Abstract
Background
Transbronchial lung biopsies are commonly performed for a variety of indications. Although generally well tolerated, complications such as bleeding do occur. Description of bleeding severity is crucial both clinically and in research trials; to date, there is no validated scale that is widely accepted for this purpose. Can a simple, reproducible tool for categorizing the severity of bleeding after transbronchial biopsy be created?
Methods
Using the modified Delphi method, an international group of bronchoscopists sought to create a new scale tailored to assess bleeding severity among patients undergoing flexible bronchoscopy with transbronchial lung biopsies. Cessation criteria were specified a priori and included reaching > 80% consensus among the experts or three rounds, whichever occurred first.
Results
Thirty-six expert bronchoscopists from eight countries, both in academic and community practice settings, participated in the creation of the scale. After the live meeting, two iterations were made. The second and final scale was vetted by all 36 participants, with a weighted average of 4.47/5; 53% were satisfied, and 47% were very satisfied. The panel reached a consensus and proposes the Nashville Bleeding Scale.
Conclusions
The use of a simplified airway bleeding scale that can be applied at bedside is an important, necessary tool for categorizing the severity of bleeding. Uniformity in reporting clinically significant airway bleeding during bronchoscopic procedures will improve the quality of the information derived and could lead to standardization of management. In addition to transbronchial biopsies, this scale could also be applied to other bronchoscopic procedures, such as endobronchial biopsy or endobronchial ultrasound-guided needle aspiration.
Key Words: biopsy (lung), bleeding, bronchoscopy, interventional bronchoscopy
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events
The use of flexible bronchoscopy to guide flexible forceps and obtain small biopsy specimens of the lung is one of the most common procedures performed by pulmonologists, interventional pulmonologists, and some thoracic surgeons. These biopsies have a low complication rate, with bleeding occurring in 0.26% to 2.8% of cases.1,2 However, current scales to measure airway bleeding in clinical trials are inadequate and have not been validated.3 The Common Terminology Criteria for Adverse Events (CTCAE) is one of the most commonly used scales to gauge bleeding after bronchoscopic biopsies and is graded from 1 to 5, generally based on the level of intervention needed to control bleeding.4 In the largest study to date evaluating the diagnostic yield of electromagnetic navigation bronchoscopy, the CTCAE was used to assess and document bleeding complications.5 Other authors have used scales based on subjective descriptions such as mild, moderate, and severe and are thus largely dependent on the bronchoscopist’s interpretation and their level of expertise or comfort. In a study performed by Ernst et al,6 assessing bleeding complications after transbronchial biopsy in patients using clopidogrel, bleeding was graded subjectively by the bronchoscopist with mild defined as need for continuous suctioning, moderate as need for wedging the bronchoscope into the affected airway, and severe as needing any additional intervention, including bronchoscopic intervention, blood product administration, or change in level of care. The 2013 British Thoracic Society guidelines for diagnostic flexible bronchoscopy adapted these criteria to assess bleeding complications while noting that subjectively estimating blood loss was difficult and unreliable.7 Although attempts at objectively quantifying bleeding during bronchoscopy have been made, they are not widely used.8 In addition, there is no agreement in the literature on what volume of blood expectorated in 24 h should be considered “massive” bleeding. Reports are widely variable and include estimates ranging from 100 mL to greater than 1,000 mL in 24 h.9, 10, 11
There is substantial variability among the bleeding definitions currently used. The heterogeneity and absence of standardization make it difficult to objectively quantify complications during clinical trials. The subjective nature of adjudication further complicates the reproducibility of these findings in other studies. In the absence of standard definitions for endobronchial bleeding following transbronchial lung biopsies, the management of these cases is highly variable. This is especially pertinent given the current clinical and research interests in electromagnetic navigation and robotic bronchoscopy for the diagnosis of peripheral lung nodules as well as the use of cryobiopsies in the diagnosis of interstitial lung disease.
In response to the need to develop, test, disseminate, and adopt standardized airway bleeding definitions for patients undergoing flexible bronchoscopy with transbronchial lung biopsies, we convened the Nashville Working Group to address this issue. This group was composed of an international panel of experts from both academic and community-based institutions with extensive experience in bronchoscopy to develop a consensus airway bleeding scale that could be used for general communication, clinical trials, and registries. This study aimed to develop a simple scale that is readily applicable at the bedside, can be validated, and is a reliable measurement tool to categorize the severity of bleeding in patients undergoing transbronchial lung biopsies via flexible bronchoscopy. In keeping with the COMET (Core Outcome Measures in Effectiveness Trials) Initiative guidelines, a stepwise process was followed to define the core outcome and develop an appropriate outcome measurement instrument.12
Methods
Thirty-six expert bronchoscopists (more than 5 years of experience and/or more than 500 procedures) were convened and asked to participate in the creation of a new, simple scale tailored for a population of patients undergoing flexible bronchoscopy with transbronchial lung biopsies. Content and face validity were examined by expert review. The plan to reach consensus was developed by “modified consensus methodology” in accordance with published recommendations, where the first method is used to generate items and subsequent methods are used for final consensus.13,14
The study had three phases: the live meeting, the Delphi surveys, and the preparation of a final manuscript. During the live meeting, a general open forum was convened where collective opinions were gathered in preparation for the initial scale. Thereafter, the general Delphi criteria were followed in two consecutive surveys sent by e-mail, engaging the participants in an anonymous iterative process involving ranking or rating of items proposed during the live session.14 The opportunity for free-texting of ideas was encouraged, and all questions had to be answered before submitting the survey. Cessation criteria were specified a priori and included reaching > 80% consensus among the experts or three rounds, whichever occurred first. We determined that any new items suggested by participants would be dropped if they were not suggested by more than one participant. The term “consensus” was defined a priori as: > 80% of participants in agreement with the proposed scale. Agreement was defined as self-reporting being “satisfied” or “very satisfied” on a five-point scale (Table 1).
Table 1.
Methodologic Criteria for Delphi Study
| Study Components | Description |
|---|---|
| Study objective | To propose and attempt to reach consensus about a new scale of severity of bleeding after transbronchial lung biopsies |
| Participant expertise, background, and geographic diversity | 45 experts in transbronchial lung biopsies from backgrounds in pulmonary medicine, interventional pulmonology, and thoracic surgery were sought. A total of 36 responded to the requests. The backgrounds of experienced participants were as follows: 61% in academic practice, 28% in community-based practice, and 11% in combined practice. A total of 28 participants were from the United States, with a diverse geographic background, and eight were from seven countries in Europe and South America |
| Consensus definition | Reaching > 80% agreement in successive iterations of a bleeding scale. (Participants expressed being satisfied or very satisfied on a 5-point scale) |
| Criteria used to determine consensus or lack of consensus | Reaching more than 80% agreement or three successive iterations, whichever event came first |
| Were terms dropped? | Yes, those items found to rank in the bottom 25%, and a shared opinion that they did not add value or degrees of separation to the scale, were dropped |
| Criteria used to drop items | Those items that ranked in the bottom 25% in weighed average were investigated further to determine their added value to the scale. The lowest ranking items were dropped |
| Method of communication anonymous among participants, so their input could be freely expressed? | Yes, only the coordinator of the surveys had knowledge of the identity of the individual submitting the survey. This allowed for free expression of ideas and minimized the “herd mentality” phenomenon |
| Was there a method to reduce the chance of a respondent submitting more than one survey? | Yes, cookie-based duplication protection was used to reduce multiple responses from a single participant. We also analyzed the individual responders and confirmed there were no duplicate responses |
| Were key guidelines provided to the participants? | No, there are no available guidelines or previous consensus statements on this issue |
The survey was distributed with SurveyMonkey, a commercial online survey tool (SurveyMonkey Inc.). Bronchoscopists were free to decline participation. Cookie-based duplication protection was used to prevent duplicate responses. This study was exempt from institutional review board review.
The survey questions were presented in multiple-choice format; eight of nine questions were text-based, and one showed a proposed scale for grading bleeding. The questions assessed level of experience in performing transbronchial lung biopsies, whether operators typically use a grading scale to assess bleeding, degree of confidence at quantifying bleeding, which pathophysiologic mechanism is responsible for death in pulmonary hemorrhage, which variables are important to include in a scale that estimates bleeding, level of satisfaction with the proposed scale, and willingness to participate in further surveys. In a follow-up survey, the questions assessed level of satisfaction with the modified proposed scale, whether this scale could be used to assess bleeding in other bronchoscopic procedures, and participant willingness to answer further survey questions.
Results
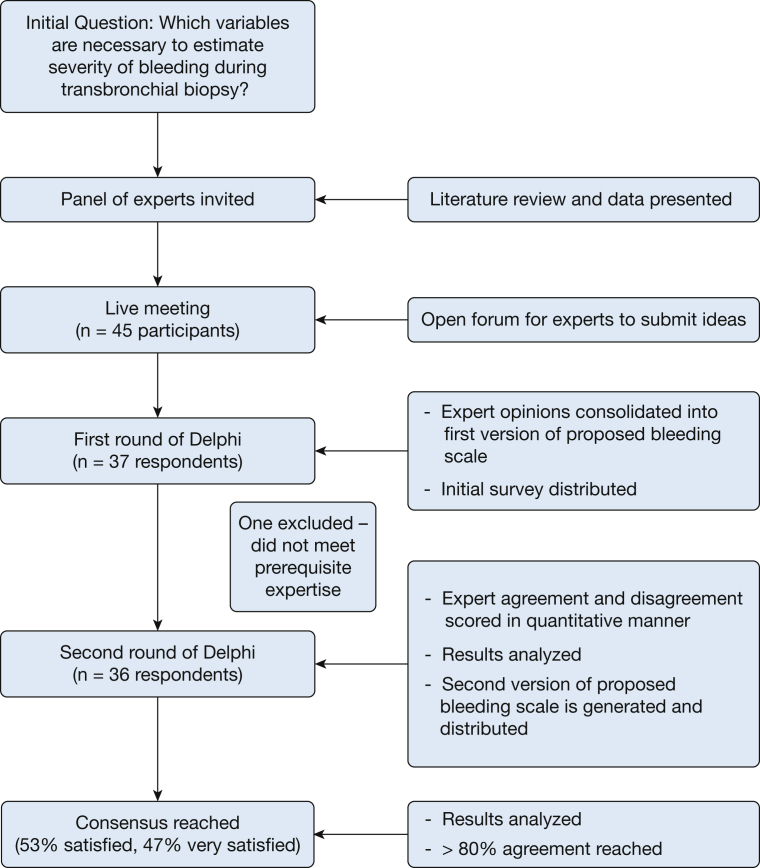
A total of 45 experts were invited to participate. Respondents numbered 37 for the first survey and 36 for the second iteration of the survey (Fig 1). One respondent to the first survey was eliminated for not meeting the prespecified criteria of expertise (had not performed bronchoscopy). The breakdown of specialties was 69% interventional pulmonology, 19% pulmonary and critical care medicine, and 11% thoracic surgery. Most respondents (94%) described not regularly using a standardized bleeding scale because of the inherent difficulties and lack of a validated scale. The two participants who reported consistently using a bleeding scale described using two different, unpublished scales. One scale was based on volumetric quantification of bleeding: mild (< 5 mL), moderate (5-50 mL), and large (> 50 mL). The other scale was based on the need for tools required to control bleeding: grade 1 (insignificant bleeding), grade 2 (bleeding requiring chemical intervention), grade 3 (bleeding requiring mechanical intervention), and grade 4 (bleeding requiring surgical intervention).
Figure 1.
Flow chart of Delphi method and consensus.
After the initial live meeting, all suggested variables were included in the questionnaire and then vetted during the first anonymous Delphi survey. The participants gave a graded weight to the following variables: use of cold saline, bronchoscopic tamponade, use of balloon to tamponade, admission to the ICU, change in level of care including admission to the hospital, blood transfusion, need for bronchial artery embolization, interruption of the procedure, need for mechanical ventilation, use of epinephrine or phenylephrine, total time needed to control bleeding, and amount of blood that was suctioned.
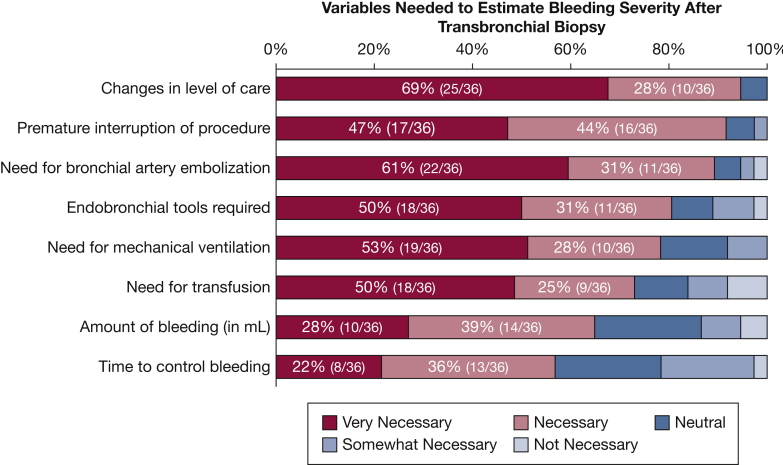
The following characteristics were considered quantitatively the most important by the surveyed group (Fig 2):
-
•
Changes in level of care required for the patient (ie, admission to the ICU)
-
•
Need for premature interruption of the procedure
-
•
Need for bronchial artery embolization to control bleeding
-
•
Need for endobronchial tools to control bleeding (ie, diluted epinephrine or phenylephrine, balloons)
-
•
Need for mechanical ventilation
-
•
Need for transfusion therapy (ie, packed RBCs)
-
•
Amount of bleeding quantified in milliliters
-
•
Time needed to control bleeding
Figure 2.
Survey data assessing which variables are necessary to estimate bleeding severity after transbronchial biopsy.
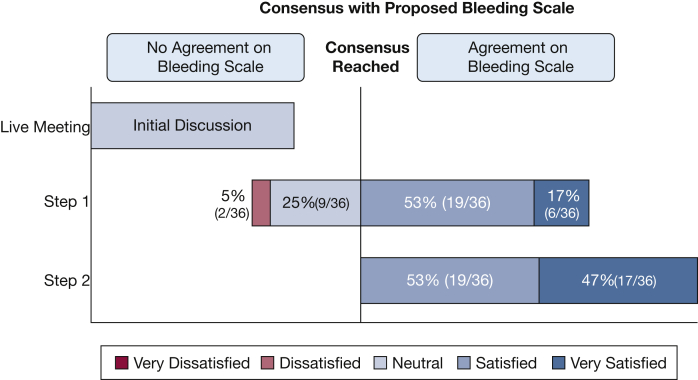
Using this initial set of variables, the group was surveyed about their level of satisfaction with a first iteration of the scale. The results showed that 17% were very satisfied, 53% were satisfied, 25% were neutral, and 5% were dissatisfied (Fig 3). In the free-text questions, 13 suggestions were received and incorporated into the second iteration of the anonymous Delphi survey. All 36 participants agreed to receive and answer the second survey. In addition, only 22% of the participants described being able to accurately quantify the amount of blood suctioned during the procedure. For this reason, quantification was eliminated from the scale. An overwhelming majority (97%) agreed that the dead space in the tracheobronchial tree is approximately 150 mL and agreed that patients with lethal airway bleeding die of asphyxia, not as a consequence of hypovolemic shock. These two statements were considered very important in the creation of the final bleeding scale.
Figure 3.
Satisfaction with proposed bleeding scale using Delphi method.
Using the feedback collected during the live meeting as well as the first iteration of the Delphi survey, a second scale was created. The second and final scale was vetted by all 36 participants, with a weighted average of 4.47 (out of a maximum score of 5), where 53% were satisfied, and 47% were very satisfied. There were no participants who felt dissatisfaction with the second scale (Fig 3). The participants were also surveyed about the specific use of this scale both in content and scope. When asked about other maneuvers done before the procedure to prevent bleeding, the majority agreed (86%) on the following statement: “Any interventions done to prevent bleeding (ie, blood product transfusion before the procedure, balloon placement for preemptive tamponade, or prophylactic intubation) are not considered within the scope of this scale. This scale only pertains to actions taken to control active bleeding.”
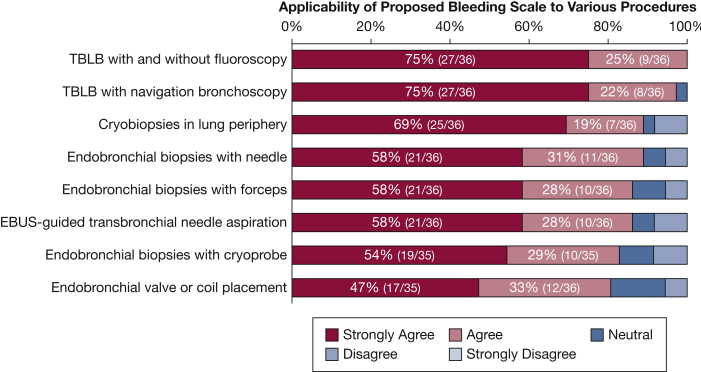
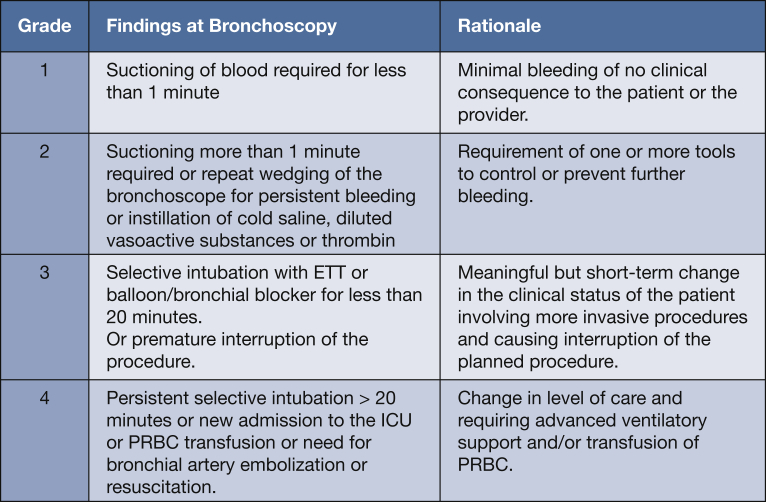
Regarding the scope of the scale, the participants deemed the scale valuable for other bronchoscopic procedures and studies (Fig 4). However, given the primary objective of the consensus, the panel believes that further research is needed to validate the use of this scale in other bronchoscopic procedures. Interestingly, during the second survey, when asked if the participants were willing to receive further surveys if the panel did not reach consensus, the overwhelming majority (97%) agreed to a third iteration. Fortunately, this was not necessary given the favorable outcome, but it does highlight the level of commitment and lack of “burnout” of the surveyed experts. Ultimately, a simplified model was created on the basis of the actions prompted by the encounter of postbiopsy endobronchial bleeding (Fig 5).
-
•
Grade 1: Requiring less than 1 min of suctioning or wedging of the bronchoscope resulting in spontaneous cessation of bleeding
-
•
Grade 2: Suctioning more than 1 min or need for rewedging of the bronchoscope or instillation of cold saline, vasoactive substances, or thrombogenic agents
-
•
Grade 3: Selective intubation with endotracheal tube or balloon/bronchial blocker for less than 20 min or premature interruption of the procedure
-
•
Grade 4: Persistent selective intubation > 20 min or new admission to the ICU or packed RBC transfusion or need for bronchial artery embolization or resuscitation
Figure 4.
Survey data regarding the applicability of the proposed scale to other bronchoscopic procedures. EBUS = endobronchial ultrasound; TBLB = transbronchial lung biopsy.
Figure 5.
Proposed scale for assessing severity of bleeding during transbronchial biopsy.
Discussion
Herein the panel has proposed the Nashville Bleeding Scale: a new, simple, objective, and hierarchically graded consensus classification for bleeding. After a review of the available data and prior definitions, this new scale was developed by consensus of a diverse group of expert bronchoscopists with experience in clinical trials and registries as well as expertise in bronchoscopy and transbronchial lung biopsies. Clinical validation of this scale is still necessary. The panel urges researchers in the field of bronchoscopy to begin reporting airway bleeding events according to these new definitions. The simple nature of the scale and its basis on meaningful changes during the procedure are likely to facilitate its implementation at the point of care. It should be noted that this scale grades response to bleeding and does not take into account any preventive measures, such as preemptive introduction of a balloon blocker or intubation. This is particularly pertinent to transbronchial cryobiopsies, where a prophylactic endobronchial blocker is often used. In these cases, we suggest that only reinflation of the balloon in response to continued bleeding should be considered grade 3.
The strengths of this study rely on its external validity based on experts from eight countries (United States, Spain, Chile, France, Great Britain, Denmark, Austria, and Italy), with representation from both community-based and academic institutions and from diverse clinical backgrounds in interventional pulmonology, thoracic surgery, and pulmonary and critical care. Regarding its internal validity, the panel adhered to a strict set of quality measures for Delphi consensus statements. These included a priori study objective; determination of a participant’s expertise, background specialty, and geographic diversity; a priori definition of consensus and criteria used to define consensus (or lack thereof); a method of communication that was anonymous among participants so that their input could be freely expressed; a method to reduce duplicate submissions; as well as the ability to “free-text” any opinions that were not previously considered. The strength of the conclusions reached also rests on the excellent response rate and continuous support of all participants.
The weaknesses of this study, similar to other Delphi consensus statements, rest on the limited ability of the participants to interact during subsequent iterations. This is done in an attempt to limit “herd behavior” and to allow each participant to express his or her opinion in isolation.
Evidently, any bleeding scale that involves instruments and medications applies only to currently available tools and thus will be outdated when newer tools, techniques, and medications are available. This scale is, in addition, ordinal in nature as opposed to interval, and therefore the progression from one grade to the next is not necessarily equal in severity.
Finally, the panel recognizes that the perfect scale to grade bleeding after transbronchial lung biopsies may never exist. However, this scale is a simple and valuable tool that can be easily integrated into daily practice. In this age of intense investigation into the bronchoscopic diagnosis of peripheral lung nodules and interstitial lung disease, the emergence of this scale is timely and practical, while providing a uniform approach to classifying an important complication for both clinical and research applications. The panel welcomes input from other experts, particularly during implementation of this scale, to identify areas needing improvement.
Acknowledgments
Author contributions: All of the authors contributed to the development of the project, data collection, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: E. E. F. serves as a scientific consultant for Medtronic as well as an education and scientific consultant for Boston Scientific. He has a research grant from Intuitive Surgical. None of these represent conflicts of interest for the content of this manuscript. A. K. M. is an educational consultant for Boston Scientific and Medtronic. This does not represent a conflict of interest for the content of this manuscript. F. M. serves as a consultant for Medtronic. This does not represent a conflict of interest for the content of this manuscript. W. S. K. is the chief medical officer for Gala Therapeutics, a consultant for Medtronic, and owns intellectual property rights for Medtronic. None of these represent conflicts of interest for the content of this manuscript. M. R. B. serves as a consultant for Medtronic, Biodesix, and Veracyte. None of these represent conflicts of interest for the content of this manuscript. S. J. K. is the co-PI for NAVIGATE. This does not represent a conflict of interest for the content of this manuscript. None declared (C. L. O., E. T., S. O., S. Be., C. M. K., A. C. M., S. F.-B., J. F., K. L., G. K., M. A. N., F. H., A. A. A.-N., E. B., S. Ba., M. Z., P. O. L., R. J. L., C. P., M. Sa., K. P., C. K., G. P. LeM., J. D. H., A. M., M. C., J. K., G. L., E. W., M. St., C. V. T.).
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Pue C.A., Pacht E.R. Complications of fiberoptic bronchoscopy at a university hospital. Chest. 1995;107(2):430–432. doi: 10.1378/chest.107.2.430. [DOI] [PubMed] [Google Scholar]
- 2.Facciolongo N., Patelli M., Gasparini S. Incidence of complications in bronchoscopy: multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71(1):8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 3.Hetzel J., Maldonado F., Ravaglia C. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy Working Group on Safety and Utility and a call for standardization of the procedure. Respiration. 2018;95(3):188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- 5.Folch E.E., Pritchett M.A., Nead M.A., NAVIGATE Study Investigators Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019;14(3):445–458. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Ernst A., Eberhardt R., Wahidi M., Becker H.D., Herth F.J. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006;129(3):734–737. doi: 10.1378/chest.129.3.734. [DOI] [PubMed] [Google Scholar]
- 7.Du Rand I.A., Blaikley J., Booton R., British Thoracic Society Bronchoscopy Guideline Group British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68(suppl 1):i1–i44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 8.Carr I.M., Koegelenberg C.F., von Groote-Bidlingmaier F. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration. 2012;84(4):312–318. doi: 10.1159/000339507. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim W.H. Massive haemoptysis: the definition should be revised. Eur Respir J. 2008;32:1131. doi: 10.1183/09031936.00080108. [DOI] [PubMed] [Google Scholar]
- 10.Hirshberg B., Biran I., Glazer M., Kramer M.R. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112(2):440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- 11.Corey R., Hla K.M. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987;294(5):301–309. doi: 10.1097/00000441-198711000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Williamson P.R., Altman D.G., Bagley H. The COMET Handbook: version 1.0. Trials. 2017;18(suppl 3):280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair R., Aggarwal R., Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011;41(2):95–105. doi: 10.1016/j.semarthrit.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond I.R., Grant R.C., Feldman B.M. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]