Abstract
Ageing is a natural phenomenon which is a fold, ridge and crease in the skin that occurs due to loss of body mass, poor hydration, disintegration of dermis and epidermis junction. The Skin ageing process involves many changes that occur due to the combination of both endogenous factors (gene mutation, cellular metabolism, and hormonal factor) and exogenous factors (U.V, pollutants, chemical, and toxins). In 1950, the number of older people were found to be almost 205 million across the globe. But this number almost got 4 times by the year 2012 and the number of older persons increased to a massive amount of 810 million. The ageing of the skin occurs due to various mechanisms like glycation, free radical, cell cycle, and cellular and molecular mechanism of skin ageing. In this review article, we have discussed the treatment, worldwide newer therapies and marketed formulation that are currently available for the reduction of skin ageing. The most promising and revolutionizing field of nanotechnology is mostly applied in the field of dermatology, cosmetics, and biomedical applications. Nanotechnology also plays a vital role in increasing the efficacy of the product.
Keywords: Treatment, nanotechnology, skin ageing, dermatology, nanocarriers, body mass
1. INTRODUCTION
In the early 1950s, nearly about 205 million people were in the age of 60s and by 2012 it continued to increase to almost 810 million. This number may be increased to double and reaching up to 2 billion by 2050. Today, 15 countries comprising about 10 million old persons and out of those 15 countries, seven are developing and do not have enough resources for the proper betterment of those people [1].
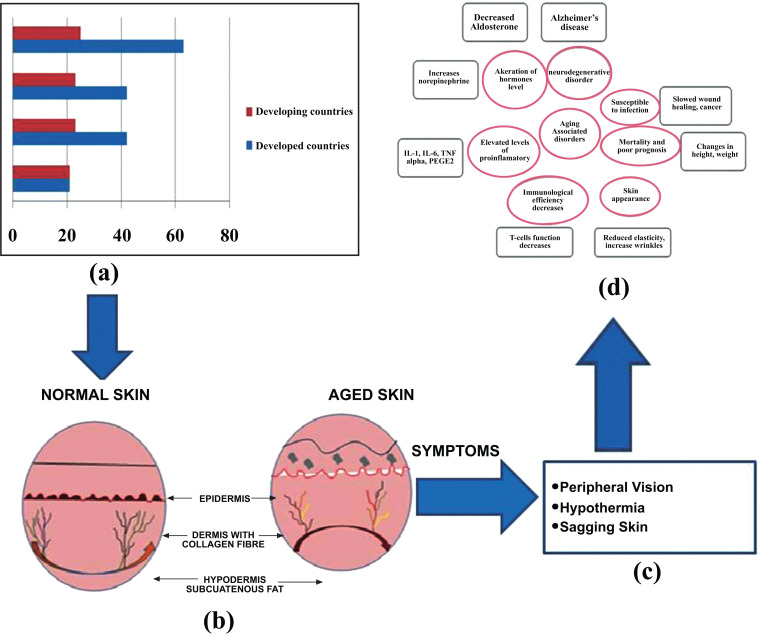
Between 2010 and 2050 the number of older individuals were in a smaller amount in the developed countries which expected to extend upto 250% compared to a 71% increase in developing countries. The world's population of people of 60 years of age and older is expected to increase by 22% from 10% (from 800 million to 2 billion) by 2050. Almost, one-fourth person would be older than the age of 60. It has been estimated that over 8% of the population of the Southeast Asian regions are above the age of 60 years [2]. Accordingly, it is expected that age-related diseases will also increase greatly in the coming decades (Fig. 1).
Fig. (1).
Schematic representation of skin ageing (a) Graphical representation of skin ageing (b) Difference between normal and aged skin (c) Symptoms of skin ageing (d) Disorders associated with skin ageing. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Skin ageing is a process in which skin quality deteriorates with age due to the synergistic effects of chronological ageing, photo-ageing, hormonal deficiency and environmental factors [3]. In skin ageing, there is a reduction in the number of fibroblasts that synthesize collagen and vessels that supply the skin which leads to an increase in laxity and hence forms wrinkles [4] (Fig. 1).
The sun-exposed skin gradually leads to sagging of skin. This leads to the loss of fibril and collagen type VII (Col-7) that retards the bond between epidermis and dermis leading to extrinsically aged skin [5]. Many theories of ageing have been proposed including DNA or genetic theory, free radical theory, neuroendocrine theory, membrane theory, Hayflick limit theory, telomerase theory and mitochondrial decline theory. In intrinsically aged skin, the histological changes occur within the basal cell layer due to the internal influences which produce skin sagging, thinning of skin [6], while in extrinsically sun-exposed aged skin due to the accumulation of abnormal elastic tissue in the mid and deep dermis, the solar elastosis or UV-irradiation, increase in elastin promoter activity which induces the elastin gene transcriptional activity and decreases the fibrillin-1 expression resulting in heavy deposition of elastic fibres, which are dystrophic and shorten. If the lysine derived cross-linked compound increases, then the photoaged skin is confirmed [7].
Generally, there are four types of skin ageing that occurs which include intrinsic ageing and characterized by unblemished, smooth, pale(r), drier, less elastic skin with fine wrinkles [8, 9] and occurs within the tissue itself via reductions in dermal mast cells, fibroblasts, collagen production (Fig. 1) and extrinsic ageing which can be caused due to extreme exposure to sun (i.e., photo-ageing) and various exogenous factors such as pro-oxidant and antioxidant influences on cell turnover via neuro-endocrine-immune biological response modifiers [10] which affects mainly the face and neck. The third one is photo-ageing which is caused by sunlight which comprises mostly infrared (52-55%), visible (44%) and 3% UV light, which is harmful to the skin and is completely absorbed by the ozone layer [11]. Hormonal ageing is caused by decreased collagen synthesis, skin thickness, skin hydration and epidermal barrier function [12, 13].
Skin ageing can occur at any layer of the skin. There are generally two factors responsible for Skin ageing namely: Internal factor and External Factor. Skin collagen and elastin synthesis decrease by 1%, each year leading to disorganization of connective tissue. This depletion of collagen and elastin cause the development of skin wrinkles in the dermis layer [14].
There are various skin disorders that are associated with the ageing process which includes susceptibility to infection, skin appearance, and neurodegenerative disorders (Fig. 1). Several factors also promote skin ageing such as internal factors that may be due to the slowdown of blood flow and when moisture level decreases ultimately develop fine lines on the skin. External factors may also be the cause of ageing which include, oxidative stress in which there is a release of such molecules in the body that are highly unstable, and capable of damaging all cellular structure. Smoking leads to a loss of skin elasticity that results in the greyish tan of the skin as well as faster skin ageing [15]. However, there are numerous signs and symptoms exist that may appear on the skin due to skin ageing like Hypothermia, Sagging of skin, Severe arthritis, and decreased visual activity (Fig. 1).
2. PATHOPHYSIOLOGY OF SKIN AGEING
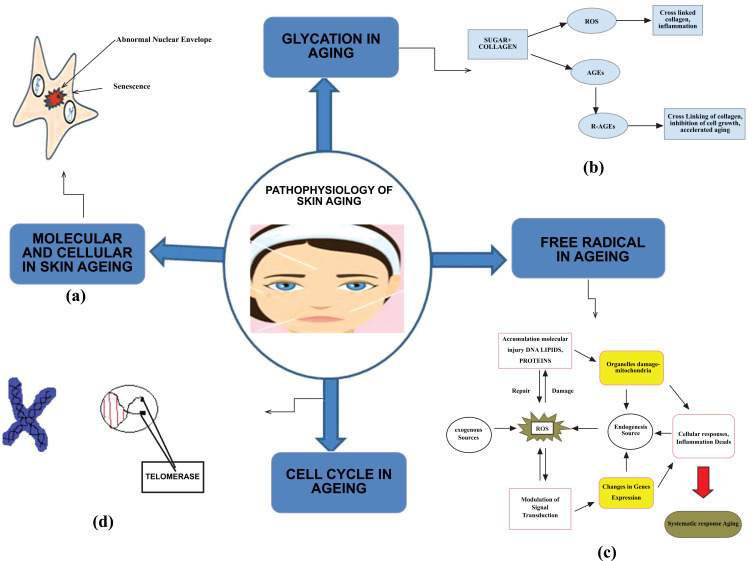
Scientific literature suggests various molecular basis that fully justifies the mechanism of skin pathophysiology for skin ageing. This process may be explained by the theory of cellular senescence or deprive the cellular DNA repair capacity and loss of telomeres, point mutations of extranuclear mitochondrial DNA, oxidative stress, or the abnormalities exist in chromosomes, single-gene mutations, reduced sugar, chronic inflammation, and many more. Research predicts that generally extrinsic factors are the main cause of skin ageing and only 3% of ageing factors contributed by intrinsic factors [16] (Fig. 2).
Fig. (2).
Schematic representations of various mechanism that associated for pathophysiology of Skin ageing (a) Molecular and cellular mechanism of skin ageing (b) Glycation in skin ageing (c) Free radical in skin ageing (d) Cell cycle in skin ageing. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Mechanism of Ageing at the Cellular and Molecular Level
The cell culture technique and molecular biological technique are modern analytical tools. The analysis of ageing at molecular level of cell culture was undertaken in 1920 by Careel, who performed an experiment on the basis of chick heart fibroblast in which cells could divide indefinitely [17]. When they were removed from in vivo mechanism and then placed in vitro environment, they became immortal. In 1960, Hayflick demonstrated that normal human fibroblast has limited capacity to proliferate in vitro [18]. He showed that normal cells could become double at the infinite number of times and after the phase of exponential growth, they stop dividing which is known as Hayflick limit. Ageing is associated with two overlapping processes which led to death one is progressive degeneration of cell and another one is Loss of regeneration capacity.
The muscle regenerative potential decline due to changes in satellite cells which further leads to depletion of proliferative and myogenic capacities. As the skin ageing phenomenon starts, the regenerative ability of skin muscles lessens down due to the gradual decrease in satellite cells, muscle stem cells, which undergo age-related declines in proliferative and myogenic capacities. Indeed, satellite cell numbers decline gradually in mammalian muscles with advancing age [19].
The proliferative degeneration and regeneration of cells are constant under normal conditions at each stage of life. However, in mitotic homeostasis there occurs replacement of damaged cells and preservation of functional integrity of cells as well as tissues. The mechanism of degeneration is due to the generation of Reactive Oxygen Species (ROS) and non- enzymatic glycosylation of proteins with loss of proliferative and regenerative telomerase shortening and apoptosis. This simplifies the role of both exogenous and endogenous factors in terms of the ageing process [20].
2.2. Glycation in Ageing
Age-related cell degeneration involves the accumulation of advanced glycosylation end products i.e., Advanced Glycation End-products (AGE) [21]. Production of AGEs on collagen leads to cross-linking such as the expansion of molecular packing, abnormalities in the extracellular matrix, impaired cell-matrix interaction. AGEs also bind to specific receptors on immune cells which triggers the release of inflammatory mediators and generation of ROS leads to increase in the production of AGEs damage (Fig. 2).
2.3. Free Radical in Ageing
In the Free Radical Theory of Ageing (FRTA), the involvement of free radicals in endogenous metabolic reactions, was proposed in 1954 [22]. The FRTA suggests that the common ageing process is the initiation of free Radical Reactions (RRs). After that some suggestions were included such as (a) Most free radical reactions were initiated by the mitochondria at an increasing rate with age, (b) The life span is determined by the rate of free radical damage to the mitochondria [23].
It became clear that improvements in general living conditions increased Average Life Expectancies at Birth (ALE-B) by decreasing the free radical reactions associated with suboptimal living conditions or by decreasing the free radical reactions associated with suboptimal living conditions or decreasing (a) The chain lengths of free radical reactions e.g., with antioxidants such as vitamin E (b) Their rates of initiation may be depressed by minimizing copper, iron, and other oxidant catalysts, can lower the rate of formation of ageing changes, and decrease the rate of ageing and of disease pathogenesis [24].
Any molecule that possesses a free electron and is highly reactive is known as “free radical”. The reduced components of the ancient oxygen-free atmosphere by free radical reactions, initiated mainly by ionizing radiation from the sun. The products of these reactions, including simple self-replicating progenitors of DNA, reflected the innate chemical properties of the atoms and molecules. The free radical reactions also provide compounds in the environment necessary for the survival and growth of the first protocells, and to produce more or less random free radical reactions mediated changes throughout the cells. Some changes were inheritable, improving the ability of the offspring to survive and function, i.e., to evolve, whereas others were ageing changes that led to cell death (Fig. 2). The intrinsic ageing process due to age-inducing free radical reactions going on continuously throughout the cells and tissues. In the beginning, the reactions were largely initiated by UV radiation from the sun and, to a lesser extent, by volcanic activity, and now almost all arise endogenously from enzymatic and non-enzymatic free radical reaction [25].
2.4. Cell Cycle and Ageing
Atrophy of organ or tissue is a the well-recognized feature of ageing which can destroy genes to build up organ due to poor circulation, loss of hormonal support, decrease inthe physical activity and less active lifestyle. In recent years, the mechanism of ageing is due to reduced regenerative capacity which involved two processes to cell cycle control which include Regulation of cell proliferation and inductionof proliferation and programmed cell death [26].
Thus, the death of cell initiated mitotic cycle in which cell division produces two daughter cells whose fate will be different in which cells, they are sometimes called stem cells which are tightly controlled by specific regulatory protein. Other alteration in Rb (retinoblastoma) pathway involves the system of cyclin, (CDK), and CDK inhibitors (CDI) whereas the process of phosphorylation depends on the activity of kinase CDK4 and CDK6 and these kinases are controlled by CDK inhibitors exfoliated by p21WAF1 protein and p21WAF1 is regulated by p53 which is a protein that guards the integrity of genome and promotes cell cycle in arrest to response DNA damage [27]. The replication of M1 barrier can be overcome by viral oncoproteins, such as SV -40T antigen or adenovirus E1A. By neutralizing the function of p53 and pRb, these stimuli cells are continuously dividing until they reach a state of crisis (M2). At this stage, chromosomal fusion breaks lead to cell death.
Telomerase is DNA fragments that form ends of chromosomes extending up-to 12,000 base pair which guards chromosome stability and protect them from degradation and rearrangement. In normal somatic cells, telomerase is shortened during DNA replication at 100 base pairs per cell division due to DNA polymerase cannot fully replicate 3’end of linear DNA called ‘end – replication problem. The shortening of telomerase decreases as human fibroblast divides, leading to wrinkle formation. The shortened telomerase damages DNA that initiates p53 and pRB pathways [28].
The shortening of telomerase in ageing leads to the contribution of apoptosis, which in the aged closely related to cell cycle regulation with a link provided by p53 protein which has capacity of inducing apoptosis when DNA damages and these damages accumulate during ageing [29].
3. TREATMENTS OF SKIN AGEING
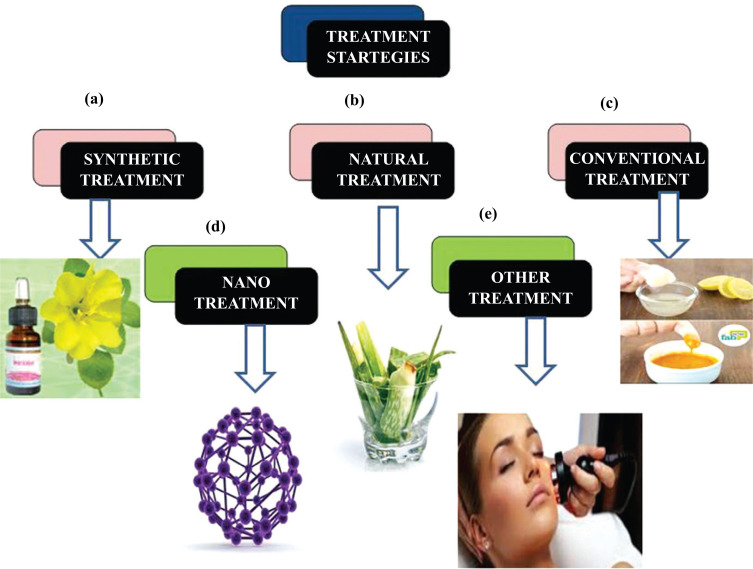
There arenumerous anti-ageing ways that dermatologists have in hand these days, as well as other preventive measurements, such as cosmetological ways, topical and systemic therapeutic agents and invasive procedures [30]. There are also various types of treatments that are available for the control of skin ageing (Fig. 3) such as current anti-ageing approaches (Table 1) and World Wide Newer Therapies which include:-
Fig. (3).
Schematic representations types of treatments for skin ageing (a) Synthetic treatment for skin ageing (b) Natural treatment for skin ageing (c) Conventional treatment for skin ageing (d) Nano treatment for skin ageing (d) Other treatment for skin ageing. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Current skin anti-ageing approaches.
| S. No. | Skin Antiaging Treatment | Techniques |
|---|---|---|
| 1. | Topical medicine agents or topical agents | Antioxidants, Cell regulator. |
| 2. | Invasive procedures | Chemical peelings, Visible light devices, Intense Pulsed Light (IPL), Ablative and non-ablative laser photo–rejuvenation, Radiofrequency (RF) Injectable skin biostimulation and rejuvenation, anatomical wrinkles.Restoration (redistribution) of fat and volume loss, skin augmentation and contouring. |
| 3. | Systemic agents | Hormone replacement therapy, Antioxidants. |
| 4. | Avoidance of exogenous factors of ageing, improvement no of lifestyle and habits | Pollution, Smoking, Solar UV irradiation. Stress, Nutrition, diet restriction and alimental supplementation, Physical activity, Management of general health. |
3.1. Cell Controllers
Nutrient A subsidiaries, polypeptides and different botanicals follow up on the collagen digestion and aides underway of collagen and flexible filaments that can anticipate for the skin maturing.
3.2. Polypeptides or Oligopeptides
At the point when applies topically, polypeptides and amino acids are useful in the arrangement of collagen and elastin that aides in anticipation of wrinkles [31].
3.3. Intrusive Procedure
Reemerge is the intrusive strategy for epidermis to evacuate damaged epidermis by the arrangement of new collagen alongside supplanting the tissues with renovated skin layers [32].
3.4. Concoction PEELS
Concoction strips are divided into:
Shallow strips α-β-lipo-Hydroxy Acids (HA), trichloroacetic corrosive (TCA) 10-30% are the shallow strips that shed epidermal layers without interacting the basal layer;
Medium-profundity strips TCA over 30 to half is the medium-profundity strip which reaches just the upper reticular dermis;
Deep strips can enter deep into the epidermis. The entrance of the strip relies upon the centralization of substance utilized, pH of the arrangement and the season of use [33].
These are the Chemical strips lead synthetic removal of deadskin layers which results in an even and tight skin.
3.5. Skin Rejuvenation or Resurfacing
“Sub surfacing” refers to a specific, heat instigated denaturalization of dermal collagen that produces a receptive combination [34]. Laser reemerging has been demonstrated to be successful in photographing maturing through epidermal removal, collagen shrinkage, incitement of neocollagenesis, broad dermal redesigning, recovery of cell organelles and intercellular connections yet are related with dangers of extreme dependable symptoms with long treatment use,, for example, determined erythematic, hypo-or hyper-pigmentation, contamination or scarring [35].
3.6. Skin Biorejuvenation and Fillers
Skin rejuvenation expands the biosynthetic limit of fibroblasts, instigating the remaking of an ideal physiologic condition, the upgrade of cell movement, hydration, and the amalgamation of collagen, elastin and HA (hyaluronic corrosive) and they all created the desired impact which could be accomplished by the microinjections in the shallow dermis of items containing just a single dynamic fixing which is biocompatible and absolutely absorbable such as: HA, nutrients, minerals, supplements, hormones, GF (growth factor), amino acids, autologous refined fibroblasts, homeopathic items, and so on [36].
Fillers are the items that are infused inside or underneath the skin to improve its physical highlights by delicate tissue growth [37]. There are autologous (fat, refined human fibroblasts), collagen (cow-like inferred, human-got from tissue culture), HA (non-creature balanced out or viscoelastic HA from bacterial maturation), engineered or pseudo-manufactured inserts (silicone, polymethacrylate microspheres, poly-L-lactic corrosive, calcium hydroxylapatite microspheres suspended in watery polysaccharide gel, alkyl-imides gel polymer) which might be gathered into brief, semi-permanent and perpetual materials.
3.7. Autologous Platelet-Rich Plasma (PRP)
PRP is obtained from whole blood containing a high centralization of platelets alongside a number of GF, including Platelet-Determined Development Factor (PDGF), Vascular Endothelial Development Factor (VEGF), changing Development Factor (TGF), and insulin-like Development factor (IGF) that are discharged from the α-granules of concentrated platelets initiated by collection inducers which are capable to control the procedures including cell movement, connection, expansion and separation, and advance extracellular lattice (ECM) aggregation by official to explicit cell surface receptors [38]. PRP may instigate the union of collagen and other network parts by animating the actuation of fibroblasts, subsequently, restoring the skin [39].
3.8. Botulinum Poison (BTX)
It has no impact on the skin surface and cannot cease the skin maturing process in light of the fact that the normal BTX infusions can hinder the unmistakable maturing process which helps in the administration of certain unique facial lines and wrinkles [40]. The component of the activity of BTX makes it a perfect specialist to focus on the real reason for these dynamic lines [41]. They are shaped by various anaerobic strains, gram-positive bacterium Clostridium botulinum [42]. BTX-subtype A (BTX-An) is considered as the strongest endotoxin that produces transitory synthetic denervation by obstructing the presynaptic arrival of Acetylcholine (Ach) at the Neuromuscular Intersection (NMJ).
3.9. Hormone Replacement Therapy
As the maturing of skin shows up, it prompts a decline in certain significant hormones for skin just as the advancement of the whole body like, development Growth Hormone (GH), insulin-like development factor-1 (IGF-1), melatonin (night time), TSH, thyroid hormones (T3), dehydroepiandrosterone (DHEA) (sulphated structure and its urinary 17-keto-metabolites), oestrogens and testosterone [43]. Hormone Replacement Therapy (HRT), oestrogen and progesterone joined together are not hostile to maturing impacts.
There is also various marketed formulations available to control skin ageing like synthetic, natural, conventional and nano forms which are shown in (Table 2a-d).
4. NANOTECHNOLOGY USED FOR SKIN AGEING
Nanotechnology is the region of innovative work which expandsthe adequacy of the item through the conveyance of inventive arrangements. Different Nano cosmeceuticals for the counteractive action of skin, hair, nail, and for lip care are being brought into the market, for the aversion from conditions like wrinkles, photograph maturing, hyperpigmentation, dandruff, and hair harm, have come into across the board use. These Novel Nano-bearers like liposomes, Niosome, Nanoemulsions, miniaturized scale emulsion, strong lipid nanoparticles, nanostructured lipid transporter, and nanospheres have supplanted the use of traditional conveyance framework and have given a lot of preferences like upgraded skin infiltration, controlled and continued medication discharge, higher soundness, site explicit focusing on, and high capture efficiency. There are several nanoformulations enlisted in Table 3 for skin ageing treatment. There are various positive aspects of nanotechnology in cosmeceuticals like increasing efficacy, site-specific targeting, active transport of active ingredient, high entrapment efficiency, physical stability. There are also various marketed formulations available for the skin ageing in Nano form like Capture Total marketed by the Dior that Removes wrinkles and dark spots and has radiance effect with sunscreen, Lumessence Eye Cream marketed by Aubrey Organics for anti-wrinkle effect [44]. Novel and innovative delivery systems are developing new products in the cosmetic field accommodating the consumers desire and need.
Table 3.
Nanotechnology used for skin ageing.
| S. No. | Nanocarrier | Drug/ API | Study Findings | References |
|---|---|---|---|---|
| 1 | Liposomes | Ascorbic acid | It act as a co-factor enzyme in the synthesis of collagen 1 and 2 type and prevents from ageing. | [45] |
| 2 | Niosome | Coenzyme Q10 | It is cellular bioenergetics also acts as an antioxidant and protects the body against ageing. | [46] |
| 3 | Solid Lipid Nanoparticles | Resveratrol | It Protect photo degradation, enhance anti-lipoperioxidative activity, appearing as a promising delivery system. | [47] |
| 4 | Silica Nanoparticles | Quercetin | They enhance the in vivo penetration of quercetin into the human stratum corneum with limiting the potential toxicological risks. | [48] |
| 5 | Ultra-small lipid nanoparticles (usNLC) | Q10 | It is the strongest reduction of the free radical formation which is non-toxic and prevent ageing. | [49] |
CONCLUSION AND FUTURE PROSPECTS OF SKIN AGEING
Maturing is one of the known natural phenomena which executes a significant zone for the advancement of a new item. This zone is quickly developing in the cosmeceuticals industry.
Ageing is the fourth most predominant healthy skin division, wherein Europe it is at the forefront. As the anti-ageing awareness isincreasing islikewise the anti ageing products are also expanding, such as liposomes, nano-emulsions, metal oxide nanoparticles, fullerenes, nano topes, nano gems etc. Nanotechnology has demonstrated to be a significant device in dermatology and cosmetology field. They provide better fairness, upgraded and delayed impact. The nutria beauty care products are likewise conceivable. Nanotechnology supersedes the ordinary products due to their, site explicitness, better steadiness, biocompatibility, delayed activity, and higher medication stacking limit. Proper guidelines should be followed in the cosmeceuticals for the recent advancements in beautifying agents.
Table 2.
Marketed formulation of skin ageing.
| S. No. | Product | Company | Uses | Duration | Limitation | ||
|---|---|---|---|---|---|---|---|
| (a). Synthetic Marketed Formulation Used in Control for Skin Ageing | |||||||
| 1 | Botox Cosmetic Vistabel, Vistabex | ALLERGAN INC. (USA) | Botulinum toxin acts at the level of the neuromuscular plate and of other cholinergic synapses. | 12 months | Skin sensitivity reactions may occur in some patients. | ||
| 2 | Skinovage | BABOR | Improves the elasticity of the skin, improves the skin pigmentation. | 30 days | Contraindication with steroidal creams. Hypersensitivity reactions. | ||
| 3 | Chemical Peels | Le- Bonheru | Increase in collagen fibre content water in the dermis has been reported improvement in skin elasticity. | 5-6 months | Dryness and scaling of the skin. | ||
| 4 | Sebamed Anti-ageing Q10 Cream | SEBAMED | Anti-oxidant, reduces wrinkles. | 6 months | Scaly skin and minor skin irritation. | ||
| 5 | Regeneration Micro sculpting | OLAY | Reduces lines and wrinkles and smoothen skin surface. | 12 months | Increased risk of disease concerning estrogen and another endocrine due to parabens. | ||
| (b). Natural Marketed Formulation Used in Control of Skin Ageing | |||||||
| 6 | Bella.vei | BELLAVEI | Increases the depletion of procollagen-1 and fibrillin rich microfibrils in aged skin. | 1 Month 8 days |
No visible side-effects. But may show allergic reactions in a few patients. | ||
| 7 | Zyderm, ZyplastCosmoderm | ZYDUS (CADILA) | It improves tissues hydration and their resistance to mechanical damage. | 12 weeks | Skin swelling, redness and itching. | ||
| 8 | BAILEY’S | BAILEYS | Reduces the number of Langerhans cells and DNA damage to skin. | 2-3 months | No observedside effects. | ||
| 9 | Nutra-Life Kyolic® |
NUTRA | Inhibits the degradation of type ? procollagenandthe expressions of MMIPs. | 1 month | Cause skin redness in some patients. | ||
| 10 | Youthrx | Lotus | Firms and tightens skin overnight to leave soft radiant young skin every morning. | 5 months | Appearance of black and brown spots, burning sensation and redness of the skin | ||
| S. No. | Product | Company | Uses | Duration | Limitation | ||
| (c). Conventional Marketed Formulation Used in Control for Skin Ageing | |||||||
| 11 | Brilliance facial oil | OILIXIA | Helps to moisturize, improve the appearance and revitalize skin. | 6 months | Skin rashes, irritation and burning sensation. | ||
| 12 | Green tea face serum | BIELENDA | Antibacterial agent for treating signs of ageing. | 12 months | Headache, nervousness, sleep problems, vomiting, diarrhoea, irritability, irregular heartbeat. | ||
| 13 | Boswellia extract | AMANDEAN | Improve skin elasticity and visibly reduce wrinkle depth, for firmer skin. | 3 months | Nausea. Stomach pain. Rash (when applied to the skin). |
||
| 14 | Aloe Vera gel | HIMALAYA | Acts an antioxidant. Ultimately, this helps to slow the ageing appearance of the skin. | 6 months | Causing skin allergies, redness in the eyes, skin rashes, irritation and burning sensation. | ||
| (d). Nano Marketed Formulation Used in Control of Skin Ageing | |||||||
| 15 | Cutanova Cream Nano Repair Q 10 |
Dr. Rimpler | Anti-wrinkle face cream which is fragrance-free and comedogenic anti-wrinkle cream for the sensitive skin type, reduce the look of fine lines. | 3 months | Skin rash, low blood pressure. | ||
| 16 | Intensive SerumNano Repair Q 10 |
Dr. Rimpler | Highly concentrated creamy serum with reconstructing and protective characteristics to -anti-ageing care. | 12 months | Upset stomach, nausea, vomiting, loss of appetite. | ||
| 17 | Cutanova CreamNano Vital Q 10 |
Dr Rimpler | It is a gentle anti-ageing product for immediately tangible toning of facial contours. | 6 months | Decreased appetite, Diarrohea. Increased levels of liver enzymes in the blood, Nausea. |
||
| 18 | SUMMER Crème Legère Nano-Protection |
Isabelle Lancray | Used as day or night cream which intensely hydrating Care cream. |
6 months | Itching, Redness |
||
| 19 | SURMER Crème Riche Nano-Restructurante |
Isabelle Lancray | Moisturizing cream containing a cocktail of exotic plant extracts. | 3 months | Sweating Irritability |
||
Acknowledgements
The authors are also thankful to Delhi Pharmaceutical Sciences and Research University, Delhi, India for providing all the resources for this project.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Miguel J.D., Carlos H.M., David M.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014;44(4):1055–1068. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez A.M., Nöbauer K., Soler L., et al. Detection of potential markers for systemic disease in saliva of pigs by proteomics: A pilot study. Vet. Immunol. Immunopathol. 2013;151(1-2):73–82. doi: 10.1016/j.vetimm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Bolognia J.L., Braverman I.M., Rousseau M.E., Sarrel P.M. Skin changes in menopause. Maturitas. 1989;11(4):295–304. doi: 10.1016/0378-5122(89)90026-1. [DOI] [PubMed] [Google Scholar]
- 4.Castelo-Branco C., Duran M., González-Merlo J. Skin collagen changes related to age and hormone replacement therapy. Maturitas. 1992;15(2):113–119. doi: 10.1016/0378-5122(92)90245-Y. [DOI] [PubMed] [Google Scholar]
- 5.Curtis R., Geesaman B.J., DiStefano P.S. Ageing and metabolism: Drug discovery opportunities. Nat. Rev. Drug Discov. 2005;4(7):569–580. doi: 10.1038/nrd1777. [DOI] [PubMed] [Google Scholar]
- 6.Makrantonaki E., Zouboulis C.C., William J. Characteristics and pathomechanisms of endogenously aged skin. Dermatology. 2007;214(4):352–360. doi: 10.1159/000100890. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein E.F., Chen Y.Q., Kopp J.B., et al. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J. Am. Acad. Dermatol. 1996;34(2 Pt 1):209–218. doi: 10.1016/S0190-9622(96)80114-9. [DOI] [PubMed] [Google Scholar]
- 8.Varani J., Spearman D., Perone P., et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am. J. Pathol. 2001;158(3):931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landau M. Exogenous factors in skin aging. Curr. Probl. Dermatol. 2007;35:1–13. doi: 10.1159/000106405. [DOI] [PubMed] [Google Scholar]
- 10.Leyden J.J. Clinical features of ageing skin. Br. J. Dermatol. 1990;122(Suppl. 35):1–3. doi: 10.1111/j.1365-2133.1990.tb16118.x. [DOI] [PubMed] [Google Scholar]
- 11.Seité S., Medaisko C., Christiaens F., et al. Biological effects of simulated ultraviolet daylight: A new approach to investigate daily photoprotection. Photodermatol. Photoimmunol. Photomed. 2006;22(2):67–77. doi: 10.1111/j.1600-0781.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Piérard-Franchimont C., Letawe C., Goffin V., Piérard G.E. Skin water-holding capacity and transdermal estrogen therapy for menopause: A pilot study. Maturitas. 1995;22(2):151–154. doi: 10.1016/0378-5122(95)00924-A. [DOI] [PubMed] [Google Scholar]
- 13.Phillips T.J., Demircay Z., Sahu M. Harmonal effects on skin ageing. Europe PMC. 2001;17(4):661–672. doi: 10.1016/s0749-0690(05)70092-6. [DOI] [PubMed] [Google Scholar]
- 14.Lovell C.R., Smolenski K.A., Duance V.C., Light N.D., Young S., Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987;117(4):419–428. doi: 10.1111/j.1365-2133.1987.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 15.Morita A. Tobacco smoke causes premature skin aging. J. Dermatol. Sci. 2007;48(3):169–175. doi: 10.1016/j.jdermsci.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Duan E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrel A., Ebeling A.H. Age and multiplication of fibroblasts. J. Exp. Med. 1921;34(6):599–623. doi: 10.1084/jem.34.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002;123(7):801–810. doi: 10.1016/S0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 19.Renault V., Throne L.E., Eriksson P.O. Gillian butler- Browne. Vincemt Mouly. 2002;1(4):188. [Google Scholar]
- 20.Shay J.W., Wright W.E. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000;1(1):72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 21.Ly D.H., Lockhart D.J., Lerner R.A., Schultz P.G. Mitotic misregulation and human aging. Science. 2000;287(5462):2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 22.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 23.Harman D. Free radical theory of aging mutation. Mutat. Res. Rev. Mutat. Res. 2003;5:5. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- 24.Commoner B., Townsend J., Pake G.E. Free radicals in biological materials. Nature. 1954;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 25.Andrew P.W. Ageing and the free radical theory. Respir. Physiol. 2001;128(3):379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 26.Golias C.H., Charalabopoulos A., Charalabopoulos K. Cell proliferation and cell cycle control: A mini review. Int. J. Clin. Pract. 2004;58(12):1134–1141. doi: 10.1111/j.1742-1241.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 27.El-Deiry W.S., Tokino T., Velculescu V.E., et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 28.Buckingham E.M., Klingelhutz A.J. The role of telomeres in the ageing of human skin. Exp. Dermatol. 2011;20(4):297–302. doi: 10.1111/j.1600-0625.2010.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyzis R.K., Buckingham J.M., Cram L.S., et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA. 1988;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganceviciene R., Liakou A., Theodoridis A., Makrantonaki E., Zouboulis C.C. Skin anti-ageing stratergies. Dermatoendocrinol. 2012;4(3):308–319. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupo M.P., Cole A.L. Cosmeceutical peptides. Dermatol. Ther. 2007;20(5):343–349. doi: 10.1111/j.1529-8019.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 32.Nelson B.R., Majmudar G., Griffiths C.E., et al. Clinical improvement following dermabrasion of photoaged skin correlates with synthesis of collagen I. Arch. Dermatol. 1994;130(9):1136–1142. doi: 10.1001/archderm.1994.01690090060008. [DOI] [PubMed] [Google Scholar]
- 33.Collins P.S. The chemical peel. Clin. Dermatol. 1987;5(4):57–74. doi: 10.1016/0738-081X(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 34.Fischer T.C., Perosino E., Poli F., Viera M.S., Dreno B. Cosmetic dermatology European expert group chemical peels in aesthetic dermatology: An update. J. Eur. Acad. Dermatol. Venereol. 2010;24(3):281–292. doi: 10.1111/j.1468-3083.2009.03409.x. [DOI] [PubMed] [Google Scholar]
- 35.Sadick N.S. Update on non-ablative light therapy for rejuvenation: A review. Lasers Surg. Med. 2003;32(2):120–128. doi: 10.1002/lsm.10127. [DOI] [PubMed] [Google Scholar]
- 36.Alster T.S., Garg S. Treatment of facial rhytides with a high-energy pulsed carbon dioxide laser. Plast. Reconstr. Surg. 1996;98(5):791–794. doi: 10.1097/00006534-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Herne K.B., Zachary C.B. New facial rejuvenation techniques. Semin. Cutan. Med. Surg. 2000;19(4):221–231. doi: 10.1053/sder.2000.18362. [DOI] [PubMed] [Google Scholar]
- 38.Biesman B.S. Fractional ablative skin resurfacing: Complications. Lasers Surg. Med. 2009;41(3):177–178. doi: 10.1002/lsm.20764. [DOI] [PubMed] [Google Scholar]
- 39.Freymiller E.G. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004;62(4):1046–1047. doi: 10.1016/j.joms.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 40.Marx R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Krasna M., Domanović D., Tomsic A., Svajger U., Jeras M. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta Dermatovenerol. Alp. Panonica Adriat. 2007;16(3):105–110. [PubMed] [Google Scholar]
- 42.Dessy L.A., Mazzocchi M., Rubino C., Mazzarello V., Spissu N., Scuderi N. An objective assessment of botulinum toxin effect on superficial skin texture. Ann. Plast. Surg. 2007;58(5):469–473. doi: 10.1097/01.sap.0000244968.16977.1f. [DOI] [PubMed] [Google Scholar]
- 43.Böni R., Kreyden O.P., Burg G. Revival of the use of botulinum toxin: Application in dermatology. Dermatology. 2000;200(4):287–291. doi: 10.1159/000018389. [DOI] [PubMed] [Google Scholar]
- 44.Coffield J.A., Considine R.B., Simpson L.L. The site and mechanism of action of botulinum neurotoxin. Therapy with botulinum toxin. New York: Dekker; 1994. pp. 3–14. [Google Scholar]
- 45.Kishimoto Y., Saito N., Kurita K., Shimokado K., Maruyama N., Ishigami A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013;430(2):579–584. doi: 10.1016/j.bbrc.2012.11.110. [DOI] [PubMed] [Google Scholar]
- 46.Yadav N.K., Nanda S., Sharma G., Katare O.P. Systematically optimized coenzyme q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: Characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J. Drug Target. 2016;24(3):257–271. doi: 10.3109/1061186X.2015.1077845. [DOI] [PubMed] [Google Scholar]
- 47.Sapino S., Ugazio E., Gallarate M., Morel S. Resveratrol in solid lipid nanoparticles. J. Dispers. Sci. Technol. 2012;33(4):465–471. doi: 10.1080/01932691.2010.548274. [DOI] [Google Scholar]
- 48.Scalia S., Franceschinis E., Bertelli D., Iannuccelli V. Comparative evaluation of the effect of permeation enhances, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Skin Pharmacol. Physiol. 2013;25(2):57–67. doi: 10.1159/000345210. [DOI] [PubMed] [Google Scholar]
- 49.Lohan S.B., Bauersachs S., Ahlberg S., et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur. J. Pharm. Biopharm. 2015;89(1):201–207. doi: 10.1016/j.ejpb.2014.12.008. [DOI] [PubMed] [Google Scholar]