Abstract
Background
Despite the use and purported benefits of balloon atrial septostomy (BAS), its safety, efficacy, and therapeutic role in the setting of advanced pulmonary arterial hypertension (PAH) are not well defined.
Objective
The goal of this study was to conduct a systematic review and meta-analysis to better determine the evidence supporting the use of BAS in PAH.
Methods
MEDLINE, Scopus, Cochrane Library, and Clinicaltrials.gov were searched from inception through May 2018 for original studies reporting outcomes with PAH prior to and following BAS. Studies comparing BAS vs other septostomy procedures were excluded. Weighted mean differences and 95% CIs were pooled by using a random effects model.
Results
Sixteen studies comprising 204 patients (mean age, 35.8 years; 73.1% women) were included. Meta-analysis revealed significant reductions in right atrial pressure (–2.77 mm Hg [95% CI, –3.50, –2.04]; P < .001) and increases in cardiac index (0.62 L/min/m2 [95% CI, 0.48, 0.75]; P < .001) and left atrial pressure (1.86 mm Hg [95% CI, 1.24, 2.49]; P < .001) following BAS, along with a significant reduction in arterial oxygen saturation (–8.45% [95% CI, –9.93, –6.97]; P < .001). The pooled incidence of procedure-related (48 h), short-term (≤ 30 day), and long-term (> 30 days up to a mean follow-up of 46.5 months) mortality was 4.8% (95% CI, 1.7%, 9.0%), 14.6% (95% CI, 8.6%, 21.5%), and 37.7% (95% CI, 27.9%, 47.9%), respectively.
Conclusions
The present analysis suggests that BAS is relatively safe in advanced PAH, with beneficial hemodynamic effects. The relatively high postprocedural and short-term survival with less impressive long-term survival suggest a bridging role for BAS; its contribution to this change needs to be verified by using a comparator group.
Key Words: balloon atrial septostomy, efficacy, meta-analysis, pulmonary arterial hypertension, safety
Abbreviations: BAS, balloon atrial septostomy; LAP, left atrial pressure; MAP, mean arterial pressure; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; RAP, right atrial pressure; RHF, right-sided heart failure; Sao2, arterial oxygen saturation
Pulmonary arterial hypertension (PAH) is a fatal disease associated with increased pulmonary vascular resistance that eventually progresses to right-sided heart failure (RHF).1 Patients who fail to respond to maximal combination therapy, including parenteral prostacyclin, are sometimes referred for lung transplantation as a last resort.2 Unfortunately, many patients with PAH are deemed to be either nontransplant candidates or experience excessively long wait times and die while on the waiting list,3 highlighting the need for alternate salvage therapies. Despite the absence of randomized controlled evidence, the recommended method of shunt creation by current guidelines is balloon atrial septostomy (BAS) (Class IIb, Level C evidence).2 BAS may be considered in patients with PAH who are awaiting lung transplantation who have insufficient response to maximal medical therapy or when medical therapy is not tolerated or unavailable.
Several studies have indeed reported improved hemodynamic parameters and reduced symptoms following BAS in patients with PAH.1, 4 However, because most reported data are from small, uncontrolled series, the safety, efficacy, and therapeutic role of BAS in the management of advanced PAH remain undefined. We therefore conducted a systematic review and meta-analysis to further explore and clarify the evidence surrounding this procedure in patients with PAH.
Materials and Methods
Data Sources and Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items of Systematic Review and Meta-Analysis reporting standards.5 MEDLINE, Scopus, Cochrane Library, and Clinicaltrials.gov were searched from inception through May 2018 for original articles reporting outcomes in patients with PAH prior to and following BAS. No restrictions were placed with respect to time of publishing. Only articles in English were considered. The complete list of search terms used in each database is outlined in e-Table 1. All citations were exported to Endnote Reference Manager version X7.5 (Clarivate Analytics), and duplicates were removed.
Study Selection
Articles were initially short-listed according to title and abstract and were finalized by reviewing full texts and applying predetermined inclusion/exclusion criteria. The study selection procedure was performed by two independent reviewers (E. A. and M. M. M.), and disagreements were resolved by consultation with a third reviewer (M. S. K.). Original articles reporting hemodynamic and clinical outcomes prior to and following BAS in patients with PAH were included. Editorials, review articles, case reports, and studies consisting of fewer than three patients were excluded.
Data Extraction and Assessment of Study Quality
Two independent investigators (E. A. and M. M. M.) extracted data onto a standardized abstraction form. Hemodynamic outcomes of interest included mean right atrial pressure (RAP), arterial oxygen saturation (Sao2), cardiac index, cardiac output, left atrial pressure (LAP), mean pulmonary artery pressure (PAP), and mean arterial pressure (MAP). We also planned to assess changes in pulmonary vascular resistance (n = 1), stroke volume (n = 0), stroke volume index (n = 0), pulmonary artery compliance (n = 0), arterial elastance (n = 0), heart rate (n = 1), systolic and diastolic BPs (n = 0), 6-min walk distance (n = 2), brain natriuretic peptide (n = 2), glomerular filtration rate (n = 0), and creatinine (n = 2) following BAS; however, these outcomes could not be analyzed because of the low number of studies (represented by “n” following each outcome).
Mortality rates and procedural complications were also extracted and pooled. Quality of included studies was assessed by using the Newcastle-Ottawa scale for observational studies.6 Although we also intended to conduct Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) risk score calculations in the included studies, none of the studies reported the minimum of seven evaluable elements that are required to maintain significant predictive power.7, 8
Statistical Analysis
RevMan version 5.3 (Cochrane Collaboration) was used for meta-analysis of continuous variables. Continuous variables were pooled by using a random effects model9 to estimate weighted mean differences with 95% CIs. Categorical variables are presented as proportions subjected to Freeman-Tukey double arcsine transformation10 and pooled to obtain pooled estimates with 95% CIs using random effects modeling. Between-study heterogeneity was quantified by using the Cochrane I2 statistic, with I2 = 25% to 50%, 50% to 75%, and > 75% indicating mild, moderate, and severe heterogeneity, respectively. Egger regression test with visual inspection of the funnel plot was used to test for publication bias. To account for differences in patient populations investigated across studies, subgroup analysis was conducted, stratifying studies with > 50% children (aged < 18 years) into pediatric populations and those with < 50% children as adult populations; the χ2 test was used to examine differences between subgroups. Multivariate random effects meta-regression analysis was performed to assess the contribution of mean age (years), female sex (percentage), percentage of patients with history of syncope, and idiopathic-type PAH (percentage) to heterogeneity in key outcomes. Open Meta-Analyst software (Brown University School of Public Health) was used to conduct meta-regression and categorical proportion meta-analyses. P values < .05 were considered statistically significant.
Results
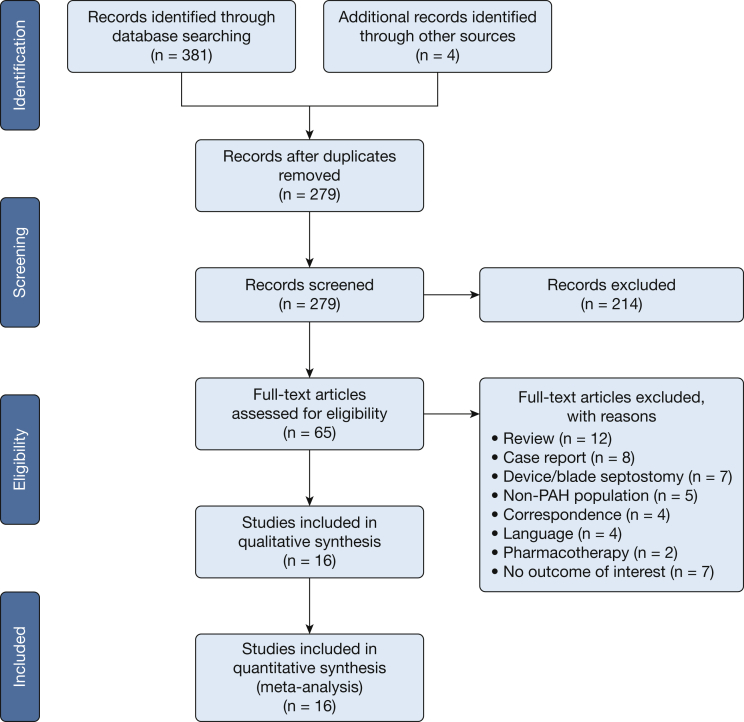
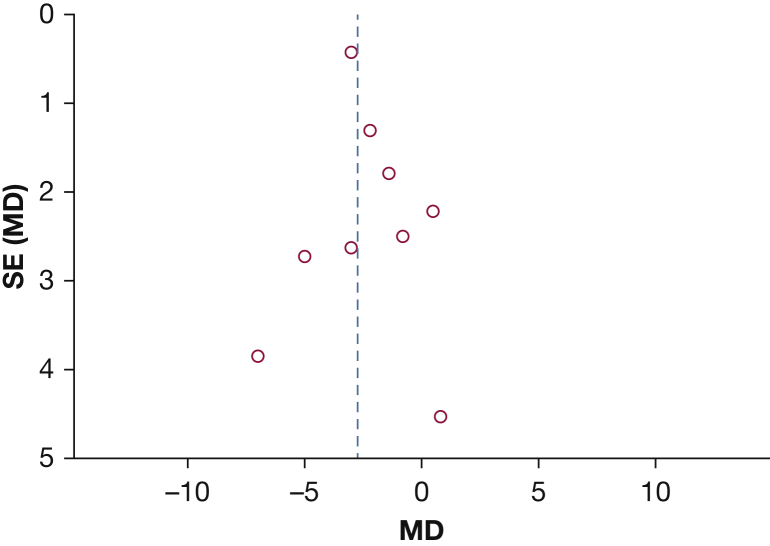
The literature search process is detailed in the PRISMA flowchart (Fig 1). Sixteen noncomparative observational studies comprising 204 patients with PAH were included in the final analysis (Table 1).1, 4, 11, 14, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 All individual studies had small (n < 35) sample sizes. The mean age of patients ranged from 6 to 56 years. On average, studies consisted of a majority (73.1%) of women with a history of syncope (50.6%) and RHF (53.4%) as the most commonly documented indications for the BAS procedure. The majority of studies (n = 10) reported either partial or complete resolution of syncopal symptoms. Visual inspection of funnel plot and Egger regression found evidence of publication bias for mean RAP (P [2-tailed] < .001) (Fig 2). The high risk of bias was suggested in methodologic quality assessment of studies, mainly due to the lack of a comparison group (Table 2).
Figure 1.
Literature search process outlined according to Preferred Reporting Items of Systematic Review and Meta-Analysis flow diagram. PAH = pulmonary arterial hypertension.
Table 1.
Study and Patient Demographic Characteristics
| Study/Year | No. of Patients | Mean Age, y/ Female Sex, % | NYHA Functional Class (I/II/III/IV) | Patient Population | RHF, % | History of Syncope, %/Syncope Relieved, % | Idiopathic PAH, % | Spontaneous Closure, No. | Post-BAS Follow-up Time, mo |
|---|---|---|---|---|---|---|---|---|---|
| Nihill et al19/1991 | 3 | 34.0/100 | NR | Adults | 66.6 | 33.3/100 | 66.7 | NR | 16.0 |
| Thanopoulos et al20/1996 | 6 | 6.1/NR | NR | Children | NR | 50.0/100 | 100.0 | NR | 48.0 |
| Hayden21/1997 | 6 | 35.0/83.3 | 6 (IV) | Children/adults | 16.7 | 83.3/100 | 100.0 | NR | 17.0 |
| Sandoval et al22/1998 | 15 | 33.0/86.7 | 1 (II), 4 (III), 10 (IV) | Adults | 20.0 | 46.7/100 | 100.0 | 4 | 36.0 |
| Rothman et al23/1999 | 12 | 37.0/83.3 | NR | Children/adults | NR | 50.0/50.0 | 75.0 | 1 | 14.0 |
| Pepke-Zaba et al24/2000 | 9 | 43.0/66.7 | NR | Adults | NR | 100.0/77.8 | 88.9 | 1 | 2.6 |
| Kothari et al14/2002 | 11 | 16.2/36.4 | NR | Children/adults | 90.9 | 27.3/100 | 64.0 | NR | 60.0 |
| Allcock et al25 /2003 | 9 | 56.4/100 | 6 (IV), 3 (III) | Adults | 0.0 | 100.0/100 | 67.0 | 2 | 36.0 |
| Reichenberger et al26/2003 | 17 | 40.0/71.0 | 7 (III), 10 (IV) | Adults | NR | 23.5/NR | 76.0 | 2 | 31.6 |
| Ciarka et al11/2007 | 11 | 48.0/54.5 | 5 (III), 6 (IV) | Adults | 90.9 | 18.2/NR | 54.5 | NR | 0.0 |
| Kurzyna et al27/2007 | 11 | 33.0/54.5 | NR | Adults | 81.8 | NR/NR | 81.8 | 6 | 20.2 |
| Sandoval et al28/2011 | 34 | 35.0/85.0 | NR | Adults | 41.0 | 26.0/NR | 85.0 | 10 | 138.0 |
| Baglini1/2013 | 11 | 42.5/45.5 | 4 (III), 7 (IV) | Adults | NR | NR/NR | 73.0 | NR | 12.0 |
| Kuhn et al29/2015 | 16 | 47.6/75.0 | NR | Adults | 100.0 | 62.5/NR | 43.8 | 4 | 61.5 |
| Chiu et al4/2015 | 23 | 23.0/74.0 | NR | Children/adults | 46.0 | 41.0/57.9 | 63.0 | NR | 184.3 |
| Martin et al30 /2016 | 10 | 43.5/80.0 | NR | Adults | 50.0 | 50.0/100 | 60.0 | 3 | 67.0 |
BAS = balloon atrial septostomy; NR = not reported; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; RHF = right-sided heart failure.
Figure 2.
Asymmetrical funnel plot indicating evidence of publication bias. MD = mean difference.
Table 2.
Quality Assessment of Included Studies According to the Newcastle-Ottawa Scale
| Study/Year | Selection |
Comparability |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | O1 | O2 | O3 | |
| Nihill et al19/1991 | … | … | * | * | * | … | … | * | * |
| Thanopoulos et al20/1996 | … | … | * | * | * | … | … | … | * |
| Hayden21/1997 | … | … | * | * | * | … | … | … | * |
| Sandoval et al22/1998 | … | … | * | * | * | … | … | * | * |
| Rothman et al23/1999 | … | … | * | * | * | … | … | … | * |
| Pepke-Zaba et al24/2000 | … | … | * | * | * | … | … | … | * |
| Kothari et al14/2002 | … | … | * | * | * | … | … | … | * |
| Allcock et al25 /2003 | … | … | * | * | * | … | … | * | * |
| Reichenberger et al26/2003 | … | … | * | * | * | … | … | … | * |
| Ciarka et al11/2007 | … | … | * | * | * | … | … | … | * |
| Kurzyna et al27/2007 | … | … | * | * | * | … | … | … | * |
| Sandoval et al28/2011 | … | … | * | * | * | … | … | * | … |
| Baglini1/2013 | … | … | * | * | * | … | … | * | * |
| Kuhn et al29/2015 | … | … | * | * | * | … | … | * | * |
| Chiu et al4/2015 | … | … | * | * | * | … | … | * | … |
| Martin et al30 /2016 | … | … | * | * | * | … | … | * | * |
The asterisks indicate that the study has accounted for that variable (ie, low risk of bias for that variable). C1 and C2 = comparability of cohorts on the basis of the design or analysis; O1 = assessment of outcome; O2 = was follow-up long enough for outcomes to occur; O3 = adequacy of follow-up of cohorts; S1 = representativeness of the exposed cohort; S2 = selection of the nonexposed cohort; S3 = ascertainment of exposure; S4 = demonstration that outcome of interest was not present at start of study.
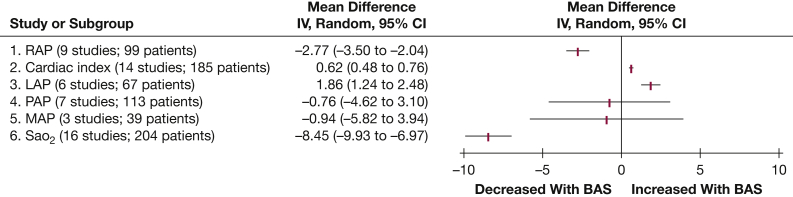
The results of the meta-analysis for hemodynamic outcomes are summarized in Figure 3.
-
1.
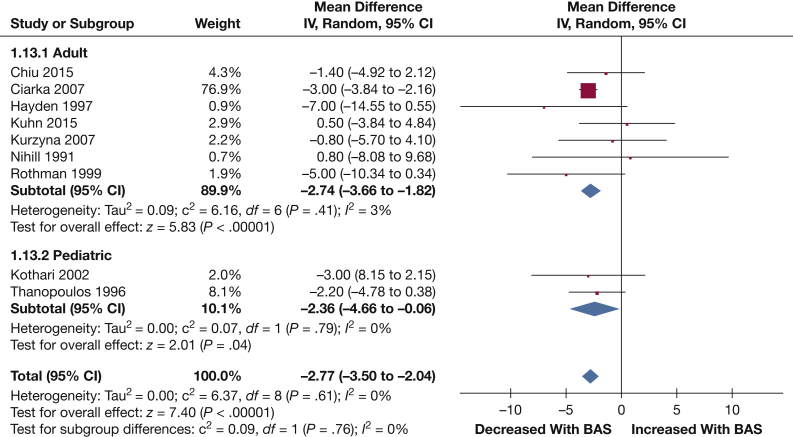
RAP: Nine studies reported data on mean RAP (99 patients). Mean RAP was significantly reduced following BAS (–2.77 mm Hg [–3.50, –2.04]; P < .001) (Fig 4).
-
2.
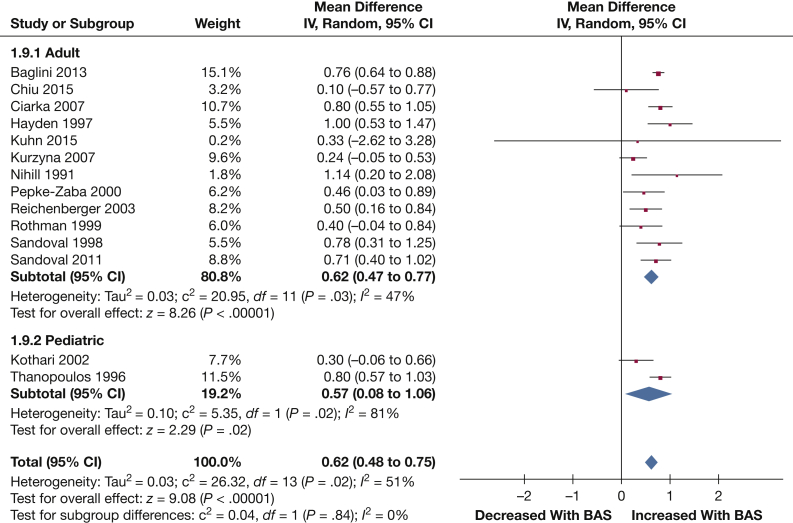
Cardiac index: Fourteen studies provided data on the cardiac index (185 patients). BAS exhibited a significant increase in cardiac index (0.62 L/min/m2 [0.48, 0.75]; P < .001) (Fig 5).
-
3.
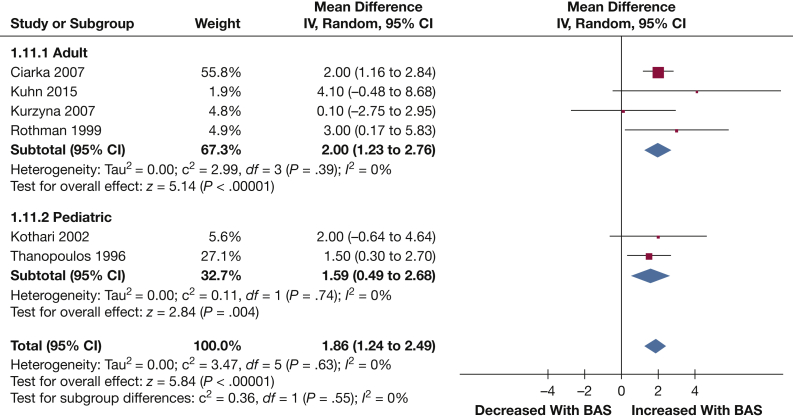
LAP: LAP was reported in six studies (67 patients). LAP was significantly decreased following BAS (1.86 mm Hg [1.24, 2.49]; P < .001) (Fig 6).
-
4.
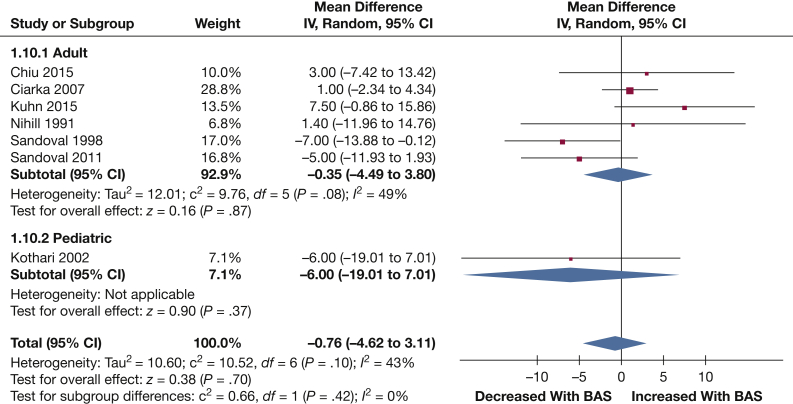
PAP: Mean PAP values were reported in seven studies (113 patients). BAS did not significantly lower mean PAP (–0.76 mm Hg [–4.62, 3.11]; P = .70) (Fig 7).
-
5.
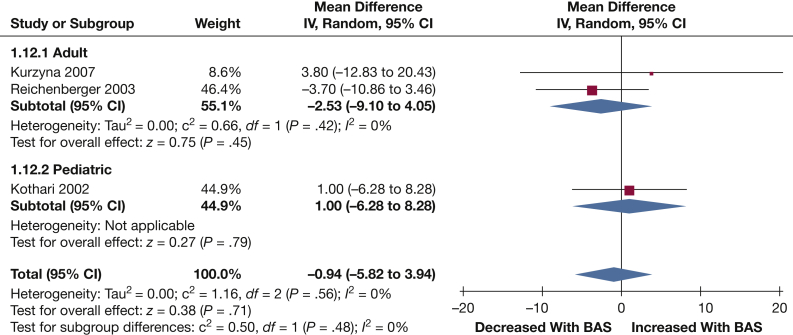
MAP: Three studies reported data on MAP following BAS (39 patients). No significant change in MAP was observed following BAS (–0.94 mm Hg [–5.82, 3.94]; P = .71) (Fig 8).
-
6.
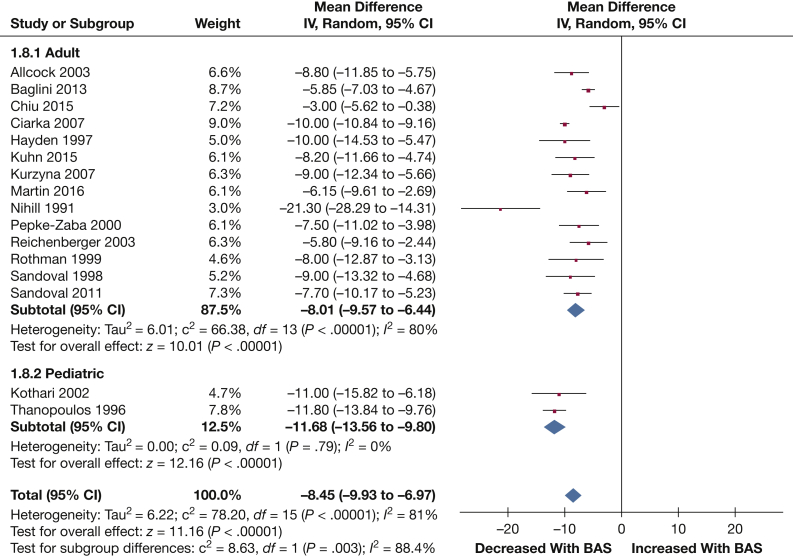
Sao2: A total of 16 studies contained adequate data on Sao2 (204 patients). A significant reduction in Sao2 was observed following BAS (–8.45% [–9.93, –6.97]; P < .001) (Fig 9).
Figure 3.
Summary of results of meta-analysis. BAS = balloon atrial septostomy; LAP = left atrial pressure; MAP = mean arterial pressure; PAP = pulmonary artery pressure; RAP = right atrial pressure; RHF = right-sided heart failure; Sao2 = arterial oxygen saturation.
Figure 4.
Forest plot outlining mean difference in mean RAP following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations.
Figure 5.
Forest plot outlining mean difference in mean cardiac index following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations.
Figure 6.
Forest plot outlining mean difference in mean PAP following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations.
Figure 7.
Forest plot outlining mean difference in MAP following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations.
Figure 8.
Forest plot outlining mean difference in LAP following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations
Figure 9.
Forest plot outlining mean difference in mean Sao2 following BAS compared with prior to BAS. See Figure 3 legend for expansion of abbreviations.
With respect to the meta-analysis of procedural complications:
-
1.
Postprocedural (48 h) complications: Fifteen studies reported complications related to the procedure up to 48 h following BAS. The most common pooled procedural complication was hypoxemia, occurring in 3.0% (0.5%-6.7%) of patients. Procedure-related mortality is reported separately.
-
2.
Spontaneous septostomy closure: Nine studies reported this outcome at a mean follow-up of 46.5 months (range, 0-184 months). The pooled incidence of spontaneous septostomy closure was 23.8% (15.5%-33.0%).
With respect to the meta-analysis of survival associated with BAS, 16 studies reported data on mortality following BAS at a mean follow-up of 46.5 months (range, 0-184 months). The pooled incidence of procedure-related (48 h), short-term (≤ 30 day), and long-term (> 30 days) mortality was 4.8% (1.7%-9.0%), 14.6% (8.6%-21.5%), and 37.7% (27.9%-47.9%), respectively.
The results of multivariate meta-regression analysis are summarized in Table 3. Meta-regression analysis could not significantly attribute heterogeneity to study-level covariates of mean age, female sex, proportion of syncopal history, or proportion of patients with idiopathic PAH for mean RAP and Sao2. Mean age, however, was found to significantly contribute to the heterogeneity observed in the cardiac index estimate (coefficient, 0.025; P < .001). Meta-regression was not performed for the other outcomes because of the low number of studies reporting these parameters.
Table 3.
Results of Random Effects Meta-Regression Analysis
| Covariate | RAP |
Sao2 |
Cardiac Index |
|||
|---|---|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | Coefficient | P Value | |
| Mean age, y | –0.106 | .129 | –0.005 | .955 | 0.025 | < .001a |
| Female sex | 0.114 | .092 | –0.046 | .497 | –0.005 | .267 |
| Syncope, % | –0.104 | .110 | 0.009 | .831 | 0.006 | .118 |
| Idiopathic PAH, % | –0.078 | .255 | 0.010 | .859 | 0.006 | .259 |
RAP = right atrial pressure; Sao2 = arterial oxygen saturation. See Table 1 legend for expansion of other abbreviation.
Statistically significant.
Subgroup analysis stratifying study populations into pediatric and adult groups did not result in significantly different results for any of the outcomes of interest. The test for interaction between adult (–8.01% [–9.57, –6.44]; P < .001) and pediatric (–11.68% [–13.56, –9.80]; P < .001) subgroups for Sao2 was, however, significant (P = .003).
Discussion
This comprehensive systematic review and meta-analysis comprising 212 patients with PAH found that BAS significantly improved important hemodynamic parameters, namely RAP, LAP, and the cardiac index, while also resulting in a reduction in Sao2. The interpretation of mortality rates associated with BAS is challenging because of the high-risk patient population and the absence of randomized or comparative studies as part of the meta-analysis. Despite these issues, approximately 50% of patients remained alive at long-term follow-up, and the overall complication rate associated with the procedure was relatively low.
The mechanism by which the BAS technique works easily explains the improvements in hemodynamic parameters. An artificially created right-to-left interatrial septal shunt, as occurs with BAS, improves left ventricular filling (reported herein as an increase in LAP) and systemic cardiac output. Although the shunt will induce systemic hypoxemia (as seen by the reported drop in Sao2), the increase in systemic cardiac output (albeit with desaturated blood) coupled with an expected reactive polycythemia often results in a net increase in oxygen tissue delivery that may prove to be clinically beneficial.11 Conversely, the BAS procedure should be generally avoided in patients with PAH with an RAP > 20 mm Hg or a preprocedural Sao2 < 90% due to concerns surrounding excessive right-to-left shunting that may result in pulmonary edema and/or profound hypoxemia.2, 12 In fact, a baseline RAP > 20 mm Hg has been associated with a > 10-fold increased risk of mortality in this subset of patients.13 Two of the studies in our analysis consisted of such patients with preprocedural RAP > 20 mm Hg, which might have contributed to the overall mortality rates, leading to contemporary guidelines suggesting caution. Other predictors of mortality included a pulmonary vascular resistance index of 55 Wood units/m2 and an expected 1-year survival < 40%.14 It is plausible that a pulmonary vascular resistance index of this extent may indicate a stage of disease that is beyond salvage even with BAS, although additional studies investigating this parameter are warranted.
Interestingly, we observed greater improvement in the cardiac index following BAS with increasing age on meta-regression analysis. This finding could possibly be explained by the general decline in cardiac index associated with aging overall (and hence the proportionately greater net increase in cardiac index with BAS).15 Conversely, the average age of patients in the meta-analysis was 26.2 years, with only one study having patients aged > 50 years. In addition, the pediatric and adult subgroups differed significantly with respect to the reduction in Sao2. The exact reason behind this finding is unclear; however, it can serve to be hypothesis-generating for future studies. Given the relatively small sample size even with pooling of data from 16 studies, these observations should be interpreted cautiously.
We also observed that nearly one-quarter of patients (23.8%) experienced spontaneous closure of the septostomy. Although additional risk is likely to be incurred with repeated procedures, the risk associated with an initial, excessively large septostomy could be devastating. To prevent sudden precarious drops in Sao2 or increases in left ventricular end-diastolic pressure,16 a graded balloon dilatation technique should be used, repeating the procedure as many times as needed to achieve the desired result. This approach of staged procedures is considered to be safer due to the stepwise increase in the diameter of the induced defect; however, as a consequence, it is also accompanied by a higher risk of spontaneous closure of the septostomy, which is much less common in other variations of septostomy creation, most notably blade and BAS.14 Our pooled analysis showing an incidence of spontaneous septostomy closure of 23.8% with BAS is significantly higher than the approximately 3% closure rate that has been reported following blade and BAS in other studies.17
Currently, BAS has an important, albeit limited role in the treatment of PAH. Our meta-analysis of survival seems to suggest a “bridging” role for septostomy at a minimum (ie, to possible future transplantation), although it could be argued that achieving an approximately 50% 4-year survival rate in a cohort of patients with PAH experiencing RHF and/or syncope is an acceptable result irrespective of future therapeutic options. Although BAS is unlikely to influence the disease process of PAH itself, the procedure generates beneficial hemodynamic changes, including decreased RAP and increased cardiac index, both of which are consistently associated with improved survival in the PAH patient population.18 In fact, it has been argued that this may be one of the reasons why survival of PAH associated with Eisenmenger syndrome is superior to that in patients with other types of PAH, including idiopathic PAH.
The true benefit of BAS is likely unable to be fully assessed from small, nonrandomized individual studies, as it is often unclear whether the change in outcomes resulted from the intervention itself or whether co-interventions or even background medical therapy were also responsible for the outcome of interest. Thus, meta-analyses such as the present one are often needed to better characterize the evidence in the absence of randomized clinical trials. In addition, the likelihood of a randomized trial of BAS occurring in the near future is low. First, the perceived benefits of BAS for a declining patient with PAH may dissuade physicians from enrolling patients into such a randomized trial in which a significant fraction of patients will ultimately not receive the intervention. Moreover, although cross-over to the BAS arm could be an adjudicated outcome, the relatively narrow therapeutic window of the safety of BAS (ie, avoidance of severe RHF) may preclude this possibility as well. In addition, the financial support for such a trial in the current era of about 15 approved drugs/formulations of targeted PAH therapies despite its orphan disease designation would be difficult. Finally, the overall rarity of the disease and the limited number of centers with operators experienced in the BAS intervention might also limit the ability to enroll in such a study. Despite such hurdles, the present meta-analysis supports the continued, albeit judicious use of BAS in select patients with advanced stages of PAH. Studies aiming to further improve BAS techniques, such as cryoplasty to freeze the margins of the newly created atrial defect to help sustain patency, are ongoing (PROPHET trial).31
The present meta-analysis is not without limitations. First, as mentioned, none of the included studies had a control group. Second, the effect of concomitant background medical therapy, including the use of parenteral prostanoid therapy, could not be assessed and could therefore result in confounding. In addition, inherent to many meta-analyses, most the included studies were small, potentially leading to imprecise estimates, including the approximation of means and SDs from median and interquartile ranges. Lastly, certain variables of interest such as changes in pulmonary vascular resistance, stroke volume, stroke volume index, heart rate, pulmonary artery compliance, arterial elastance, 6-min walk distance, brain natriuretic peptide, glomerular filtration rate, creatinine, and REVEAL risk calculation8 could not be assessed due to the limited number of studies reporting such variables. Thus, the results of this meta-analysis should be interpreted with caution, keeping in mind its inherent limitations.
Conclusions
BAS seems to be a relatively safe procedure associated with largely favorable hemodynamic outcomes in carefully selected patients with PAH. Short-term survival supports its consideration as a bridging procedure (ie, to lung transplantation), and longer term survival may rival contemporary medical treatments in patients with advanced stages of this uniformly fatal disease.
Acknowledgments
Author contributions: M. S. Khan: Study inception, study design, data interpretation, and critical review; M. M. M. and E. A.: Study inception, study design, literature search, data collection, statistical analysis, figure creation, data interpretation, writing, and critical review; N. Y., S. U. Khan, V. M. F., S. D., J. D. R., R. L. B., and R. A. K.: Study design, data interpretation, writing, and critical review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. A. K. receives research funding from and has served as a consultant to Actelion Pharmaceuticals. He also serves as an investigator for Edwards Lifesciences and as an unfunded member of the scientific advisory board for Ventripoint. None declared (M. S. Khan, M. M. M., E. A., N. Y., S. U. Khan, V. M. F., S. D., J. D. R., R. L. B.).
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
The abstract of this study was presented at the American Heart Association Scientific Sessions, November 10-12, 2018, Chicago, IL.
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Baglini R. Atrial septostomy in patients with end-stage pulmonary hypertension. No more needles but wires, energy and close anatomical definition. J Interven Cardiol. 2013;26(1):62–68. doi: 10.1111/j.1540-8183.2012.00759.x. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Shiboski S.C., Golden J.A. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Critic Care Med. 2009;180(5):468–474. doi: 10.1164/rccm.200810-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu J.S., Zuckerman W.A., Turner M.E. Balloon atrial septostomy in pulmonary arterial hypertension: effect on survival and associated outcomes. J Heart Lung Transplant. 2015;34(3):376–380. doi: 10.1016/j.healun.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells G.A., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 7.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 8.Benza R.L., Miller D.P., Barst R.J., Badesch D.B., Frost A.E., McGoon M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Miller J.J. The Inverse of the Freeman–Tukey Double Arcsine Transformation. The American Statistician. 1978;32(4):138. [Google Scholar]
- 11.Ciarka A., Vachiery J.L., Houssiere A. Atrial septostomy decreases sympathetic overactivity in pulmonary arterial hypertension. Chest. 2007;131(6):1831–1837. doi: 10.1378/chest.06-2903. [DOI] [PubMed] [Google Scholar]
- 12.Yuan J.J., Garcia J., West J., Hales C., Rich S., Archer S. Springer; Boston, MA: 2011. Textbook of Pulmonary Vascular Disease. [Google Scholar]
- 13.Sandoval J., Torbicki A. Atrial septostomy. In: Voelkel N., Schranz D., editors. The Right Ventricle in Health and Disease. Humana Press, Springer; New York, NY: 2015. pp. 419–437. [Google Scholar]
- 14.Kothari S.S., Yusuf A., Juneja R., Yadav R., Naik N. Graded balloon atrial septostomy in severe pulmonary hypertension. Indian Heart J. 2002;54(2):164–169. [PubMed] [Google Scholar]
- 15.Carlsson M., Andersson R., Bloch K.M. Cardiac output and cardiac index measured with cardiovascular magnetic resonance in healthy subjects, elite athletes and patients with congestive heart failure. J Cardiovasc Magnetic Resonance. 2012;14(1):51. doi: 10.1186/1532-429X-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval J. Interventional therapies in pulmonary hypertension. Revista Española de Cardiología (English Edition) 2018;71(7):565–574. doi: 10.1016/j.rec.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Ali Khan M.A., Bricker J.T., Mullins C.E., al Yousef S., Nihill M.R., Vargo T.A. Blade atrial septostomy: experience with the first 50 procedures. Cathet Cardiovasc Diagn. 1991;23(4):257–262. doi: 10.1002/ccd.1810230406. [DOI] [PubMed] [Google Scholar]
- 18.D'Alonzo G.E., Barst R.J., Ayres S.M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 19.Nihill M.R., O'Laughlin M.P., Mullins C.E. Effects of atrial septostomy in patients with terminal cor pulmonale due to pulmonary vascular disease. Cathet Cardiovasc Diagn. 1991;24(3):166–172. doi: 10.1002/ccd.1810240305. [DOI] [PubMed] [Google Scholar]
- 20.Thanopoulos B.D., Georgakopoulos D., Tsaousis G.S., Simeunovic S. Percutaneous balloon dilatation of the atrial septum: immediate and midterm results. Heart. 1996;76(6):502–506. doi: 10.1136/hrt.76.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden A.M. Balloon atrial septostomy increases cardiac index and may reduce mortality among pulmonary hypertension patients awaiting lung transplantation. J Transpl Coord. 1997;7(3):131–133. doi: 10.7182/prtr.1.7.3.b5v5j80353hx0716. [DOI] [PubMed] [Google Scholar]
- 22.Sandoval J., Gaspar J., Pulido T. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998;32(2):297–304. doi: 10.1016/s0735-1097(98)00238-1. [DOI] [PubMed] [Google Scholar]
- 23.Rothman A., Sklansky M.S., Lucas V.W. Atrial septostomy as a bridge to lung transplantation in patients with severe pulmonary hypertension. Am J Cardiol. 1999;84(6):682–686. doi: 10.1016/s0002-9149(99)00416-6. [DOI] [PubMed] [Google Scholar]
- 24.Pepke-Zaba J.M.K., Satchithanada D.K., Doughty N., Shapiro L. Atrial septostomy as a successful procedure improving symptoms of right heart failure in patients with severe pulmonary hypertension. Thorax. 2000;55(A48) [Google Scholar]
- 25.Allcock R.J., O'Sullivan J.J., Corris P.A. Atrial septostomy for pulmonary arterial hypertension. Heart. 2003;89(11):1344–1347. doi: 10.1136/heart.89.11.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichenberger F., Pepke-Zaba J., McNeil K., Parameshwar J., Shapiro L.M. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax. 2003;58(9):797–800. doi: 10.1136/thorax.58.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzyna M., Dabrowski M., Bielecki D. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest. 2007;131(4):977–983. doi: 10.1378/chest.06-1227. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval J., Gaspar J., Pena H. Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J. 2011;38(6):1343–1348. doi: 10.1183/09031936.00072210. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn B.T., Javed U., Armstrong E.J. Balloon dilation atrial septostomy for advanced pulmonary hypertension in patients on prostanoid therapy. Catheter Cardiovasc Interv. 2015;85(6):1066–1072. doi: 10.1002/ccd.25751. [DOI] [PubMed] [Google Scholar]
- 30.Martín M.V., González-Trevilla A.A., López-Guarch C.J., Tejada J.G., Asenjo R.M., Subías P.E. Use of atrial septostomy to treat severe pulmonary arterial hypertension in adults. Revista espanola de cardiologia (English ed) 2016;69(1):78–81. doi: 10.1016/j.rec.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 31.National Library of Medicine. The Prophet Trial - Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Patients With Pulmonary Hypertension. https://clinicaltrials.gov/ct2/show/NCT03022851. Accessed April 13, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.