Abstract
The systematic development of early age talent in sports academies has led to the professionalization of pediatric sport and the sports physician need to be aware of pediatric cardiological problems. Research into the medical cardiac care and assessment of the pediatric athlete are accumulating, but specific pediatric international guidelines are not available yet and reference data for ECG and echocardiography are incomplete, in particular for the age group <12 years of age. This article is an introduction to the physiological and diagnostics specifics of the pediatric athlete. The focus lies in the differences in presentation and diagnosis between pediatric and adult athletes for the most common pathologies. Reference data for electrical and structural adaptations to intensive exercise are sparse particularly in athletes aged below 12 years old. Training related changes include decrease of resting heart rate, increase of cardiac output, ventricular cavity size, and wall thickness. Cardiac hypertrophy is less pronounced in pediatric athletes, as HR mediated cardiac output increase to endurance exercise is the dominant mechanism in peripubertal children. As in adults, the most pronounced cardiovascular adaptations appear in classical endurance sports like rowing, triathlon, and swimming, but the specifics of pediatric ECG and echocardiographic changes need to be considered.
Keywords: adolescence, athletic adaptation, pediatric arrhythmia, pediatric athlete, pediatric cardiomyopathy, pediatric exercise, sports cardiology
1. INTRODUCTION
1.1. General activity recommendations for children
Healthy children are more active than adults and exercise and sports are a central experience of childhood and are essential for a normal physical and mental development in health and also chronic disease. Although overall cardiorespiratory fitness has been falling in teenagers for many years, this decline has stabilized since 2000 with the greatest improvement in countries with the least income inequality. 1 , 2 The importance of integration of sports and exercise into the daily lives of children is reflected in the international activity recommendations for children and adolescents, modified from References 3, 4.
At least 60 minutes of moderate to vigorous physical activity daily, >60 minutes provide additional health benefits. Most physical activity should be aerobic.
Vigorous‐intensity activities at least 3 days a week
Activities that strengthen muscle and bone
These recommendations reflect the overwhelming evidence of health benefits from regular physical exercise and are intended to counteract the alarming sedentary lifestyle in children worldwide. Data from the United Kingdom, suggests, that only 14% of 13 to 15 year old boys and 9% of girls achieve physical activity targets—a figure which has fallen from 28% in boys and 14% in girls in 2008. 5
In contrast to the sedentary majority, there is an emerging subpopulation of children and adolescents that train to a very high level. Moreover, there is robust evidence to suggest that in children and adolescents, higher amounts of physical activity are associated with multiple health benefits including cardiorespiratory and muscular fitness, bone health, and weight status or adiposity. 6 Although the term “pediatric athlete” is usually defined as 12 to 16 years old, 7 regular training can begin from the age of 6 years onward for some sports disciplines (eg, gymnastics). Specific strategies for athlete development from a young age may include “professional” level training intensity and volumes. 8 However, in addition to some positive adaptive responses, intensive training can have a detrimental effect on the developing musculoskeletal system leading to overuse injuries, overtraining syndrome and burnout. 9 , 10 Hence, training the elite pediatric athlete will need to be accompanied by evidenced based medical care for the pediatric athlete.
1.2. Epidemiology of disease and sudden cardiac death
The etiology of sudden cardiac death (SCD) in pediatric athletes is comparable to those in young adult athletes. Available data show for example that 2000 children die each year from SCD in the US, the incidence ranging from 0.6‐8/100000. 11 , 12 A recent study of 11 168 adolescent footballers, who were undergoing cardiac screening, identified disorders associated with SCD in 0.38% and an incidence of SCD in 6.8 per 100 000 athletes during follow up. 13 While available data supports the concept that cardiac screening can pick up cardiac disease in pediatric and adolescent athletes, 14 , 15 , 16 the optimal screening strategy is still debated. 17 , 18 Additionally its efficacy in preventing SCD in this population is still a matter of debate. The causes of SCD in the pediatric athlete population 19 , 20 include 44% structural heart disease such as congenital cardiac anomalies or cardiomyopathies, nonstructural heart disease, primarily channelopathies, accounting for the remaining 56%. The variable etiology, incidence and age of presentation of common causes of SCD such as cardiomyopathies and inherited arrhythmia disorders needs to be taken into account when assessing the true incidence and etiology of SCD in childhood. 21 , 22 Alarmingly, sudden cardiac arrest is the first presenting symptom in approximately 50% of cases, providing a strong argument for early detection of disease by preparticipation screening. 23 , 24
1.3. Maturation, growth, and adaption of the cardiovascular system in childhood
Knowledge of the physiological changes in the pediatric athlete caused by growth, maturation, and training are incomplete but have been increasingly investigated over the last decade. This has informed training and talent development 8 However, it is still difficult to predict adult athletic performance before puberty. 25 Many of the most talented young athletes are simply those with those most advanced pubertal development. Trainability and training effects have been investigated in pediatric athletes, 26 but physiological gender related maturation and development particular during puberty with growth, hormone dependent bone and muscle mass maturation, changes in endurance, speed, strength, balance, age and weight related heart rate, blood pressure and cardiac output, respiratory capacity and peak oxygen uptake need to be taken into account when differentiating training progress from growth and maturation or indeed pathology. Although some sports specialization is important to develop elite‐level skills, it is recommended that intense training in a single sport should be delayed until late adolescence to minimize injury, psychological stress, and burnout. 27 Exercise training effects in childhood include improvements in bone strength, kinetic skills, biological maturity, lung and endocrine function, academic performance and mental health. 28
The complex interaction between growth, training, gender and maturity is also true and must be taken into account when assessing the cardiovascular system of pediatric athletes. During puberty, significant changes occur which affect both cardiac size and function and are reflected in screening tests such as echocardiography and the ECG. Importantly, this is also the time where inherited arrhythmia disorders or cardiomyopathies can present for the first time as growth and hormonal changes unmask genetic cardiac disease. This is seen in electrical diseases such as channelopathies and in structural diseases such as the cardiomyopathies. Thus, 23% of children with a family history of Brugada syndrome and a negative ajmaline challenge in early childhood will have a positive (diagnostic) ajmaline challenge if repeated after puberty. 29 Similarly, in a large multicenter review of young people with catecholaminergic polymorphic ventricular tachycardia (CPVT), the average onset of symptoms was 10.8 years with diagnosis delayed for up to 2.5 years. 30 This is also relevant for cardiomyopathies (eg, hypertrophic cardiomyopathy [HCM]), where a clear prepubertal phenotype is more difficult to recognize than in adult life. 31 For children with a family history of HCM, 1 to 2 yearly screening is recommended from aged 10 years 32 as presentation can occur in childhood. 33 These factors make the assessment of normality of cardiac size and function challenging, and during childhood, cardiac chamber sizes should be referenced to somatic size using z scores or relating cardiac dimensions to height or body surface area. Pubertal stage should also be taken into account, although this is rarely formalized due to the impracticality of assessing pubertal stage in teenagers which requires either an assessment of secondary sexual characteristics or x‐ray imaging such as bone age. Size/gender specific pediatric centiles for both echocardiographic measurements and cardiac MRI in the nonathletic pediatric population are available. 34 , 35 , 36
Prior and La Gerche defined athlete's heart as “the complex of structural and functional electrical remodeling that accompanies athletic training”. 37 Knowledge and data on physiological changes to athletic training in childhood are well documented in adults but less is known in children and in particular the developing pediatric athlete. 1 , 38 , 39 Studies reporting training‐related cardiovascular changes in peripubertal athletes are summarized in Table 1. Adaptation in cardiac chamber size and myocardial mass have been described, 41 but are less pronounced in pediatric athletes, which may reflect the different effect of exercise on the immature heart but it may also be due to the reduced intensity and volume of exercise training participated in by younger children. Interestingly, overtraining can occur in pediatric athletes, for example described by elevation in microvolt T wave alternans as a specific sign of overtraining in elite child athletes. 42 When cardiac adaptations to training occur in the pediatric athlete, the phenotype is different to adults, 43 These are summarized in Table 2 and include a more pronounced chamber dilatation and less ventricular hypertrophy than in adults. 44 , 45
TABLE 1.
Cardiovascular remodeling in peripubertal child athletes
| Study | Number of athletes/sport | Age range | Evaluation technique | Pubertal status described | Conclusions and effect of exercise | Reference |
|---|---|---|---|---|---|---|
| Rowland | 14 competitive swimmers; matched active nontrained controls | 8.8‐13.5 y (mean 11) | ECG/Echocardiogram | Yes | Lower resting heart rates and LV volume overload in athletes | Pediatrics 1987;79(5):800‐804 |
| Telford | 85 trained child athletes (mixed) compared with skeletal age matched controls | 11‐12 y | Echocardiogram | No | No difference in ventricular dimensions or mass | J Sports Sci 1988;6:49‐57 |
| Rowland | 10 male runners, matched with active, nontrained controls | 11‐13 y | ECG/Echocardiogram/Metabolic exercise testing | yes–described as prepubertal | No clinically significant differences in ECG or LV mass and wall thickness | Int J SportsMed 1994;15;515‐519 |
| Ozer | 82 swimmers with mean 32 months swim training; 41 sedentary control group | 7‐14 y (mean 11.2) | Echocardiography | No | Athletes had increased LV dimensions, wall thickness, aortic root size and LV mass compared to controls | Jpn Heart J 1994 |
| Rowland | 7 competitive cyclists compared with control group. | 11.9 y |
Metabolic exercise testing Echocardiography |
No | Maximal stroke volume determines VO2 max. Lower resting heart rate and higher stroke volume than controls. | Med Sci Sports Exerc 2000;32(4):747‐52 |
| Obert | 29 boys and girls. 3 months aerobic training/detraining for 2 months (nonexercised control group 26) | 10‐11 y | Echocardiography | No | LV internal dimensions increased 4.6% and wall thickness decreased (10.7%) returned to normal after detraining. Heart rate slowed with training. No change in systolic function with training or detraining. | Int J Sports Med 2001;22(2):90‐96 |
| Triposkiadis | 25 elite swimmers 12‐14 h training per week compared with sedentary controls | 11.5 y |
Heart rate variability (HRV) Echocardiography |
No | Increased vagal dominance, LV and LA dimensions increased. No change in wall thickness or HRV | Eur J Clin Invest 2002;32:16‐23 |
| Nottin | 12 boy cyclists, 11 untrained controls; 10 adult cyclists and 13 sedentary adults | 11–13 y (adults 20‐26 y) | Echocardiography | Yes; Tanner stages. Post pubertal boys excluded. | Increased LV relaxation in adult and child cyclists but no LV hypertrophy in children | Med Sci Sports Med 2004;36(9);1507‐1513 |
| Ayabakan | 22 male pubertal swimmers compared with 21 age‐matched, sedentary controls. Mean 10 h training per week. | 11 y |
Echocardiography Including tissue Doppler imaging |
Yes (described as prepubertal) | No differences in tissue Doppler but increased concentric LV wall thickness in athletes compared to controls. No change in diastolic dimensions. | Cardiol in Young 2006;16:61‐66. |
| Rowland | 7 girls, 7 boys trained swimmers (5 h/week Prone swim simulation. Compared to nontrained controls | 12 y (=/− 0.5 y) |
Metabolic exercise testing Exercise Echocardiography |
No | No rise in stroke volume during exercise implying peripheral factors (increased filling) and heart rate are main determinants of cardiac output on exercise. Minor increase in LV diastolic dimension and mass in trained group. | J Sci Med Sport 2009;12:266‐272 |
| Zdravkovic | 94 highly trained male footballers | 12.85 +/− 0.84 y | Echocardiography | No | Significant increase in LV dimensions, aortic root and LA size | J Sci Sports Med 2010;13:602‐606 |
| Koch | 342 elite athletes at Sports Schools. Multiple disciplines | 10‐15 y‐old | ECG/ echocardiogram | No |
LV upper limits described Age 11: boys 10 mm, girls 9 Age 13: boys/girls 10 mm Age 15: boys 11 mm/girls 10 mm. No ECG gender differences |
Eur J Prev Cardiol 2014;21(6):774‐781 |
| Binnetoglu | 140 athletes; 6 Sports minimum 3 h per week for 2 y, sedentary controls | 10‐16 y | ECG/echocardiogram including strain imaging | No | Normal systolic and diastolic indices in athletes. 16% concentric remodeling; 28% eccentric remodeling. Strain lower in athletes. Myocardial deformation more evident in mixed sports participants. | Pediatr Cardiol 2014;35:126‐139 |
| Agrebi | Elite male national handball players; male. 3 groups of 12 | Mean age 12/16/25 y | ECG/echocardiogram | No | Chamber dilatation occurred in younger athletes but less hypertrophy compared to older athletes. | PLoS ONE 2015;10(12):e0143609. doi:10.1371/journal.pone.0143609 |
| Calo | 2261Caucasian male soccer players | Mean age 12.4 y | ECG/Echocardiogram | No | Anterior T wave inversion (>2 leads) associated with cardiac disease in 4.8%: T wave inversion (inferolateral leads) associated with disease in 60% | Heart 2015;101;193–200 |
Note: Reproduced with permission from Reference 40.
TABLE 2.
Cardiovascular adaption to exercise training in child athletes‐comparison with adults
| Cardiovascular change in child | Comparison with adult athletes | Comment |
|---|---|---|
| Resting heart rate falls | Resting heart rate remains higher than in adult | Age‐dependent. Younger athletes have higher resting heart rates |
| Dilatation of left atrium | Similar pattern | Considerable variation between children in the same exercise group and in different studies. Some studies have demonstrated concentric hypertrophy, others predominantly dilatation. If LV dilates above 60 mm in diastole consider pathology. |
| Left ventricle dilates, mild LV hypertrophy | Less chamber dilatation and more hypertrophy occurs in adults | |
| Mild concentric hypertrophy with prolonged vigorous training | Eccentric hypertrophy tends to occur in adults with athletes heart | |
|
Increased LV relaxation Improved diastolic function |
Similar pattern | Occurs in prepubertal and post pubertal children |
| Raised VO2 max in comparison to untrained | Lower VO2 max relative to body size in comparison to adult athletes | Reflects lower maximal stroke volume and maturity related increase in diastolic filling |
| Reduced vascular stiffness | Similar pattern | Acute effect known but long‐term effects not studied in children |
| No differences between the sexes | Female athletes have higher resting heart rates, smaller cardiac chambers, and less hypertrophy | Prepubertal changes present but change to adult pattern post puberty |
Note: Reproduced with permission from Reference 40.
2. DIAGNOSTIC ASSESSMENT OF THE PEDIATRIC ATHLETE
Current data are insufficient to provide evidence‐based guidance on when and how often to screen the pediatric athlete. In Italy, it has been the practice for 40 years to screen all child athletes over 13 years old every 2 years using an ECG and since 2005, the European Society of Cardiology have recommended that two yearly screening should take place using a preparticipation questionnaire, physical examination, and ECG from the start of competitive activity—“usually aged 12‐14 years”. 46 However, although the overall outcome of such programs has been to significantly reduce the incidence of athletic related death there are few data on the efficacy of this screening process in younger athletes. It is established, that while SCD is a rare event, inherited diseases can present and be diagnosed or indeed excluded by experienced pediatric cardiologists. Although the current recommendations are based on overwhelmingly adult data, 47 general guidance is provided by the Association for European Pediatric and Congenital Cardiology (AEPC) working group on Sports Cardiology, Physical Activity, and Prevention document, 48 additionally it is consensus that the current international recommendations for adult athlete ECG screening should be followed. 49 Despite age and gender specific data on cardiac adaptation it remains particularly important to have an individualized approach not only to training, 8 but also to cardiac assessment of the pediatric athlete as pace of growth and maturation of the cardiovascular system show significant interindividual differences.
2.1. Medical, Family history, and physical examination
Medical and family history should include an accurate documentation of sports and exercise training history. It is important to include any school or college ‐based sports activities which often co‐exist with the child athlete's main sport. An assessment of training volume and intensity and sporting discipline should be made. Family history is very important to help detect congenital, inherited or acquired cardiac disease and risk factors (dyslipidemia, hypertension, and diabetes). Medical history will detect symptoms such as palpitations, exercise dependent respiratory symptoms, dizziness, and syncope and recent infections. Based on physician experience, pyrexia, should result in a training break, whereas in simple upper airway only infection light endurance training can be continued 50 , 51 Nutritional assessment should be included, also as anorexia is relatively common especially in sports with a female predominance, 52 and nutritional supplements such as energy drinks can be arrhythmogenic by adrenergic overstimulation. 53 Examination will investigate for congenital lesions (specific heart murmurs or pulse, BP differences in hand and feet (eg, coarctation). Additionally, particularly relevant in paralympic athletes is the assessment for syndromes, many are associated with cardiac disease (eg, signs of connective tissue disease, Marfan syndrome, Williams syndrome, and Turner syndrome).
2.2. 12‐lead ECG
The 12‐lead ECG is the primary cardiac screening tool in athletes. 49 Moreover, even in the USA where the ECG is less commonly used as an athletic screening tool, many pediatricians will use the ECG selectively for assessment rather than relying on clinical examination and preparticipation questionnaire alone, 54 therefore detailed knowledge of the specifics of the pediatric ECG is required when assessing pediatric athletes. For healthy children, interpretation follows routine age and height dependent reference values 55 with respect to heart rate, time intervals, heart axis, negative precordial T waves, and signs of hypertrophy. The ECG during early childhood gradually moves from the right dominant infancy pattern to the typical adult appearance at the end of puberty. Normal centiles for the childhood ECG are available. 56 Prepubertal T wave inversion (TWI) in right precordial leads is common and while anterior TWI (V1‐V3) in an adult is often abnormal and may suggest pathology such as arrhythmogenic right ventricular cardiomyopathy, identical T wave inversion can be normal in a young teenager and was found in 8.4% of under 14 years old but only in 1.7% over 14 years olds with the only predictor being incomplete pubertal status. 57 Other studies in caucasian soccer players (mean 12 years, range 8‐18) found TWI present in 136 (6%), virtually always in anterior leads (>90%). Anterior TWI was associated with mild cardiac disease in 4.8% but infero‐lateral TWI was associated with LH hypertrophy or cardiomyopathy in 60%. 58 TWI in V1 to V3 is common in adolescent caucasian athletes (<16 years) but only 0.1% had TWI beyond V2 after 16 years . 59 Recent meta‐analysis data show that ECG adaptation such as voltage criteria for atrial and ventricular hypertrophy and repolarization changes occur in athletes as young as 12 years and TWI in chest leads beyond V3 (extended anterior leads to V4) is only found in a minority of healthy 14 to 16 year old athletes. 60 No specific recommendations for the ECG interpretation in pediatric athletes exist, and the current ECG interpretation guidelines for adult athletes 49 should be followed. McClean demonstrated these recommendations to have an acceptable overall accuracy in pediatric athletes compared to previous adult ECG recommendations 61 (Figure 1 ). This was confirmed by Malhotra although in this study older teenagers were investigated with mean age of 16.4 years. 62 The international recommendations, however, are valid only from 14 years of age and while chronological age of the athlete helps to differentiate between a physiological juvenile and pathological T wave morphology, biological age is a more reliable denominator. 63
FIGURE 1.
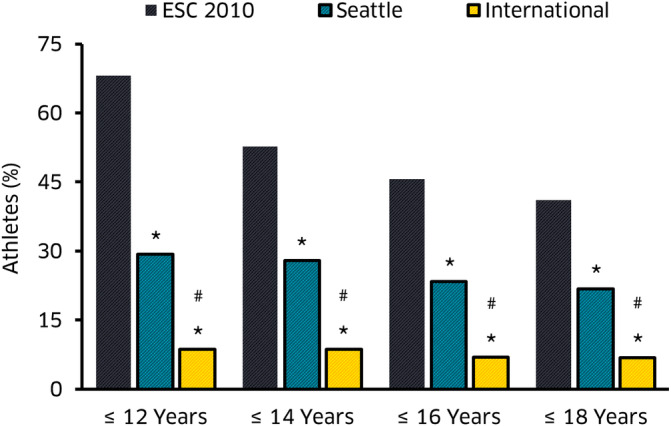
Bar chart shows the percentage of false‐positive ECG findings according to the 3 ECG interpretation criteria by chronological age. *P < .05, significantly reduced prevalence to ESC 2010 recommendations. #P < .05, significantly reduced prevalence to Seattle Criteria, reproduced with permission from Reference 61
2.3. Echocardiography
Echocardiography is the first line diagnostic imaging tool also for the pediatric athlete. Pediatric echocardiographic examinations should include a morphological assessment 64 and include a coronary artery assessment. 65 Normative reference values exist for cardiac morphology 66 and function 67 in the nonathlete pediatric population, values should be indexed for BSA and z‐scored. Reference data for the healthy paediatric population for advanced echocardiographic parameters are available for 2‐D strain 68 or exercise stress echocardiography. 69 , 70 Recently, studies have investigated pediatric athlete specific morphologic echocardiographic parameters for the LV, 71 RV, 72 , 73 but also atria 74 and complement previous studies in adolescents athletes providing evidence of quantitative wall thickness and cavity size athletic adaptation. 45 , 75 , 76 Comprehensive data correlating echocardiographic parameters to sports discipline, training volume and intensity, ethnicity, gender and age are still missing for the pediatric age group.
2.4. Cross sectional imaging
Cross sectional imaging to investigate pediatric athletes for a cardiac pathology should follow adult recommendations, 77 but protocols (higher incidence of undiagnosed congenital heart disease in children) and the relevance of specific diagnostic parameters (different diagnostic criteria for cardiomyopathies) differ. Size and gender specific pediatric centiles are available for cardiac magnetic resonance imaging (CMR) parameters. 34 , 35 , 36 CMR has recently been used as a primary screening tool for cardiac anomalies, in particular coronary artery anomalies. 78 Cardiac CT is the imaging modality of choice in the delineation of small anatomical structures such as coronary arteries and collateral arteries and for imaging parenchymal lung pathology.
2.5. Exercise assessment
Exercise testing, in particular cardio‐pulmonary exercise testing (CPET) is also a core diagnostic tool in children to investigate metabolic, respiratory, or cardiac pathology 79 , 80 as it can investigate pulmonary vascular disease, dysfunction of the autonomic nervous system, peripheral defects in oxygen transport, oxygen utilization at the working muscle and indirectly cardiac function. It can be safely performed in children above the age of 8 years. 81 CPET should always be combined with exercise 12‐lead ECG testing to allow assessment of underlying exercise‐related arrhythmias. It is paramount that supervising personnel should be trained and familiar with the pediatric population to encourage maximal effort and deliver reliable test results. 82 , 83 Exercise metabolism in pediatric athletes differs from adults in that children do not always attain a true VO2 max, 84 and submaximal measures such as ventilatory anaerobic threshold 85 or oxygen uptake efficiency slope 86 can be equally good markers for endurance performance. CPET is also ‐ used to indirectly investigate stroke volume response in pediatric patients with ventricular dysfunction, cardiomyopathies or congenital heart disease and is a core part in assessing sports participation eligibility also in the pediatric athlete. Exercise stress echocardiography can aid in detecting early systolic and diastolic dysfunction, assess valve function, myocardial exercise reserve, subtle wall motion abnormalities or dyssynchrony. Myocardial exercise reserve to investigate borderline or low cardiac function at rest, often seen in athletes can be determined by relatively load independent parameters such myocardial deformation (strain) and Tissue Doppler where, contrary to the adult population, normative reference data exist. 69 , 70 No studies exist to date using exercise stress CMR to assess cardiac volumes, function and wall motion abnormalities during exercise in athletes as is now performed in adult athletes. 87 Cableless mobile and app based devices to monitor heart rate and rhythm during exercise have been piloted in children and are a credible alternative to laboratory testing. 88
3. SPECIFIC DIAGNOSTIC CONSIDERATIONS IN THE PEDIATRIC ATHLETE
The spectrum of hereditary and acquired arrhythmia disorders and cardiomyopathies as a cause of SCD is very similar to that seen in adult athletes, and thus, adult competitive sports participation guidelines should be adhered to in the diagnosis and management of arrhythmias 89 and cardiomyopathies. 90 In pediatric athletes however, age and mode of presentation can be different from that in adult for arrhythmias and cardiomyopathies. Genetic testing in index cases and family cascade genotyping in pediatric cardiomyopathies should be performed when clinically indicated. However, age of independent‐ and legal‐capacity to consent to genetic testing is debated and a close collaboration with a geneticist and genetic counselor is recommended. No specific pediatric guidelines for pediatric cardiomyopathy genetic testing exist, but there is emerging evidence, that genotyping can help in risk stratification (reviewed in Reference 91). In all pediatric athletes with a suspected cardiomyopathy, serial annual evaluation is paramount and should include ECG, echocardiogram, exercise testing, and CMR.
3.1.1. Hypertrophic cardiomyopathy
Family and medical history are central as the majority of cases remain phenotypically dormant in childhood and first line 12‐lead ECG screening should follow the published international ECG criteria for athletes. While anterior T wave inversion is related to mild cardiac disease in 4.8%, infero‐lateral T wave inversion in 60% with cardiomyopathy or LVH. 58 Family history, ECG, Echocardiography, and CMR are imaging tools of choice. LV hypertrophy should be categorized as abnormal if the LV end‐diastolic wall thickness z‐score >2. End‐diastolic LV diameter is reduced in the majority of phenotypic pediatric HCM, 92 however, more common are mild and developing or subclinical phenotypes in childhood. As in adults, abnormal echocardiographic diastolic tissue Doppler parameters or myocardial systolic strain can be first indicators of an evolving phenotype. 92 Data on the use of de‐training in the pediatric athlete population to ascertain a diagnosis of HCM is still absent. Risk factors for cardiac events in the nonathlete pediatric HCM population are those of adults, but a pediatric risk stratification model has been recently suggested. 93
3.1.2. Arrhythmogenic ventricular cardiomyopathy
Disease is often concealed in childhood and mimics changes seen in the healthy young athletic population. 94 Diagnostic criteria are less sensitive and specific in pediatric ARVC 95 , 96 as they are based on data from affected adults 97 Echocardiographic revised task force criteria in particular rarely trigger suspicion in children and adolescents as they rely on adult RV diameters and are less sensitive and specific in pediatric ARVC 98 and enlarged RV diameters in pediatric athletes can mimic cardiomyopathic changes. 73 Recent data suggest that additional modalities such as 2‐D strain are more sensitive in assessing adolescents with ARVC. 96 CMR is the diagnostic imaging modality of choice also in children, but CMR findings in adult arrhythmogenic ventricular cardiomyopathy (AVC) such as fatty infiltration and fibrosis are of limited value in children and focus lies on ventricular function, regional wall motion abnormalities, and z‐scores of RV and LV dimensions. 95 Arrhythmic activity is highly variable in adolescent patients with AVC, 99 serial arrhythmia monitoring and exercise testing is important, LV involvement in adolescents is less frequent. Exercise can unmask or exacerbate AVC disease and pediatric athletes with phenotypic AVC should be restricted following adult recommendations. 90
3.1.3. Left‐ventricular noncompaction cardiomyopathy
Left‐ventricular noncompaction cardiomyopathy (LVNC) in childhood shows an undulating and heterogeneous phenotype of different severity. 100 LV hypertrabeculation is not uncommon in the healthy adult athlete, 101 and this is similar in the pediatric population, 78 however, a definite differentiation between physiological trabeculation and LVNC disease in this age group remains a challenge. Mild hypertrabeculation in the setting of normal function, without CMR features such as wall motion abnormalities or fibrosis, and without evidence of rhythm abnormalities can be regarded as a normal phenomenon, but serial monitoring is advised. Advanced echocardiographic imaging tools such as speckle tracking strain imaging can help detect disease in childhood, 102 but cross section al imaging and arrhythmia monitoring is warranted.
3.1.4. Pre‐excitation in the pediatric athlete
Ventricular pre‐excitation appearing as a delta wave on the ECG, occurs in children and may be symptomatic (Wolff Parkinson White syndrome) or asymptomatic and may be constant or intermittent. Although 65% of adolescents are asymptomatic, in comparison to 40% of adults, even asymptomatic young people may experience a sudden death event with no prior symptoms. 103 , 104 Moreover, although it used to be thought that intermittent pre‐excitation was a good prognostic finding, it is now clear that intermittent preexcitation does not imply a low risk pathway in children. 105 Consequently, most pediatric electrophysiologists would now carry out an invasive electrophysiology study to establish risk and the presence of pre‐excitation on the ECG of a child athlete should lead to a referral to a pediatric electrophysiologist for further risk stratification. 106
3.2. Congenital heart disease
Congenital heart disease (CHD) is the most common birth defect with a good survival rate and there are now many more adults than children with congenital heart disease—approximately 1 in 150 young adults have some form of CHD. 107 Recently, exercise advise in children and adolescents has shifted away from a more restrictive approach. It is now recommended that exercise advice and prescription should be an integral component of every patient encounter. 108 The assessment and follow up of the pediatric athlete should be in the hands of experienced congenital cardiologists, the ‐ recommendations on individual exercise and eligibility assessments in ‐ patients with CHD help risk stratify individuals in terms of type, intensity and level of exercise 109 but these relate to athletes over the age of 16 years of age.
3.3. Care of the pediatric athlete in the future
The rapid development and professionalization of youth sports academies demands increased diligence to safeguard the development of the pediatric athlete. The significant progress achieved in adult sports cardiology can guide assessment of the pediatric athlete, but adult cardiac preparticipation screening and management recommendations cannot be unequivocally applied to the pediatric heart. Somatic growth, psycho‐cognitive maturation with particular communicational and also ethical and legal considerations require an interdisciplinary approach involving pediatricians, pediatric cardiologists and sports medicine professionals as well as coaching staff with the young athlete in the center.
Future research should focus on the development of gender, growth, and age specific normative assessment criteria for ECG and echocardiogram to increase diagnostic accuracy. Most importantly, development of training pathways and a stronger engagement of pediatric and sports governing bodies is required, as too few pediatricians and pediatric cardiologists are sufficiently trained to provide expert opinion on the pediatric athlete. 110 Consequently, an approach requiring synergy between pediatric and sports cardiologists, exercise physiologists, policy makers, and sports organizations is required to develop pediatric cardiac monitoring tools and protocols, eventually working towards a child athlete centered specialty (pediatric sports cardiology) that matches the sports professionalism of the current and future pediatric athlete.
CONFLICT OF INTEREST
Dr. Guido E. Pieles is director of the sports cardiology consulting company “Cardiac Health and Performance Ltd”, Prof. A Graham Stuart is Medical Director of “Sports Cardiology UK”—a company which specializes in the assessment and management of athletes with cardiovascular problems.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
Pieles GE, Stuart AG. The adolescent athlete's heart; A miniature adult or grown‐up child? Clin Cardiol. 2020;43:43:852–862. 10.1002/clc.23417
REFERENCES
- 1. Pieles GE, Horn R, Williams CA, Stuart AG. Paediatric exercise training in prevention and treatment. Arch Dis Child. 2014;99:380‐385. [DOI] [PubMed] [Google Scholar]
- 2. Tomkinson GR, Carver KD, Atkinson F, et al. European normative values for physical fitness in children and adolescents aged 9‐17 years: results from 2 779 165 Eurofit performances representing 30 countries. Br J Sports Med. 2018;52:1445‐14563. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Global recommendations on physical activity for health. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 4.UK Chief Medical Officers' Physical Activity Guidelines. London: Pubslishing Services UK Gov; 2019.
- 5. Townsend MWK, Williams J, Bhatnagar P, Rayner M. Physical Activity Statistics 2015. London: British Heart Foundation; 2015. [Google Scholar]
- 6. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Araujo CG, Scharhag J. Athlete: a working definition for medical and health sciences research. Scand J Med Sci Sports. 2016;26:4‐7. [DOI] [PubMed] [Google Scholar]
- 8. Ford P, De Ste Croix M, Lloyd R, et al. The long‐term athlete development model: physiological evidence and application. J Sports Sci. 2011;29:389‐402. [DOI] [PubMed] [Google Scholar]
- 9. Brenner JS, American Academy of Pediatrics Council on Sports Medicine and Fitness . Overuse injuries, overtraining, and burnout in child and adolescent athletes. Pediatrics. 2007;119:1242‐1245. [DOI] [PubMed] [Google Scholar]
- 10. Gustafsson H, Hill AP, Stenling A, Wagnsson S. Profiles of perfectionism, parental climate, and burnout among competitive junior athletes. Scand J Med Sci Sports. 2016;26(10):1256‐1264. [DOI] [PubMed] [Google Scholar]
- 11. Atkins DL, Everson‐Stewart S, Sears GK, et al. Epidemiology and outcomes from out‐of‐hospital cardiac arrest in children: the Resuscitation outcomes Consortium Epistry‐cardiac arrest. Circulation. 2009;119:1484‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drezner JA, Rao AL, Heistand J, Bloomingdale MK, Harmon KG. Effectiveness of emergency response planning for sudden cardiac arrest in United States high schools with automated external defibrillators. Circulation. 2009;120:518‐525. [DOI] [PubMed] [Google Scholar]
- 13. Malhotra A, Dhutia H, Finocchiaro G, et al. Outcomes of cardiac screening in adolescent soccer players. N Engl J Med. 2018;379:524‐534. [DOI] [PubMed] [Google Scholar]
- 14. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593‐1601. [DOI] [PubMed] [Google Scholar]
- 15. Harmon KG, Zigman M, Drezner JA. The effectiveness of screening history, physical exam, and ECG to detect potentially lethal cardiac disorders in athletes: a systematic review/meta‐analysis. J Electrocardiol. 2015a;48:329‐338. [DOI] [PubMed] [Google Scholar]
- 16. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085‐1092. [DOI] [PubMed] [Google Scholar]
- 17. De Wolf D, Matthys D. Sports preparticipation cardiac screening: what about children? Eur J Pediatr. 2014;173:711‐719. [DOI] [PubMed] [Google Scholar]
- 18. Fudge J, Harmon KG, Owens DS, et al. Cardiovascular screening in adolescents and young adults: a prospective study comparing the pre‐participation physical evaluation monograph 4th edition and ECG. Br J Sports Med. 2014;48:1172‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finocchiaro G, Papadakis M, Robertus JL, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67:2108‐2115. [DOI] [PubMed] [Google Scholar]
- 20. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association Athletes: a decade in review. Circulation. 2015b;132:10‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berger S, Kugler JD, Thomas JA, Friedberg DZ. Sudden cardiac death in children and adolescents: introduction and overview. Pediatr Clin North Am. 2004;51:1201‐1209. [DOI] [PubMed] [Google Scholar]
- 22. Maron BJ, Epstein SE, Roberts WC. Causes of sudden death in competitive athletes. J Am Coll Cardiol. 1986;7:204‐214. [DOI] [PubMed] [Google Scholar]
- 23. Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512‐1520. [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199‐204. [PubMed] [Google Scholar]
- 25. Costa MJ, Marinho DA, Bragada JA, Silva AJ, Barbosa TM. Stability of elite freestyle performance from childhood to adulthood. J Sports Sci. 2011;29:1183‐1189. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong N, Barker AR. Endurance training and elite young athletes. Med Sport Sci. 2011;56:59‐83. [DOI] [PubMed] [Google Scholar]
- 27. Jayanthi N, Pinkham C, Dugas L, Patrick B, Labella C. Sports specialization in young athletes: evidence‐based recommendations. Sports Health. 2013;5:251‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school‐age youth. J Pediatr. 2005;146:732‐737. [DOI] [PubMed] [Google Scholar]
- 29. Conte G, De Asmundis C, Ciconte G, et al. Follow‐up from childhood to adulthood of individuals with family history of Brugada syndrome and normal electrocardiograms. JAMA. 2014;312:2039‐2041. [DOI] [PubMed] [Google Scholar]
- 30. Roston TM, Vinocur JM, Maginot KR, et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol. 2015;8:633‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norrish G, Field E, Mcleod K, et al. Clinical presentation and survival of childhood hypertrophic cardiomyopathy: a retrospective study in United Kingdom. Eur Heart J. 2019a;40:986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Authors/Task Force Members , Elliott PM, Anastasakis A, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733‐2779. [DOI] [PubMed] [Google Scholar]
- 33. Lafreniere‐Roula M, Bolkier Y, Zahavich L, et al. Family screening for hypertrophic cardiomyopathy: is it time to change practice guidelines? Eur Heart J. 2019;40:3672‐3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarikouch S, Peters B, Gutberlet M, et al. Sex‐specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady‐state free‐precession and phase‐contrast MRI flow. Circ Cardiovasc Imaging. 2010;3:65‐76. [DOI] [PubMed] [Google Scholar]
- 35. Dallaire F, Slorach C, Hui W, et al. Reference values for pulse wave Doppler and tissue Doppler imaging in pediatric echocardiography. Circ Cardiovasc Imaging. 2015;8:e002167. [DOI] [PubMed] [Google Scholar]
- 36. Overbeek LI, Kapusta L, Peer PG, De Korte CL, Thijssen JM, Daniels O. New reference values for echocardiographic dimensions of healthy Dutch children. Eur J Echocardiogr. 2006;7:113‐121. [DOI] [PubMed] [Google Scholar]
- 37. Prior DL, LA Gerche A. The athlete's heart. Heart. 2012;98:947‐955. [DOI] [PubMed] [Google Scholar]
- 38. Weiner RB, Baggish AL. Cardiovascular adaptation and remodeling to rigorous athletic training. Clin Sports Med. 2015;34:405‐418. [DOI] [PubMed] [Google Scholar]
- 39. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221‐1238. [DOI] [PubMed] [Google Scholar]
- 40. Stuart AG, Pieles GE. The Athlete's heart in children and adolescents ESC Textbook of Sports Cardiology. Oxford: Oxford University Press; 2019. [Google Scholar]
- 41. Ayabakan C, Akalin F, Mengutay S, Cotuk B, Odabas I, Ozuak A. Athlete's heart in prepubertal male swimmers. Cardiol Young. 2006;16:61‐66. [DOI] [PubMed] [Google Scholar]
- 42. Komoliatova VV, Makarov LM, Fedina NN, Kiseleva II. Microvolt T‐wave alternans in adolescent elite athletes. Kardiologiia. 2015;55:43‐46. [PubMed] [Google Scholar]
- 43. Rowland T. Morphologic features of the "Athlete's heart" in children: a contemporary review. Pediatr Exerc Sci. 2016;28:345‐352. [DOI] [PubMed] [Google Scholar]
- 44. Nottin S, Nguyen LD, Terbah M, Obert P. Left ventricular function in endurance‐trained children by tissue Doppler imaging. Med Sci Sports Exerc. 2004;36:1507‐1513. [DOI] [PubMed] [Google Scholar]
- 45. Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, Mckenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete's heart and hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:1431‐1436. [DOI] [PubMed] [Google Scholar]
- 46. Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiovascular pre‐participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus statement of the study Group of Sport Cardiology of the working Group of Cardiac Rehabilitation and Exercise Physiology and the working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516‐524. [DOI] [PubMed] [Google Scholar]
- 47. Drezner JA, O'connor FG, Harmon KG, et al. AMSSM position statement on cardiovascular Preparticipation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med. 2017;51(3):153‐167. [DOI] [PubMed] [Google Scholar]
- 48. Fritsch P, Ehringer‐Schetitska D, Dalla Pozza R, et al. Cardiovascular pre‐participation screening in young athletes: recommendations of the Association of European Paediatric Cardiology. Cardiol Young. 2017;27:1655‐1660. [DOI] [PubMed] [Google Scholar]
- 49. Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057‐1075. [DOI] [PubMed] [Google Scholar]
- 50. Dick NA, Diehl JJ. Febrile illness in the athlete. Sports Health. 2014;6:225‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scharhag J, Meyer T. Return to play after acute infectious disease in football players. J Sports Sci. 2014;32:1237‐1242. [DOI] [PubMed] [Google Scholar]
- 52. Joy E, Kussman A, Nattiv A. 2016 update on eating disorders in athletes: a comprehensive narrative review with a focus on clinical assessment and management. Br J Sports Med. 2016;50:154‐162. [DOI] [PubMed] [Google Scholar]
- 53. Enriquez A, Frankel DS. Arrhythmogenic effects of energy drinks. J Cardiovasc Electrophysiol. 2017;28:711‐717. [DOI] [PubMed] [Google Scholar]
- 54. Clark BC, Hayman JM, Berul CI, Burns KM, Kaltman JR. Selective use of the electrocardiogram in pediatric preparticipation athletic examinations among pediatric primary care providers. Ann Noninvasive Electrocardiol. 2017;22:e12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park MK, Guntheroth WG. How to Read Pediatric ECG's. Philadelphia, PA: Mosby; 2006. [Google Scholar]
- 56. Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA. New normal limits for the paediatric electrocardiogram. Eur Heart J. 2001;22:702‐711. [DOI] [PubMed] [Google Scholar]
- 57. Migliore F, Zorzi A, Michieli P, et al. Prevalence of cardiomyopathy in Italian asymptomatic children with electrocardiographic T‐wave inversion at preparticipation screening. Circulation. 2012;125:529‐538. [DOI] [PubMed] [Google Scholar]
- 58. Calo L, Sperandii F, Martino A, et al. Echocardiographic findings in 2261 peri‐pubertal athletes with or without inverted T waves at electrocardiogram. Heart. 2015;101:193‐200. [DOI] [PubMed] [Google Scholar]
- 59. Papadakis M, Basavarajaiah S, Rawlins J, et al. Prevalence and significance of T‐wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J. 2009;30:1728‐1735. [DOI] [PubMed] [Google Scholar]
- 60. McClean G, Riding NR, Ardern CL, et al. Electrical and structural adaptations of the paediatric athlete's heart: a systematic review with meta‐analysis. Br J Sports Med. 2018;52(4):230. [DOI] [PubMed] [Google Scholar]
- 61. McClean G, Riding NR, Pieles G, et al. Diagnostic accuracy and Bayesian analysis of new international ECG recommendations in paediatric athletes. Heart. 2019b;105:152‐159. [DOI] [PubMed] [Google Scholar]
- 62. Malhotra A, Dhutia H, Yeo TJ, et al. Accuracy of the 2017 international recommendations for clinicians who interpret adolescent athletes' ECGs: a cohort study of 11 168 British white and black soccer players. Br J Sports Med. 2020;54:739‐745. [DOI] [PubMed] [Google Scholar]
- 63. McClean G, Riding NR, Pieles G, et al. Prevalence and significance of T‐wave inversion in Arab and black paediatric athletes: should anterior T‐wave inversion interpretation be governed by biological or chronological age? Eur J Prev Cardiol. 2019a;26:641‐652. [DOI] [PubMed] [Google Scholar]
- 64. Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric Council of the American Society of echocardiography. J Am Soc Echocardiogr. 2006;19:1413‐1430. [DOI] [PubMed] [Google Scholar]
- 65. Gerling S, Loose O, Zant R, et al. Echocardiographic diagnosis of congenital coronary artery abnormalities in a continuous series of adolescent football players. Eur J Prev Cardiol. 2019;12:988‐994. [DOI] [PubMed] [Google Scholar]
- 66. Foster BJ, Khoury PR, Kimball TR, Mackie AS, Mitsnefes M. New reference centiles for left ventricular mass relative to lean body mass in children. J Am Soc Echocardiogr. 2016;29:441‐447. [DOI] [PubMed] [Google Scholar]
- 67. Lopez L, Colan S, Stylianou M, et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the pediatric heart Network Normal echocardiogram database. Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marcus KA, Mavinkurve‐Groothuis AM, Barends M, et al. Reference values for myocardial two‐dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr. 2011;24:625‐636. [DOI] [PubMed] [Google Scholar]
- 69. Cifra B, Mertens L, Mirkhani M, et al. Systolic and diastolic myocardial response to exercise in a healthy pediatric cohort. J Am Soc Echocardiogr. 2016;29:648‐654. [DOI] [PubMed] [Google Scholar]
- 70. Pieles GE, Gowing L, Forsey J, et al. The relationship between biventricular myocardial performance and metabolic parameters during incremental exercise and recovery in healthy adolescents. Am J Physiol Heart Circ Physiol. 2015;309:H2067‐H2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cavarretta E, Maffessanti F, Sperandii F, et al. Reference values of left heart echocardiographic dimensions and mass in male peri‐pubertal athletes. Eur J Prev Cardiol. 2018;25:1204‐1215. [DOI] [PubMed] [Google Scholar]
- 72. D'Ascenzi F, Pelliccia A, Valentini F, et al. Training‐induced right ventricular remodelling in pre‐adolescent endurance athletes: the athlete's heart in children. Int J Cardiol. 2017;236:270‐275. [DOI] [PubMed] [Google Scholar]
- 73. Popple E, George K, Somauroo J, et al. Right ventricular structure and function in senior and academy elite footballers. Scand J Med Sci Sports. 2018;28:2617‐2624. [DOI] [PubMed] [Google Scholar]
- 74. D'Ascenzi F, Solari M, Anselmi F, et al. Atrial chamber remodelling in healthy pre‐adolescent athletes engaged in endurance sports: a study with a longitudinal design. The CHILD study. Int J Cardiol. 2016;223:325‐330. [DOI] [PubMed] [Google Scholar]
- 75. Basavarajaiah S, Makan J, Naghavi SH, Whyte G, Gati S, Sharma S. Physiological upper limits of left atrial diameter in highly trained adolescent athletes. J Am Coll Cardiol. 2006;47:2341‐2342. author reply 2342. [DOI] [PubMed] [Google Scholar]
- 76. Makan J, Sharma S, Firoozi S, Whyte G, Jackson PG, Mckenna WJ. Physiological upper limits of ventricular cavity size in highly trained adolescent athletes. Heart. 2005;91:495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pelliccia A, Caselli S, Sharma S, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. Eur Heart J. 2017;1‐27. [DOI] [PubMed] [Google Scholar]
- 78. Angelini P, Cheong BY, Lenge De Rosen VV, et al. Magnetic resonance imaging‐based screening study in a general population of adolescents. J Am Coll Cardiol. 2018;71:579‐580. [DOI] [PubMed] [Google Scholar]
- 79. Astrand PO. Methods of ergometry in children. Definitions, testing procedures, accuracy and reproduceability. Acta Paediatr Scand Suppl. 1971;217:9‐12. [PubMed] [Google Scholar]
- 80. Wasserman K. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 81. Rhodes J, Ubeda Tikkanen A, Jenkins KJ. Exercise testing and training in children with congenital heart disease. Circulation. 2010;122:1957‐1967. [DOI] [PubMed] [Google Scholar]
- 82. Paridon SM, Alpert BS, Boas SR, et al. Clinical stress testing in the pediatric age group: a statement from the American Heart Association Council on cardiovascular disease in the young, Committee on Atherosclerosis, hypertension, and obesity in youth. Circulation. 2006;113:1905‐1920. [DOI] [PubMed] [Google Scholar]
- 83. Rowland TW, American college of sports medicine & north american society of pediatric exercise medicine . Cardiopulmonary Exercise Testing in Children and Adolescents. Human Kinetics: Champaign, IL; 2018. [Google Scholar]
- 84. Rowland TW. Aerobic exercise testing protocols In: Rowland TW, ed. Pediatric Laboratory Exercise Testing Clincal Guidelines. Champaign, IL: Human Kinetics; 1992. [Google Scholar]
- 85. Buchheit M, Solano R, Millet GP. Heart‐rate deflection point and the second heart‐rate variability threshold during running exercise in trained boys. Pediatr Exerc Sci. 2007;19:192‐204. [DOI] [PubMed] [Google Scholar]
- 86. Akkerman M, Van Brussel M, Bongers BC, Hulzebos EH, Helders PJ, Takken T. Oxygen uptake efficiency slope in healthy children. Pediatr Exerc Sci. 2010;22:431‐441. [DOI] [PubMed] [Google Scholar]
- 87. La Gerche A, Claessen G, Van De Bruaene A, et al. Cardiac magnetic resonance imaging: a new gold standard for ventricular volume quantification during high‐intensity exercise. Circ Cardiovasc Imaging. 2013;6(2):329‐338. [DOI] [PubMed] [Google Scholar]
- 88. Macinnes M, Martin N, Fulton H, Mcleod KA. Comparison of a smartphone‐based ECG recording system with a standard cardiac event monitor in the investigation of palpitations in children. Arch Dis Child. 2019;104:43‐47. [DOI] [PubMed] [Google Scholar]
- 89. Zipes DP, Link MS, Ackerman MJ, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 9: arrhythmias and conduction defects: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2412‐2423. [DOI] [PubMed] [Google Scholar]
- 90. Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the sport cardiology section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019;40:19‐33. [DOI] [PubMed] [Google Scholar]
- 91. Richard P, Denjoy I, Fressart V, Wilson MG, Carre F, Charron P. Advising a cardiac disease gene positive yet phenotype negative or borderline abnormal athlete: is sporting disqualification really necessary? Br J Sports Med. 2012;46(Suppl 1):i59‐i68. [DOI] [PubMed] [Google Scholar]
- 92. Lipshultz SE, Orav EJ, Wilkinson JD, et al. Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: an analysis of data from the pediatric cardiomyopathy registry. Lancet. 2013;382:1889‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Norrish G, Ding T, Field E, et al. Development of a Novel Risk Prediction Model for Sudden Cardiac Death in Childhood Hypertrophic Cardiomyopathy (HCM Risk‐Kids). JAMA Cardiol. 2019;4(9):918‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bauce B, Frigo G, Benini G, et al. Differences and similarities between arrhythmogenic right ventricular cardiomyopathy and athlete's heart adaptations. Br J Sports Med. 2010;44:148‐154. [DOI] [PubMed] [Google Scholar]
- 95. Etoom Y, Govindapillai S, Hamilton R, et al. Importance of CMR within the task force criteria for the diagnosis of ARVC in children and adolescents. J Am Coll Cardiol. 2015;65:987‐995. [DOI] [PubMed] [Google Scholar]
- 96. Pieles GE, Grosse‐Wortmann L, Hader M, et al. Association of Echocardiographic Parameters of right ventricular remodeling and myocardial performance with modified task force criteria in adolescents with Arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2019;12:e007693. [DOI] [PubMed] [Google Scholar]
- 97. Marcus FI, Mckenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Steinmetz M, Krause U, Lauerer P, et al. Diagnosing ARVC in pediatric patients applying the revised task force criteria: importance of imaging, 12‐Lead ECG, and genetics. Pediatr Cardiol. 2018;39:1156‐1164. [DOI] [PubMed] [Google Scholar]
- 99. Sequeira IB, Kirsh JA, Hamilton RM, Russell JL, Gross GJ. Utility of exercise testing in children and teenagers with arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2009;104:411‐413. [DOI] [PubMed] [Google Scholar]
- 100. Pignatelli RH, Mcmahon CJ, Dreyer WJ, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108:2672‐2678. [DOI] [PubMed] [Google Scholar]
- 101. Gati S, Chandra N, Bennett RL, et al. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non‐compaction in athletes? Heart. 2013;99:401‐408. [DOI] [PubMed] [Google Scholar]
- 102. Sabatino J, Di Salvo G, Krupickova S, et al. Left ventricular twist mechanics to identify left ventricular noncompaction in childhood. Circ Cardiovasc Imaging. 2019;12:e007805. [DOI] [PubMed] [Google Scholar]
- 103. Etheridge SP, Escudero CA, Blaufox AD, et al. Life‐threatening event risk in children with Wolff‐Parkinson‐white syndrome: a multicenter international study. JACC Clin Electrophysiol. 2018;4:433‐444. [DOI] [PubMed] [Google Scholar]
- 104. Pediatric and Congenital Electrophysiology Society , Heart Rhythm Society , American College of Cardiology Foundation , et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff‐Parkinson‐white (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the pediatric and congenital electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9:1006‐1024. [DOI] [PubMed] [Google Scholar]
- 105. Kiger ME, Mccanta AC, Tong S, Schaffer M, Runciman M, Collins KK. Intermittent versus persistent Wolff‐Parkinson‐white syndrome in children: Electrophysiologic properties and clinical outcomes. Pacing Clin Electrophysiol. 2016;39:14‐20. [DOI] [PubMed] [Google Scholar]
- 106. Chubb H, Campbell RM, Motonaga KS, Ceresnak SR, Dubin AM. Management of asymptomatic Wolff‐Parkinson‐White pattern by pediatric electrophysiologists. J Pediatr. 2019;213:88‐95. [DOI] [PubMed] [Google Scholar]
- 107. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749‐756. [DOI] [PubMed] [Google Scholar]
- 108. Longmuir PE, Brothers JA, De Ferranti SD, et al. Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147‐2159. [DOI] [PubMed] [Google Scholar]
- 109. Budts W, Borjesson M, Chessa M, et al. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. European Heart Journal. 2013;34 (47):3669‐3674. 10.1093/eurheartj/eht433. [DOI] [PubMed] [Google Scholar]
- 110. Drezner JA, Levine BD, Vetter VL. Reframing the debate: screening athletes to prevent sudden cardiac death. Heart Rhythm. 2013;10:454‐455. [DOI] [PubMed] [Google Scholar]


