Abstract
The nuclear lamina is essential for the structural integration of the nuclear envelope. Nuclear envelope rupture and chromatin externalization is a hallmark of the formation of neutrophil extracellular traps (NETs). NET release was described as a cellular lysis process; however, this notion has been questioned recently. Here, we report that during NET formation, nuclear lamin B is not fragmented by destructive proteolysis, but rather disassembled into intact full‐length molecules. Furthermore, we demonstrate that nuclear translocation of PKCα, which serves as the kinase to induce lamin B phosphorylation and disassembly, results in nuclear envelope rupture. Decreasing lamin B phosphorylation by PKCα inhibition, genetic deletion, or by mutating the PKCα consensus sites on lamin B attenuates extracellular trap formation. In addition, strengthening the nuclear envelope by lamin B overexpression attenuates NET release in vivo and reduces levels of NET‐associated inflammatory cytokines in UVB‐irradiated skin of lamin B transgenic mice. Our findings advance the mechanistic understanding of NET formation by showing that PKCα‐mediated lamin B phosphorylation drives nuclear envelope rupture for chromatin release in neutrophils.
Keywords: lamin B disassembly, NET formation, nuclear envelope rupture, PKCα
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton; Immunology; Signal Transduction
Nuclear envelope rupture is crucial for chromatin externalization during NET formation. Nuclear lamin B disassembly, but not cleavage, regulated by PKCα‐mediated phosphorylation, is responsible for nuclear envelope rupture and NET release.
Introduction
Neutrophils are the most common leukocytes. Increasing evidence from many studies (Brinkmann et al, 2004; Lood et al, 2016; Soehnlein et al, 2017; Boeltz et al, 2019), including our own (Folkesson et al, 2015), indicates the importance of neutrophils in acute or chronic inflammation in variety of human diseases (Brinkmann et al, 2004; Lood et al, 2016; Soehnlein et al, 2017; Boeltz et al, 2019). NET formation is characterized by nuclear chromatin decondensation (Brinkmann et al, 2004) through peptidyl arginine deiminase type IV (PAD4)‐catalyzed histone citrullination (Neeli et al, 2008; Wang et al, 2009) or neutrophil elastase‐mediated histone cleavage (Pieterse et al, 2018), followed by chromatin extrusion via the ruptured nuclear envelope. The externalized nuclear chromatin serves as the backbone of NETs that mix with cytosolic contents, i.e., granule proteins and oxidized mitochondrial DNA, and form the extracellular trap structure (Boeltz et al, 2019). Several signaling pathways may be important in the development of NET formation, i.e., production of reactive oxygen species (ROS) through NADPH oxidase (NOX2)‐dependent or NADPH oxidase (NOX2)‐independent pathways (Douda et al, 2015; Pieterse et al, 2018; Boeltz et al, 2019), activation of protein kinase C (PKC), extracellular‐signal‐regulated kinase (ERK/MAPK), and phosphatidylinositide 3‐kinase (PI3K)/Akt (Boeltz et al, 2019).
ROS is thought to be required for the release of myeloperoxidase and neutrophil elastase from granules in activated neutrophils. Then, neutrophil elastase is translocated to the nucleus for chromatin decondensation by histone cleavage in the early stage of NET formation (Fuchs et al, 2007; Papayannopoulos et al, 2010). However, a very recent consensus statement paper by experts in the field suggests that the direct connection between lysosomal membrane instability and NET formation is still under discussion (Boeltz et al, 2019). Pieterse et al (2018) reported that neutrophil elastase may be associated with cleavage of the N‐terminal tail, the proteolytic sensitive region, of histones in the late stage of NET formation. Another paper reported that neutrophils from neutrophil elastase‐deficient mice could still undergo NET formation (Martinod et al, 2016). In addition to ROS‐dependent NET formation, a number of recent studies also reported ROS‐independent NET formation induced by certain stimuli (Rochael et al, 2015; Tatsiy & McDonald, 2018).
Nuclear envelope rupture is the hallmark and a prerequisite step in the multi‐step process of NET formation (Boeltz et al, 2019). However, the relevant cellular and molecular mechanism of nuclear envelope rupture during NET formation is still unclear. NET formation was described as a lytic cell death mechanism (Fuchs et al, 2007; Papayannopoulos et al, 2010; Yipp & Kubes, 2013). However, this notion has been questioned by several recent observations (Amulic et al, 2017; Neubert et al, 2018). Rupture of the nuclear envelope appears to be a distinct process from previously described lysis or dissolution of the nuclear envelope (Amulic et al, 2017; Neubert et al, 2018). In addition, Pilsczek et al (2010) described vital, but not lytic, NET formation in which NETs are released via nuclear budding or vesicle release by an unknown mechanism.
The nuclear envelope consists of outer and inner lipid nuclear membranes (ONM and INM) and nuclear lamina, a protein filament meshwork that provides structural scaffold to reinforce nuclear envelope integrity (Goldberg et al, 2008). Nuclear lamins are categorized as either A‐type (A, C) or B‐type (B1, B2) lamins (Goldberg et al, 2008). A‐type lamins form thick filament bundles that contribute to the mechanical stiffness of nuclei (Lammerding et al, 2006; Goldberg et al, 2008; Rowat et al, 2013), whereas B‐type lamins form thin but highly organized meshworks that are crucial to the integrity and elasticity of the nuclear envelope (Vergnes et al, 2004; Goldberg et al, 2008), but not stiffness (Lammerding et al, 2006; Swift et al, 2013). Shin et al reported that nuclear stiffness increases with the lamin A:B ratio (Shin et al, 2013), and increasing the lamin A:B ratio by downregulation of lamin B promotes nuclear stiffness in hematopoietic cells (Shin et al, 2013). Lamin B is anchored underneath the INM through the lamin B receptor (LBR) (Olins et al, 2008; Singh et al, 2016). Nuclear envelope breakdown (Hatch & Hetzer, 2014) is a common cellular event in nuclear fragmentation during cell apoptosis (Shimizu et al, 1998), in nuclear division during cell mitosis (Collas et al, 1997; Mall et al, 2012), and in viral nuclear access during viral infection (Park & Baines, 2006). In the cellular processes described above, nuclear lamin B is either proteolytically degraded by caspases (Slee et al, 2001) or disassembled through phosphorylation by protein kinase C (PKC) (Collas et al, 1997; Muranyi et al, 2002).
Mature neutrophils are terminally differentiated cells thought to have an unusual nucleus with a paucity of lamin A/C (Olins et al, 2008) and a reduced amount of lamin B (Olins et al, 2008). Recent studies, however, reported that neutrophils do have A‐type (Amulic et al, 2017) and B‐type (Moisan & Girard, 2006; Rowat et al, 2013) lamins. The role of lamin B in neutrophil biology is unclear and was ignored, and the involvement of lamin B in NET formation has not been investigated. In the current study, we investigated the importance of lamin B for the integrity of the nucleus in neutrophils and cellular mechanisms that regulate nuclear envelope rupture during NET formation in neutrophils from human and mice. We also investigated the effect of strengthening the nuclear envelope by lamin B overexpression on NET formation in vivo and on the presence of NET‐associated inflammatory cytokines in UVB‐irradiated skin of lamin B transgenic mice.
Results
Nuclear lamin B is a substantial component of the nuclear envelope that is involved in NET formation
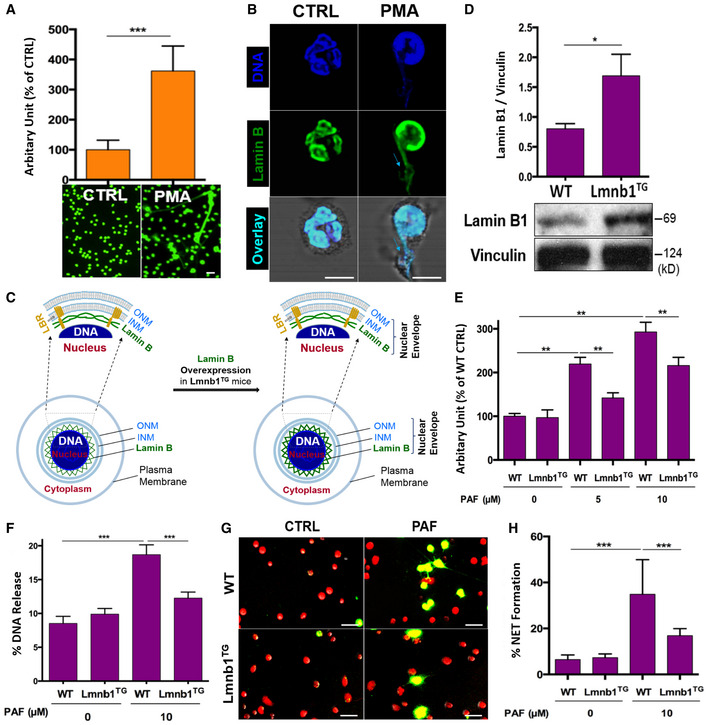
Neutrophils are terminally differentiated cells, and the role of nuclear lamina in neutrophil biology is not well understood (Olins et al, 2008). However, nuclear envelope breakdown is required for nuclear chromatin externalization during NET formation. To explore the role of nuclear lamin B, we detected the expression of lamin B in primary human polymorphonuclear neutrophils (pPMNs) and the involvement of lamin B in NET formation (Fig 1A and B). NET formation can be triggered by PMA, the most commonly used NET inducer (Fig 1A; Brinkmann et al, 2004), or by platelet activating factor (PAF), a UVB‐induced lipid mediator and naturally existing stimulus of NETs (Damiani & Ullrich, 2016). Confocal microscopy analyses indicated that nuclear lamin B is a substantial component of the nuclear envelope (Fig 1B and C), which is ruptured during NET release (Fig 1B), indicating the involvement of lamin B in the process of NET formation.
Figure 1. Nuclear lamin B is a substantial component of nuclear envelope that is involved in NET formation.
-
ASummary and representative analysis of NET formation of pPMNs stimulated by 50 nM PMA for 3 h, detected by fluorescent microplate reader and confocal microscopy, respectively. Scale bar 20 μm.
-
BRepresentative confocal microscopy images of pPMNs that were treated without (control) or with 50 nM PMA for 3 h and stained for lamin B and DNA as described in panel (B). The light blue arrows indicate the release of decondensed DNA associated with lamin B molecules from ruptured nuclear envelope. Scale bar 10 μm.
-
CSchematic cross‐section of a cell and portion of the nucleus and the nuclear envelope, as well as the ones with corresponding overexpression of lamin B. The two lipid bilayers of the nuclear envelope are the inner and outer nuclear membranes (INM and ONM, respectively). The meshwork, nuclear lamin B is anchored to INM through lamin B receptor (LBR).
-
DRepresentative and summary of immunoblots of lamin B of bone marrow neutrophils from WT and Lmnb1TG mice. Vinculin served as loading control.
-
ESummary analysis of PAF‐induced NET formation in mPMNs from WT vs. Lmnb1TG mice that were stimulated without or with 10 μM PAF for 3 h and then fixed by 2% PFA and stained by SYTOX Green, followed by detection with fluorescent microplate reader.
-
FThe endpoint analysis of NET‐DNA release index was detected by coincubation of primary mouse peritoneal mPMNs from WT and Lmnb1TG mice without (control) or with 10 μM PAF in medium containing 1 μM SYTOX Green dye with recording by a microplate reader at the 3‐h time point. The NET‐DNA release index was reported in comparison with an assigned value of 100% for the total DNA released by neutrophils lysed by 0.5% (v/v) Triton X‐100.
-
G, HRepresentative images (G) and summary analysis (H) of PAF‐induced NET formation in mPMNs from WT vs. Lmnb1TG mice that were stimulated without or with 10 μM PAF for 3 h and stained with both cell‐permeable SYTO Red and cell‐impermeable SYTOX Green, without fixation. Images were taken by Olympus confocal microscopy, followed by automated quantification of NETs on 5–6 non‐overlapping area per well using ImageJ for calculation of % cells with NET formation. (G) Scale bars, 40 μm.
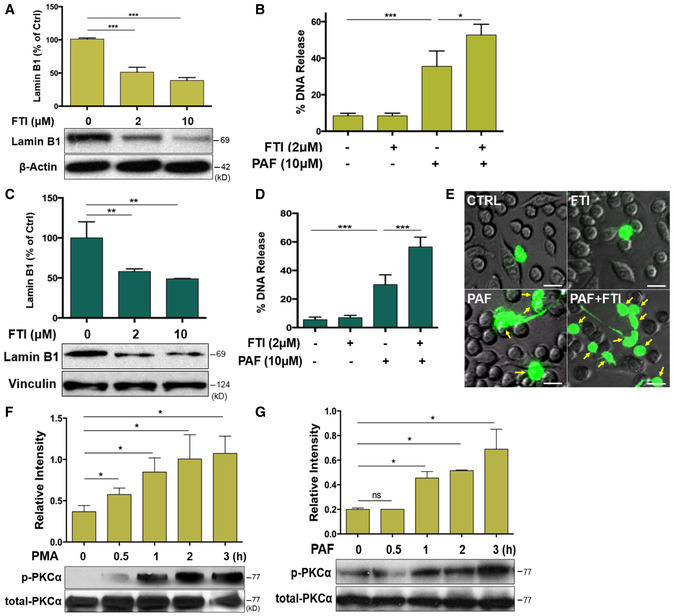
Lamin B is assembled as a filament meshwork that lies beneath the INM, and the two are associated by an integral protein, lamin B receptor (Fig 1C). Given the importance of lamin B in nuclear envelope integration (Vergnes et al, 2004; Goldberg et al, 2008), one may propose that lamin B may be important in regulating nuclear envelope rupture during NET formation. To test this hypothesis, we first explored the effects of lamin B overexpression on NET formation by using neutrophils from lamin B transgenic Lmnb1TG mice (Fig 1D). We found attenuated NET formation in peritoneal neutrophils from Lmnb1TG mice as compared to those from their WT littermates (Fig 1E–H), analyzed by fluorometric NET quantification (Fig 1E; Sollberger et al, 2016), endpoint analysis of NET‐DNA release index (Fig 1F; Douda et al, 2015; Khan et al, 2018), and immunofluorescent imaging analysis of NET formation (Fig 1G and H; Sollberger et al, 2016). On the other hand, lamin B maturation is regulated by farnesylation (Adam et al, 2013), and long‐term inhibition of protein farnesylation with farnesyltransferase inhibitor (FTI) can reduce the amount of mature lamin B (Adam et al, 2013). To explore the effects of decreased lamin B, we found that coincubation with FTI can reduce the amount of mature lamin B both in differentiated HL‐60 neutrophils (dPMNs) (Fig EV1A) and in RAW 264.7 macrophages (Fig EV1C). Most importantly, we found that decreased lamin B in FTI‐pretreated cells could enhance PAF‐induced extracellular trap formation both in dPMNs and in RAW264.7 cells (Fig EV1B, D and E). Taken together, the levels of mature lamin B expression in the nucleus affected extracellular trap formation in a negative dose‐dependent manner both in neutrophils and macrophages.
Figure EV1. Decreased lamin B expression enhances extracellular traps formation in vitro and time course of PKCα phosphorylation during NET formation (Related to Figs 1 and 3).
-
A, CSummary and representative immunoblot analysis for the mature lamin B expression in HL‐60 dPMNs (A) or RAW264.7 cells (C) that were treated without (0) or with 2 or 10 μM farnesyltransferase inhibitor (FTI) L‐744,832 for 48 h.
-
B, D, ESummary analysis (B, D) and representative images (E) of extracellular trap release in HL‐60 dPMNs (B) RAW264.7 cells (E) that were pretreated without or with 2 μM for 48 h, followed by treatment or not with 10 μM PAF for 3 h and then stained with cell‐impermeable SYTOX Green, without fixation. Fluorescent and phase‐contrast images were taken by Olympus confocal microscopy. The yellow arrows indicate neutrophils with NET formation. Scale bars, 20 μm (E).
-
F, GRepresentative immunoblots and the summary analyses of total and phosphorylated PKCα (p‐PKCα), in human dPMNs that were treated either by PMA (F) or PAF (G) for 0, 0.5, 1, 2, 3 h.
Therefore, our results demonstrated that nuclear lamin B is a substantial component of the nuclear envelope in neutrophils, similar to other cell types (Vergnes et al, 2004; Goldberg et al, 2008). Nuclear lamin B is important in regulating nuclear envelop breakdown and release of extracellular traps.
Nuclear lamin B disassembly, but not proteolytic cleavage, is responsible for nuclear envelope rupture during NET formation
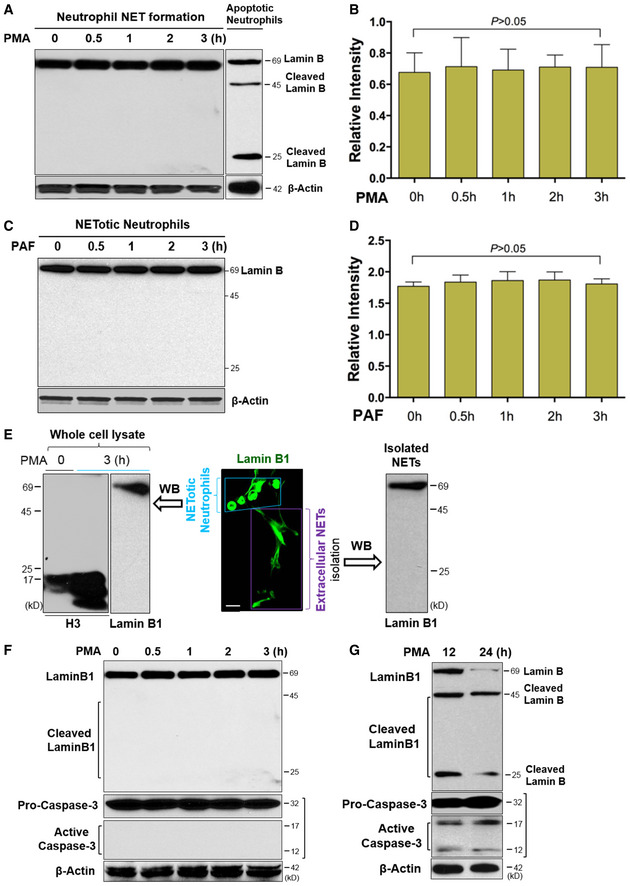
Next, we sought to elucidate how lamin B is involved in nuclear envelope rupture during NET formation. Unexpectedly, immunoblot analysis of the time‐course studies demonstrated that nuclear lamin B remained as an intact full‐length molecule during NET formation in human dPMNs 0–3 h after stimulation by PMA (Fig 2A and B) or PAF (Fig 2C and D), in contrast to the destructively cleaved and fragmented lamin B in apoptotic neutrophils (Fig 2A). Importantly, immunoblot analysis of isolated extracellular NETs (Fig 2E) showed that nuclear lamin B is also released with NETs to the extracellular space and NET‐associated lamin B was not degraded, but remained as an intact full‐length molecule (Fig 2E), confirming the findings from the whole‐cell lysates. In contrast to uncleaved nuclear lamin B, we observed cleaved histone H3 in the neutrophils with NET formation (Fig 2E). Neutrophil elastase may contribute to the cleavage of histone H3 and decondensation of chromatin during NET formation (Konig & Andrade, 2016; Pieterse et al, 2018). Therefore, our results suggested potential disassembly, but not the destructive cleavage, of lamin B is responsible for nuclear envelope rupture and extracellular trap formation during NET formation in neutrophils.
Figure 2. Nuclear lamin B disassembly, but not proteolytic cleavage, is responsible for nuclear envelope rupture in neutrophils with NET formation.
-
A, BRepresentative and summary of immunoblots of full‐length lanes of lamin B in human dPMNs that were treated with PMA for 0, 0.5, 1, 2, 3 h during NET formation, or apoptotic dPMNs that were induced by PMA for longer term (12 h) treatment (A).
-
C, DRepresentative and summary immunoblots of lamin B in human dPMNs that were treated with PAF for 0, 0.5, 1, 2, 3 h during NET formation.
-
ERepresentative confocal microscopy image of a group of pPMNs with NET formation (enclosed by a light blue trapezoid) and their extracellular NETs (indicated by a purple rectangle square) released by these cells, as well as the immunoblot analysis of uncleaved lamin B and cleaved histone H3 with the whole‐cell lysates, as well as immunoblot analysis of uncleaved lamin B with lysate of the NETs isolated from conditioned medium of pPMNs with NET formation that were induced by 3 h PMA treatment.
-
FRepresentative immunoblots with full‐length lanes for lamin B analysis, and pro‐caspase‐3 and its activated form caspase‐3 in human dPMNs with NET formation that were treated with PMA for 0, 0.5, 1, 2, 3 h.
-
GRepresentative immunoblots display full‐length lamin B (69 kDa) and their cleaved fragments (45 and 25 kDa), and pro‐caspase‐3 and its activated form caspase‐3 in the apoptotic human dPMNs that were treated with PMA for 12, 24 h.
Caspase‐3 mediates apoptosis and cleaves lamin B (Slee et al, 2001) during apoptotic nuclear fragmentation. In the current study, we found that caspase‐3 remained inactive as pro‐caspase‐3 over a 3‐h period of time during NET formation (Fig 2F), while activated caspase‐3 was detected in apoptotic dPMNs induced by extended PMA treatment (Arroyo et al, 2002) for 12–24 h (Fig 2G). Furthermore, we saw that lamin B was cleaved into 25 and 45 kDa fragments, corresponding to caspase‐3 activation during apoptosis (Fig 2G). These experiments further confirmed that destructive proteolytic cleavage is not responsible for lamin B disintegration during nuclear envelope rupture in neutrophils with NET formation.
Nuclear translocation and phosphorylation of PKCα during NET formation
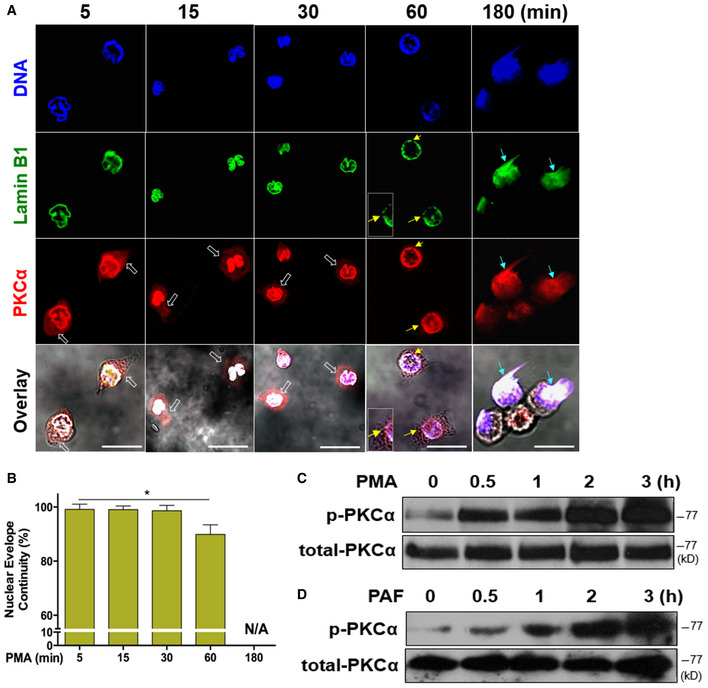
To address how nuclear lamin B was disassembled during nuclear envelope rupture, we found that cytosolic PKCα was translocated to the nuclear membrane where it may mediate lamin B phosphorylation and nuclear envelope disintegration, similar to the role of PKCα during mitosis (Mall et al, 2012), and viral infection (Park & Baines, 2006). The immunofluorescent image analysis (Fig 3A) demonstrated that total PKCα was gradually translocated from the cytosol into the nucleus over the time during the early period of PMA stimulation from 5 to 30 min (analysis started after 5 min PMA stimulation to allow the neutrophils to adherer for microscopic analysis). PKCα accumulated in the nucleus and resulted in nuclear envelope discontinuity/rupture at 60‐min time point (Fig 3A and B), followed by the consequent extrusion of decondensed chromatin from the ruptured/collapsed nuclear envelope at 180 min (Fig 3A), and the subsequent NET formation. Correspondingly, PKCα phosphorylation was gradually increased over the 3‐h stimulation period in human primary pPMNs (Fig 3C and D) and dPMNs (Fig EV1F and G) treated with PMA or PAF.
Figure 3. Nuclear translocation and phosphorylation of PKCα is accompanied by nuclear envelope rupture during NET formation.
-
ARepresentative images for the time course of PKCα nuclear translocation, subsequent nuclear envelope rupture, and DNA release in human dPMNs exposed to 50 nM PMA for 5, 15, 30, 60, and 180 min and then stained concomitantly for DNA (DAPI), nuclear lamin B (primary anti‐lamin B, and FITC‐labeled secondary antibody), and PKCα (primary anti‐human PKCα, and PE‐labeled secondary antibody), followed by confocal fluorescent microscopy analysis. White empty arrows indicate cytoplasmic distribution of PKCα at 5, 15, and 30 min, yellow arrows indicate site of discontinuity/rupture of nuclear envelope at 60 min, light blue arrows display the sites of nuclear envelope rupture and chromatin release at 180 min (A). Scale bars, 20 μm. The time course started 5 min after PMA stimulation in order to allow the adherence of neutrophils on the bottom of dish for immunocytostaining.
-
BSummary analysis of the nuclear envelope continuity was analyzed based on staining of the nuclear envelope with primary anti‐lamin B, and FITC‐labeled secondary antibody.
-
C, DRepresentative immunoblots of total PKCα and p‐PKCα in primary human pPMNs that were treated by PMA (C) or PAF (D) for 0, 0.5, 1, 2, 3 h.
Accumulation of phosphorylated PKCα in the nucleus results in lamin B phosphorylation, disassembly, and nuclear envelope rupture during NET formation
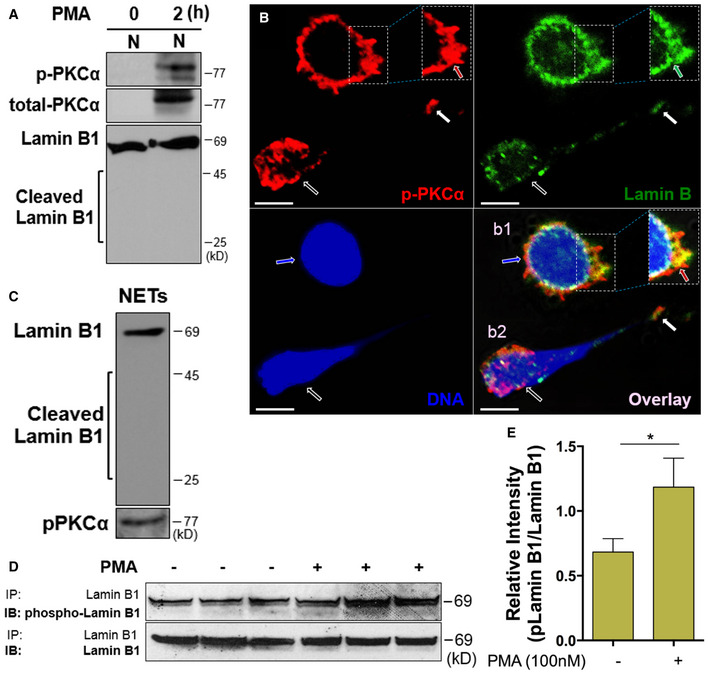
To explore the potential role of phosphorylated PKCα (p‐PKCα) in nuclear envelope rupture, we first isolated the nucleus of neutrophils that were stimulated by PMA at 0 and 2 h (Fig 4A). We found accumulation of p‐PKCα and full‐length lamin B in the nuclear fraction of neutrophils with 2‐h PMA stimulation (Fig 4A), while there was no detectable total PKCα in the nuclear fraction of neutrophils at time 0 (Fig 4A) in immunoblot analysis. These data are in line with the results obtained by fluorescent microscopy (Fig 3A). Confocal microscopy analysis of a neutrophil in the early stage of NET formation further confirmed the accumulation of p‐PKCα in the nucleus, particularly at the rupture site of the nuclear envelope, which had decondensed/swollen chromatin but not yet released to form extracellular traps (b1 cell, Fig 4B). In contrast, another neutrophil in the same image showed a ruptured nuclear envelope with release of decondensed chromatin as the backbone of NETs that contained the mixture of p‐PKCα and disassembled nuclear lamin B (b2 cell, Fig 4B). Furthermore, p‐PKCα and full‐length lamin B were also detected in the immunoblot of the extracellular NETs isolated from cultured human primary pPMNs (Fig 4C). The results of concomitant detection of these two molecules both by immunofluorescent microscopy (Fig 4B) and immunoblots (Fig 4C) indicate a potential active interaction between p‐PKCα and lamin B during nuclear envelope rupture.
Figure 4. Accumulation of phosphorylated PKCα in nucleus results in nuclear lamin B phosphorylation, disassembly, and the consequent nuclear envelope rupture during NET formation.
-
ARepresentative immunoblots of p‐PKCα and total PKCα of isolated nuclei and the corresponding status of lamin B in human dPMNs that were treated with PMA for 0 or 2 h.
-
BRepresentative sectional images of nuclei of two neutrophils treated by PMA, stained for lamin B (primary anti‐lamin B, and FITC‐labeled secondary antibody), p‐PKCα (primary anti‐human p‐PKCαS657, and PE‐labeled secondary antibody), and nuclear DNA (DAPI). The accumulation of p‐PKCα (red arrow pointed) at the site of ruptured/interrupted (green arrow) nuclear lamin B, and swollen/decondensed DNA (blue arrow) were indicated (b1). A neutrophil (white empty arrow) with NET formation that contained the mixture of DNA with p‐PKCα and lamin B (white arrow) was also demonstrated (b2). Scale bars, 10 μm.
-
CImmunoblot images of lamin B, then stripped and probed for p‐PKCα, of the homogenates of NETs isolated from conditioned medium of primary human pPMNs with NET formation that were induced by PMA.
-
D, ERepresentative and summary immunoblot (IB) detection of phospho‐lamin B and total lamin B with the lamin B protein purified by immunoprecipitation (IP) with anti‐lamin B from human dPMNs that were treated either by PMA (D, E) for 0 or 3 h. Data represent mean ± SD (n = 3–5 biological replicates) for (E). *P < 0.05 between groups as indicated. Comparison between two groups was analyzed by the Student t test.
Source data are available online for this figure.
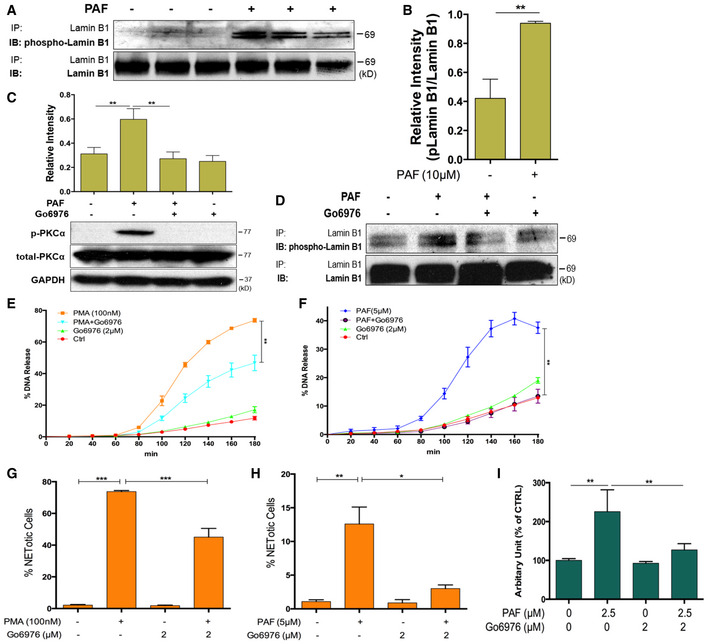
In several other contexts of nuclear envelope rupture (Shimizu et al, 1998; Machowska et al, 2015), nuclear lamin B is disassembled through lamin B phosphorylation. There is no relevant anti‐phosphorylated lamin B (phospho‐lamin B) antibody available for the direct detection of phospho‐lamin B. Here, we purified lamin B through immunoprecipitation and analyzed the protein phosphorylation status with anti‐p‐Ser/Thr/Tyr antibody. Interestingly, increased phospho‐lamin B was detected among total lamin B, purified through immunoprecipitation with anti‐lamin B antibody, from neutrophils with NET formation stimulated by PMA (Fig 4D and E) or PAF (Fig EV2A and B), as compared to those from control neutrophils.
Figure EV2. Inhibition of PKCα phosphorylation attenuated Lamin B1 phosphorylation and alleviated extracellular trap formation. (Related to Figs 4 and 5).
-
A, BRepresentative and summary immunoblot (IB) detection of phosphor‐lamin B and total lamin B with the lamin B protein purified by immunoprecipitation (IP) with anti‐lamin B from human dPMNs that were treated either by PAF (A, B) for 0 or 3 h.
-
CSummary and representative immunoblots of the total PKCα and p‐PKCα in dPMNs that were pretreated without or with PKC inhibitor Go6976 for 1 h and then treated without or with PAF (B) for 3 h.
-
DRepresentative immunoblot (IB) detection of the phospho‐lamin B and total lamin B with lamin B protein purified by immunoprecipitation (IP) with anti‐lamin B from human dPMNs that were pretreated without or with PKC inhibitor Go6976 for 1 h and then treated either by PAF (D) for 3 h.
-
E, FThe kinetic analysis of NET‐DNA release index was determined by coincubation of primary human pPMNs that were pretreated without or with PKCα inhibitor Go6976 for 1 h and then treated without (control) or with 100 nM PMA (E) or 5 μM PAF (F) in medium containing 1 μM SYTOX Green dye for 3 h with recording by a microplate reader for every 20 min. The NET‐DNA release index was reported in comparison with an assigned value of 100% for the total DNA released by neutrophils lysed by 0.5% (v/v) Triton X‐100.
-
G, HSummary analysis of PMA (G)‐ or PAF (H)‐induced NET formation in pPMNs that were stimulated without or with 100 nM PMA or 5 μM PAF for 3 h and stained with both cell‐permeable SYTO Red and cell‐impermeable SYTOX Green, without fixation. Images were taken by Olympus confocal microscopy, followed by automated quantification of NETs on 5–6 non‐overlapping area per well using ImageJ for calculation of % NETotic cells.
-
FSummary analyses of extracellular trap formation in RAW264.7 cells that were pretreated without or with PKCα inhibitor Go6976 for 1 h and then treated without or with PAF (I) for 3 h. Then, the plates were analyzed with microplate reader for fluorometric NET quantification.
Taken together these results indicate that nuclear accumulation of p‐PKCα (Fig 4A and B) may be responsible for nuclear lamin B phosphorylation (Figs 4D and E, and EV2A and B), phosphorylation‐dependent lamin B disassembly, and nuclear envelop rupture that allows the release of full‐length lamin B with decondensed nuclear chromatin during NET formation (Fig 4B).
Decreasing lamin B phosphorylation by PKCα inhibition, or mutation of the phosphorylation sites in lamin B, attenuates NET release
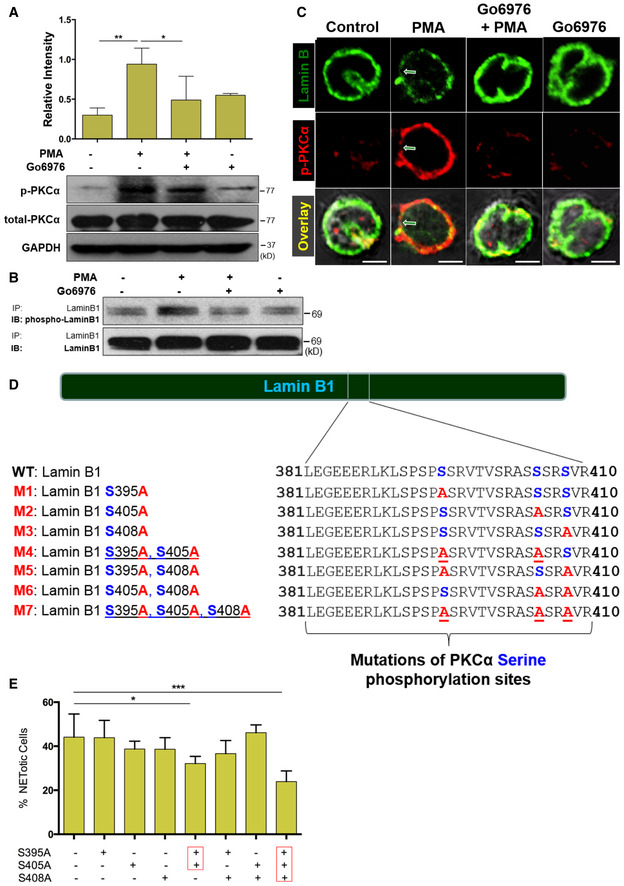
To explore the causal role of lamin B phosphorylation, we found that pretreatment of human neutrophils with Go6976, a selective inhibitor of conventional PKC, attenuated PKCα phosphorylation in human neutrophils that were stimulated by PMA (Fig 5A) or PAF (Fig EV2C). Importantly, inhibition of PKCα by Go6976 also decreased lamin B phosphorylation in neutrophils that were treated with PMA (Fig 5B) or PAF (Fig EV2D), as compared to controls. Furthermore, confocal microscopy analysis showed that pretreatment of neutrophils with Go6976 decreased the nuclear accumulation of p‐PKCα and prevented the nuclear envelope disintegration in PMA‐stimulated neutrophils (Fig 5C). Consequently, inhibition of PKCα significantly attenuated NET formation of primary human pPMNs that were treated by PMA or PAF (Fig EV2E–H). In addition, pretreatment of RAW264.7 macrophages with the PKCα inhibitor Go6976 attenuated PAF‐induced extracellular trap formation (Fig EV2I).
Figure 5. Inhibition of lamin B phosphorylation, or mutation of phosphorylation sites in lamin B, attenuates nuclear envelope rupture and NET release.
-
ASummary and representative immunoblots of total PKCα and p‐PKCα in dPMNs that were pretreated without or with PKC inhibitor Go6976 for 1 h and then treated without or with PMA (A) for 3 h.
-
BImmunoblot (IB) detection of the phospho‐lamin B and total lamin B with lamin B protein purified by immunoprecipitation (IP) with anti‐lamin B from human dPMNs that were pretreated without or with PKC inhibitor Go6976 for 1 h and then treated by PMA (B) for 3 h.
-
CConfocal microscopy images of human dPMNs that were pretreated without or with PKCα Go6976 for 1 h and then treated without or with PMA for 3 h, followed by staining of lamin B (primary anti‐lamin B, and FITC‐labeled secondary antibody), and phosphorylated PKCα (primary anti‐human p‐PKCαS657, and PE‐labeled secondary antibody). The green/white arrows indicate the site of nuclear envelope rupture. Scale bars, 10 μm.
-
DSchematic representation of lamin B domain structure and several mitotic serine phosphorylation sites (indicated by blue font) that exhibit PKC consensus motifs (Mall et al, 2012) and overview of lamin B reporters containing serine (S) to alanine (A) mutations at PKC consensus phosphorylation sites.
-
EThe endpoint analysis of % cells with NET formation was detected with dPMNs that were transfected with plasmids of either wild‐type lamin B (WT control) or mutants with single or multiple defined point mutations at PKCα‐consensus phosphorylation sites (S395A, S405A, S408A) of lamin B, and treated for 3 h by 10 μM PAF in medium containing cell‐permeable dye SYTO Red (500 nM) for the total cell count, while the cells with NET formation were stained by cell‐impermeable dye SYTOX Blue (5 μM). Then, the images were taken with Olympus confocal microscopy, followed by automated quantification of NETs using ImageJ for quantification of % cells with NET formation.
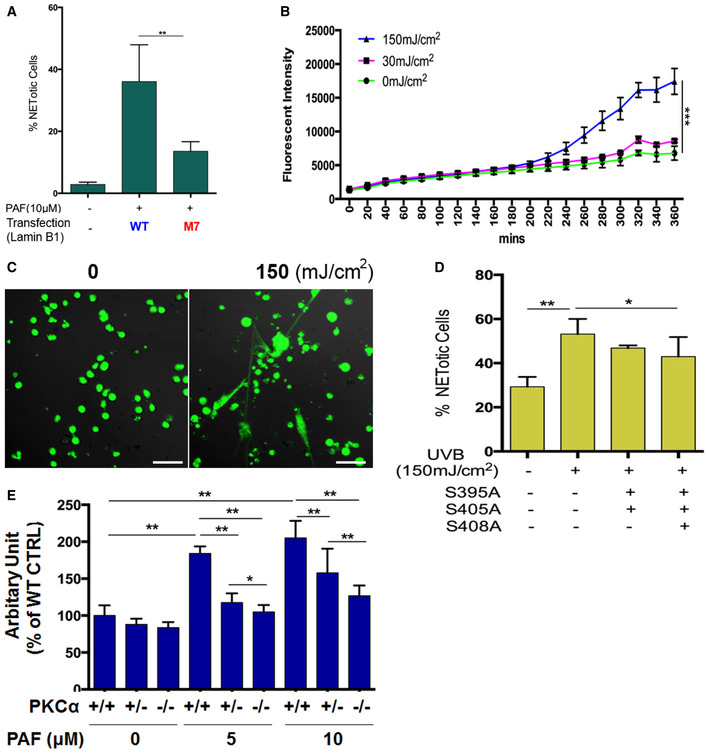
To further define the causal role of PKCα‐mediated lamin B phosphorylation in NET formation, we introduced mutations at PKCα‐consensus serine phosphorylation sites (S395, S405, S408) of lamin B (Hocevar et al, 1993; Mall et al, 2012; Machowska et al, 2015) by replacement of serine (S) with alanine (A) to prevent PKCα‐mediated serine phosphorylation. Mutants were made either with single mutations, or with different combinations of mutations (Fig 5D). We found that PAF‐induced NET formation was significantly impaired in dPMNs which were transfected with plasmids containing mutations at all three (S395A/S405A/S408A) or two (S395A/S405A), but not other (S395A, S405A, S408A, S395A/S408A, S405A/S408A), PKCα‐consensus serine phosphorylation sites of lamin B, as compared to those transfected with WT lamin B (Fig 5E). In addition, PAF‐induced extracellular trap formation was also attenuated in RAW264.7 macrophages that were transfected with plasmids containing mutations at all three (S395A/S405A/S408A), but not fewer (S395A, S395A/S405A), as compared to those transfected with WT lamin B controls (Fig EV3A).
Figure EV3. Mutation of PKCα phosphorylation site in lamin B or PKCα deficiency impaired extracellular trap formation‐induced by PAF or UVB irradiation (Related to Figs 5 and 6).
-
ASummary analysis of extracellular trap formation in the RAW264.7 cells that were transfected with plasmids of either wild‐type lamin B (WT control), or the M7 mutant of PKCα‐consensus phosphorylation sites (S395A/S405A/S408A) of lamin B, and treated by 10 μM PAF for 3 h. The cells were stained by cell‐impermeable dye SYTOX Green (500 nM), and the total cells were detected by staining with cell‐permeable dye SYTO Red (500 nM). Images were taken by Olympus confocal microscopy, followed by automated quantification of NETs using ImageJ for quantification of % cells with extracellular trap release. RAW264.7 cells without transfection and without PAF treatment were served as basal control.
-
B, CSummary (B) and representative (C) analysis of NET formation in dPMNs that were irradiated without or with UVB at 30 or 150 mJ/cm2, in medium containing 1 μM SYTOX Green dye for 6 h with recording every 20 min by a microplate reader for up to 6 h. Then, the plates were analyzed with microplate reader for fluorometric NET quantification. Scale bar, 50 μm.
-
DSummary analyses of NET formation in the dPMNs that were transfected with plasmids of either wild‐type lamin B (WT control for the left two columns), or the M4 or M7 mutants and then were irradiated without or with UVB at 150 mJ/cm2. Then, the staining and quantification of % NETotic cells were conducted as described in panel (A).
-
ESummary analyses of NET formation in mouse bone marrow mPMNs, from WT, heterozygous, or homozygous PKCα‐deficient mice, which were treated without or with either 5 or 10 μM of PAF for 3 h, following by fluorometric NET quantification analysis. Data information: The panels (A, B, D) were calculated based on at least 3 independent biological replicates. Panel (E) is a summary analysis that was calculated based on the arbitrary fluorescent unit from 3 independent biological replicates. Data are given as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 between groups as indicated. Comparisons among three or more groups were analyzed using ANOVA, followed by Student–Newman–Keuls test.
Ultraviolet light exposure is related to various human diseases, including lupus and skin cancer (Damiani & Ullrich, 2016). Recent studies have reported that UV radiation may induce NET formation in vitro (Azzouz et al, 2018; Zawrotniak et al, 2019). To explore if UVB‐induced NET formation contributes to UVB‐induced skin inflammation in vivo in the current study, we set out to study it in vitro. We found that exposure of dPMNs to UVB at the dose of 150, but not 30 mJ/cm2 significantly induced NET formation in vitro (Fig EV3B and C). Very interestingly, UVB‐induced NET formation was also attenuated in dPMNs that were transfected with plasmids containing mutations at all three (S395A/S405A/S408A), but not two (S395A/S405A), PKCα‐consensus serine phosphorylation as compared to WT lamin B controls (Fig EV3D).
All of the above experiments highlight a causal link between PKCα and lamin B phosphorylation, suggesting that PKCα may serve as a lamin kinase to mediate lamin B phosphorylation. This consequently results in lamin B disassembly and nuclear envelope rupture, followed by release of extracellular traps in neutrophils or macrophages upon stimulation.
Genetic deficiency of PKCα decreases lamin B phosphorylation and NET formation in primary mouse neutrophils
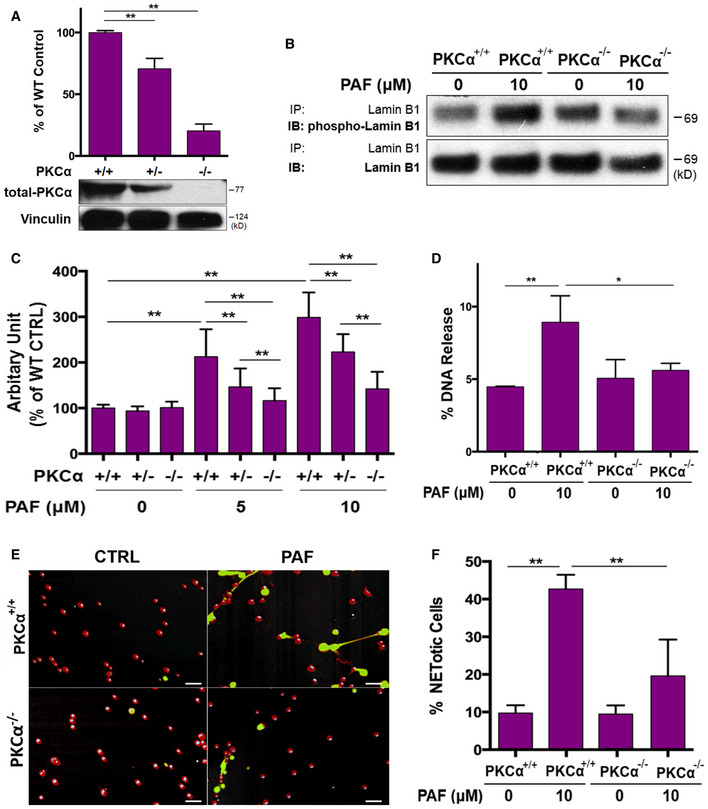
Next, we sought to further explore the causal effect of PKCα in lamin B phosphorylation and NET formation by using primary mouse neutrophils from both heterozygous and homozygous PKCα deficient mice (Fig 6A). Firstly, the repeated experiments have shown decreased lamin B phosphorylation of immunopurified lamin B by precipitation from the PKCα−/− bone marrow mPMNs as compared to the WT mPMNs (Fig 6B) without or with PAF treatment. These results further defined that PKCα may serve as a lamin kinase to phosphorylate lamin B during NET formation. Most importantly, we found that both peritoneal (Fig 6C–F) and bone marrow (Fig EV3E) mPMNs from either heterozygous (Fig 6C) or homozygous (Fig 6C–F) PKCα‐deficient mice had significantly impaired NET formation detected by fluorometric NET quantification (Fig 6C), endpoint analysis of NET‐DNA release index (Fig 6D), and fluorescent imaging analysis (Fig 6E and F). In addition, the protective effects of PKCα deficiency were dose‐dependent as less NET formation was seen in neutrophils from homozygous PKCα KO mice as compared to those from heterozygous PKCα‐deficient mice (Figs 6C and EV3E). Our results from the genetic PKCα deficient mice provide important evidence that PKCα is crucial to NET formation, at least in part, by serving as a lamin kinase to regulate phosphorylation and disassembly of lamin B during nuclear envelope rupture and NET formation in neutrophils.
Figure 6. Genetic deficiency of PKCα attenuates lamin B phosphorylation and NET formation in primary neutrophils from PKCα knockout mice.
-
ASummary and representative immunoblots of PKCα expressions in mouse bone marrow neutrophils from PKCα−/−, PKCα+/−, and their littermate WT (PKCα+/+) mice. Vinculin served as loading control.
-
BRepresentative immunoblot (IB) detection of the phospho‐lamin B (Ser) and total lamin B with lamin B protein purified by immunoprecipitation (IP) with anti‐lamin B from mouse peritoneal mPMNs from WT vs. PKCα−/− mice, stimulated without or with PAF for 3 h.
-
CSummary analyses of NET formation in peritoneal mPMNs, from WT, PKCα+/−, or PKCα−/− mice, which were treated without or with either 5 μM or 10 μM PAF for 3 h following by 2% PFA fixation and fluorometric microplate analysis.
-
DThe endpoint analysis of NET‐DNA release index was detected by coincubation of primary mouse peritoneal mPMNs from WT and PKCα−/− mice without (control) or with 10 μM PAF in medium containing 1 μM SYTOX Green dye with recording by a microplate reader at the end of the 3 h time point. The NET‐DNA release index was reported in comparison with an assigned value of 100% for the total DNA released by neutrophils lysed by 0.5% (v/v) Triton X‐100.
-
E, F(E) Representative and (F) summary analysis of NET formation of mouse peritoneal mPMNs from WT vs. PKCα−/− mice stimulated without or with 10 μM PAF for 3 h and stained with both cell‐permeable SYTO Red dye and cell‐impermeable SYTOX Green dye, without fixation. Images were taken by Olympus confocal microscopy, followed by automated quantification of NETs on 5–6 non‐overlapping area per well using ImageJ for calculation of % cells with NET formation. (E) Scale bars, 50 μm.
Strengthening nuclear envelope integrity by lamin B overexpression attenuates NET release in vivo and reduces NET‐associated proinflammatory cytokines in UVB‐irradiated skin of lamin B transgenic mice
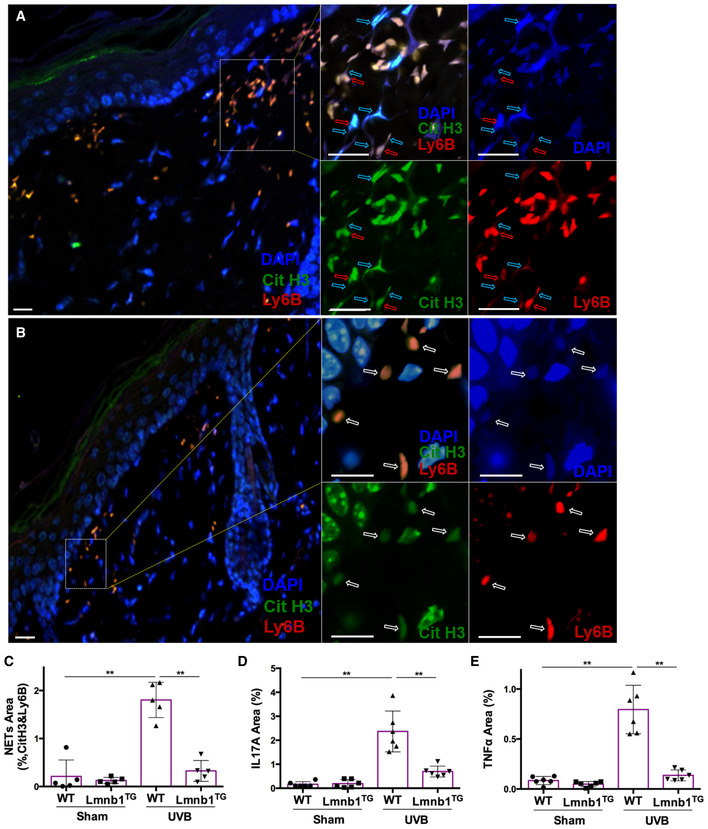
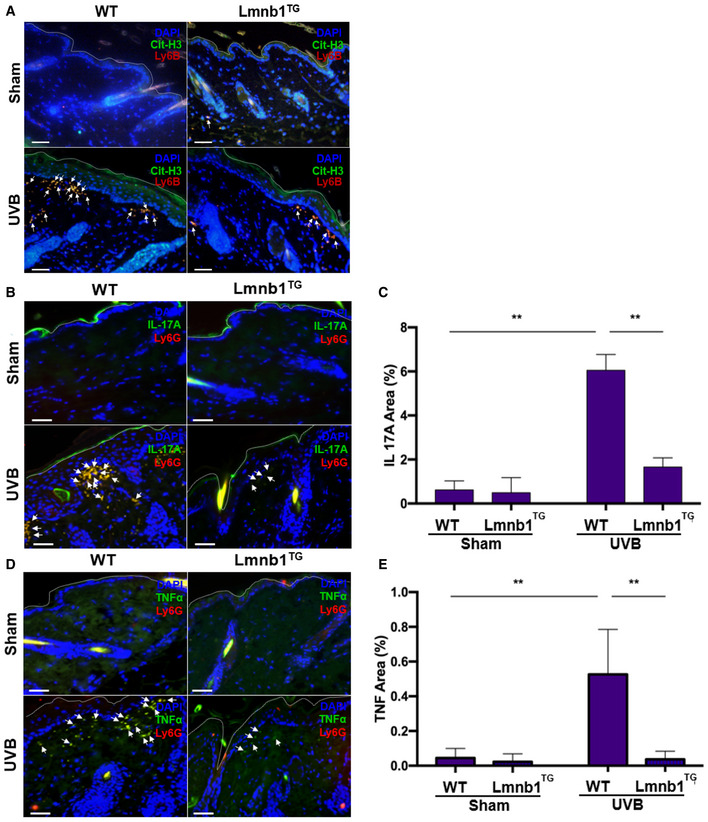
After identifying a crucial role of lamin B integrity in NET formation in vitro (Fig 1E–H), we next sought to examine if the same mechanism is also acting in vivo. IHC staining demonstrated extensive extrusion of decondensed chromatin in neutrophils in skin tissues of UVB‐irradiated WT mice (Fig 7A). In contrast, we observed fewer neutrophils, particularly neutrophils without externalization of citrullinated chromatin (anti‐Cit‐H3 staining), in UVB‐irradiated skin of Lmnb1TG mice (Fig 7B). Therefore, these in vivo results confirm the findings from the in vitro experiments (Fig 1E–H), in which strengthening nuclear envelope integrity by lamin B overexpression attenuated NET formation. The summary analysis demonstrates that NET formation and neutrophil infiltration was attenuated in UVB‐irradiated skin of Lmnb1TG mice as compared to those of WT littermates (Figs 7C and EV4A). Furthermore, neutrophils with NET formation exhibited proinflammatory cytokines, IL‐17A and TNFα, in UVB‐irradiated skin of WT littermates (Figs 7D and E, and EV4B–E). Very interestingly, we found attenuated NET release with decreased levels of NET‐associated cytokines in UVB‐irradiated skin of Lmnb1TG mice (Figs 7C–E and EV4A–E) compared to WT littermates. In the current study, we used both anti‐Ly6B (Nakazawa et al, 2017; Fig 7A–E) and anti‐Ly6G (Amulic et al, 2017; Fig EV4A–E) to detect neutrophils as reported by other publications (Amulic et al, 2017; Nakazawa et al, 2017).
Figure 7. Strengthening nuclear envelope integrity by lamin B overexpression decreases NET release in vivo and alleviates NET‐associated proinflammatory cytokine accumulation in UVB‐irradiated skin of the lamin B transgenic mice.
-
A–C(A, B) representative (C) summary analysis of confocal fluorescent microscopy images of neutrophils with NET formation in the skin tissue of WT (A) vs. Lmnb1TG (B) mice with UVB exposure, in which DNA was stained by DAPI, citrullinated histone H3 was probed by rabbit anti‐mouse citrullinated histone H3, following stained by Alexa Fluor‐488‐labeled donkey anti‐rabbit secondary antibody, while neutrophil surface marker Ly6B was probed by rat anti‐mouse Ly6B Ab following stained by Alexa Fluor‐647‐conjugated goat anti‐rat secondary antibody. The images of different staining and their overlays are shown (A, B). The parental neutrophils (stained by neutrophil membrane surface marker Ly6B) and their released extracellular NET structures (co‐stained by anti‐cit H3 and DAPI) are indicated by red arrows and light blue arrows, respectively. The white arrows indicated neutrophils without NET release. Scale bar, 50 μm.
-
D, ESummary analysis for staining area of neutrophils with NET formation and their exhibition of IL‐17A (D) or TNFα (E), in the skin tissue of WT vs. Lmnb1TG mice without (sham) or with UVB exposure, by co‐staining of Ly6B (stained as described in panels A–C) with IL‐17A (D) or TNFα (E) using FITC‐conjugated rat anti‐mouse IL‐17A, or FITC‐conjugated rat anti‐mouse TNFα Abs.
Figure EV4. Lamin B overexpression decreased NET release and alleviated exhibition of NET‐associated proinflammatory cytokines in skin of lamin B1 transgenic mice after UVB irradiation (Related to Fig 7).
-
A–EFluorescent staining of the skin tissues from Lmnb1TG mice and their WT littermates that were irradiated or not (sham) by UVB. (A) The citrullinated histone H3 was probed by rabbit anti‐mouse citrullinated histone H3, following stained by Alexa Fluor‐488‐labeled donkey anti‐rabbit secondary antibody, while neutrophil marker was probed by rat anti‐mouse Ly6B Ab following stained by Alexa Fluor‐647 conjugated goat anti‐rat secondary antibody. (B–E) The representative (B, D) or summary (C, E) analysis of neutrophil surface marker Ly6G was stained by PE‐conjugated rat anti‐mouse Ly6G Ab. FITC‐labeled rat anti‐mouse IL‐17A antibody was used to detect IL‐17A. FITC‐labeled rat anti‐mouse TNF‐α antibody was used to detect TNF‐α. DNA was stained by DAPI for or panel (A–E).
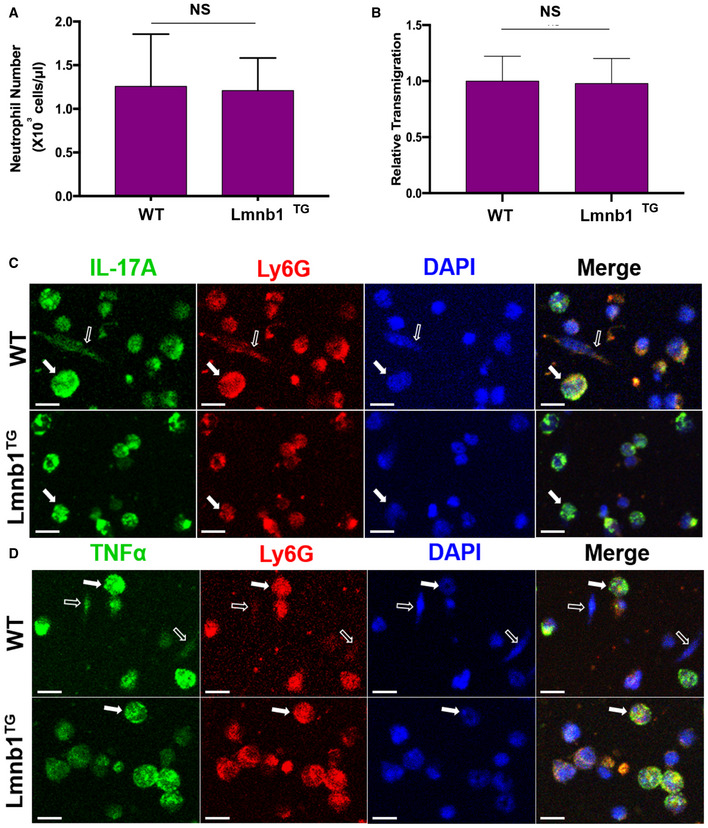
To understand the decreased numbers of neutrophils with NET formation in skin of UVB‐irradiated Lmnb1TG mice, we also examined if nuclear lamin B overexpression affects the neutrophil counts in the peripheral blood and the neutrophil transmigration ability, which may influence the amount of neutrophil recruitment to inflamed skin. No significant differences were detected for either neutrophil counts in peripheral blood (Fig EV5A), or pore transmigration ability of neutrophils (Fig EV5B), from the Lmnb1TG vs. WT mice. Therefore, neither the normal neutrophil counts, nor the unaffected transmigration ability, may contribute to the decreased neutrophil accumulation in the skin tissues of UVB‐irradiated Lmnb1TG mice as compared to those of WT control mice (Fig 7C).
Figure EV5. Features of neutrophil counts in peripheral blood, transmigration ability and cytokine expression in neutrophils from Lmnb1TG vs WT mice.
-
ANeutrophil counts in peripheral blood from Lmnb1TG mice and their WT littermates (n = 4 biological replicates).
-
BRelative transmigration was measured using Boyden chamber with pore size of 3.0 μm. The pore transmigration ability in neutrophils from the Lmnb1TG mice was expressed as relative transmigration compared to that in neutrophils from WT littermates (n = 3 biological replicates).
-
C, DThe bone marrow neutrophils from Lmnb1TG mice and their WT littermates were treated with 10 μM PAF for 16 h and then fixed with 2% PFA and permeabilized with 0.1% Triton X‐100, following immunocytochemistry staining. The neutrophil surface marker Ly6G was stained by PE‐conjugated rat anti‐mouse Ly6G Ab. FITC‐labeled rat anti‐mouse IL‐17A antibody or FITC‐labeled rat anti‐mouse TNF‐α antibody was used to detect IL‐17A or TNF‐α. DNA was stained by DAPI for or panels (C, D).
It has been known that IL‐17A and TNFα can synergistically work to sustain neutrophil recruitment during inflammatory responses (Liu et al, 2011; Griffin et al, 2012). Thus, one may propose that the exhibition of IL‐17A and TNFα by NETs may propagate neutrophil recruitment to inflamed skin of UVB‐irradiated mice. To address this point, we found reduced exhibition of NET‐associated IL‐17A and TNFα in skin tissue of UVB‐irradiated Lmnb1TG mice as compared to those of WT mice (Figs 7D and E, and EV4B–E). In the in vitro study, we found the expression of IL‐17A and TNFα in PAF‐stimulated neutrophils from both Lmnb1TG vs. WT mice, and the exhibition of these cytokines by NETs of neutrophils from WT mice (Fig EV5C and D). These in vitro experimental results indicate that neutrophils from both Lmnb1TG vs. WT mice can express IL‐17A and TNFα that are known to diffuse freely from local tissue to the circulation and then diluted. In contrast, NET‐associated cytokines can be retained in local microenvironment with a relatively high concentration, thus synergistically contributing to the sustained neutrophil recruitment to inflamed skin. Thus, reduced NET formation with decreased extracellular display of IL‐17A and TNFα may, at least in part, explain the attenuated neutrophil recruitment to UVB‐irradiated skin in Lmnb1TG mice as compared to that in WT littermates.
The results from in vitro and in vivo studies have verified our hypothesis that lamin B is crucial to nuclear envelope integrity, and strengthening nuclear envelope integrity by lamin B overexpression attenuated NET release in lamin B transgenic mice.
Discussion
Externalization of decondensed chromatin from the ruptured nuclear envelope forms the backbone of NETs. Lamin B filamentous meshwork is a substantial structural component of the nuclear envelope. Given the nature of their structural arrangement, lamin B filaments are assembled laterally to form a highly organized meshwork underneath the inner nuclear membrane. The two are vertically associated by the integral membrane protein LBR (Fig 1C; Vergnes et al, 2004; Goldberg et al, 2008). The role of nuclear lamin B in neutrophil biology and its involvement in nuclear envelope rupture and NET formation has not been investigated, although LBR has been used as a marker of the nuclear envelope in neutrophil biology studies (Fuchs et al, 2007; Singh et al, 2016). Here, we verified that nuclear lamin B is involved, and the disassembled lamin B is also released and decorated on the surface of externalized NET structures. To test the effects of nuclear lamin B, we found that overexpression of lamin B strengthened the nuclear envelope integrity and blunts NET release in vitro. In contrast, the reduced amount of mature lamin B both in neutrophils and macrophages, by inhibition of protein farnesylation with farnesyltransferase inhibitor (FTI) (Adam et al, 2013), can enhance NET release in these cells. Taken together, the levels of nuclear lamin B expression affect nuclear envelope integrity and NET formation in a negative dose‐dependent manner. Therefore, our findings provide compelling evidence for the importance of lamin B in the nuclear envelope integrity and NET formation. In line with our findings, a recent study reported that nuclear lamin A/C is also involved in NET formation (Amulic et al, 2017).
During nuclear envelope rupture, nuclear lamin B is either cleaved or disassembled (Hocevar et al, 1993; Collas et al, 1997; Slee et al, 2001) in different cellular events. According to the lytic cell death mechanism of NET formation described before (Fuchs et al, 2007; Papayannopoulos et al, 2010; Yipp & Kubes, 2013), we expected to see the destructive proteolytic cleavage of lamin B during NET formation. However, we only found the intact full‐length lamin B both in whole‐cell lysates and the isolated NETs. These unexpected results indicated that this nuclear structural protein was not destructively cleaved during NET formation. In contrast, lamin B in apoptotic neutrophils was indeed fragmented, through proteolytic cleavage by the activated caspase‐3 (Slee et al, 2001). In our study, the pro‐caspase‐3 remained as an inactive molecule throughout the period of NET formation. Our results are in line with a previous report in which NET formation is a caspase‐independent process (Remijsen et al, 2011). All of the above results support the proteolytic‐independent mechanism of lamin B disassembly during NET formation. Cyclin‐dependent kinases (CDK) 4/6 have been reported to be involved in NET formation through lamin A/C phosphorylation (Amulic et al, 2017). In addition, nuclear swelling and chromatin decondensation via histone citrullination (Wang et al, 2009) or acetylation (Hamam et al, 2019) provides physical force to drive nuclear envelope rupture and chromatin release (Neubert et al, 2018). Furthermore, a few recent studies reported that Gasdermin D may also contribute to nuclear expansion, nuclear envelope rupture, and NET release (Chen et al, 2018; Sollberger et al, 2018). Taken together, these latest novel findings from our and other groups (Amulic et al, 2017; Chen et al, 2018; Neubert et al, 2018; Sollberger et al, 2018; Hamam et al, 2019) suggest that nuclear envelope rupture appears to be a distinct process from previously described (Fuchs et al, 2007; Papayannopoulos et al, 2010; Yipp & Kubes, 2013).
Assembly of the monomeric lamin proteins (lamin A/C, B) is required for the formation of fibrous meshworks and maintenance of the nuclear envelope integrity (Vergnes et al, 2004; Goldberg et al, 2008). To explore the potential mechanisms of lamin B disassembly, we found that cytosolic PKCα was translocated to nucleus where it became activated and phosphorylated, probably by diacylglycerol (DAG) derived from the nuclear membrane (Neri et al, 1998; Martelli et al, 2006). Nuclear DAG may act as a chemoattractant to induce nuclear translocation and activation of PKCα (Neri et al, 1998; Martelli et al, 2006), while nuclear lamin B serves as a PKCα substrate for its nuclear localization (Neri et al, 1998; Martelli et al, 2006). Nuclear accumulation of activated PKCα, i.e., p‐PKCαSer657 (Bornancin & Parker, 1997), is responsible for lamin B phosphorylation, phosphorylation‐dependent disassembly (Hocevar et al, 1993), and consequent nuclear envelope rupture. In the current study, co‐localization of p‐PKCα and lamin B in the nucleus would make it possible for their direct interaction and phosphorylation of lamin B, while phosphorylation results in lamin B disassembly and efficient nuclear envelope breakdown (Collas et al, 1997; Mall et al, 2012). In addition, the coexistence of p‐PKCα and lamin B in the released extracellular NETs further indicate their possible interaction happened in the nucleus.
To determine the causal role of PKCα, we found that either pharmacological inhibition, or genetic deficiency, of PKCα inhibited lamin B phosphorylation, resulting in attenuated nuclear envelope rupture and NET formation. Most importantly, mutation at three PKCα‐consensus serine phosphorylation sites of lamin B impaired the release of extracellular traps both in neutrophils and macrophages, indicating a causal role of PKCα‐mediated lamin B phosphorylation in lamina disassembly and nuclear envelope rupture during NET formation. The above findings with independent experimental approaches provide strong evidence for the causal role of PKCα‐mediated lamin B phosphorylation in NET formation. In addition to PKCα, PKCβII may also be involved in NET formation as Go6976 is known to inhibit both PKCα and PKCβ, and PKCβII is known to phosphorylate lamin B (Martelli et al, 2006). In line with us, the PKCβ‐specific inhibitor LY333531 is also reported to inhibit NET formation in vitro (Chen et al, 2018).
PKCs regulate the function of proteins through phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins (Fontayne et al, 2001). The current study indicates that PKCα can phosphorylate several serine sites (S395/S405/S408) of lamin B, resulting in lamin B disassembly. Fontayne et al (2001) reported that several PKCs (i.e., PKCα) can indirectly induce ROS production through NOX activation by phosphorylation of serine (probably S328) of the NOX p47phox subunit. So, both lamin B and p47phox are substrates for PKC‐mediated phosphorylation (Collas et al, 1997; Fontayne et al, 2001). Lamin B phosphorylation is the direct effect of PKCα in the nucleus, while ROS generation is an indirect effect of PKCα through activation of NOX2 in cell membrane (Fontayne et al, 2001); thus, the two distinct cellular events seem to take place in different cellular compartments during the multi‐step process of NET formation. Given PKCα‐mediated nuclear lamina disassembly may be essential to nuclear chromatin extrusion from the broken nuclear envelope during NET formation, while NOX2‐mediated ROS is only involved in some (Fuchs et al, 2007; Papayannopoulos et al, 2010; Douda et al, 2015), but not all (Rochael et al, 2015; Pieterse et al, 2018; Tatsiy & McDonald, 2018), types of NET formation through different mechanisms (Fuchs et al, 2007; Papayannopoulos et al, 2010; Douda et al, 2015). Thus, one may propose that the direct effects of PKCα on nuclear lamin B phosphorylation is required, but the indirect effects of PKCα through NOX‐ROS pathway may not be necessary, to the nuclear envelop rupture during NET formation. Similarly, Zychlinsky's group reported that CDK4/6 can control NET formation through phosphorylation of lamin A/C (Amulic et al, 2017), while lamin A/C can still be phosphorylated in NOX2‐deficient neutrophils from patients with chronic granulomatous disease (Amulic et al, 2017). Thus, they suggest that the cell‐cycle signaling CDK4/6 may function in a parallel pathway to ROS generation during NET formation (Amulic et al, 2017). Taken together, nuclear lamina phosphorylation (for lamin A/C, and B) by either CDK4/6 or PKCα, and cytosolic ROS generation by NOX2, might be independent cellular events during NET formation (Amulic et al, 2017).
In addition to identifying the crucial role of lamin B in NET formation in vitro, we also tested it in vivo by studying NET formation in skin inflammation induced by UVB exposure, a risk factor for lupus and skin cancer (Damiani & Ullrich, 2016). We confirmed the crucial role of lamin B in nuclear envelope integrity and NET formation in vivo as neutrophils in UVB‐irradiated skin of Lmnb1TG mice exhibited a lack of NET formation. Regarding the relevant mechanisms, UVB exposure is known to stimulate skin keratinocytes to release PAF (Damiani & Ullrich, 2016), a natural proinflammatory lipid mediator that can induce NET formation (Etulain et al, 2015). The current study suggests that PAF might be a potential natural stimulus for NET formation in UVB‐irradiated skin. Furthermore, the current study and few recent publications demonstrated that UV could induce NET formation in vitro in cultured neutrophils (Azzouz et al, 2018; Neubert et al, 2019; Zawrotniak et al, 2019). Therefore, UVB‐induced NET formation in vivo may be attributed to the indirect effect of UVB through PAF (Etulain et al, 2015; Damiani & Ullrich, 2016) and the direct effects of UVB light on neutrophils that have been recruited to the inflamed skin (Zawrotniak et al, 2019).
The externalization of NETs may be important for the propagation of inflammation by induction of proinflammatory cytokines (Lood et al, 2016). Studies have shown that UVB irradiation can induce IL‐17A (Li et al, 2015) and TNFα (Sharma et al, 2011), both of which can be expressed by neutrophils (Lin et al, 2011; Giambelluca et al, 2014). In the current study, we found that NETs exhibited both IL‐17A and TNFα in the skin of UVB‐irradiated WT mice. Co‐presence of IL‐17A and TNFα on NETs may synergistically propagate inflammatory responses through different mechanisms (Ruddy et al, 2004; Liu et al, 2011; Griffin et al, 2012). Interestingly, the current study showed that strengthening nuclear envelope integrity attenuated NET formation in vivo in Lmnb1TG mice. Those neutrophils enclosed IL‐17A and TNFα within the cells, thus decreasing the opportunity for their exhibition by NETs to the extracellular milieu. Therefore, the intracellular retention, but not extensive extracellular display, of cytokines by neutrophils without NET release may prevent further propagation of inflammatory responses in UVB‐irradiated Lmnb1TG mice. On the other hand, the neutrophil numbers in blood may not contribute to the decreased leukocyte infiltration in the skin of UVB‐irradiated Lmnb1TG mice since lamin B overexpression does not affect neutrophil counts in blood.
It is known that the stiffness of nuclei may affect the transmigration capability of cells (Lammerding et al, 2006; Goldberg et al, 2008; Rowat et al, 2013). Published papers demonstrated that A‐type lamins, but not B‐type lamins, contribute to the mechanical stiffness of nuclei (Lammerding et al, 2006; Goldberg et al, 2008; Rowat et al, 2013), and the nuclear stiffness increases with the lamin A:B ratio (Shin et al, 2013). Increasing the lamin A:B ratio by downregulation of lamin B promotes nuclear stiffness in hematopoietic cells (Shin et al, 2013). Thus, elevated lamin B expression may not increase nuclear stiffness in neutrophils and influence their migration to inflamed skin in Lmnb1TG mice. Therefore, reduced NET release with decreased extracellular cytokine exhibition may explain, at least in part, the attenuated skin inflammation in UVB‐irradiated Lmnb1TG mice. Nevertheless, the current study was mainly focused on the novel molecular and cellular mechanism, while the animal study was designed to test the mechanism in vivo. To thoroughly understand the role of NET formation in driving skin inflammation in Lmnb1TG mice following UVB irradiation, an additional study may be needed to examine NET‐stimulated production of cytokines other than IL‐17A and TNFα, and their effects on skin inflammation, as well as the neutrophil‐independent changes, i.e., keratinocytes and other inflammatory cells. In addition to UVB‐irradiation models, other animal models (Brinkmann et al, 2004; Lood et al, 2016; Martinod et al, 2016) may also be used to test the role of nuclear lamina integration in NET formation in vivo in the future.
In conclusion, our novel findings identified that (i) lamin B is crucial to the nuclear envelope integrity in neutrophils, (ii) PKCα serves as lamin kinase that mediates lamin B phosphorylation, resulting in consequent nuclear envelope rupture and NET formation, and (iii) strengthening nuclear envelope integrity by lamin B overexpression attenuates NET formation in vitro and in vivo, thus alleviating the release of NET‐associated cytokines in the skin of UVB‐irradiated lamin B transgenic mice. These findings, combined with other recent studies (Amulic et al, 2017; Neubert et al, 2018), advance our mechanistic understanding of NET formation. The current work highlights a cellular mechanism in nuclear envelope rupture during NET formation, which may provide avenues towards new therapeutic strategies for NET‐associated human diseases.
Materials and Methods
Mice
Lamin B1 transgenic C57BL/6J‐Tg (Lmnb1TG) 1Yfu/J mice (JAX stock #023083) (Heng et al, 2013), PKCα‐deficient mice (B6;129‐Prkca tm1Jmk/J mice, JAX stock #009068) (Braz et al, 2004), and their corresponding littermate controls were housed in a pathogen‐free environment and given food and water ad libitum. All the animal experiments were approved by the Animal Care and Use Committee of Philadelphia VA Medical Center.
Cell culture and treatment
Primary human polymorphic nuclear granulocytes (pPMNs) or neutrophils were isolated by dextran (250,000) sedimentation of red blood cells (RBC) followed by Ficoll‐Paque PLUS H‐1077 (GE Healthcare) gradient separation as described before with modification (Denny et al, 2010). Primary mouse peritoneal and bone marrow neutrophils (mPMNs) were isolated according to the published protocols (Boxio et al, 2004). Mouse peritoneal mPMN isolation was conducted with 3% thioglycollate broth (TGB) medium induction and then isolated with neutrophil isolation kit (Miltenyl) according to manufacturer's instruction. Mouse bone marrow mPMNs were isolated from the femur and tibia bone marrow with a neutrophil isolation kit (Miltenyl). PKCα‐deficient mPMNs were isolated from heterozygous (PKCα+/−) or homozygous (PKCα−/−) PKCα‐deficient mice (B6;129‐Prkca tm1Jmk/J mice, JAX stock #009068) (Boxio et al, 2004).
The mPMNs with lamin B overexpression (Lmnb1TG) were isolated from lamin B1 transgenic C57BL/6J‐Tg (LMNB1) 1Yfu/J mice (JAX stock #023083) (Heng et al, 2013). Neutrophils from their littermate wild‐type (WT) control mice for both PKCα deficient and lamin B1 transgenic strains served as controls. Mouse peripheral blood neutrophil counts for Lmnb1TG vs. WT mice were conducted using May–Grunwald–Giemsa staining of the peripheral blood smear slides, and total white blood count was analyzed with methylene blue and acetic acid, with commercial kits according to manufacturers’ protocols. The pore transmigration study of the neutrophils from Lmnb1TG vs. WT mice was conducted as described before (Justus et al, 2014) by using Boyden chamber, 24‐well transwell plates with 3 μm pore inserts, under stimulation of PAF (10 μM). The pore transmigration ability in neutrophils from the Lmnb1TG mice was expressed as relative transmigration compared to that in neutrophils from WT littermates.
Human HL‐60 cell line was differentiated into neutrophil‐like or polymorphic nuclear granulocytes (dPMNs) in RPMI‐1640 with 10% FBS and 1.2% DMSO for 5–6 days as described in our published work (Folkesson et al, 2015). RAW264.7 cells (ATCC) were cultured in DMEM medium supplemented with 10% FBS and were also used in certain experiments. All of the above pPMNs, dPMNs, mPMNs, or RAW264.7 were treated with PMA (50 or 100 nM) or PAF (5 or 10 μM) for 0–3 h without or with 1‐h pretreatment with an inhibitor of conventional PKC Go6976, followed by detection of NET formation, or lysis for immunoblot analysis. Since lamin B maturation is regulated by farnesylation (Adam et al, 2013), the reduced mature lamin B expression in HL‐60 dPMNs or RAW264.7 cells was achieved by treatment without (0) or with 2 or 10 μM farnesyltransferase inhibitor (FTI) L‐744,832 for 48 h. In addition, we also examined the effects of UVB radiation on NET formation in vitro (Azzouz et al, 2018; Zawrotniak et al, 2019), using our published protocol (Bashir et al, 2009) with modifications. In brief, dPMNs were placed into a poly‐d‐lysine‐coated 48‐well plate that was irradiated at the top of the plate with a dose of 30 (Bashir et al, 2009) or 150 mJ/cm2 with two FS40T12‐UVB bulbs (Bashir et al, 2009). Then, NET formation was determined either by kinetic analysis for up to 6 h or by immunofluorescent imaging quantification analysis of the fixed cells 6 h later.
NET formation assessment and quantification
All of the above pPMNs, dPMNs, or mPMNs were treated with either PMA (50 or 100 nM) or PAF (5 or 10 μM), for 0–3 h. Three currently validated methodologies were used for NET formation assessment and quantification in the current study. (i) In the conventional fluorometric NET quantification (Sollberger et al, 2016), the above treated or untreated cells were fixed with 2% PFA after the stimulation and stained with 1 μM SYTOX Green, following by fluorescent readout with a microplate reader at 485/518 nm as described before with modification (Sollberger et al, 2016). NET formation in each experiment was also confirmed by fluorescent microscopy. (ii) For measuring the NET‐DNA release index under different conditions (Douda et al, 2015; Khan et al, 2018), cells were grown without (control) or with PMA or PAF treatment for 3 h in medium containing 1 μM SYTOX Green dye in 48‐well plates. The experiments were recorded by a microplate reader either at the end of the 3‐h treatment for endpoint analysis, or every 20 min for up to 3 h for kinetic analysis. The NET‐DNA release index was reported in comparison with an assigned value of 100% for the total DNA released from cells lysed by 0.5% (v/v) Triton X‐100 as described before with modification (Douda et al, 2015; Khan et al, 2018). To calculate the kinetic NET‐DNA release index in each condition, the fluorescence intensity at time 0 min was subtracted from the fluorescence at each time point and was then divided by the fluorescence values of cell lysed with 0.5% (v/v) Triton X‐100. All the experimental values were standardized to total DNA released by cell lysis. Each condition was tested with a technical triplicate. (iii) For the immunofluorescent imaging quantification analysis of NET formation (Sollberger et al, 2016), the equal number of cells was treated by stimuli for 3 h. Then, cell‐impermeable DNA dye SYTOX Green (500 nM) was used to detect cells with NET formation, while the total number of cells were determined by staining with cell‐permeable DNA dye SYTO Red and phase‐contrast imaging. All images were acquired with Fluoview 4.2 software by Olympus confocal microscopy, followed by automated quantification of NETs on 5–6 non‐overlapping area per well using ImageJ for quantification of % cells with NET formation.
NET isolation
NETs isolation was processed according to the published literature (Najmeh et al, 2015) with modification. Briefly, the conditioned medium of neutrophils with NET formation were gently aspirated and discarded after NETs generation. Cold PBS without Ca2+ and Mg2+ was used to collect solution of neutrophils with NET formation. All cells were removed by centrifuge at 450 × g for 10 min at 4°C; the cell‐free NET‐rich supernatant was collected and followed by centrifuge at 18,000 × g for 10 min at 4°C. The isolated NETs lysed by RIPA lysis buffer containing phosphatase inhibitor cocktail and protease inhibitor cocktail (Roche) and the NET lysates were used for immunoblot assay.
DNA constructs and cell transfection
The pEGFP‐C1‐lamin B1 was used to generate phosphorylation site mutants. All plasmids for PKCα‐consensus phosphorylation site mutant isoforms with single or multiple mutations (pEGFP‐C1‐lamin B1 S395A, pEGFP‐C1‐lamin B1 S405A, pEGFP‐C1‐lamin B1 S408A, pEGFP‐C1‐lamin B1 S395A/S405A, pEGFP‐C1‐lamin B1 S395A/S408A, pEGFP‐C1‐lamin B1 S405A/S408A, pEGFP‐C1‐lamin B1 S395A/S405A/S408A) were generated as described before (Mall et al, 2012). HL‐60 (dPMNs) cells were cultured in RPMI‐1640 and transfected with HL‐60 Cell Avalanche™ (EZ Biosystems) transfection reagent according to the manufacturer's instructions. RAW264.7 cells (ATCC) were cultured in DMEM supplemented with 10% FBS and were transfected with TransIT‐Jurket (Mirus) according to the manufacturer's instructions. Forty‐eight hours after HL‐60 (dPMNs) cell transfection or 24 h after RAW264.7 cell transfection, cells were treated by 10 μM PAF for 3 h and evaluated for induction of extracellular traps, which were then detected as described above.
Confocal fluorescent microscopy analysis
Neutrophils and NETs were fixed with 100% methanol and then stained with primary anti‐total PKCα, or anti‐phospho‐PKCαS657, or anti‐lamin B Abs, followed by their matched secondary Abs with FITC or PE conjugation. DNA was stained with DAPI. For measurement of nuclear envelope continuity, the circumferences/perimeters of the cell nucleus, and the lengths of the discontinuity of ruptured nuclear envelope (lamin B staining), of the cells from confocal microscopy images were measured with ImageJ software with modification. Then, the percentages of the discontinuity were calculated according to the above measurements. Confocal fluorescent images were analyzed with an Olympus Fluoview 1000 confocal microscope.
Nuclear protein extraction
To further study the nuclear accumulation of PKCα and its association with nuclear lamin B in neutrophils, the nuclei were extracted using hypotonic buffer according to the published protocol (Shaiken & Opekun, 2014) with modification. In brief, the collected neutrophils were resuspended in ice‐cold buffer (pH 7.4) containing 10 mM Tris–HCl, 10 mM NaCl, and 5 mM magnesium acetate for 30 min. NP‐40 (0.3%) detergent was then added and homogenized. The mixture homogenates were centrifuged at 1,200 g for 10 min at 4°C, to collect sedimented nuclei; these were then resuspended in high volumes of buffer containing 880 mM sucrose and 5 mM magnesium acetate. The suspension was then centrifuged at 2,000 g for 20 min at 4°C and nuclei were resuspended in buffer containing 340 mM sucrose and 5 mM magnesium acetate. The recovered nuclei were dissolved in 8 M urea, followed by sonication, and centrifugation by 10,000 g for 10 min at 4°C.
Immunoprecipitation (IP)
Immunoprecipation was carried out with direct magnetic IP kit (Pierce) by coupling lamin B antibody (66095‐1‐Ig, Proteintech) to N‐hydroxysuccinimide (NHS)‐activated magnetic beads, followed by incubating the lysate with the coupled magnetic beads at 4°C overnight. The immunoprecipitates were then washed twice with cold washing buffer and once with cold ultra‐pure water and then bound lamin B was eluted for immunoblot detection of phosphorylated and total lamin B, with anti‐p‐Ser/Thr/Tyr Ab and anti‐lamin B Ab, respectively, in neutrophils that were treated without or with PMA or PAF for 3 h.
Immunoblot analysis
For immunoblot analysis, the cells, that were treated or not, were harvested and washed in ice‐cold PBS, and then lysed with RIPA lysis buffer containing phosphatase inhibitor cocktail and protease inhibitor cocktail (Roche). Equal amounts of protein were loaded on SDS–PAGE gel and electrophoresed and then transferred to PVDF membranes. After membrane blocking, they were probed with primary antibodies and matched HRP‐conjugated secondary antibodies and then detected using an enhanced chemiluminescent detection kit (Millipore). In some of our immune‐blot results, full‐length lanes were shown to display the status of lamin B that was either intact or fragmented. Relative densitometric values of protein bands were quantified by the ImageJ 1.51f software (NIH, USA).
Exposure of mice to ultraviolet B (UVB)
For the experiments with UVB exposure, the dorsal skin was exposed to UVB with two FS40T12‐UVB bulbs (LIGHT SOURCES) according to our published protocol (Sharma et al, 2011) with modification. Female lamin B transgenic Lmnb1TG mice and their female WT littermates in 8 weeks of age were randomly assigned to receive UVB exposure without/with 150 mJ/cm2 for five consecutive days under anesthetization by peritoneal injected Ketamine/Xylazine. Animals were sacrificed 24 h after the last exposure. The whole dorsal skin samples, including epidermis, dermis, and subcutaneous fat were collected. Tissue sample sectioning was performed by the Penn Skin Biology and Diseases Resource‐based Center.
Fluorescent immunohistochemistry
For immunohistochemistry staining, the paraffin‐embedded skin tissue samples were cut into 5 μm sections and placed on glass slides. Slides were placed in a 60°C oven overnight, deparaffinized with CitriSolv (Fisher Scientific) and rehydrated with serial ethanol dilutions. Samples underwent antigen retrieval using Target Retrieval Solutions (DAKO). Peroxidase was deactivated using 0.3% H2O2 dissolved in deionized H2O for 5 min. Sections were blocked using DAKO protein block (Carpintera). The neutrophil markers Ly6G (Amulic et al, 2017) and Ly6B (Nakazawa et al, 2017) were used for detection of neutrophils as described in other publications in NETosis research. Samples were incubated with primary antibodies overnight at 4°C. Primary antibodies used for staining included Ly6B [Rat anti‐neutrophil (7/4), 1:500, Abcam, ab53457], Citrullinated histone H3 (citrulline R2 + R8 + R17) (Rabbit anti‐citrullinated Histone H3, 1:300, Abcam, ab5103), IL‐17A (Rabbit anti‐IL‐17A, 1:100, Abcam, ab79056). Goat anti‐rat IgG secondary antibody conjugated with Alexa Fluor® 647 (1:500, Invitrogen) and donkey anti‐rabbit IgG secondary antibody conjugated with Alexa Fluor®488 (1:500, Invitrogen) were used to detect primary antibodies. In separate experiments, PE‐conjugated rat anti‐mouse Ly6G antibody (BioLegend) was used for neutrophil detection. FITC anti‐mouse TNFα antibody (Rat anti‐TNFα, 1:100, BioLegend, 506304) was also used for immunofluorescent staining. Isotype‐matched IgG was used instead of primary antibody as a negative control of the staining. Counterstaining with DAPI was performed and slides were mounted with SlowFade Gold Antifade Mountant (Invitrogen). Images were obtained using the Nikon Eclipse fluorescent microscope, and the fluorescent staining area percentage was analyzed by Nikon NIS‐Elements Advanced Research software, according to previous publication (Brinkmann et al, 2016).
Quantification and statistical analysis
Prism 6 (GraphPad Software) was used to perform statistical analysis. Normally distributed data are shown as means ± standard deviations (SD). Comparisons between two groups were conducted with the Student t test. Comparisons among three or more groups were performed using ANOVA, followed by Student–Newman–Keuls test. Statistical significance was considered at a level of P‐value < 0.05.
Author contributions
M‐LL conceived and designed the study. YL, ML, M‐LL performed the laboratory experiments and analyzed the experimental data. For the confocal image analysis, M‐LL took the images, and YL and MHL conducted further quantification and statistical analysis. MM and BW made the plasmids for the lamin B mutations. M‐LL, YL, VPW, MM wrote/drafted and finalized the paper. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
The authors would like to acknowledge Penn Skin Biology and Diseases Resource‐based Center for their skin histology service. The authors would like to thank Debra A. Pawlowski (animal facility, Philadelphia VA Medical Center) for her kindly help with our animal experiments. The authors would also thank Dr. Iain W. Mattaj for his support on the lamin B mutation work. This work was supported by Lupus Research Alliance (416805) and NIH R21AI144838 (to MLL), and the Veterans Affairs Merit Review Award (to VPW), ERC StG No 804710 and the Hector Stiftung II gGmbH (to MM), and a Helmholtz International Graduate School Fellowship (BW).
EMBO Reports (2020) 21: e48779
Data availability
No primary datasets have been generated and deposited.
References
- Adam SA, Butin‐Israeli V, Cleland MM, Shimi T, Goldman RD (2013) Disruption of lamin B1 and lamin B2 processing and localization by farnesyltransferase inhibitors. Nucleus 4: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Knackstedt SL, Abu Abed U, Deigendesch N, Harbort CJ, Caffrey BE, Brinkmann V, Heppner FL, Hinds PW, Zychlinsky A (2017) Cell‐cycle proteins control production of neutrophil extracellular traps. Dev Cell 43: 449–462 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Modrianský M, Serinkan FB, Bello RI, Matsura T, Jiang J, Tyurin VA, Tyurina YY, Fadeel B, Kagan VE (2002) NADPH oxidase‐dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO‐differentiated HL‐60 cells. Role in phagocytic clearance. J Biol Chem 277: 49965–49975 [DOI] [PubMed] [Google Scholar]
- Azzouz D, Khan MA, Sweezey N, Palaniyar N (2018) Two‐in‐one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov 4: 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir MM, Sharma MR, Werth VP (2009) UVB and proinflammatory cytokines synergistically activate TNF‐alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol 129: 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeltz S, Amini P, Anders HJ, Andrade F, Bilyy R, Chatfield S, Cichon I, Clancy DM, Desai J, Dumych T et al (2019) To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ 26: 395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ (1997) Phosphorylation of protein kinase C‐alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase‐resistant state. J Biol Chem 272: 3544–3549 [DOI] [PubMed] [Google Scholar]
- Boxio R, Bossenmeyer‐Pourie C, Steinckwich N, Dournon C, Nube O (2004) Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 75: 604–611 [DOI] [PubMed] [Google Scholar]
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB et al (2004) PKC‐alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10: 248–254 [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535 [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Abu Abed U, Goosmann C, Zychlinsky A (2016) Immunodetection of NETs in paraffin‐embedded tissue. Front Immunol 7: 513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K (2018) Noncanonical inflammasome signaling elicits gasdermin D‐dependent neutrophil extracellular traps. Sci Immunol 3: eaar6676 [DOI] [PubMed] [Google Scholar]
- Collas P, Thompson L, Fields AP, Poccia DL, Courvalin JC (1997) Protein kinase C‐mediated interphase lamin B phosphorylation and solubilization. J Biol Chem 272: 21274–21280 [DOI] [PubMed] [Google Scholar]
- Damiani E, Ullrich SE (2016) Understanding the connection between platelet‐activating factor, a UV‐induced lipid mediator of inflammation, immune suppression and skin cancer. Prog Lipid Res 63: 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, McCune WJ, Kaplan MJ (2010) A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 184: 3284–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douda DN, Khan MA, Grasemann H, Palaniyar N (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase‐independent NETosis induced by calcium influx. Proc Natl Acad Sci USA 112: 2817–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD (2015) P‐selectin promotes neutrophil extracellular trap formation in mice. Blood 126: 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson M, Li C, Frebelius S, Swedenborg J, Wagsater D, Williams KJ, Eriksson P, Roy J, Liu ML (2015) Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb Haemost 114: 1165–1174 [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang P, Gougerot‐Pocidalo M, El Benna J (2001) Phosphorylation of p47phox sites by PKC α, βII, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41: 7743–7750 [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambelluca MS, Bertheau‐Mailhot G, Laflamme C, Rollet‐Labelle E, Servant MJ, Pouliot M (2014) TNF‐alpha expression in neutrophils and its regulation by glycogen synthase kinase‐3: a potentiating role for lithium. FASEB J 28: 3679–3690 [DOI] [PubMed] [Google Scholar]
- Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R (2008) Filaments made from A‐ and B‐type lamins differ in structure and organization. J Cell Sci 121: 215–225 [DOI] [PubMed] [Google Scholar]
- Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto‐Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ et al (2012) IL‐17 and TNF‐alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188: 6287–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamam H, Khan M, Palaniyar N (2019) Histone acetylation promotes neutrophil extracellular trap formation. Biomolecules 9: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch E, Hetzer M (2014) Breaching the nuclear envelope in development and disease. J Cell Biol 205: 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Lin ST, Verret L, Huang Y, Kamiya S, Padiath QS, Tong Y, Palop JJ, Huang EJ, Ptacek LJ et al (2013) Lamin B1 mediates cell‐autonomous neuropathology in a leukodystrophy mouse model. J Clin Investig 123: 2719–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Burns DJ, Fields AP (1993) Identification of protein kinase C (PKC) phosphorylation sites on human lamin B. Potential role of PKC in nuclear lamina structural dynamics. J Biol Chem 268: 7545–7552 [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz‐Echevarria M, Yang LV (2014) In vitro cell migration and invasion assays. J Vis Exp: 51046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Philip LM, Cheung G, Vadakepeedika S, Grasemann H, Sweezey N, Palaniyar N (2018) Regulating NETosis: increasing pH promotes NADPH oxidase‐dependent NETosis. Front Med 5: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig MF, Andrade F (2016) A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front Immunol 7: 461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT (2006) Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 281: 25768–25780 [DOI] [PubMed] [Google Scholar]
- Li H, Prasad R, Katiyar SK, Yusuf N, Elmets CA, Xu H (2015) Interleukin‐17 mediated inflammatory responses are required for ultraviolet radiation‐induced immune suppression. Photochem Photobiol 91: 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT (2011) Mast cells and neutrophils release IL‐17 through extracellular trap formation in psoriasis. J Immunol 187: 490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S et al (2011) IL‐17A and TNF‐alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro . J Immunol 186: 3197–3205 [DOI] [PubMed] [Google Scholar]
- Lood C, Blanco LP, Purmalek MM, Carmona‐Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ (2016) Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus‐like disease. Nat Med 22: 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machowska M, Piekarowicz K, Rzepecki R (2015) Regulation of lamin properties and functions: does phosphorylation do it all? Open Biol 5: 150094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Walter T, Gorjanacz M, Davidson IF, Nga Ly‐Hartig TB, Ellenberg J, Mattaj IW (2012) Mitotic lamin disassembly is triggered by lipid‐mediated signaling. J Cell Biol 198: 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Nyakern M, Manzoli FA (2006) Nuclear protein kinase C. Biochem Biophys Acta 1761: 542–551 [DOI] [PubMed] [Google Scholar]
- Martinod K, Witsch T, Farley K, Gallant M, Remold‐O'Donnell E, Wagner DD (2016) Neutrophil elastase‐deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost 14: 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan E, Girard D (2006) Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol 79: 489–498 [DOI] [PubMed] [Google Scholar]
- Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH (2002) Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297: 854–857 [DOI] [PubMed] [Google Scholar]
- Najmeh S, Cools‐Lartigue J, Giannias B, Spicer J, Ferri LE (2015) Simplified human neutrophil extracellular traps (NETs) isolation and handling. J Vis Exp: 52687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW et al (2017) Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 28: 1753–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeli I, Khan SN, Radic M (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180: 1895–1902 [DOI] [PubMed] [Google Scholar]
- Neri LM, Borgatti P, Capitani S, Martelli AM (1998) Nuclear diacylglycerol produced by phosphoinositide‐specific phospholipase C is responsible for nuclear translocation of protein kinase C‐alpha. J Biol Chem 273: 29738–29744 [DOI] [PubMed] [Google Scholar]
- Neubert E, Meyer D, Rocca F, Gunay G, Kwaczala‐Tessmann A, Grandke J, Senger‐Sander S, Geisler C, Egner A, Schon MP et al (2018) Chromatin swelling drives neutrophil extracellular trap release. Nat Commun 9: 3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert E, Bach KM, Busse J, Bogeski I, Schon MP, Kruss S, Erpenbeck L (2019) Blue and long‐wave ultraviolet light induce in vitro neutrophil extracellular trap (NET) formation. Front Immunol 10: 2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins AL, Zwerger M, Herrmann H, Zentgraf H, Simon AJ, Monestier M, Olins DE (2008) The human granulocyte nucleus: unusual nuclear envelope and heterochromatin composition. Eur J Cell Biol 87: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R, Baines JD (2006) Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J Virol 80: 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse E, Rother N, Yanginlar C, Gerretsen J, Boeltz S, Munoz LE, Herrmann M, Pickkers P, Hilbrands LB, van der Vlag J (2018) Cleaved N‐terminal histone tails distinguish between NADPH oxidase (NOX)‐dependent and NOX‐independent pathways of neutrophil extracellular trap formation. Ann Rheum Dis 77: 1790–1798 [DOI] [PubMed] [Google Scholar]
- Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M et al (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus . J Immunol 185: 7413–7425 [DOI] [PubMed] [Google Scholar]
- Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21: 290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochael NC, Guimaraes‐Costa AB, Nascimento MT, DeSouza‐Vieira TS, Oliveira MP, GarciaeSouza LF, Oliveira MF, Saraiva EM (2015) Classical ROS‐dependent and early/rapid ROS‐independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci Rep 5: 18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J (2013) Nuclear envelope composition determines the ability of neutrophil‐type cells to passage through micron‐scale constrictions. J Biol Chem 288: 8610–8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL (2004) Functional cooperation between interleukin‐17 and tumor necrosis factor‐alpha is mediated by CCAAT/enhancer‐binding protein family members. J Biol Chem 279: 2559–2567 [DOI] [PubMed] [Google Scholar]
- Shaiken TE, Opekun AR (2014) Dissecting the cell to nucleus, perinucleus and cytosol. Sci Rep 4: 4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MR, Werth B, Werth VP (2011) Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH1 and Balb/c mice. Photochem Photobiol 87: 690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Cao CX, Shao RG, Pommier Y (1998) Lamin B phosphorylation by protein kinase calpha and proteolysis during apoptosis in human leukemia HL60 cells. J Biol Chem 273: 8669–8674 [DOI] [PubMed] [Google Scholar]
- Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE (2013) Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci USA 110: 18892–18897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Johnstone DB, Martin KA, Tempera I, Kaplan MJ, Denny MF (2016) Alterations in nuclear structure promote lupus autoimmunity in a mouse model. Dis Models Mech 9: 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ (2001) Executioner caspase‐3, ‐6, and ‐7 perform distinct, non‐redundant roles during the demolition phase of apoptosis. J Biol Chem 276: 7320–7326 [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Steffens S, Hidalgo A, Weber C (2017) Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 17: 248–261 [DOI] [PubMed] [Google Scholar]
- Sollberger G, Amulic B, Zychlinsky A (2016) Neutrophil extracellular trap formation is independent of de novo gene expression. PLoS One 11: e0157454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B et al (2018) Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3: eaar6689 [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M et al (2013) Nuclear lamin‐A scales with tissue stiffness and enhances matrix‐directed differentiation. Science 341: 1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsiy O, McDonald PP (2018) Physiological stimuli induce PAD4‐dependent, ROS‐independent NETosis, with early and late events controlled by discrete signaling pathways. Front Immunol 9: 2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K (2004) Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA 101: 10428–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA et al (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184: 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp BG, Kubes P (2013) NETosis: how vital is it? Blood 122: 2784–2794 [DOI] [PubMed] [Google Scholar]
- Zawrotniak M, Bartnicka D, Rapala‐Kozik M (2019) UVA and UVB radiation induce the formation of neutrophil extracellular traps by human polymorphonuclear cells. J Photochem Photobiol B 196: 111511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Data Availability Statement
No primary datasets have been generated and deposited.


