Abstract
Schizophrenia and bipolar disorder overlap considerably in terms of symptoms, familial patterns, risk genes, outcome, and treatment response. This article provides an overview of the specificity and continuity of schizophrenia and mood disorders on the basis of biomarkers, such as genes, molecules, cells, circuits, physiology and clinical phenomenology. Overall, the discussions herein provided support for the view that schizophrenia, schizoaffective disorder and bipolar disorder are in the continuum of severity of impairment, with bipolar disorder closer to normality and schizophrenia at the most severe end. This approach is based on the concept that examining biomarkers in several modalities across these diseases from the dimensional perspective would be meaningful. These considerations are expected to help develop new treatments for unmet needs, such as cognitive dysfunction, in psychiatric conditions.
Keywords: Schizophrenia, bipolar disorder, psychosis, differential diagnosis, RDoC, spectrum, cognitive dysfunction
1. Introduction
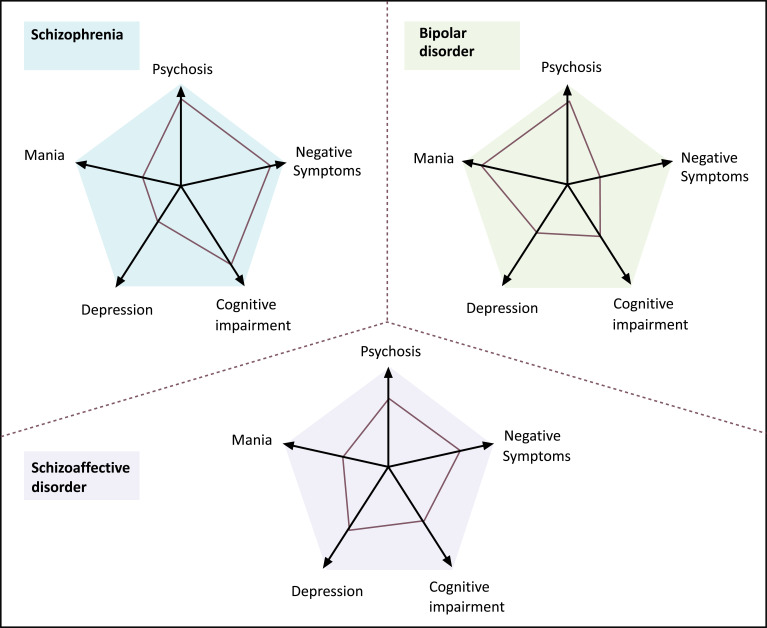
Schizophrenia and bipolar disorder, operationally defined by clinical features, overlap considerably in terms of symptoms, familial patterns, risk genes, outcome and treatment response [1]. Although Kraepelin differentiated between schizophrenia and bipolar disorder as two forms of psychoses on the basis of the clinical course, he also pointed out that both disorders shared certain symptoms, such as hallucinations, delusions, and mood symptoms [2]. To interpret the overlap of schizophrenia and bipolar disorder, Kasanin [3] introduced a concept of schizoaffective disorder that elicits clinical features of both diseases. Nevertheless, it was sometimes difficult to clearly distinguish between schizophrenia, schizoaffective disorder and bipolar disorder solely by phenomenological features (Fig. 1) [4]. Accordingly, there has been a growing need for a valid diagnostic system based on biological indicators.
Fig. (1).
Typical profile of each disease diagnosed with a combination of psychopathological categories and phenomenological features. Categorical diagnoses of schizophrenia (blue), bipolar disorder (green), and schizoaffective disorder (violet) are accompanied by a patient's quantitative scores (connected by red lines) on five main dimensions of psychopathology [4]. Copyright (2009), with permission from Elsevier. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Operational diagnostic criteria, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the International Statistical Classification of Diseases and Related Health Problems (ICD), have been developed to improve the reliability of symptom-based diagnosis. However, these diagnostic criteria may not be valid enough because they do not incorporate neuroscience and genetics information, or resolve the issues of coexistence and heterogeneity. Thus, the National Institute of Mental Health (NIMH) has proposed the Research Domain Criteria (RDoC) system, as a new evaluation system to study mental illnesses [5, 6]. RDoC provides a framework that excludes categorical diagnoses, and adopts dimensional evaluation based on genetic, neural and behavioral indicators. The system consists of six research domains and eight analysis units (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml, 21/2/2019). The research domains include Negative Valence Systems, Positive Valence Systems, Cognitive Systems, Systems for Social Processes, Arousal and Regulatory Systems, and Sensorimotor Systems. For each research domain, genes, molecules, cells, circuits, physiology, behavior, self-reports, and paradigm are provided as analysis units (Fig. 2) [7].
Fig. (2).
Schematic diagram of the RDoC framework.
This review article is intended to provide an up-to-date insight into the specificity and continuity of schizophrenia and bipolar disorder based on the concept of RDoC.
2. Specificity and continuity in genes
Genetic research of schizophrenia and bipolar disorder includes quantitative genetics, such as family, twin and adoption studies, while molecular genetics concerns common risk variants and variants of rare chromosomal structures. Results of quantitative genetics show notable similarities across these disorders [8]. Conventional studies show a substantial familial aggregation with sibling relative risks of around 8-10 for schizophrenia [9-11], bipolar disorder [12, 13], and schizoaffective disorder [14]. Twin studies show concordances of around 40-45% in monozygotic and 0-10% in dizygotic twin pairs for schizophrenia [15], bipolar disorder, and schizoaffective disorder [16]. In a meta-analysis [17], first-degree relatives of schizophrenia patients were shown to possess a higher risk of developing bipolar disorder compared to other relatives. These findings support the genetic link between schizophrenia and bipolar disorder.
Molecular genetic research includes large-scale genome-wide association studies (GWAS), aimed at detecting commonly occurring genetic variants which by themselves have a small effect on the risk for diseases. On the other hand, large chromosomal structural variants, particularly copy number variants (CNV), produce rare but large effects on the risk [8]. GWAS typically deals with more than a million of genetic markers residing in each chromosome, with sample sizes (cases and controls combined) of tens of thousands in recent years [18, 19].
Genetic markers include single-nucleotide polymorphisms (SNPs) that are used to determine whether one of the variants occurs more frequently than expected in affected cases compared with control subjects. An association indicates the presence of a causal genetic variant nearby or, less commonly, i.e., the genetic marker variant itself may have a causal effect [8]. In a review [20] that summarized major GWAS findings for schizophrenia, bipolar disorder, and both disorders combined, the associations indicate a small effect on risk (ORs around 1.1), consistent with a partial overlap of genetic influences from commonly occurring variants on the two disorders (Table 1). These findings show that both diseases share a similar genetic sensitivity.
Table 1.
Genome-wide association study (GWAS) findings for schizophrenia and bipolar disorder from the review by Sullivan et al. [20]. Based on studies with large samples (minimum of around 10000 cases and 10000 controls) and SNP markers showing associations at genome-wide level of statistical significance (p<5×10-8). Odds Ratio; OR. Copyright (2012), with permission from Springer Nature.
| Phenotype | Chromosome where Marker is Located | Nearest Gene | OR |
|---|---|---|---|
| Schizophrenia | 1 | MIR137 | 1.12 |
| 2 | VRK2 | 1.09 | |
| 2 | ZNF804A | 1.10 | |
| 2 | PCGEM1 | 1.20 | |
| 6 | MHC | 1.22 | |
| 8 | MMP16 | 1.10 | |
| 8 | CSMD1 | 1.11 | |
| 8 | LSM1 | 1.19 | |
| 10 | CNNM2 | 1.10 | |
| 10 | NT5C2 | 1.15 | |
| 11 | AMBRA1 | 1.25 | |
| 11 | NRGN | 1.12 | |
| 18 | CCDC68 | 1.09 | |
| 18 | TCF4 | 1.20 | |
| Bipolar disorder | 11 | ODZ4 | 1.14 |
| 12 | CACNA1C | 1.14 | |
| 19 | NCAN | 1.17 | |
| Schizophrenia and Bipolar disorder | 2 | ZNF804A | 1.11 |
| 3 | ITIH3-ITIH4 | 1.12 | |
| 10 | ANK3 | 1.22 | |
| 12 | CACNA1C | 1.11 |
3. Issues of neurometabolites
Proton magnetic resonance spectroscopy (1H -MRS) is a non-invasive technique that detects magnetic resonance signals produced by atomic nuclei located within molecules in living tissues and measures their chemical composition, energy metabolism, neurotransmitter levels, and neuronal integrity in vivo. 1H -MRS has increasingly been applied to characterize tissue-based chemical or metabolic abnormalities in psychiatric disorders [21]. This is done by evaluating concentrations of N-acetylaspartate (NAA), creatine (Cr), choline (Cho) and related chemicals [21]. NAA is a metabolite thought to reflect neuronal integrity, and is present exclusively in the brain. Cr is a marker of phosphate metabolism, while Cho indicates the breakdown of cell membranes and cellular turnover [22]. Abnormalities of neurometabolites in various regions of the brain have been implicated in the pathophysiology of schizophrenia and bipolar disorder. For example, both mental disorders show decreased.
NAA levels in the hippocampus and frontal lobes (gray and white matter) [23, 24]. A decrease in NAA concentrations is thought to reflect neuronal or axonal loss, or mitochondrial dysfunction [25], indicating structural abnormalities on a molecular level in schizophrenia and bipolar disorder. Both disorders also show decreased Cr levels in the dorsolateral prefrontal cortex (DLPFC), hippocampus and basal ganglia [26-29], suggesting alterations in the cellular energy metabolism. Conflicting results have been reported for Cho levels in the basal ganglia, hippocampus and DLPFC of schizophrenia patients [21]. In bipolar disorder, increased, decreased, or unaltered Cho levels have been reported in the DLPFC, hippocampus and anterior cingulate cortex [21]. Results of meta-analysis indicate schizophrenia and bipolar disorder share a decline of NAA concentrations and steady-state transition of Cho and Cr.
NAA levels in the thalamus and frontal lobes of schizophrenia patients are significantly decreased, while it is so in the basal ganglia of patients with bipolar disorder [21]. These observations suggest that the changes of several metabolites in the brain may also represent the notion of specificity and continuity pertinent to some psychiatric illnesses (Table 2).
Table 2.
Changes in concentrations of neurometabolites in schizophrenia and bipolar disorder [21]. N-acetylaspartate; NAA, Creatine; Cr, Choline; Cho, and dorsolateral prefrontal cortex; DLPFC.
| Phenotype | Neurometabolites | Concentration Change | Region |
|---|---|---|---|
| Schizophrenia | NAA | ↓↓ | Thalamus, Frontal lobe |
| Bipolar disorder | NAA | ↓↓ | Basal ganglia |
| Schizophrenia and Bipolar disorder | NAA | ↓ | Hippocampus, Frontal lobe |
| Cr | ↓~±0 | DLPFC, Basal ganglia | |
| Cho | ±0 | DLPFC, Hippocampus |
4. Key proteins in postmortem brain regions
Proteins are major targets for many types of medicine to treat psychiatric disorders [30]. In particular, schizophrenia and bipolar disorder have been associated with aberrant blood cytokine levels. For example, Goldsmith et al. [31] performed meta-analysis of blood cytokines in acutely and chronically ill patients with these disorders, and found increased levels of cytokines (interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)), a cytokine receptor (sIL-2R), and its antagonist (IL-1RA) (Table 3). Overall, there were cross-diagnostic similarities in the direction of alterations in cytokine levels throughout the course of illness, suggesting common underlying immune dysfunctions. The association between peripheral levels of cytokines and C-reactive protein (CRP) and cognition was also reviewed [32], which indicates worse cognitive performance in schizophrenia patients with higher CRP levels. By contrast, better cognitive functioning was associated with higher concentrations of tumor necrosis factor-α (TNF-α) [32].
Table 3.
Abnormal proteins in schizophrenia and bipolar disorders. Brain-derived neurotrophic factor; BDNF, synaptosomal-associated protein-25; SNAP-25, cyclic-AMP response element binding protein; CREB, amyloid beta A4 precursor protein; APP, striatal-enriched protein tyrosine phosphatase; STEP, growth associated protein 43; GAP43, ras-related C3 botulinum toxin substrate1; RAC1, interleukin; IL, tumor necrosis factor-α; TNF-α, acute phase; AP, chronic phase; CP.
| Region | Marker | Schizophrenia | Bipolar Disorder |
|---|---|---|---|
| Hippocampus | Reelin | ↓↓ | ↓ |
| BDNF | ↓↓ | ↓↓ | |
| Complexin1 | ↓↓ | ↓↓ | |
| Complexin2 | ↓ | ↓ | |
| SNAP-25 | - | ↓ | |
| Parvalbumin | ↓↓ | ↓ | |
| Glucocorticoid receptor | ↓ | ↓ | |
| Dopamine 5 receptor | - | ↑ | |
| Serotonin 2A receptor | ↓↓ | ↓↓ | |
| Prefrontal cortex | Reelin | ↓↓ | ↓ |
| Kainate receptor KA2 subunit | - | ↓ | |
| Glucocorticoid receptor | ↓ | - | |
| Dopamine D2 receptor | ↓ | - | |
| CREB | - | ↓ | |
| Homer1 | ↓ | ↓ | |
| APP | ↓ | ↓ | |
| STEP | ↓ | - | |
| Anterior cingulate cortex | Synaptophysin | - | ↓↓ |
| Neuromodulin (GAP43) | - | ↓↓ | |
| Complexin2 | - | ↓ | |
| Calbindin | ↓ | ↓ | |
| CREB | ↓ | ↓ | |
| Lateral cerebellum | RAC1 | - | ↑ |
| Blood | IL-1β | ↑ (AP)↑ (CP) | ↑ (CP) |
| IL-1RA | ↑ (AP) | ↑ (AP) ↓ (CP) | |
| sIL-2R | ↑ (AP) ↑ (CP) | ↑ (AP) ↑ (CP) | |
| IL-6 | ↑ (AP) ↑ (CP) | ↑ (AP) ↑ (CP) | |
| TNF-α | ↑ (AP) ↑ (CP) | ↑ (AP) |
Aberrant regulation of synaptic function is thought to play a role in the etiology of schizophrenia and bipolar disorder. Specifically, normal neurotransmitter release is dependent on a complex group of presynaptic proteins that regulate synaptic vesicle docking, membrane fusion and fission, such as synaptophysin, syntaxin, synaptosomal-associated protein-25 (SNAP-25), vesicle-associated membrane protein (VAMP), α-synuclein and dynamin I. In addition, structural and signaling proteins, such as neural cell adhesion molecule (NCAM), maintain the integrity of the synapse [33]. Postmortem brain studies suggest impaired neuroplasticity. Therefore, much attention has been paid to the imbalance of intracellular signaling systems. For example, Ren et al. [34] reported that cyclic-AMP (cAMP) response element binding protein (CREB), its mRNA expression levels, and CRE-DNA binding activity were decreased in the nuclear fraction of the dorsolateral prefrontal cortex (DLPFC) and cingulate gyrus (CG) in postmortem specimens from subjects with bipolar disorder. On the other hand, these intracellular indicators were decreased in the CG, but not DLPFC of subjects with schizophrenia. These results indicate region-specific abnormalities of expression and function of CREB in both disorders.
Fragile X mental retardation protein (FMRP) is an RNA binding protein with 842 target mRNAs in the mammalian brain. Silencing of the fragile X mental retardation 1 (FMR1) gene leads to the loss of expression of FMRP and upregulated metabotropic glutamate receptor 5 (mGluR5) signaling, resulting in multiple physical and cognitive deficits associated with fragile X syndrome (FXS). Reduced FMRP expression has been reported in subjects with schizophrenia and bipolar disorder who do not carry the mutation for FMR1. Specifically, Folsom et al. [35] investigated the expression of four downstream targets of FMRP-mGluR5 signaling, i.e. homer1, amyloid beta A4 precursor protein (APP), ras-related C3 botulinum toxin substrate1 (RAC1), and striatal-enriched protein tyrosine phosphatase (STEP), in the brains of subjects with either disorder. In the frontal cortex, expressions of APP and homer1 were reduced in both disorders, whereas expressions of STEP were reduced only in subjects with schizophrenia. By contrast, expressions of RAC1 in the lateral cerebellum were, increased only in subjects with bipolar disorder. Overall, proteins involved in the FMRP-mGluR5 signaling pathway are altered in both disorders, consistent with the specificity/continuity concept (Table 3) [35-38].
5. Specificity and continuity in brain morphology
Schizophrenia and bipolar disorder exhibit considerable overlaps in terms of morphological brain changes including ventricular enlargement and global reduction in the brain volume [39]. For example, whole-brain voxel-based morphometry (VBM) studies report the reduction of grey matter volumes, mainly in bilateral insula and anterior cingulate cortex, in both mental disorders [40]. On the other hand, generalized grey matter deficits are greater in schizophrenia compared with bipolar disorder [41]. Indeed, schizophrenia patients show smaller grey matter volumes than bipolar disorder patients in fronto-temporal cortex, thalamus, hippocampus and amygdala [41], suggesting that morphological changes are subtler in bipolar disorder. In addition, atrophy of the hippocampus, amygdala and thalamus, brain areas associated with cognitive function [43], is more prominent in schizophrenia as compared with bipolar disorder [41, 42]. These characteristics are consistent with abnormal expressions of proteins and neurometabolites in both diseases (Tables 2 and 3). Especially, morphological changes in the hippocampus and prefrontal cortex are conspicuous in conjunction with the decrease in NAA concentrations, which is thought to reflect neuronal or axonal loss, or mitochondrial dysfunction in these regions [25]. Moreover, the hippocampus and prefrontal cortex constitute key neural circuits responsible for cognitive impairment [54], a clinical manifestation representing specificity and continuity for schizophrenia and mood disorders.
Advances in neuroimaging also support the hypothesis that schizophrenia and bipolar disorder have common changes in a series of functional connectivity. For example, there are some lines of evidence for white matter alterations shared by the two disorders, in contrast to the case for grey matter deficits [44, 45]. Abnormalities of white matter microstructures, identified from diffusion tensor imaging, have been collated in meta-analyses, supporting altered white matter connectivity as one of the shared features [45].
The dysconnectivity hypothesis [46] suggests that both illnesses arise not from the regionally specific focal pathophysiology in the brain, but rather from impaired integration between neuroanatomical regions. For example, results of meta-analyses indicate pervasive reductions in organization among all major brain regions of white matter of patients with schizophrenia, while patients with bipolar disorder elicit decreased organization, specifically in the left limbic and right temporal-parietal white matter [47-49].
In view of the connectivity in the brain, both psychiatric disorders share white matter alterations incorporating prefrontal, cortico-thalamic, and callosal fibers this potentially contributes to aberrant executive and cognitive function, a common feature across the psychosis spectrum albeit to a lesser degree in bipolar disorder (Table 4) [50-53].
Table 4.
Brain morphology changes in grey matter volume and white matter (WM) connectivity between schizophrenia (SZ) and bipolar disorder (BP).
| Phenotype | Morphology | Volume/Connectivity Change | Region |
|---|---|---|---|
| Schizophrenia | Grey matter | ↓↓ | Insula |
| Grey matter | ↓↓ | Anterior cingulate cortex | |
| Schizophrenia and Bipolar disorder | Grey matter | ↓↓ (SZ), ↓ (BP) | Fronto-temporal cortex |
| Grey matter | ↓↓ (SZ), ↓ (BP) | Thalamus | |
| Grey matter | ↓↓ (SZ), ↓ (BP) | Hippocampus | |
| Grey matter | ↓↓ (SZ), ↓ (BP) | Amygdala | |
| Schizophrenia | White matter | ↓↓ | Fronto-temporal WM |
| Bipolar disorder | White matter | ↓ | Left limbic WM |
| White matter | ↓ | Right temporal-parietal WM | |
| Schizophrenia and Bipolar disorder | White matter | ↓↓ (SZ), ↓ (BP) | Prefrontal WM |
| White matter | ↓↓ (SZ), ↓ (BP) | Cortico-thalamic WM | |
| White matter | ↓↓ (SZ), ↓ (BP) | Callosal fiber |
In schizophrenia, the hippocampus is supposed to be hyperactive, leading to overdrive in the responsivity of midbrain dopamine (DA) neurons that project to the associative striatum, which is proposed to underlie positive symptoms [54]. Additionally, hyperactivity of the hippocampus may interfere with the function of other circuits. Thus, the hippocampal projection to the prefrontal cortex (PFC) may lead to disruption of activity and rhythmicity of the PFC, leading to cognitive impairment [54]. Moreover, the hippocampal projection to basolateral amygdala (BLA) may interfere with the BLA-limbic cortical control of emotional responses, possibly leading to negative symptoms [54]. In this way, altered hippocampal function potentially disrupts multiple interconnected circuits, and causes major symptoms of schizophrenia and bipolar disorders (Figs. 1 and 3) [54].
Fig. (3).
Circuitry of dopamine system regulation and its disruption in schizophrenia. Hyperactive, dysrhythmic limbic hippocampus potentially disrupts multiple interconnected circuits, and could contribute to all 3 symptom classes of schizophrenia [54]. Hipp: Hippocampus, PFC: Prefrontal cortex, BLA: Basolateral amygdala, VP: Ventral pallidum, DA: Dopamine. Copyright (2019), with permission from Oxford University Press.
6. Specificity and continuity in brain connectivity
The medial prefrontal cortex (MPFC) plays a crucial role in the psychophysiology of schizophrenia and bipolar disorders [55]. The ventral and orbital parts of the MPFC are extensively and reciprocally connected to the limbic circuit and surrounding prefrontal cortical regions [56]. Abnormalities of these neural systems may be responsible for the emotional dysregulation of bipolar disorder [57]. For example, patients with bipolar disorders exhibit increased functional connectivity between MPFC and the amygdala compared with healthy controls [58]. MPFC is also associated with internal, self-referential processing [59], and has been suggested to underlie the impairments in reality monitoring of schizophrenia [60].
The MPFC is a major hub of the default mode network, which is typically more active during the resting state than during the performance on tasks that demand external attention, and thought to mediate internal mental activity [61]. Chai et al. [55] examined functional connectivity between MPFC and other brain regions in schizophrenia and bipolar disorder using resting-state functional magnetic resonance imaging (fMRI). The schizophrenia group did not exhibit any resting-state correlations between the MPFC and the ventral lateral prefrontal cortex (VLPFC) or insula. In contrast, the bipolar disorder group exhibited positive correlations between the MPFC vs. insula and VLPFC. Under the same conditions, the control group exhibited negative correlations between these regions. Moreover, the decoupling of dorsal lateral prefrontal cortex (DLPFC) with MPFC in both disorders was observed, consistent with the impaired executive functioning. In sum, functional connectivity between MPFC and insula/VLPFC may distinguish between bipolar disorder and schizophrenia, whereas both diseases share the decoupling DLPFC from MPFC, which may provide a common pathogenesis.
Recent fMRI studies have shown altered brain dynamic functional connectivity (DFC) in mental disorders. Thus, Du et al. [62] examined DFC across a spectrum of symptomatically-related disorders, including schizophrenia, schizoaffective disorder and bipolar disorder with psychosis. They conducted group information guided independent component analysis to estimate both group-level and subject-specific connectivity states from DFC, using fMRI data from patients and healthy control subjects. Regarding the dominant state, widespread group differences were found in 166 functional connectivity, which mainly involved the thalamus and cerebellum, as well as frontal, temporal, occipital, fusiform, postcentral, cuneus, supramarginal and calcarine cortices. Specifically, 22 functional connectivity associated with the postcentral, frontal, and cerebellar cortices were weakened across health control, bipolar disorder with psychosis, schizoaffective disorder, and schizophrenia groups, while 34 functional connectivity associated with the insular, temporal, frontal, fusiform, lingual, occipital, supramarginal cortices, as well as thalamus and cerebellum, were strengthened across those groups (Fig. 4). The degree of these abnormalities, i.e., hypo-connectivity and hyper-connectivity, was in the ascending order of bipolar disorder with psychosis, schizoaffective disorder, and schizophrenia relative to healthy controls. These results are consistent with those in previous studies that observed more severe gray matter deficits [63] and functional impairments [64] in these disorders. These findings support the view that schizophrenia, schizoaffective disorder and bipolar disorder with psychosis are in a continuum of severity, with bipolar disorder with psychosis closer to normality and schizophrenia at the most severe end.
Fig. (4).
The mean static functional connectivity matrix across subjects and its visualized pattern for health control (HC), bipolar disorder with psychosis (BPP), schizoaffective disorder (SAD) and schizophrenia (SZ) group, respectively [62]. The red and blue lines represent positive and negative connectivity strengths, respectively. Copyright (2017), with permission from John Wiley and Sons. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
7. Specificity and continuity in neurocognition
Neurocognitive impairment has long been recognized as a core feature of schizophrenia [65]. In contrast, the importance of cognitive problems in bipolar disorder has been recognized more recently [68]. Individuals with bipolar disorder also demonstrate persistent and trait-like cognitive deficits during remission, while there are some effects of mood state on cognition with acute manic or depressed patients demonstrating profound cognitive deficits [66]. The impairment is most notable in attention, verbal learning and executive function [67], with performance falling 0.5-1 standard deviation (SD) below average. Moreover, these cognitive deficits significantly contribute to functional disability in both schizophrenia and bipolar disorder [65, 68].
Schizophrenia shows cognitive heterogeneity [69], and generally has four subgroups; one with almost normal and one with profoundly impaired cognitive performance, and two intermediate subgroups [70]. Similarly, bipolar disorder has cognitive heterogeneity, with some subgroups whose cognitive deficits are less severe than those reported in schizophrenia [71]. Accordingly, Burdick et al. conducted a cluster analysis of data from the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) cognitive battery test in 136 bipolar disorder patients, and found three distinct subgroups, as follows [72] (Fig. 5):
Fig. (5).
Neurocognitive profiles of bipolar disorder clusters and the schizophrenia sample. The X-axis indicates the MATRICS Consensus Cognitive Battery (MCCB) domains. The Y-axis depicts a Z-scale score with a mean of 0 and a standard deviation of 1. Z scores were computed based upon the healthy control sample. Patients are divided into lines based on scoring for each cognitive domain [72]. Copyright (2014), with permission from Cambridge University Press. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Cluster 1, global impairment group, presenting diffuse and severe cognitive dysfunction, with performance falling between 1 and 2 SD below the mean of healthy controls (Global Group);
Cluster 2, selective impairment group, presenting modest deficits on specific domains, with performance ranging between normal and -1 SD below average (Selective Group);
Cluster 3, intact group, performing comparably to healthy controls on all domains, with superior performance vs. healthy controls on social cognition (Intact Group).
In this way, these subtypes of bipolar disorder were based on degree and pattern of cognitive decline, with the Global Group demonstrating cognitive deficits comparable to those of schizophrenia [72]. This supports the concept of continuity between bipolar disorder and schizophrenia on the basis of behavioral paradigms.
Conclusion
Information from multiple modalities of measures, herein reviewed, support the notion that schizophrenia and bipolar disorder share several biological substrates responsible for the clinical manifestations. These considerations suggest the utility of dimensional perspectives to develop new therapeutics for unmet needs, such as cognitive dysfunction, which may compensate the limitations of categorical diagnostic classifications for psychiatric disorders.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was partly supported by Japan Society for the Promotion of Science (JSPS) KAKENHI No. 17K10321, Intramural Research Grant (29-1, 30-1, 30-8) and Young Investigator Encouragement Grant for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP), and AMED under Grant Numbers 18dk0307069 and 18dk0307081, Japan.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Pearlson G.D., Clementz B.A., Sweeney J.A., Keshavan M.S., Tamminga C.A. Does biology transcend the symptom-based boundaries of psychosis? Psychiatr. Clin. North Am. 2016;39(2):165–174. doi: 10.1016/j.psc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebert A., Bär K.J. Emil kraepelin: a pioneer of scientific understanding of psychiatry and psychopharmacology. Indian J. Psychiatry. 2010;52(2):191–192. doi: 10.4103/0019-5545.64591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasanin J. The acute schizoaffective psychoses. 1933. Am. J. Psychiatry. 1994;151(6) Suppl.:144–154. doi: 10.1176/ajp.151.6.144. [DOI] [PubMed] [Google Scholar]
- 4.van Os J., Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 5.Insel T.R., Cuthbert B.N. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol. Psychiatry. 2009;66(11):988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Insel T., Cuthbert B., Garvey M., et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 7.Clark L.A., Cuthbert B., Lewis-Fernández R., Narrow W.E., Reed G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the national institute of mental health’s research domain criteria (RDoC). Psychol. Sci. Public Interest. 2017;18(2):72–145. doi: 10.1177/1529100617727266. [DOI] [PubMed] [Google Scholar]
- 8.Cardno A.G., Owen M.J. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr. Bull. 2014;40(3):504–515. doi: 10.1093/schbul/sbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendler K.S., Diehl S.R. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr. Bull. 1993;19(2):261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Kendler K.S., McGuire M., Gruenberg A.M., O’Hare A., Spellman M., Walsh D. The roscommon family study. I. methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch. Gen. Psychiatry. 1993;50(7):527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein P., Yip B.H., Björk C., et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuang M.T., Winokur G., Crowe R.R. Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression and surgical conditions. Br. J. Psychiatry. 1980;137:497–504. doi: 10.1192/bjp.137.6.497. [DOI] [PubMed] [Google Scholar]
- 13.Maier W., Lichtermann D., Minges J., et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch. Gen. Psychiatry. 1993;50(11):871–883. doi: 10.1001/archpsyc.1993.01820230041004. [DOI] [PubMed] [Google Scholar]
- 14.Bertelsen A., Gottesman I.I. Schizoaffective psychoses: genetical clues to classification. Am. J. Med. Genet. 1995;60(1):7–11. doi: 10.1002/ajmg.1320600103. [DOI] [PubMed] [Google Scholar]
- 15.Cardno A.G., Gottesman I.I. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am. J. Med. Genet. 2000;97(1):12–17. doi: 10.1002/(SICI)1096-8628(200021)97:1<12:AID-AJMG3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Cardno A.G., Marshall E.J., Coid B., et al. Heritability estimates for psychotic disorders: the maudsley twin psychosis series. Arch. Gen. Psychiatry. 1999;56(2):162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 17.Van Snellenberg J.X., de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch. Gen. Psychiatry. 2009;66(7):748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- 18.Ripke S., O’Dushlaine C., Chambert K., et al. Multicenter genetic studies of schizophrenia consortium; psychosis endophenotypes international consortium; wellcome trust case control consortium 2. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013;45(10):1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psychiatric GWAS consortium bipolar disorder working group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan P.F., Daly M.J., O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraguljac N.V., Reid M., White D., et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203(2-3):111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshavan M.S., Stanley J.A., Pettegrew J.W. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings-part II. Biol. Psychiatry. 2000;48(5):369–380. doi: 10.1016/S0006-3223(00)00940-9. [DOI] [PubMed] [Google Scholar]
- 23.Steen R.G., Hamer R.M., Lieberman J.A. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 24.Yildiz-Yesiloglu A., Ankerst D.P. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(6):969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Sager T.N., Topp S., Torup L., Hanson L.G., Egestad B., Møller A. Evaluation of CA1 damage using single-voxel 1H-MRS and un-biased stereology: Can non-invasive measures of N-acetyl-asparate following global ischemia be used as a reliable measure of neuronal damage? Brain Res. 2001;892(1):166–175. doi: 10.1016/S0006-8993(00)03274-1. [DOI] [PubMed] [Google Scholar]
- 26.Deicken R.F., Pegues M.P., Anzalone S., Feiwell R., Soher B. Lower concentration of hippocampal N-acetylaspartate in familial bipolar I disorder. Am. J. Psychiatry. 2003;160(5):873–882. doi: 10.1176/appi.ajp.160.5.873. [DOI] [PubMed] [Google Scholar]
- 27.Ohrmann P., Siegmund A., Suslow T., et al. Evidence for glutamatergic neuronal dysfunction in the prefrontal cortex in chronic but not in first-episode patients with schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr. Res. 2005;73(2-3):153–157. doi: 10.1016/j.schres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Frey B.N., Stanley J.A., Nery F.G., et al. Abnormal cellular energy and phospholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disord. 2007;9(Suppl. 1):119–127. doi: 10.1111/j.1399-5618.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 29.Rüsch N., Tebartz van Elst L., Valerius G., et al. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr. Res. 2008;99(1-3):155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Sharif-Barfeh Z., Beigoli S., Marouzi S., Sharif-Rad A., Asoodeh A., Chamani J. Multi-spectroscopic and HPLC studies of the interaction between estradiol and cyclophosphamide with human serum albumin: binary and ternary systems. J. Solution Chem. 2017;46:488–504. doi: 10.1007/s10953-017-0590-2. [DOI] [Google Scholar]
- 31.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misiak B., Stańczykiewicz B., Kotowicz K., Rybakowski J.K., Samochowiec J., Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr. Res. 2018;192:16–29. doi: 10.1016/j.schres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Gray L.J., Dean B., Kronsbein H.C., Robinson P.J., Scarr E. Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res. 2010;178(2):374–380. doi: 10.1016/j.psychres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Ren X., Rizavi H.S., Khan M.A., Bhaumik R., Dwivedi Y., Pandey G.N. Alteration of cyclic-AMP response element binding protein in the postmortem brain of subjects with bipolar disorder and schizophrenia. J. Affect. Disord. 2014;152-154:326–333. doi: 10.1016/j.jad.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folsom T.D., Thuras P.D., Fatemi S.H. Protein expression of targets of the FMRP regulon is altered in brains of subjects with schizophrenia and mood disorders. Schizophr. Res. 2015;165(2-3):201–211. doi: 10.1016/j.schres.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leber S.L., Llenos I.C., Miller C.L., Dulay J.R., Haybaeck J., Weis S. Homer1a protein expression in schizophrenia, bipolar disorder, and major depression. J. Neural Transm. (Vienna) 2017;124(10):1261–1273. doi: 10.1007/s00702-017-1776-x. [DOI] [PubMed] [Google Scholar]
- 37.Torrey E.F., Barci B.M., Webster M.J., Bartko J.J., Meador-Woodruff J.H., Knable M.B. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Novikova S.I., He F., Cutrufello N.J., Lidow M.S. Identification of protein biomarkers for schizophrenia and bipolar disorder in the postmortem prefrontal cortex using SELDI-TOF-MS ProteinChip profiling combined with MALDI-TOF-PSD-MS analysis. Neurobiol. Dis. 2006;23(1):61–76. doi: 10.1016/j.nbd.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Arnone D., Cavanagh J., Gerber D., Lawrie S.M., Ebmeier K.P., McIntosh A.M. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br. J. Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 40.Ellison-Wright I., Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Maggioni E., Bellani M., Altamura A.C., Brambilla P. Neuroanatomical voxel-based profile of schizophrenia and bipolar disorder. Epidemiol. Psychiatr. Sci. 2016;25(4):312–316. doi: 10.1017/S2045796016000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nenadic I., Maitra R., Langbein K., et al. Brain structure in schizophrenia vs. psychotic bipolar I disorder: a VBM study. Schizophr. Res. 2015;165(2-3):212–219. doi: 10.1016/j.schres.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla P., Perlini C., Rajagopalan P., et al. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br. J. Psychiatry. 2013;202(1):50–55. doi: 10.1192/bjp.bp.111.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altamura A.C., Bertoldo A., Marotta G., et al. White matter metabolism differentiates schizophrenia and bipolar disorder: a preliminary PET study. Psychiatry Res. 2013;214(3):410–414. doi: 10.1016/j.pscychresns.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 45.O’Donoghue S., Holleran L., Cannon D.M., McDonald C. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: a selective review of structural network analyses using diffusion MRI. J. Affect. Disord. 2017;209:217–228. doi: 10.1016/j.jad.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150(2):192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 49.Vederine F.E., Wessa M., Leboyer M., Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(8):1820–1826. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Baker J.T., Holmes A.J., Masters G.A., et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bullmore E. Functional network endophenotypes of psychotic disorders. Biol. Psychiatry. 2012;71(10):844–845. doi: 10.1016/j.biopsych.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 52.De Peri L., Crescini A., Deste G., Fusar-Poli P., Sacchetti E., Vita A. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr. Pharm. Des. 2012;18(4):486–494. doi: 10.2174/138161212799316253. [DOI] [PubMed] [Google Scholar]
- 53.Skudlarski P., Schretlen D.J., Thaker G.K., et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am. J. Psychiatry. 2013;170(8):886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- 54.Grace A.A., Gomes F.V. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr. Bull. 2019;45(1):148–157. doi: 10.1093/schbul/sbx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chai X.J., Whitfield-Gabrieli S., Shinn A.K., et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ongür D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 57.Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(9):829–857. doi: 10.1038/mp.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Versace A., Thompson W.K., Zhou D., et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol. Psychiatry. 2010;67(5):422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert S.J., Spengler S., Simons J.S., et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- 60.Vinogradov S., Luks T.L., Schulman B.J., Simpson G.V. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb. Cortex. 2008;18(11):2532–2539. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 62.Du Y., Pearlson G.D., Lin D., et al. Identifying dynamic functional connectivity biomarkers using GIG-ICA: Application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum. Brain Mapp. 2017;38(5):2683–2708. doi: 10.1002/hbm.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivleva E.I., Bidesi A.S., Keshavan M.S., et al. Gray matter volume as an intermediate phenotype for psychosis: bipolar-schizophrenia network on intermediate phenotypes (B-SNIP). Am. J. Psychiatry. 2013;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Argyelan M., Ikuta T., DeRosse P., et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40(1):100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 66.Daban C., Martinez-Aran A., Torrent C., et al. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychother. Psychosom. 2006;75(2):72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- 67.Bora E., Yucel M., Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J. Affect. Disord. 2009;113(1-2):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Burdick K.E., Goldberg J.F., Harrow M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatr. Scand. 2010;122(6):499–506. doi: 10.1111/j.1600-0447.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reichenberg A., Harvey P.D., Bowie C.R., et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009;35(5):1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill S.K., Ragland J.D., Gur R.C., Gur R.E. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J. Clin. Exp. Neuropsychol. 2002;24(6):765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burdick K.E., Goldberg T.E., Cornblatt B.A., et al. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology. 2011;36(8):1587–1592. doi: 10.1038/npp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burdick K.E., Russo M., Frangou S., et al. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol. Med. 2014;44(14):3083–3096. doi: 10.1017/S0033291714000439. [DOI] [PMC free article] [PubMed] [Google Scholar]