA according to what are they proposed to be at position 34 are listed in Table 1. Surprisingly, compared to that of WT cells, only the Gm34 quantity of tRNAPhe(GAA) was significantly decreased in both ftsj1 KO and wdr6 KO cells (Table 1; Fig 6). The Cm level of tRNAArg(CCG) decreased in ftsj1 KO cells, but showed no significant difference in wdr6 KO cells (Table 1). One explanation for this modification level change of tRNAArg(CCG) (which has U32 and C34) could be that the probe also isolated another isodecoder (tRNAArg(CCG)‐2‐1 which has C32) 31,33. Due to high sequence similarity, the change may come from C32 of tRNAArg(CCG)‐2‐1 that was modified by FTSJ1. All the other tested tRNAs did not show a decrease in Nm34 when wdr6 was knocked out. All 4 tested A37‐tRNAs, including elongator tRNAMet(CAU), were not substrates of FTSJ1‐WDR6 (Table 1; Fig 6), which was consistent with our discovery that FTSJ1‐WDR6 modification to Nm34 depends on the pre‐existence of m1G37.
Table 1.
Quantification of the expected Nm34/A% ratios in 13 G37‐tRNAs and 4 A37‐tRNAs isolated from WT, ftsj1 KO, and wdr6 KO cells by UPLC‐MS/MS analysis
| tRNAs | Expected Nm34 | WT (Nm/A%) | ftsj1 KO1 (Nm/A%) | ftsj1 KO2 (Nm/A%) | wdr6 KO1 (Nm/A%) | wdr6 KO2 (Nm/A%) |
|---|---|---|---|---|---|---|
| G37 | ||||||
| tRNAArg(ACG) | Am | 2.52 ± 0.11 | 2.65 ± 0.05 | 2.79 ± 0.17 | 2.88 ± 0.14 | 2.67 ± 0.10 |
| tRNAArg(CCG) | Cm | 2.73 ± 0.66 | 0.29 ± 0.04 | 0.40 ± 0.04 | 2.48 ± 0.52 | 2.93 ± 0.35 |
| tRNAArg(UCG) | Um | 1.39 ± 0.12 | 1.35 ± 0.15 | 1.53 ± 0.11 | 1.50 ± 0.08 | 1.32 ± 0.06 |
| tRNAHis(GUG) | Gm | n.d. | n.d. | n.d. | n.d. | n.d. |
| tRNALeu(AAG) and ‐(UAG) |
Am Um |
0.75 ± 0.01 10.61 ± 0.91 |
0.88 ± 0.02 7.42 ± 0.84 |
0.80 ± 0.03 7.38 ± 1.12 |
0.86 ± 0.02 16.18 ± 1.11 |
0.78 ± 0.07 14.86 ± 1.51 |
| tRNALeu(CAG) | Cm | 1.01 ± 0.01 | 0.64 ± 0.02 | 0.41 ± 0.03 | 0.77 ± 0.03 | 1.15 ± 0.03 |
| tRNALeu(UAA) | Um | 4.48 ± 0.39 | 4.74 ± 0.22 | 5.55 ± 0.63 | 6.64 ± 0.50 | 6.60 ± 0.14 |
|
tRNAPro(AGG), ‐(CGG) and ‐(UGG) |
Am Cm Um |
1.04 ± 0.07 n.d. 15.10 ± 1.09 |
0.90 ± 0.03 n.d. 16.19 ± 1.59 |
1.07 ± 0.03 n.d. 14.70 ± 0.62 |
0.95 ± 0.03 n.d. 15.90 ± 1.03 |
0.69 ± 0.04 n.d. 16.64 ± 0.90 |
| tRNAPhe(GAA) | Gm | 4.14 ± 0.06 | 0.30 ± 0.02 | 0.27 ± 0.02 | 0.25 ± 0.01 | 0.28 ± 0.01 |
| tRNATrp(CCA) | Cm | 6.93 ± 0.44 | 0.58 ± 0.04 | 0.67 ± 0.02 | 6.37 ± 0.38 | 6.80 ± 0.56 |
| A37 | ||||||
| elongator tRNAMet(CAU) | Cm | 4.74 ± 0.26 | 7.60 ± 0.42 | 7.83 ± 0.37 | 6.50 ± 0.41 | 6.33 ± 0.41 |
| tRNAAla(AGC) | Am | n.d. | n.d. | n.d. | n.d. | n.d. |
| tRNAGly(CCC) | Cm | 3.84 ± 0.54 | 3.89 ± 0.72 | 2.97 ± 0.39 | 5.57 ± 1.01 | 6.28 ± 0.94 |
| tRNAGly(GCC) | Gm | 1.80 ± 0.10 | 1.92 ± 0.12 | 1.00 ± 0.06 | 2.33 ± 0.12 | 1.95 ± 0.15 |
The results are the average of three independent repeats with standard deviation indicated. n.d., not detected.
Figure 6. The Nm32/A% and Nm34/A% ratios of some tRNAs.
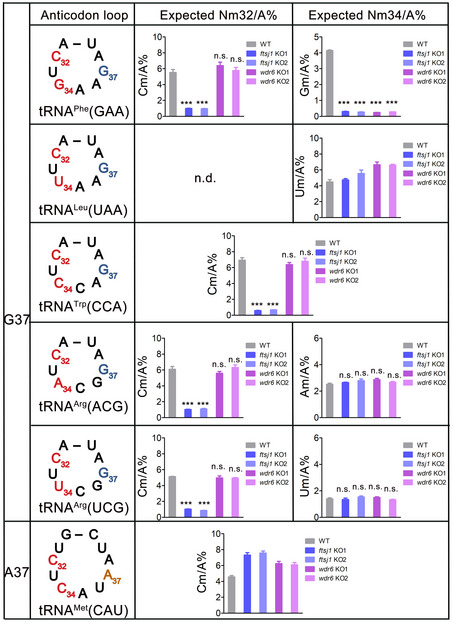
The representative graphs for quantification of the expected Nm32/A% and Nm34/A% ratios of G37‐tRNAPhe(GAA), tRNALeu(UAA), tRNATrp(CCA), tRNAArg(ACG), tRNAArg(UCG), and A37‐tRNAMet(CAU), which were isolated from WT, ftsj1 KO, and wdr6 KO cells by UPLC‐MS/MS analysis. Error bars represent the standard deviation of three independent experiments. P values were determined using two‐tailed Student's t‐test for paired samples. ***P < 0.001. n.s., no significance. n.d., not detected.
We also performed the in vitro enzymatic assays of FTSJ1‐WDR6 for all 16 G37‐tRNAs except for tRNALeu(CAA) that was already reported as the substrate of FTSJ1 (Kawarada et al, 2017). The results from these tRNAs with m1G37 obtained by transcription with T7 RNA polymerase and modification of TrmD showed that only m1G37_tRNAPhe(GAA) is the substrate of FTSJ1‐WDR6 in vitro (Appendix Table S4).
Collectively, our results together with a previous report (Kawarada et al, 2017) suggest that two tRNAs, tRNAPhe(GAA) and tRNALeu(CAA), are the substrates of FTSJ1‐WDR6 for catalyzing 2′‐O‐methylation at position 34.
tRNA substrates of FTSJ1 at position 32
For tRNAPhe(GAA), knockout of ftsj1 leads to the decrease in both Cm32 and Gm34, while knockout of wdr6 only leads to the decrease in Gm34 without affecting Cm32 (Figs 3D and E, and EV3). These results suggested that the processes of FTSJ1 catalyzing Nm34 and Nm32 are two independent events. Therefore, it raised the question of whether FTSJ1 has numerous tRNA substrates at position 32. Does the Nm32 formation depend on m1G37? Interestingly, an A37‐tRNAGln(UUG) contains Cm32 according to the tRNA database (Boccaletto et al, 2018). To address the above questions, we tested the Nm level of 7 G37‐tRNAs and 2 A37‐tRNAs according to what are they proposed to be at position 32 (Table 2). Interestingly, when ftsj1 was knocked out, 5 tRNAs among the 7 G37‐tRNAs had significantly decreased Ym32 quantity (Y=C, U): Cm/A for tRNAArg(ACG), Um/A for tRNAArg(CCG), Cm/A for tRNAArg(UCG), Cm/A for tRNATrp(CCA), and Cm/A for tRNAPhe(GAA), which did not change when wdr6 was knocked out (Table 2; Fig 6), indicating that these 5 tRNAs are substrates of FTSJ1 at position 32. Intriguingly, for the 2 tested A37‐tRNAs, compared to that of WT cells, the Cm/A ratio of tRNAGln(UUG) obviously decreased in ftsj1 KO cells, but did not decrease in wdr6 KO cells (Table 2), while for tRNAGly(GCC), no significant change in Cm/A ratio could be observed in knockouts of ftsj1 or wdr6 (Table 2). These results showed that A37‐tRNAGln(UUG), but not A37‐tRNAGly(GCC), is the substrate of FTSJ1 catalyzing 2′‐O‐methylation at position 32.
Table 2.
Quantification of the expected Nm32/A% ratios in 7 G37‐tRNAs and 2 A37‐tRNAs isolated from WT, ftsj1 KO, and wdr6 KO cells by UPLC‐MS/MS analysis
| tRNAs | Expected Nm32 | WT (Nm/A%) | ftsj1 KO1 (Nm/A%) | ftsj1 KO2 (Nm/A%) | wdr6 KO1 (Nm/A%) | wdr6 KO2 (Nm/A%) |
|---|---|---|---|---|---|---|
| G37 | ||||||
| tRNAArg(ACG) | Cm | 6.08 ± 0.50 | 1.02 ± 0.06 | 1.09 ± 0.10 | 5.57 ± 0.36 | 6.31 ± 0.46 |
| tRNAArg(CCG) | Um | 4.23 ± 0.38 | 0.24 ± 0.02 | 0.49 ± 0.03 | 3.54 ± 0.34 | 3.65 ± 0.27 |
| tRNAArg(UCG) | Cm | 5.13 ± 0.03 | 1.03 ± 0.04 | 0.86 ± 0.03 | 4.73 ± 0.14 | 4.95 ± 0.10 |
| tRNAHis(GUG) | Um | n.d. | n.d. | n.d. | n.d. | n.d. |
| tRNALeu(UAA) | Cm | n.d. | n.d. | n.d. | n.d. | n.d. |
| tRNAPhe(GAA) | Cm | 5.52 ± 0.65 | 1.00 ± 0.06 | 0.95 ± 0.08 | 6.39 ± 0.74 | 5.77 ± 0.64 |
| tRNATrp(CCA) | Cm | 6.93 ± 0.44 | 0.58 ± 0.04 | 0.67 ± 0.02 | 6.37 ± 0.38 | 6.80 ± 0.56 |
| A37 | ||||||
| tRNAGln(UUG) | Cm | 6.27 ± 0.10 | 0.21 ± 0.02 | 0.02 ± 0.01 | 4.69 ± 0.11 | 9.38 ± 0.27 |
| tRNAGly(GCC) | Cm | 1.24 ± 0.11 | 1.18 ± 0.06 | 0.76 ± 0.67 | 1.68 ± 0.09 | 1.32 ± 0.12 |
The results are the average of three independent repeats with standard deviation indicated. n.d., not detected.
Collectively, we found that FTSJ1 could catalyze Ym32 formation of tRNAs with either G37 or A37 and has more substrates than at position 34.
Knockout of ftsj1 inhibits cell proliferation and affects translation efficiency for the UUU codon
Compared to WT cells, a noticeable decrease in cell proliferation was observed in ftsj1 KO cells (Fig 7A). Remarkably, when cultured with 0.2 mg/ml paromomycin, an antibiotic of aminoglycoside family which binds to ribosomes and interferes with protein synthesis, the growth rates of ftsj1 KO cells were slower than those of WT cells (Fig 7B), suggesting that ftsj1 KO cells were more sensitive to paromomycin. To investigate the role of tRNAPhe(GAA) for FTSJ1, the WT and ftsj1 KO cells were transfected with mature tRNAPhe(GAA). Intriguingly, tRNAPhe(GAA) had no effect on the growth of WT HEK293T cells under normal culture condition (Fig 7C) or in the presence of paromomycin (Fig 7D), while tRNAPhe(GAA) could significantly promote the growth of ftsj1 KO cells under both conditions (Fig 7E and F). These results indicated that tRNAPhe(GAA) serves as the main functional executor of FTSJ1. In yeast, KO of trm7 caused a similar phenomenon in both S. cerevisiae and S. pombe, and overexpression of only tRNAPhe(GAA) could rescue this phenotype, suggesting a conserved and critical role of tRNAPhe(GAA) for Trm7/FTSJ1 (Pintard et al, 2002; Guy et al, 2012; Guy & Phizicky, 2015).
Figure 7. FTSJ1 affects cell proliferation and translation efficiency of the UUU codon.
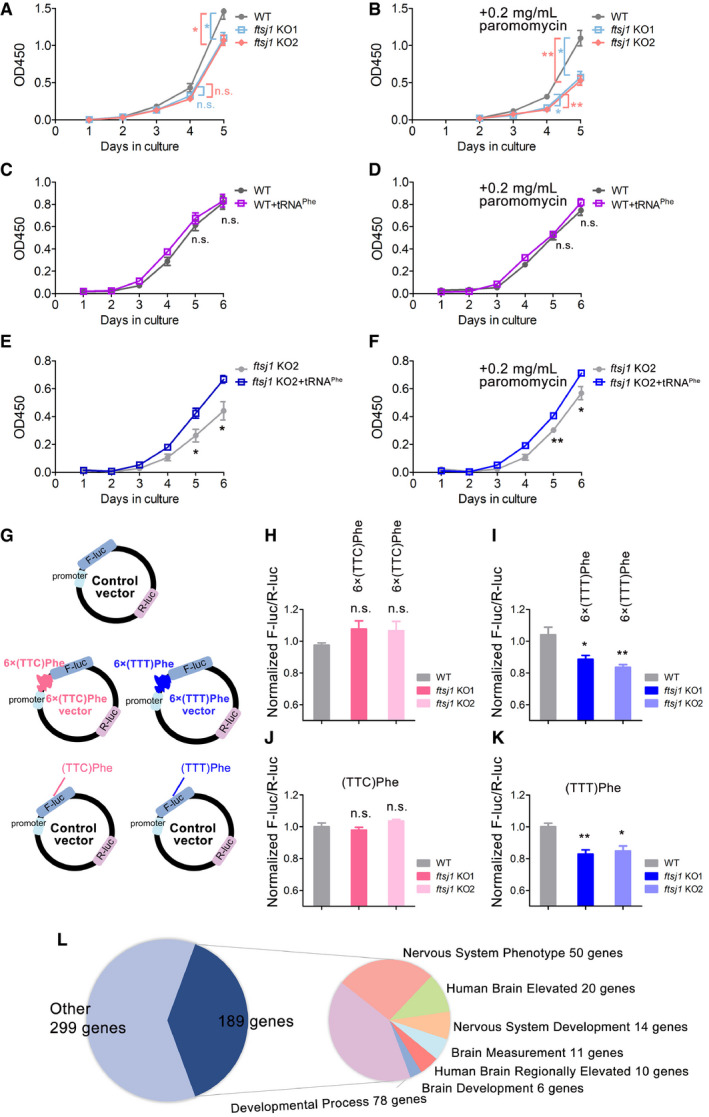
-
A, BThe growth curve of WT and two ftsj1 KO HEK293T cell lines under normal culture or in the culture condition with 0.2 mg/ml paromomycin assayed by Cell Counting Kit‐8 proliferation analysis.
-
C, DThe growth curve of WT with or without overexpressing tRNAPhe(GAA) under normal culture or with 0.2 mg/ml paromomycin.
-
E, FThe growth curve of ftsj1 KO cells with or without overexpressing tRNAPhe(GAA) under normal culture or with 0.2 mg/ml paromomycin.
-
GThe RNA reporter vector encodes F‐luc as the primary reporter and R‐luc on the same plasmid as the internal transfection control. The effect of tRNAPhe(GAA) in protein translation was revealed by a reporter assay. 6 × TTC (Phe) or 6 × TTT (Phe) were inserted after the PGK promoter region of F‐luc as the positive reporter.
-
H, IThe translation efficiency of F‐luc with the 6 × UUC codons or 6 × UUU codons.
-
J, KThe translation efficiency of F‐luc with all UUC codons or all UUU codons.
-
LThe frequency usage analysis of UUU and UUC codons showed that in 488 genes, at least one transcript was found to use the UUU codon for more than 80% of Phe codons. Among the 488 genes, 189 genes are involved in the nervous system and brain functions.
Considering that tRNAPhe(GAA) has to decode both UUU and UUC codons in most organisms (Chan & Lowe, 2016) including yeast and humans, we investigated the effect of FTSJ1‐mediated tRNAPhe(GAA) methylation on the translation efficiency of UUU and UUC codons. 6 × TTT or 6 × TTC were inserted into the promoter of the F‐luc gene in pmirGlo vector (Fig 7G). Since 6 × codons in a row are rarely found in nature, to mimic the situation in vivo, instead of using 6 × codons, we constructed the F‐luc gene with all the Phe codons using either TTT or TTC (Fig 7G). R‐luc was used to normalize the expression efficiency. To further normalize translation differences between ftsj1 KO cells and WT cells introduced by any other factors, the WT reporter (F‐luc plus R‐luc) was also transfected. Surprisingly, the translation efficiency of 6 × UUC codons was not affected (Fig 7H), but the translation efficiency of the 6 × UUU codons obviously decreased in ftsj1 KO cells (Fig 7I). Moreover, the translation efficiency of F‐luc with all UUC codons for Phe was not affected (Fig 7J), but the translation efficiency of F‐luc with all UUU codons for Phe showed significant decrease in ftsj1 KO cells (Fig 7K). Thus, these results suggested that the loss of modifications on tRNAPhe(GAA) caused by knockout of ftsj1 mainly affects the translation efficiency of the UUU codon. G34 in tRNAPhe(GAA) has non‐Watson‐Crick base pairing with the third nucleotide in the UUU codon during decoding.
Thus, it is noteworthy to investigate the codon usage bias of UUU and UUC in the human transcriptomes to provide information about the expression level of potential genes that are likely regulated by FTSJ1‐mediated tRNAPhe(GAA) methylation. To this end, we downloaded protein‐coding transcript sequences from GENCODE (Harrow et al, 2012). For each sequence, we extracted coding sequences by GENCODE's annotation and ignored sequences that did not start with “AUG”. Then, we counted the frequency of codon usage. Interestingly, at least one transcript of 488 genes was found to use more than 80% UUU in transcript encoding codon of Phe (either UUU or UUC), as the list in Dataset EV1. Since FTSJ1 is associated with NSXLID, we focused on collecting nervous system‐ and brain‐related genes list from various resources. We found 111 genes (22.7%) that might be involved in nervous system and brain functions (Fig 7L). 78 additional genes were annotated as related to developmental processes, which might also have upstream impacts on nervous system and brain functions (Fig 7L). The 189 genes are listed in Dataset EV1.
Collectively, we found that the loss of modifications on tRNAPhe(GAA) caused by knockout of ftsj1 mainly affects the translation efficiency of the UUU codon. About 189 genes of the 488 UUU codon‐biased genes in the human genome are involved in the nervous system and brain function, and we propose that the expression level of these genes may be regulated by FTSJ1‐mediated tRNAPhe(GAA) modifications. Our findings lay the foundation for further study of the relationship between the modifications of tRNAPhe(GAA) mediated by FTSJ1 and nervous system development.
Discussion
Enzymatic activity and substrate recognition of FTSJ1
Over the past years, the intellectual disability‐associated protein FTSJ1 has been proposed to be a tRNA 32 and 34 2′‐O‐MTase (Guy & Phizicky, 2015). However, the enzymatic activity of FTSJ1 had not yet been confirmed. In this study, for the first time, we successfully reconstituted the 2′‐O‐methylation activity of FTSJ1 in vitro. We found that FTSJ1 together with WDR6 could catalyze Gm34 formation on human cytoplasmic tRNAPhe(GAA) and identified that m1G37 is one of the prerequisites for this process. In the binary FTSJ1‐WDR6 complex, FTSJ1 is the SAM‐binding catalytic subunit with weak tRNA‐binding capacity, while WDR6 mainly plays a more prominent role in binding tRNA substrates.
Combined with previous report (Kawarada et al, 2017), we found that among 49 human cytoplasmic tRNA species, only 2 G37‐tRNAs, tRNAPhe(GAA) and tRNALeu(CAA), are modified by FTSJ1‐WDR6 at position 34 (Fig 8A). We showed that tRNATrp(CCA) and tRNALeu(UAA) are not the substrates of FTSJ1‐WDR6, even though in yeast they are the substrates of Trm7‐Trm734. In vitro methylation assays from tRNAPhe(GAA) mutants showed that C35 is an anti‐recognition element, which may explain why tRNATrp(CCA) is not the substrate of FTSJ1‐WDR6. However, the underlying mechanism of why tRNALeu(UAA) is not the substrate of FTSJ1‐WDR6 and the distinct tRNA substrate specificity of FTSJ1‐WDR6 and Trm7‐Trm734 remains to be investigated. The elongator tRNAMet(CAU) with A37 has the Cm34 modification (Boccaletto et al, 2018), but our results showed that it is not a substrate of FTSJ1‐WDR6. Actually, few months ago, a report identified that this modification was catalyzed by a specific box C/D RNP (Vitali & Kiss, 2019), which is consistent with our findings.
Figure 8. The working model of FTSJ1.
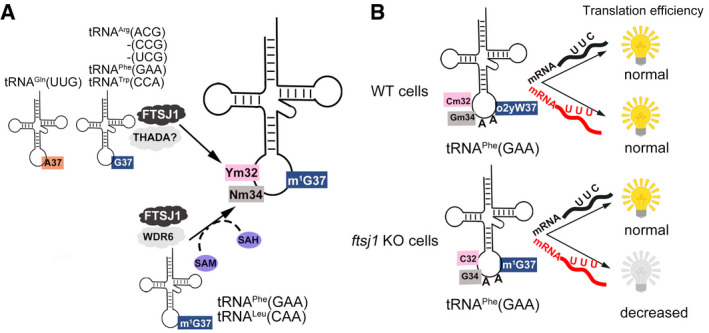
-
ASchema showing the identified tRNA substrates and recognition mechanism of FTSJ1.
-
BThe translation efficiency of UUU and UUC codons regulated by the interdependence of tRNAPhe(GAA) modifications at positions 32, 34, and 37.
Our results also showed that FTSJ1 has broader tRNA substrates for position 32 than for position 34. Among the 9 tRNAs we identified, 6 of them were substrates of FTSJ1 at position 32 (Fig 8A). Interestingly, FTSJ1‐mediated Ym32 modification did not depend on m1G37; both A37‐ and G37‐tRNAs could be modified by FTSJ1, which suggests that FTSJ1 recognizes tRNA substrates for positions 32 and 34 using different strategies. Standalone FTSJ1 failed to catalyze Cm32 formation even using pre‐modified tRNAPhe(GAA) as a substrate, indicating that auxiliary proteins may be involved in substrate recognition. Although THADA is predicted to be the human homolog of yeast Trm732 (Guy & Phizicky, 2015), it is not in the list of potential FTSJ1‐interacting proteins identified by IP‐MS or proximity labeling IP‐MS. The exact recognition mechanism for Ym32 remains to be investigated in the future.
During the submission of our work, the biochemical and structural studies of S. cerevisiae Trm7‐Trm734 (PDB ID: 6JP6) have been published online (Hirata et al, 2019). The structural model shows that Trm7 is a class I‐type Rossmann‐fold MTase, and Trm734 comprises three WD40 repeat domains (Hirata et al, 2019). They showed that Trm7‐Trm734 are able to catalyze both G37‐ and A37‐tRNAs for Nm34 formation and that m1G37 is not an essential element for catalysis (Hirata et al, 2019). Conversely, the presence of m1G37 could accelerate Nm34 formation (Hirata et al, 2019). Therefore, FTSJ1‐WDR6 may have strict substrate discrimination mechanisms compared to Trm7‐Trm734. To reveal the accurate tRNA substrate recognition mechanism, the complex structure of Trm7‐Trm734 bound with tRNA and the structural information of FTSJ1‐WDR6 are required. Recently, two Trm7 homologs were identified in Drosophila (Angelova et al, 2020). One is responsible for Nm34 modification, and the other one is in charge of Nm32 modification, suggesting that the formation of tRNA 2′‐O‐methylations at positions 32 and 34 is complicated and distinct in different species.
Intricate network of interdependent modifications on tRNA
Our results together with previous studies in yeast suggest an interdependent relationship of tRNA modification at positions 32, 34, and 37. In our study, for human cytoplasmic tRNAPhe(GAA), m1G37 is the prerequisite for Gm34 formation; Cm32 and Gm34 modifications will promote o2yW37 formation from m1G37. These results suggested that modifications in vivo occur in a hierarchical order: (i) m1G37; (ii) Cm32 and Gm34; and (iii) o2yW37.
The influence of modification at site 37 on modification at site 34 of tRNA has been demonstrated for three cases. First, our previous study has revealed that Cm/Um34 formation in E. coli tRNALeu relies on prior i6A37 (Zhou et al, 2015). Second, the terminal methylation in 5‐carboxy‐methoxy modification of U34 (mcmo5U34) of E. coli tRNAPro(UGG) depends on the primary m1G37 formation (Masuda et al, 2018). Third, N6‐threonylcarbamoyladenosine (t6A) at site 37 might serve as the determinant for lysidine formation at site 34 by tRNAIle‐lysidine synthase (TilS) (Thiaville et al, 2015). All the three cases revealed that modification at site 37 is prerequisite for modification at site 34 in E. coli, which are also in agreement with our findings.
However, these three cases all point out the influence of modification at site 37 on modification at site 34, but not vice versa. In yeast and human tRNAPhe(GAA), Cm32 and Gm34 will further enhance the hypermodification at site 37 from m1G (Guy et al, 2012, 2015; Guy & Phizicky, 2015), which demonstrates that an intricate network of interdependent modifications exists at the ASL region. We suggested that the “crosstalk” between modifications at the ASL region ensures that the tRNA participates in the cellular process, but the mechanism of how these modifications influence each other and the exact role of modification enzymes require further biochemical, structural, and functional studies to elucidate.
FTSJ1 in translational regulation and other functions
In this study, we showed that the translation efficiency of the UUU but not the UUC codon decreased in ftsj1 KO cells, suggesting that the interdependence of tRNAPhe(GAA) modifications at ASL region may have more impact on non‐Watson‐Crick wobble base (G34) pairs with U than the classical wobble base (G34) pairs with C (Fig 8B). Intriguingly, in the human transcriptomes, we found that 189 genes from the total 488 UUU codon‐biased (> 80% use UUU for Phe) genes are related to the nervous system and brain functions or development. In addition, mutations in human ftsj1 are associated with intellectual disability (Freude et al, 2004; Ramser et al, 2004). Thus, the correlation of FTSJ1, interdependence modifications on tRNAPhe(GAA) at ASL region, and nervous system development requires further cellular biological and mechanistic studies.
In addition, some reports demonstrated that FTSJ1 and WDR6 participate in several cellular processes aside from neurological development. FTSJ1 is a target of p53‐mediated repression in murine and human hepatocellular carcinoma (Holzer et al, 2019); WDR6 is linked to cell growth (Xie et al, 2007) and innate immunity (Sivan et al, 2015). However, the underlying mechanism of FTSJ1 and WDR6 involved in these processes is still unknown. Recently, many tRNA MTases have been found to methylate other RNA substrates beside tRNAs (Hussain et al, 2013; Chen et al, 2019; Ringeard et al, 2019; Shinoda et al, 2019). Therefore, whether or not FTSJ1 has other RNA substrates besides tRNAs awaits to be determined. These crucial questions need to be addressed with future studies.
Materials and Methods
Materials
Cytidine (C), adenosine (A), guanosine (G), uridine (U), Am, Cm, Um, Gm, m1G, m5C, 5′‐guanosine monophosphate (GMP), ammonium acetate (NH4OAc), Tris base, β‐mercaptoethanol (β‐Me), S‐adenosyl‐L‐homocysteine (SAH), benzonase, RNase A, pyrophosphate, and phosphodiesterase I were purchased from Sigma‐Aldrich Co. LLC. (St. Louis, MO, USA). Tris–HCl, tryptone, yeast extract, MgCl2, NaCl, ATP, CTP, GTP, UTP, and isopropyl‐D‐thiogalactoside (IPTG) were purchased from Sangon Biotech (Shanghai, at Shanghai Information Center for Life Sciences, China). DNA fragment rapid purification and plasmid extraction kits were purchased from Yuanpinghao Biotech (Tianjin, China). KOD‐Plus Mutagenesis Kit and KOD‐Plus‐Neo Kit were from TOYOBO. Dynabeads protein G, Lipofectamine 2000 and RNAiMAX transfection reagent, TRIzol, bacterial alkaline phosphatase, streptavidin‐conjugated agarose beads, Tris(2‐carboxyethyl)phosphine hydrochloride (TCEP) solution pH 7.0, T4 DNA ligase, 4′,6‐diamidino‐2‐phenylindole (DAPI), ribonuclease inhibitor, all restriction endonucleases, polyvinylidene fluoride (PVDF) membranes, and chemiluminescent substrates were obtained from Thermo Scientific (Waltham, MA, USA). Qproteome Nuclear Protein Kits and Ni2+‐NTA Superflow resin were purchased from Qiagen Inc. (Hilden, Germany). [Methyl‐3H] SAM (78.0 Ci/mmol) was purchased from PerkinElmer Inc. (Waltham, MA, USA). SAM was purchased from New England BioLabs, Inc. PCR primers were synthesized by BioSune (Shanghai, China). Glutathione HiCap matrix was purchased from GE Healthcare (Fairfield, CT, USA). T7 RNA polymerase (Li et al, 1999) was purified from an overproduction strain in our laboratory.
Antibodies were obtained from different companies. The antibodies used in this study were as follows: HRP‐conjugated anti‐mouse/anti‐rabbit IgG (A9044/A9169, Sigma‐Aldrich), anti‐FTSJ1 antibody (ab227259, Abcam), anti‐β‐actin antibody (AC004, ABclonal), anti‐lamin A/C antibody (4777, Cell Signaling Technology), anti‐α‐tubulin antibody (3873, Cell Signaling Technology), anti‐Flag antibody (8146, Cell Signaling Technology), anti‐Flag antibody (F7425, Sigma‐Aldrich), anti‐HA antibody (H3663, Sigma‐Aldrich), anti‐Flag M2 affinity gel (A2220, Sigma), and anti‐GAPDH antibody (30201ES20, Yeasen). Alexa Fluor 488‐conjugated goat anti‐mouse IgG was purchased from Jackson ImmunoResearch.
Plasmids
The coding sequences of ftsj1 (NM_012280.3) and wdr6 (NM_018031.4) were amplified from cDNA, which was obtained by RT–PCR from total RNA extracted from Hela cells. The plasmids for protein purification in E. coli Rosetta (DE3) and in insect cells, for immunoprecipitation and co‐immunoprecipitation, and for immunofluorescence experiments were constructed as shown in Appendix Table S5. Gene mutagenesis was performed according to the protocol provided with the KOD‐Plus Mutagenesis Kit.
Cell culture
HEK293T cells were purchased from the cell resource center of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China. They were cultured at 37°C incubator with 5% CO2 in Dulbecco's modified Eagle's medium (high glucose) (Corning) supplemented with 10% fetal bovine serum (Gibco). The viable cell numbers were counted by trypan blue staining assays. Insect cells, Spodoptera frugiperda Sf9 and High Five cells, were cultured on a shaking table at 26°C and 110 rpm in ESF921 medium (Expression Systems, USA).
Confocal immunofluorescence microscopy
HEK293T cells were transfected with pcDNA3.1‐ftsj1‐Flag plasmid. After transfection for 24 h, the cells were fixed in 4% paraformaldehyde for 30 min and then permeated in 0.2% Triton X‐100 for 5 min on ice. After washing with phosphate‐buffered saline (PBS), fixed cells were blocked in PBS plus 0.1% Triton X‐100 buffer containing 5% BSA and incubated with mouse anti‐Flag antibodies with 1:400 dilution overnight at 4°C. The cells were then immunolabeled with Alexa Fluor 488‐conjugated goat anti‐mouse IgG in PBS with 1:1,000 dilution for 2 h and the nuclear counterstain DAPI for 5 min at room temperature. Fluorescent images were taken and analyzed using an FV1000 confocal microscope (Olympus).
Immunoprecipitation or co‐immunoprecipitation
For immunoprecipitation, dishes (10‐cm2) of HEK293T cells were transfected with 5 μg pcDNA3.1‐ftsj1‐Flag. For co‐immunoprecipitation, dishes (10‐cm2) of HEK293T cells were transfected with 5 μg pcDNA3.1‐ftsj1‐Flag (or the plasmids for ftsj1 mutants) and 10 μg pcDNA3.1‐wdr6‐HA. Lipofectamine 2000 was used for transfection at a ratio of 1:2.5, according to the manufacturer's protocol. After transfection for 24 h, the cells were washed with 5 ml of ice‐cold PBS twice and lysed with 0.8 ml of ice‐cold lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 20 mM NaF, 1% NP‐40) supplemented with a ProteinSafe™ Protease Inhibitor Cocktail (TransGen Biotech, Beijing, China). The supernatant was collected by centrifugation at 12,000 ×g for 10 min. Subsequently, the supernatant was incubated with the anti‐Flag antibody with gentle agitation overnight, and then, the mixture was incubated with Dynabeads™ protein G for 2 h at 4°C. Recovered immune complexes were washed three times with ice‐cold PBS and 0.05% Tween‐20 (PBST) buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.5% Tween‐20). All procedures were performed at 4°C. Proteins were eluted by incubating with 2× protein loading buffer (100 mM Tris–HCl, pH 6.8, 4% sodium dodecyl sulfate, 0.2% bromophenol blue, 20% glycerol, and 200 mM DTT) for Western blotting.
Construction of knockout cell lines
Sense and anti‐sense oligonucleotides for a guide RNA (sgRNA) were computationally designed for the selected genomic targets (http://crispr.mit.edu) and were cloned into vector pX330‐mcherry (Addgene, 98750) which expresses red fluorescence protein (Wu et al, 2013). Two sgRNAs were designed for ftsj1 and wdr6, respectively. The sequences and targeting sites are shown in Fig 3. For generating KO cell lines, 6 μg sgRNA plasmids were transfected in dishes (6‐cm2) of HEK293T cells using Lipofectamine 2000 as transfection reagent. After transfection for 24 h, HEK293T cells expressing red fluorescent protein were enriched by Fluorescence‐Activated Cell Sorting Aria II (BD Bioscience) and plated into a well of a 10‐cm2 dish at a low density. After 5–8 days, single colonies were picked and plated into a well of a 96‐well plate. Genotyping of the stable cell lines was performed by sequencing cloned PCR products based upon the following primers:
FTSJ1‐identify‐F: TCAGGCCCTATAAGGTCAGTGGGGT
FTSJ1‐identify‐R: CCACCACGTGGCCGGACCCTTGGCCC
WDR6‐identify‐F: TTTATTGTGTACTGACTCCATCTGC
WDR6‐identify‐R: CTGGTCTCTGGGTCTACCTTAACCA
The knockout efficiency of ftsj1 and wdr6 was measured by Western blotting and qRT–PCR, respectively. The qRT–PCR primers for wdr6 were wdr6‐PF (AAATTAGCTGGGGACAGGGC) and wdr6‐PR (CGGTCAGGTGCTACAGGTTT). The qRT–PCR primers for gapdh were gapdh‐PF (AGAAGGCTGGGGCTCATTTG) and gapdh‐PR (AGGGGCCATCCACAGTCTTC).
Western blotting
Cell lysates, different cell fraction extracts, and immune complexes were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to 0.2‐μm PVDF membranes, and incubated with different antibodies. After blocking with 3% (w/v) non‐fat dried milk, the membranes with targeted proteins were incubated with the corresponding primary antibodies overnight at 4°C. Membranes were then washed three times with PBST and incubated with HRP‐conjugated secondary antibody at room temperature for 30 min. After washing three times with PBST, the membranes were treated with the chemiluminescent substrates, and imaging was performed using the LAS‐4000 system (GE, CA, USA) or MiniChemi 610 (Sage Creation, Beijing, China).
Quantitative Real‐Time PCR (qRT–PCR)
Total cellular RNAs were isolated using TRIzol according to the manufacturer's instructions. The first‐strand cDNA synthesis using total cellular RNAs as the template was performed with PrimeScript™ RT Reagent Kit (TAKARA, Japan). qRT–PCR was performed using the relative standard curve method in QuantStudio 7 (Life Technology, USA) with SYBR Green I (TOYOBO, Japan) as the dsDNA fluorescence dye. The reactions were performed under the following conditions: 95°C for 2 min; 40 cycles of 95°C for 30 s, 62°C for 20 s, and 72°C for 30 s; and a melting curve from 50 to 95°C.
Isolation of a given tRNA by biotinylated DNA probes
Total RNA was extracted using TRIzol. The endogenous tRNAs used in this study were isolated by their own biotinylated DNA probe and were purified by Streptavidin Agarose Resin, according to the previous method developed by our laboratory (Huang et al, 2012). The biotinylated DNA probes were designed to complement the 5′ or 3′ sequences of the tRNAs. Some tRNA isoacceptors, such as tRNALeu(AAG) and tRNALeu(UAG); tRNAPro(AGG), tRNAPro(CGG), and tRNAPro(UGG), differing only by one or two nucleotides, are too difficult to distinguish from each other with fishing probes. Thus, we fished the mixture of these two groups of tRNAs to identify their modification level. The various tRNAs and their probes for tRNA selection used in this study are listed in Appendix Table S6. 20 μl of high‐capacity streptavidin‐conjugated agarose beads was washed with buffer A (10 mM Tris–HCl, pH 7.5) and suspended in buffer B (100 mM Tris–HCl, pH 7.5). Subsequently, 200 μM biotinylated oligonucleotides were mixed and incubated at room temperature for 90 min. After the incubation, the oligonucleotide‐coated beads were then washed four times in buffer A and equilibrated in 6× NTE solution (1× NTE: 200 mM NaCl, 5 mM Tris–HCl, pH 7.5, 2.5 mM EDTA). The oligonucleotide‐coated beads and total RNAs in 6× NTE solutions were heated for 5 min at 70°C and cooled down to 30°C. Then, the beads were washed with 3× NTE for three times and 1× NTE for twice. The specific tRNA retained on the beads was eluted with 0.1× NTE at 70°C and precipitated using 75% ethanol.
Quantitative analysis of tRNA modification using UPLC‐MS/MS
400 ng of specific tRNAs purified by the biotinylated DNA probe was hydrolyzed with 0.5 μl benzonase, 0.5 μl phosphodiesterase I, and 0.5 μl bacterial alkaline phosphatase in a 100 μl solution including 4 mM NH4OAc at 37°C overnight. After complete hydrolysis, 10 μl of the solution was applied to ultra‐performance liquid chromatography–mass spectrometry/mass spectrometry (UPLC‐MS/MS). The nucleosides were separated on a C18 column (Agilent ZORBAX Eclipse Plus C18, 2.1 × 50 mm, 1.8‐μm) and then detected by a triple quadrupole mass spectrometer (Agilent 6495 QQQ or SCIEX 6500 QTRAP) in the positive ion multiple reaction monitoring (MRM) mode. The nucleosides were quantified using the nucleoside‐to‐base ion mass transitions of 268.1 to 136.2 (A), 284.1 to 152.2 (G), 244.1 to 112.1 (C), 245.0 to 113.1 (U), 282.1 to 136.2 (Am), 298.1 to 152.1 (Gm), 258.1 to 112.1 (Cm), 259.1 to 113.1 (Um), 298.1 to 166.1 (m1G), 258.1 to 126.1 (m5C), and 541.2 to 409.0 (o2yW). Quantification was performed by comparison with the standard curve obtained from pure nucleoside standards running in the same batch. The ratio of Cm, Gm, m1G, m5C, and o2yW to the sum of A was determined based on the calculated concentrations.
Preparation of transcript tRNAs
The DNA sequences of the T7 promoter and the tRNAArg(ACG), tRNAArg(CCG), and tRNAArg(UCG); tRNACys(GCA); tRNAHis(GUG); tRNALeu(UAA); tRNALeu(AAG), tRNALeu(UAG), and tRNALeu(CAG); tRNAPro(AGG), tRNAPro(CGG), and tRNAPro(UGG); tRNATrp(CCA); tRNAPhe(GAA); and tRNATyr(AUA) and tRNATyr(GUA) were obtained from the MODOMICS database (Chan & Lowe, 2016; Boccaletto et al, 2018) and ligated into pTrc99b (pre‐cleaved with EcoRI/BamHI) to construct pTrc99b‐T7‐tRNAs. All tRNAs were generated via in vitro transcription using T7 RNA polymerase, as described previously (Li et al, 1999). The tRNA concentrations were determined by UV absorbance at 260 nm, and the molar absorption coefficient was calculated according to the sequence of each tRNA (Kibbe, 2007).
Protein expression and purification
The gene encoding FTSJ1 fused with a C‐terminal His6‐tag (FTSJ1‐His) was expressed in baculovirus‐mediated transduction of High Five insect cells. At 72 h postinfection, the cells were harvested by centrifugation and frozen at −80°C until purification. After thawing, cells were lysed by sonication in buffer C containing 20 mM Tris–HCl, pH 7.5, 500 mM NaCl, 10% glycerol, and 2 mM DTT with 1 mM PMSF. The supernatant was collected by centrifugation at 100,000 ×g at 4°C for 30 min and then loaded onto a Ni2+‐NTA Superflow column (QIAGEN) equilibrated with buffer C. After washing with two column volumes, the protein was eluted in buffer C with 250 mM imidazole and concentrated. The concentrated FTSJ1 was dialyzed in a buffer containing 20 mM Tris–HCl pH 7.5, 500 mM NaCl, and 2 mM DTT and concentrated for the follow‐up experiments.
The gene containing WDR6 fused with an N‐terminal GST‐tag (GST‐WDR6) was expressed in baculovirus‐mediated transduction of Sf9 insect cells. At 72 h postinfection, the cells were harvested by centrifugation and frozen at −80°C until purification. After thawing, cells were lysed by sonication in buffer C containing 20 mM Tris–HCl, pH 7.5, 500 mM NaCl, 10% glycerol, and 2 mM DTT with 1 mM PMSF. The collected supernatant was loaded onto the glutathione HiCap matrix columns, eluted in buffer C with 20 mM reduced glutathione. Then, the WDR6 fused with GST was dialyzed in a buffer containing 20 mM Tris–HCl pH 7.5, 500 mM NaCl, and 2 mM DTT.
The gene containing the E. coli TrmD, which catalyzes formation of m1G37 (Hou et al, 2017), fused with an N‐terminal His6‐tag was expressed in the E. coli Rosetta (DE3) cells (TIANGEN). The transformants were cultured in LB medium and induced with 200 μM IPTG at 18°C overnight. The N‐terminal His6‐tagged TrmD was purified from the cell lysate using Ni‐NTA Superflow resin according to the manufacturer's protocol.
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) measurements were performed at 25°C, using an iTC200 Microcalorimeter (MicroCal Inc.). Experiments included 20 injections of 2 μl of SAM (1 mM) into the sample cell containing 100 μM FTSJ1, which was purified from insect cells. The SAM and FTSJ1 were kept in the same buffer (20 mM Tris–HCl, pH 7.5, 500 mM NaCl, and 0.5 mM TCEP). SAM was titrated in the same buffer and was used as a control. Binding isotherms were fit by non‐linear regression using Origin Software version 7.0 (MicroCal Inc.). The ITC data were fitted to a one‐site binding model using Origin Software version 7.0 (MicroCal Inc.).
Gel mobility shift assay
FTSJ1 (final concentrations, 0, 0.25, 0.5, 1, 1.5, 2, 4, and 8 μM) and 250 nM tRNAPhe transcript were incubated in 20 μl of buffer D (50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 5 mM MgCl2, and 2 mM DTT) with 0.5 mM SAM at 37°C for 20 min. For another reaction, 0.2 μΜ GST, 0.2 μΜ GST‐WDR6, 1 μM FTSJ1, and the mixture of 1 μM FTSJ1 with 0.2 μΜ GST‐WDR6 or 0.2 μΜ GST were incubated in 20 μl buffer D with 0.5 mM SAM and 250 nM tRNAPhe transcript at 37°C for 20 min. After incubation, 1 μl of loading solution (0.25% bromophenol blue and 30% glycerol) was added into each sample and loaded immediately onto a 6% polyacrylamide native gel. Electrophoresis was carried out at 4°C at a constant voltage of 60 V for 80 min, using 50 mM Tris‐glycine buffer. The gel was stained with ethidium bromide for detection of RNA. The RNA bands were quantified by using a FujiFilm imaging analyzer.
tRNA methyl transfer assay
In vitro assays of 2′‐O‐methylation at position 34 on tRNAs were carried out at 37°C for 2 h in a 100 μl reaction mixture containing 50 mM Tris–HCl, pH 7.5, 200 mM NaCl, 10 mM MgCl2, 100 μg/ml BSA, 5 mM DTT, 5 μM tRNA, and 100 μM SAM with 1 μM FTSJ1, 0.2 μM WDR6, or a mixture of 1 μM FTSJ1 and 0.2 μM WDR6, respectively. The tRNAs were extracted with phenol/chloroform and precipitated using a threefold volume of ethanol. Subsequently, the tRNAs were digested with benzonase, phosphodiesterase I, and bacterial alkaline phosphatase and then subjected to UPLC‐MS/MS analysis to detect and quantify Nm34 formation.
To obtain tRNAs carrying the m1G37 modification, reactions were performed at 37°C in a mixture comprised of 0.5 μM TrmD, 100 mM Tris–HCl, pH 8.0, 6 mM MgCl2, 24 mM NH4Cl, 100 μg/ml BSA, 4 mM DTT, 200 μM SAM, and tRNAs. The wild‐type tRNAs were prepared in the same mixture by adding the TrmD‐restored buffer. The reaction was performed for 1 h and stopped using phenol/chloroform extraction. The tRNAs were precipitated using a threefold volume of ethanol.
Cell counting kit‐8 assay
Cell proliferation was determined using the Cell Counting Kit‐8 (Beyotime, Shanghai, China). Briefly, 2 × 103 WT HEK293T and ftsj1 knockout cells were seeded in a 96‐well flat‐bottomed plate under normal culture or adding 0.2 mg/ml paromomycin. At days 1, 2, 3, 4, and 5, 10 μl of CCK‐8 (5 mg/ml) was added into each well and the cells were incubated for 2 h. The optical density at 450 nm (OD450) was measured using a microplate reader (BioTek EON, USA). The experiments were repeated at three times. To verify the effect of tRNAPhe(GAA) on the growth of WT and ftsj1 KO cells, we isolated tRNAPhe(GAA) from WT HEK293T and transfected 1.5 μl tRNAPhe(GAA) (10 μM) into per well of 6‐well WT HEK293T or ftsj1 knockout cells using Lipofectamine RNAiMAX (Thermo Fisher, USA) according to the manufacturer's protocol. We used the same amount of Lipofectamine RNAiMAX but without tRNAPhe(GAA) transfection as a negative control. After transfection for 6 h, 2 × 103 WT HEK293T and ftsj1 knockout cells were seeded in a 96‐well flat‐bottomed plate and assayed the growth curve as above.
Luciferase reporter assay
pmirGlo luciferase expression vector (Promega) containing a firefly luciferase (F‐luc) and a Renilla luciferase (R‐luc) was used to construct the reporter plasmid. The F‐luc‐6 × Phe(TTT) reporter plasmid was obtained by inserting TTTTTTTTTTTTTTTTTT before the F‐luc coding region; the F‐luc‐6 × Phe(TTC) reporter plasmid was obtained by inserting TTCTTCTTCTTCTTCTTC before the F‐luc coding region. The F‐luc genes with all the Phe codons using either TTT or TTC were synthesized by TsingKe (Beijing, China) and constructed into the reporter plasmids. Basic setting: 40 ng of reporter plasmids (pmirGlo empty vector or pmirGlo mutated vector) was transfected into WT HEK293T cells and ftsj1 knockout cells in a 24‐well plate using Lipofectamine 2000. After 24 h, the cells in the 24‐well plate were assayed by Dual‐Glo Luciferase Assay System (Promega). R‐luc was used to normalize F‐luc activity to evaluate the translation efficiency of the reporter. We employed the pmirGlo mutated reporter normalized to the pmirGlo empty vector.
Author contributions
R‐JL, E‐DW, and JL conceived the ideas for this work. JL, Y‐NW, and YN generated FTSJ1 and GST‐WDR6 proteins. B‐SX provided bioinformatics analysis data. Y‐PL and PRC provided DIZPK and technological support. JL performed all the other experiments with technical help from R‐JL, MZ, TL, HL, and HD. R‐JL, E‐DW, and JL wrote the manuscript, which was reviewed by all authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Source Data for Expanded View
Review Process File
Acknowledgements
We thank the National Center for Protein Sciences at Peking University in Beijing, China, for assistance with the ultra‐performance liquid chromatography–mass spectrometry (UPLC‐MS) work, and we would be grateful to Dr. Hui Li for her help of data acquisition and analysis. We thank the staff members (Dr. Chao Peng and Mr. Zhi‐Hong Li) of the ultra‐performance liquid chromatography–mass spectrometry (UPLC‐MS) at the National Facility for Protein Science Shanghai (NFPS), Zhangjiang Lab, China, for providing technical support and assistance in data collection and analysis). This work was supported by the National Key Research and Development Program of China [2017YFA0504000]; the National Natural Science Foundation of China [81471113, 31770842, 31971230, 31870811]; the Strategic Priority Research Program of the Chinese Academy of Sciences [XDB19000000]; and the Youth Innovation Promotion Association (Chinese Academy of Sciences) (to R‐J.L.) [Y319S21291].
EMBO Reports (2020) 21: e50095
Contributor Information
En‐Duo Wang, Email: edwang@sibcb.ac.cn.
Ru‐Juan Liu, Email: liurj@shanghaitech.edu.cn.
References
- Abedini SS, Kahrizi K, de Pouplana LR, Najmabadi H (2018) tRNA methyltransferase defects and intellectual disability. Arch Iran Med 21: 478–485 [PubMed] [Google Scholar]
- Agris PF, Vendeix FA, Graham WD (2007) tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 366: 1–13 [DOI] [PubMed] [Google Scholar]
- Ai HW, Shen W, Sagi A, Chen PR, Schultz PG (2011) Probing protein‐protein interactions with a genetically encoded photo‐crosslinking amino acid. ChemBioChem 12: 1854–1857 [DOI] [PubMed] [Google Scholar]
- Angelova MT, Dimitrova DG, Da Silva B, Marchand V, Jacquier C, Achour C, Brazane M, Goyenvalle C, Bourguignon‐Igel V, Shehzada S et al (2020) tRNA 2′‐O‐methylation by a duo of TRM7/FTSJ1 proteins modulates small RNA silencing in Drosophila . Nucleic Acids Res 48: 2050–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, de Crecy‐Lagard V, Ross R, Limbach PA, Kotter A et al (2018) MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46: D303–D307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugl H, Fauman EB, Staker BL, Zheng F, Kushner SR, Saper MA, Bardwell JC, Jakob U (2000) RNA methylation under heat shock control. Mol Cell 6: 349–360 [DOI] [PubMed] [Google Scholar]
- Chan PP, Lowe TM (2016) GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res 44: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, Chen RX, Wei WS, Liu Y, Gao CC et al (2019) 5‐methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol 21: 978–990 [DOI] [PubMed] [Google Scholar]
- Dixit S, Henderson JC, Alfonzo JD (2019) Multi‐substrate specificity and the evolutionary basis for interdependence in tRNA editing and methylation enzymes. Front Genet 10: 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi B, Bailly M, de Crecy‐Lagard V (2012) Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet 46: 69–95 [DOI] [PubMed] [Google Scholar]
- Freude K, Hoffmann K, Jensen LR, Delatycki MB, des Portes V, Moser B, Hamel B, van Bokhoven H, Moraine C, Fryns JP et al (2004) Mutations in the FTSJ1 gene coding for a novel S‐adenosylmethionine‐binding protein cause nonsyndromic X‐linked mental retardation. Am J Hum Genet 75: 305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Jaffrey SR, Pan T, Rechavi G, Suzuki T (2016) RNA modifications: what have we learned and where are we headed? Nat Rev Genet 17: 365–372 [DOI] [PubMed] [Google Scholar]
- Garber K (2019) Epigenetics comes to RNA. Science 365: 16–17 [DOI] [PubMed] [Google Scholar]
- Guy MP, Podyma BM, Preston MA, Shaheen HH, Krivos KL, Limbach PA, Hopper AK, Phizicky EM (2012) Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA 18: 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Phizicky EM (2014) Two‐subunit enzymes involved in eukaryotic post‐transcriptional tRNA modification. RNA Biol 11: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Phizicky EM (2015) Conservation of an intricate circuit for crucial modifications of the tRNAPhe anticodon loop in eukaryotes. RNA 21: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Shaw M, Weiner CL, Hobson L, Stark Z, Rose K, Kalscheuer VM, Gecz J, Phizicky EM (2015) Defects in tRNA anticodon loop 2′‐O‐methylation are implicated in nonsyndromic X‐linked intellectual disability due to mutations in FTSJ1 . Hum Mut 36: 1176–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Phizicky EM (2018) A rationale for tRNA modification circuits in the anticodon loop. RNA 24: 1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Guy MP, Kon Y, Phizicky EM (2018) Lack of 2′‐O‐methylation in the tRNA anticodon loop of two phylogenetically distant yeast species activates the general amino acid control pathway. PLoS Genet 14: e1007288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S et al (2012) GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res 22: 1760–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C (2010) Grand challenge commentary: RNA epigenetics? Nat Chem Biol 6: 863–865 [DOI] [PubMed] [Google Scholar]
- Hirata A, Okada K, Yoshii K, Shiraishi H, Saijo S, Yonezawa K, Shimizu N, Hori H (2019) Structure of tRNA methyltransferase complex of Trm7 and Trm734 reveals a novel binding interface for tRNA recognition. Nucleic Acids Res 47: 10942–10955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer K, Ori A, Cooke A, Dauch D, Drucker E, Riemenschneider P, Andres‐Pons A, DiGuilio AL, Mackmull MT, Bassler J et al (2019) Nucleoporin Nup155 is part of the p53 network in liver cancer. Nat Commun 10: 2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YM, Matsubara R, Takase R, Masuda I, Sulkowska JI (2017) TrmD: a methyl transferase for tRNA methylation with m1G37. Enzymes 41: 89–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YM, Masuda I, Gamper H (2018) Codon‐specific translation by m1G37 methylation of tRNA. Front Genet 9: 713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Yao P, Eriani G, Wang ED (2012) In vivo identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain. Nucleic Acids Res 40: 10463–10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J, Gold M, Anders M (1964) The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. 3. purification of soluble ribonucleic acid‐methylating enzymes. J Biol Chem 239: 3462–3473 [PubMed] [Google Scholar]
- Hussain S, Sajini AA, Blanco S, Dietmann S, Lombard P, Sugimoto Y, Paramor M, Gleeson JG, Odom DT, Ule J et al (2013) NSun2‐mediated cytosine‐5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep 4: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarada L, Suzuki T, Ohira T, Hirata S, Miyauchi K, Suzuki T (2017) ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res 45: 7401–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbe WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35: W43–W46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschziner GD, Coffey AJ, Andrew T, Gregorio SP, Dias‐Neto E, Calafato M, Bentley DR, Kinton L, Sander JW, Johnson MR (2011) Q8IYL2 is a candidate gene for the familial epilepsy syndrome of partial epilepsy with pericentral spikes (PEPS). Epilepsy Res 96: 109–115 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen JF, Wang ED, Wang YL (1999) T7 RNA polymerase transcription of Escherichia coli isoacceptors tRNALeu . Sci China C Life Sci 42: 185–190 [DOI] [PubMed] [Google Scholar]
- Maraia RJ, Arimbasseri AG (2017) Factors that shape eukaryotic tRNAomes: processing, modification and anticodon‐codon use. Biomolecules 7: 26 [Google Scholar]
- Masuda I, Takase R, Matsubara R, Paulines MJ, Gamper H, Limbach PA, Hou YM (2018) Selective terminal methylation of a tRNA wobble base. Nucleic Acids Res 46: e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Hartmann M, Schuster I, Bender S, Thuring KL, Helm M, Katze JR, Nellen W, Lyko F, Ehrenhofer‐Murray AE (2015) Dynamic modulation of Dnmt2‐dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res 43: 10952–10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova DD, Leidel SA (2015) Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161: 1606–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T (2018) Modifications and functional genomics of human transfer RNA. Cell Res 28: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perche‐Letuvee P, Molle T, Forouhar F, Mulliez E, Atta M (2014) Wybutosine biosynthesis: structural and mechanistic overview. RNA Biol 11: 1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintard L, Lecointe F, Bujnicki JM, Bonnerot C, Grosjean H, Lapeyre B (2002) Trm7p catalyses the formation of two 2′‐O‐methylriboses in yeast tRNA anticodon loop. EMBO J 21: 1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramser J, Winnepenninckx B, Lenski C, Errijgers V, Platzer M, Schwartz CE, Meindl A, Kooy RF (2004) A splice site mutation in the methyltransferase gene FTSJ1 in Xp11.23 is associated with non‐syndromic mental retardation in a large Belgian family (MRX9). J Med Genet 41: 679–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringeard M, Marchand V, Decroly E, Motorin Y, Bennasser Y (2019) FTSJ3 is an RNA 2′‐O‐methyltransferase recruited by HIV to avoid innate immune sensing. Nature 565: 500–504 [DOI] [PubMed] [Google Scholar]
- Rubio MA, Ragone FL, Gaston KW, Ibba M, Alfonzo JD (2006) C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei . J Biol Chem 281: 115–120 [DOI] [PubMed] [Google Scholar]
- Rubio MA, Gaston KW, McKenney KM, Fleming IM, Paris Z, Limbach PA, Alfonzo JD (2017) Editing and methylation at a single site by functionally interdependent activities. Nature 542: 494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S, Kitagawa S, Nakagawa S, Wei FY, Tomizawa K, Araki K, Araki M, Suzuki T, Suzuki T (2019) Mammalian NSUN2 introduces 5‐methylcytidines into mitochondrial tRNAs. Nucleic Acids Res 47: 8734–8745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan G, Ormanoglu P, Buehler EC, Martin SE, Moss B (2015) Identification of restriction factors by human genome‐wide RNA interference screening of viral host range mutants exemplified by discovery of SAMD9 and WDR6 as inhibitors of the Vaccinia Virus K1L–C7L‐ mutant. mBio 6: e01122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF (2008) Diversity of WD‐repeat proteins. Subcell Biochem 48: 20–30 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Bond MR, Kohler JJ (2008) Photocrosslinkers illuminate interactions in living cells. Mol BioSyst 4: 473–480 [DOI] [PubMed] [Google Scholar]
- Thiaville PC, El Yacoubi B, Kohrer C, Thiaville JJ, Deutsch C, Iwata‐Reuyl D, Bacusmo JM, Armengaud J, Bessho Y, Wetzel C et al (2015) Essentiality of threonylcarbamoyladenosine (t6A), a universal tRNA modification, in bacteria. Mol Microbiol 98: 1199–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali P, Kiss T (2019) Cooperative 2′‐O‐methylation of the wobble cytidine of human elongator tRNAMet(CAT) by a nucleolar and a Cajal body‐specific box C/D RNP. Genes Dev 33: 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YX, Liang D, Wang YH, Bai MZ, Tang W, Bao SM, Yan ZQ, Li DS, Li JS (2013) Correction of a genetic disease in mouse via use of CRISPR‐Cas9. Cell Stem Cell 13: 659–662 [DOI] [PubMed] [Google Scholar]
- Xiang JF, Yang Q, Liu CX, Wu M, Chen LL, Yang L (2018) N6‐methyladenosines modulate A‐to‐I RNA editing. Mol Cell 69: 126–135 [DOI] [PubMed] [Google Scholar]
- Xie XD, Wang ZZ, Chen Y (2007) Association of LKB1 with a WD‐repeat protein WDR6 is implicated in cell growth arrest and p27(Kip1) induction. Mol Cell Biochem 301: 115–122 [DOI] [PubMed] [Google Scholar]
- Zhang M, Lin SX, Song XW, Liu J, Fu Y, Ge X, Fu XM, Chang ZY, Chen PR (2011) A genetically incorporated crosslinker reveals chaperone cooperation in acid resistance. Nat Chem Biol 7: 671–677 [DOI] [PubMed] [Google Scholar]
- Zhou M, Long T, Fang ZP, Zhou XL, Liu RJ, Wang ED (2015) Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2′‐O‐methyltransferase. RNA Biol 12: 900–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Source Data for Expanded View
Review Process File


