Abstract
Pancreatic neuroendocrine tumors (PNETs) are known to be the second most common epithelial malignancy of the pancreas. PNETs can be listed among the slowest growing as well as the fastest growing human cancers. The prevalence of PNETs is deceptively low; however, its incidence has significantly increased over the past decades. According to the American Cancer Society’s estimate, about 4032 (> 7% of all pancreatic malignancies) individuals will be diagnosed with PNETs in 2020. PNETs often cause severe morbidity due to excessive secretion of hormones (such as serotonin) and/or overall tumor mass. Patients can live for many years (except for those patients with poorly differentiated G3 neuroendocrine tumors); thus, the prevalence of the tumors that is the number of patients actually dealing with the disease at any given time is fairly high because the survival is much longer than pancreatic ductal adenocarcinoma. Due to significant heterogeneity, the management of PNETs is very complex and remains an unmet clinical challenge. In terms of research studies, modest improvements have been made over the past decades in the identification of potential oncogenic drivers in order to enhance the quality of life and increase survival for this growing population of patients. Unfortunately, the majority of systematic therapies approved for the management of advanced stage PNETs lack objective response or at most result in modest benefits in survival. In this review, we aim to discuss the broad challenges associated with the management and the study of PNETs.
Keywords: Pancreatic neuroendocrine tumors, Gastroenteropancreatic neuroendocrine tumors, Management, Limitation, Novel Agents, Emerging targets
Core tip: Pancreatic Neuroendocrine Tumors (PNETs) can cause severe morbidity due to excessive hormones production and overall tumor mass. The majority of approved therapeutic options in PNETs lack objective response suggesting that there is still a void in the understanding of the biology of this neoplasia. With the rising incidence and the underestimated prevalence of PNETs in the United States, it is paramount to discuss the challenges associated with the study and the management of this intractable disease for better patient outcomes. In this paper we elaborate on the comprehensive challenges and discuss novel and emerging therapeutic target in PNETs.
INTRODUCTION
Physiologically, neuroendocrine cells receive neurotransmitter signals from the nervous system to secrete hormones in the blood to control many body functions[1]. These specialized cells can be found in almost every organ of the body including the thymus, kidneys, prostate, skin, cervix, ovaries, testicles, stomach, colon, esophagus, appendix, small intestine, rectum, gallbladder, liver, and the pancreas[2]. The pancreas is an essential organ involved in the digestive system and the endocrine system[3]. In the endocrine system, pancreatic islet cells release hormones and polypeptides (including insulin, glucagon, somatostatin, amylin, pancreatic peptide, gastrin, incretin, and secretin) needed to regulate blood sugar level and multiple other body functions[4]. When these hormonal producing cells of the pancreas become cancerous, they are termed Pancreatic Neuroendocrine tumors (PNETs)[5].
Due to the advances in diagnostic modalities and the increase in awareness by oncologists and the general population, the incidence of PNETs is significantly increasing. According to the National Cancer Institute registry, the incidence of PNETs is estimated at 1000 new cases every year in the United States (https:// www.cancer.gov/types/pancreatic/hp/pnet-treatment-pdq). However, the American Cancer Society has predicted that about 4,032 people will be diagnosed with PNETs in 2020 (https://www.cancer.org/cancer/pancreatic-neuroendocrine-tumor/about/key-statistics.html). In the past decades, PNETs have often been diagnosed at a later stage when the disease is already advanced or metastatic[6]. In recent years, gastrointestinal oncologists are increasingly seeing patients diagnosed accidentally at an early stage[7,8]. In this scenario, the tumor is further diagnosed using gallium 68 DOTATATE PET imaging coupled with a diagnostic quality contrast-enhanced MRI of the upper abdomen[9]. The prevalence of the PNETs, that is the number of patients actually dealing with the disease at any given time is fairly high because the survival is much longer than pancreatic ductal adenocarcinoma (PDAC). In a retrospective study on about 1074 histopathological pancreatic specimens, Partelli et al[10] examined whether the real prevalence of PNETs was underestimated. After excluding 284 patients who were diagnosed with PNETs as the main lesion, they found an incidental associated diagnosis of PNETs in 4% of the remaining specimens and they concluded that the frequency of incidental histological diagnosis of PNETs is considerably high and its prevalence is probably underestimated[10].
In general, tumors grade and classification are the fundamental basis for neuroendocrine tumors (NETs) therapeutic decisions[11]. Tumor grade is a system used to predict how fast tumors would grow/spread and differentiation is a key feature to predict their behavior[12]. Ki-67 (MIB1) only stains actively dividing cells and not resting cells, is most commonly used to establish the grade of the tumor; thus, more dividing cells implies more aggressive PNETs. The world health organization classifies PNETs into three main categories based on the Ki67 proliferation index and/or mitotic count per 10 high power fields. Well-differentiated PNETs (also known as panNETs) are classified as Grade 1 (low grade), Grade 2 (intermediate grade), and Grade 3 (high grade) with a Ki67 index of < 2%, 2%-20%, and > 20% respectively. Poorly differentiated PNETs (also referred to as panNEC) are categorized as grade 3 (high grade) with a Ki67 index greater than 20%[13]. Also, tumor grade strongly predicts outcomes such as how fast the tumor will grow and how long it can be controlled with therapy. For well-differentiated grade 1, meaning PNETs patients who have small low-grade tumors, oncologists often wait and do not operate (watchful waiting protocol) and most recently treat with the PRRT (Peptide receptor radionuclide therapy), a treatment that is well tolerated and very safe (the latter drug will be further discussed in this manuscript)[14]. Well-differentiated grade 3 PNETs are fairly indolent but often have an unpredictable course and behave similarly to grade 2 panNETs; poorly differentiated grade 3 panNEC are aggressive[15]. It is important to note that there exists an additional category for PNET termed: Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN)[16,17].
Recently, cross-species analysis of mice and human panNET tissues illustrate the existence of three molecular subtypes of PNETs including Islet/Insulinoma tumors [IT (less aggressive, and express genes associated with differentiated mature β-cells)]; metastasis-like/primary [MLP (invasive and express genes associated with immature β-cells, and stem cells)], and intermediate (express genes similar to IT and are moderately invasive)[18]. Next-Gen sequencing illustrates that commonly mutated genes associated with neoplasia pathogenesis are not significantly implicated in PNET development and progression[19]. However, hyperactivation of PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways have been well documented to be the main regulators of proliferation in NETs[20]. Frequent mutations in multiple endocrine neoplasia 1 (MEN1; 44%), death domain-associated protein (DAXX)/chromatin remodeler (ATRX; 43%), mTOR (15%) pathway genes, and Von Hippel Lindau (VHL) alongside several other hereditary disorders are observed in PNETs[21]. Loss of function of the tumor suppressor gene PTEN is frequently found in PanNETs and is responsible for the over-activation of the PI3K-Akt-mTOR cascade[22]. A new examination using whole-genome sequencing of 102 primary PNETs illustrates that germline mutations in DNA repair genes such as MUTYH, CHECK2, and BRCA2 were noted in sporadic PNETs[23].
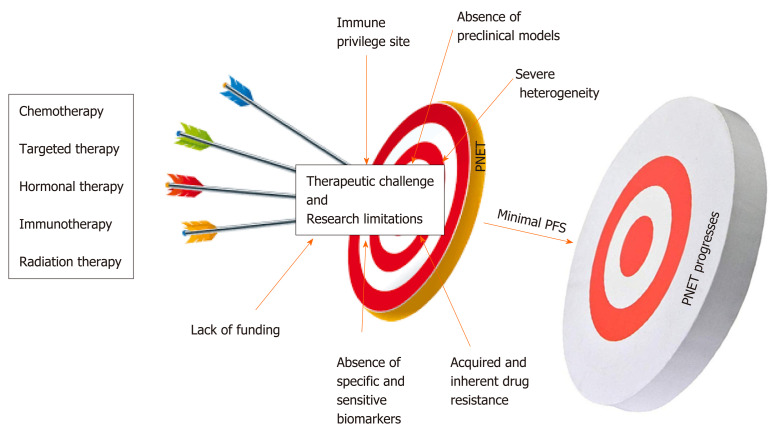
Compared to other gastroenteropancreatic neuroendocrine tumors (GEP-NETs), PNETs are a very heterogeneous subtype of cancers with unique pathophysiological features that constitutes a major challenge in the management of this neoplasia[24]. Multiple factors impede the management and the study of PNETs. As mentioned above, the majority of patients are diagnosed at a later stage when the disease is already advanced due to the lack of specific biomarkers and disease-associated symptoms[6]. Systemic treatments for PNETs only stabilize the disease most likely because of inherent and acquired drug resistance. Another challenge in drug development is related to the poor delivery of therapeutic agents related to the location of the pancreas[25]. Lack of reliable preclinical models (mostly cell lines) limits the ability to rapidly test promising therapies. The small population of relevant candidates with PNETs is a major challenge for conducting larger clinical trials[26]. Immunotherapy is not an option for this patient population given that the pancreas appears to be an immunologically coldsite[27,28]. Despite significant increase in the incidence of PNETs in the United States, this disease remains an understudied and underfunded area of research. This review intends to discuss the major challenges associated with the management of PNETs in the clinic and highlight research limitations associated with its study.
CURRENT THERAPEUTIC OPTIONS
The ultimate question in the management of PNETs is when to give specific treatment to a patient; keeping in mind that one size cannot fit all. This is left to the clinician’s own assessment as to who should have surgery? Who are the ideal candidates for drug X? And who are the ideal candidates for chemotherapy? Systemic therapeutic decisions for the management of PNETs must be personalized and rely on various considerations including functional imaging and molecular profiling in addition to clinical considerations such as hormonal secretion, tumor grade, disease burden, and the rate at which tumor progresses[29]. These considerations predict whether systemic or locoregional treatment will benefit patients[30]. The impact of therapies on the quality of the life of the patient must be considered prior to making any therapeutic decision. More than 80-90% of pancreatic islet tumors express somatostatin receptors (SSTRs). These SSTRs are G protein-coupled seven transmembrane receptors that control cellular proliferation and hormone production by PNET cells and as such are targets for diagnostics and therapeutics (theranostics)[31-33]. The somatostatin analogs (SSRA) Octreotide and Lanreotide (targeting specifically SSTR2 and SSTR5) are commonly used for initial treatment of advanced stage well-differentiated grade 1 or 2 PNETs[34]. Somatostatin analogs can inhibit hormone production from PNETs. For example Octreotide and Lanreotide can be used to prevent hypoglycemia in patients with positive SSTR2; however, these drugs could worsen hypoglycemia in patients not expressing SSTR2[35]. In addition, SSRA are also used as palliative treatments to slow down the progression and stabilize the disease burden[36]. However, somatostatin analogs do not cause tumor shrinkage. Peptide Receptor Radionuclide Therapy (PRRT), everolimus (mTOR inhibitor), chemotherapy or sunitinib (multi RTK inhibitor) are used to manage well-differentiated PNETs that have progressed on SSRA[37,38]. A combination of chemotherapeutics agents (such as Capecitabine + Temozolomide or platinum-based regimens) constitutes the first-line treatment for panNEC, MiNEN, and metastatic disease[39]. PRRT is also relevant for metastatic disease. Unfortunately, most of these treatment strategies used by GI oncologists to overcome tumor burden lack objective response and PNETs remain a serious unmet problem in the clinic. At most, these therapies stabilize the tumors and do not enhance the overall survival of patients (Figure 1).
Figure 1.
Graphical abstract. PFS: Progression free survival; PNET: Pancreatic neuroendocrine tumor.
THERAPEUTIC OPTIONS IN PNETS ONLY STABILIZE THE DISEASE
The management of NETs and PNETs, in particular, is greatly personalized and requires expert multidisciplinary strategies including surgery, medical oncology, endocrinology, radiation oncology, cardiology, gastroenterology, pathology, interventional radiology, diagnostic radiology, and nuclear medicine[40]. The majority of FDA approved drugs for the management of PNETs lack objective response characterized by meager progression free survival (PFS) and inability to shrink tumors in the clinic[41]. Yao et al[42] showed that the median progression free survival with everolimus as a single agent treatment for PNET patients is estimated at 11.0 mo relative to 4.6 mo with placebo. The overall survival (OS) with everolimus was estimated at 44.0 mo relative to 37.7 mo with placebo[43]. This means everolimus stabilizes PNETs progression for an average of 6.4 mo. Similarly, Faivre et al[44] and Vinik et al[45] showed that the PFS with sunitinib is evaluated at 11.4 mo relative to 5.5 mo with placebo. The CLARINET study designed to evaluate the response of Lanreotide in metastatic enteropancreatic neuroendocrine tumors showed that this drug can only stabilize the progression of neuroendocrine tumors[46-49]. In the same manner, the PROMID study shows that Octreotide can only lengthen the time to tumor progression in functional and metastatic NET patients[50]. Exner et al recently showed that Octreotide does not inhibit the growth of multiple NET cell lines including BON-1 and QGP-1 the commonly available PNET cellular models[51]. The CAPTEM study (Capecitabine in combination with Temozolomide) shows that PNET patients achieved a median PFS of 13 mo[52]. Dilz and colleagues analyzed data from 96 advanced PNET patients treated with Streptozocin + 5 FU; they found that 40.6% of patients showed stable disease while 16.7% showed progressive disease[53]. Nevertheless, in terms of treatment strategy, the PRRT, appear to be the most promising treatment for PNETs. PRRT is a drug coupled with therapeutic radionuclide lithium 177 and is injected intravenously irradiates PNET cells directly and radiates[54]. PRRT treatment results in PNET shrinkage; nevertheless, this treatment also stabilizes the tumor for a long period of time[55]. The side effects of PRRT are very mild, there can be some nausea with the treatment at the time of the administration which is much related to the IV fluid that is given to protect the patient’s kidneys[56].
HETEROGENEITY IN PNETS
PNETs’ heterogeneity is considered the major challenge in the management of this specific type of neoplasia in the clinic[57,58]. As mentioned above, pancreatic islet cells are specialized entities that participate in the endocrine function of the pancreas by releasing hormones and peptides necessary to maintain body homeostasis. PNETs can be functional or non-functional depending on whether they release these hormones[59,60]. Functional PNETs release excess hormones leading to a variety of hormonal associated symptoms. For example, Insulinoma can release excessive insulin, which results in hypoglycemia and related symptoms[61]. Insulinomas are mostly benign, < 10% are malignant; this subtype of PNETs can mostly be removed by surgery, but liver metastasis patients have < 2% survival[62]. In Gastrinoma (representing 30% of all PNET), excessive gastrin release would cause Zollinger-Ellison syndrome characterized by increased acidity of the stomach and could eventually result in severe peptic ulcer disease and chronic diarrhea (surgery is the only potential cure for tumor > 2 cm followed with PPI)[63]. It is important to note that gastrinoma can also be found in the duodenum but there are much smaller than those from the pancreas. The last example will be VIPoma, in which vasoactive intestinal peptide is aberrantly released causing severe diarrhea and associated symptoms; however, this specific type of functional PNET is very unusual[64]. Thus PNET release hormones, which make the patients sick not the tumor itself. Meanwhile, non-functioning PNETs do not develop hormonal symptoms because they produce insignificant amount of hormones that lack clinical implication. The majority of PNET is nonfunctional and is often diagnosed when the disease is unresectable, advanced or metastatic[6]. Attempts to manage tumor burden and hormonal symptoms concomitantly may constitute a challenge in the clinic. PNETs can also form a large mass, which causes pain by pushing on nerves at the pancreas. Additionally, patients could have a small tumor that has spread to the liver and the liver starts to fill up with metastasis and that may cause severe pain. Liver metastasis is the most significant prognosis factor in PNETs progression setting[65]. The poor quality of life due to the severity of pain in these patients could also alter the effectiveness and the treatment outcomes. As described above a wide range of malignant phenotypes characterizes PNETs’ clinical heterogeneity. Malignant phenotypes in PNETs range from slow-growing (almost indolent), noninvasive tumors, locally invasive and metastatic tumors. Slow-growing tumors are often observed (watchful and waiting) and do not require any therapeutic intervention; however, PNETs have the potential to acquire aggressive phenotype when they reach a certain size and monitoring these tumors for prompt intervention is not an easy task[66].
PNETs can be sporadic or associated with a genetic syndrome. Genetic syndromes associated with PNETs include Multiple Endocrine Neoplasia type 1 (MEN 1), Von Hippel Lindau (VHL), Neurofibromatosis (NF), and Tuberous Sclerosis Complex (TSC)[67,68]. Syndrome associated PNETs demonstrate a significant challenge in the clinic when considering how to best manage patients. For instance, MEN1 functional PNETs patients can undergo tumor resection with a high cure potential; meanwhile, surgery is not a therapeutic option for MEN1 nonfunctional PNETs[69]. As mentioned above, nonfunctional PNETs are often diagnosed at a later stage, with multiple sites within the pancreas (when they are not metastatic); they are small tumors, thus, require resection. Moreover, MEN1 nonfunctional PNETs are often associated with diabetes; therefore, pancreatic resection is not advised especially in young patients[70].
PNET LACK SPECIFIC AND SENSITIVE BIOMARKERS
A biomarker is a measurable biological indicator of the presence or severity of diseases. In cancer management biomarkers have critical importance; they are necessary for prognostication and highly essential to ease early diagnosis[71]. More importantly, biomarkers are necessary to predict and monitor response to specific treatment including recurrence after surgical intervention[72,73]. Lack of adequate biomarkers is another fundamental problem in the management of this disease in the clinic. Current PNET serum based biomarkers such as chromogranin A (CgA), pancreatic peptide (PP), and neuron specific enolase (NSE) have limited sensitivity and specificity[74,75]. The sensitivity and the specificity of a good biomarker should be greater or equal to 90%. The sensitivity of CgA range from 60 to 83% and its specificity ranged from 72 to 85%[76,78]. Meanwhile, the sensitivity of PP range from 31 to 63% and its specificity is approximately 67%[79,80]. The sensitivity of NSE is 33% and its specificity is 73%[81,82]. This poor sensitivity and specificity could be an explanation for the use of PNETs grade and stage as prognostic biomarkers for this neoplasia[83]. Additionally, CgA is an unspecific biomarker given that it can be released by non-neuroendocrine tumors including gastric disorder, inflammatory bowel disease, end-stage renal disease (ESRD), and obstruction of blood vessels (cardiovascular disease)[84,85]. NSE is also a non-specific biomarker because increase level of this molecule is associated with brain injury[86]. Nevertheless, vasostatin-1 (VS-1): The N-terminal fragment of chromogranin A (CgA), has recently been identified to be more accurate than CgA as neuroendocrine biomarker and the plasma levels of VS-1 are not altered by proton pump inhibitors (PPI) used in gastrinoma[87].
PNETS LACK SIZABLE NUMBER OF PRE-CLINICAL CELLULAR MODELS
The development of novel anticancer drugs necessitates the development and use of appropriate and relevant representative in vitro and in vivo models. Lack of reliable PNETs’ cell lines holds back meaningful research and has significantly disadvantaged the management of PNETs for decades[88,89]. Significant strides have been made over the past four decades to develop cellular and mouse models of PNETs. Currently, there are only a few PNET cellular models available for biomedical research[90,91]. BON-1, QGP-1, and CM are the available PNET cell lines often used in research to study this disease. Twenty-five years ago, Townsend et al[92] established BON-1 cell line from the lymph node of a 28-year-old male. QGP-1 is a functioning PNET cell line established in the 1980s from a 61-year-old male[93]. BON-1 and QGP-1 cells were recently authenticated to belong to neuroendocrine and epithelial lineage, but their molecular characterizations do not often resemble those seen in patients’ primary cancers. For instance, exome sequencing and genome-wide copy number analysis reveal that BON-1 and QGP-1 do not harbor PNET-associated mutations such as mTOR, DAXX/ATRX, MEN1, VHL, and NF; questioning the relevance of using these models for PNET study[94,95]. The fast growing potential of these two cell lines does not reflect the slow growth phenotype of most PNETs[96]. In general neuroendocrine cancers are characterized by high expression levels of somatostatin receptors; however, BON-1 and QGP-1 define a very low expression of somatostatin receptors[51]. Kim, B.L. and colleagues have recently shown that BON-1 and QGP-1 illustrate similar characteristics of immature/non-functional pancreatic β/δ-cells or pancreatic endocrine progenitors. They show that BON-1 and QGP-1 display high expression levels of NEUROG3 and FOXA2 two genes associated with immature/non-functional pancreatic β/δ-cells and pancreatic endocrine progenitor, respectively[97]. The latter suggests that these two cell lines have acquired malignant transformation at an early stage of their development. The latter also suggests that QGP-1 may not be functioning (gastrinoma) PNETs as previously characterized. Benten et al[98] established and characterized a novel lymph node-derived cell line (NT-3) from a male patient with well-differentiated PNETs. NT-3 cells are specifically insulinoma (the most common functional PNETs) and express neuroendocrine characteristics that surpass the phenotype observed in BON-1 and QGP-1. Even though NT-3 could become a relevant model for functioning PNETs, this cell line has not yet made any meaningful impact in the study of this intractable disease and only two studies has been published using these cells hitherto.
Several mouse models of PNETs have been developed throughout the years. It has been well established that MEN1 syndrome is associated with the development of PNETs. Therefore conventional MEN1 loss mouse model has been developed to successfully characterized PNETs. For instance, Bertolino and his team have demonstrated that heterozygous MEN1 mutant mice develop a range of endocrine tumors often seen in multiple endocrine neoplasia type 1 patients[99]. Moreover, Shen and colleagues have developed the MEN1-PDXCre mouse model to illustrate that loss of the expression of menin via knockout of MEN-1 in mature pancreatic endocrine cells resulted in tumor development[100]. Here, they confirm an association between MEN1 syndrome and the development of PNET lesions. Likewise, Li et al[101] have developed Men1f/f-RipCre+ mouse model in which MEN1 ablation in pancreatic β-cell decreased the expression of critical transcription factor and resulted in the development of glucagonoma one of the rarest PNET subtypes. The latter mouse models have been significantly important to successfully characterize PNETS. The RIP1-TAG2 mouse in which PNETs are induced by expression of SV40 T-antigen in the beta cells of Langerhans has been used as a relevant mouse model for PNETS[18]. However, there is a significant concern with RIP1-TAG2 mice because the viral system used to induce PNETs abrogates TP53 and RB genes that are rarely seen in PNETs. To complement the RIP1-TAG2 mouse model, Chung Wong et al[102], proposed [GEMMs-MPR (Men1flox/flox Ptenflox/flox RIP-Cre)] and MPM (Men1flox/flox Ptenflox/flox MIP-Cre) as novel mouse models for PNETs. At this point it is too early to assert the relevance of these two models for PNETs therapeutic examination. Here, we argue that there is a need to invest more in developing PNETs cellular models in order to enhance our understanding of the progression of PNETs. The study and analysis of patients’ tissue by researchers are fundamental for cancer research in general. Research on patient tissue could offer critical information necessary to prevent, diagnose, and more importantly treat cancer patients. However, lack of access to patient tissues also constitutes a barrier to study PNETs.
EMERGING NEW THERAPEUTIC TARGETS IN PNETS
Cysteine-rich angiogenic inducer 61
The CCN1-6 is a family of six extracellular associated proteins known to play a critical role in cellular processes including cell adhesion, migration, proliferation, differentiation, survival, apoptosis, and senescence[103]. This family of matricellular proteins contains: Cysteine-rich angiogenic inducer 61 (CYR61) or CCN1, CTGF or CCN2, NOV or CCN3, WISP1 or CCN4, WISP2 or CCN5, and WISP6 or CCN6[102]. Upon secretion in the extracellular matrix, CYR61 binds directly to various integrin receptors in a cell type-dependent manner[104]. It is important to note that human’s and mouse CYR61 protein share a 98.2% sequence identity[105]. Several studies have suggested the implication of CYR61 in tumorigenicity and progression. For instance, Huang et al have shown that CYR61 promotes breast cancer lung metastasis through tumor cell extravasation and suppression of anoikis[106]. The authors argued that CYR61 support lung metastasis by regulating two critical events relevant to the late steps of metastatic dissemination including enhancement of extravasation of cancer cells into the lung and, secondly, inhibition of process of anoikis via the activation of AMPKα pathway but not through AKT, FAK or ERK1/2 signaling. Recently, Habel and colleagues have illustrated that CYR61 induces metastatic spreading through IGF1Rβ-dependent EMT-like process in osteosarcoma[107]. It is well known that a large number of PNETs are metastatic at presentation (40-80%) and liver metastasis (about 40-90%) is the most significant prognosis factor in PNETs progression. Thus, targets associated with metastasis/invasion could be an attractive area to manage this disease. Also, relevant to pancreatic cancers, it has been shown that CYR61/CCN1 signaling facilitates pancreatic carcinogenesis via activation of mechanisms of EMT and stemness[108]. In this study, Haque and colleagues illustrate that in PDAC, CYR61 transcripts and proteins increase as the disease progresses. More significantly, Maity et al[109] have recently shown that CYR61 regulates dCK and CTGF causing Gemcitabine-resistance in PDAC. First, they show that CYR61 in highly activated in PDAC and correlates with Gemcitabine resistance. They also show that ablation of CYR61 sensitizes PDAC cellular models to Gemcitabine in 2D and 3D culture. The latter suggest that CYR61 is implicated in PDAC drug resistance, which is a major factor for therapeutic failure in PNETs. Thus, what is the implication of this target in the setting of PNETs development and progression? A novel study has suggested that CYR61 may be a tumor-promoting gene in PNETs. Notably, Huang and colleagues have newly shown that CYR61 interferes with normal pancreatic architecture and promotes PNETs progression[110]. They crossed Rip1CYR mice with Rip1-TAG2; the resulting Rip1Tag2CRY mice developed β-tumors significantly larger, more invasive and more vascularized compared to β-tumors in the Rip1-Tag2 mice (keeping in mind that CYR61 is highly conserved in human and mice). The latter study demonstrates that CYR61 is viable target in the complex to treat PNETs and required further clinical examination.
Forkhead box protein M1
Forkhead box protein M1 (FOXM1) is a critical proliferation-associated transcription factor found to be increasingly and spatiotemporally expressed during the highly regulated cell cycle events[111]. Several studies have suggested that FOXM1 is closely involved with the processes of cell proliferation, self-renewal, and tumorigenesis[112-114]. FOXM1 is differentially expressed in typical carcinoids relative to atypical carcinoids cells and more importantly, FOXM1 expression was significantly different in large cell neuroendocrine carcinomas compared to small cell lung cancers[115,116]. In a recent study, Franziska et al[117] have shown that FOXM1 expression is linked to proliferation, differentiation and metastasis in GEP-NETs and that inhibition of FOXM1 is a potential new therapeutic option for these intractable subtypes of cancers. Utilizing Genome-wide expression profiling on biopsies from well-differentiated neuroendocrine tumors of the distal ileum and metastatic disease at the time of diagnostic, Ellinor Andersson et al[118] have shown that FOXM1 expression is upregulated in small intestinal neuroendocrine tumors. The latter studies illustrate that FOXM1 has a significant implication in the development and progression of NETs. This is also true for PNETs, as De Rycke et al[119] have shown that FOXM1 expression defines highly proliferative group of tumors in pancreatic neuroendocrine tumors and pulmonary neuroendocrine tumors. They also showed that the Thiostrepton (FOXM1 specific inhibitor) display a strong anti-tumor effect in (BON-1, and QGP-1), and H-227, pancreatic and pulmonary neuroendocrine cell lines, respectively.
UPR coordinator proteins IREα and PERK
It has been well established that increased protein translation, accumulation of unfolded/misfolded proteins, and several other dynamic changes in the cells microenvironment can activate endoplasmic reticulum (ER) stress and promotes the unfolded protein response (UPR) that aide cell survival[120]. Nevertheless, sustained ER stress could lead to ER-associated programmed cell death. Inositol-requiring enzyme 1 α(IRE1α) and protein kinase R-like endoplasmic reticulum kinase(PERK) are two of the major coordinators of the UPR response[121]. Activation of the latter has been shown in several cancers and linked to oncogenesis, tumor growth, metastasis and chemoresistance. Croft A et al[122] have illustrated that mutant BRAF (V600E) promotes IRE1 and ATF6 activation in melanoma cellular models. Additionally, Hart et al[123] have shown that activation of c-MYC in mouse embryonic fibroblasts induces IRE1 and PERK activation. Moreover, Blazanin et al[124] have recently demonstrated that RAS activation was followed with UPR activation in melanocytes and keratinocytes. All these studies suggest that UPR coordination proteins are very much likely to promote cancer progression. Another recent publication demonstrates that secretory factors from endoplasmic-stressed cells aided survival of nearby cells to cytotoxic agents vie UPR activation[125]. It is well known that islet cells in the pancreas secrete hormones and polypeptides that could sensitize these cells to elevated ER stress. Therefore, sustain ER stress coupled with hyper activation of UPR could be a major mechanism regulating PNET tumor growth and/or drug resistance. More importantly, a recent study has revealed that the expression level of key proteins such as BiP, CHOP, ATF4 involved ER stress are significantly upregulated in PNET and that this hyperactivation was associated with advanced clinicopathological features[126]. The authors of the latter study used immunohistochemical analysis by tissue microarray of 49 human PNET tissues and found that BiP, CHOP, ATF4 were significantly upregulated compared to normal tissues. They also show that high expression of Bip was significantly associated with high grade tumor, proliferation and poor survival. Finally, Moore and colleagues published an excellent paper illustrating the implication of UPR signaling in PNETs growth and survival. Using available mouse models for PNETs including RIP1-TAG2 mouse model, they specifically show that UPR is upregulated in this disease and inhibition of UPR cascade significantly reduces tumor growth[127].
Nicotinamide phosphoribosyltransferase
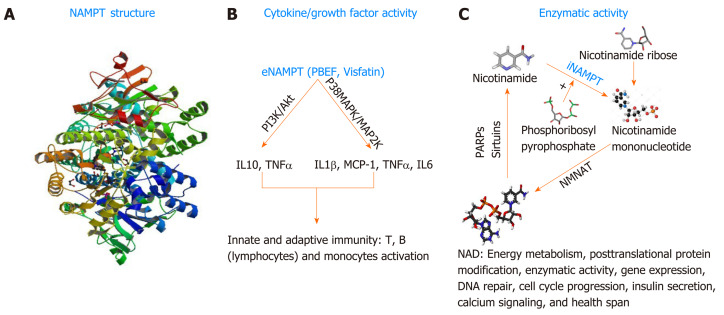
In general, cancer cells often develop strategies to promote their survival under stressful conditions caused by the administration of anticancer therapeutics. As mentioned above, PNETs are known to be equipped with intrinsic drug resistance mechanisms that alter the efficacy of personalized or systemic therapies. The protein nicotinamide pho-sphoribosyltransferase (NAMPT), best known as the rate-limiting enzyme involved in the salvage pathway of Nicotine Adenine Dinucleotide (NAD) biosynthesis in mammals[128] could become a novel target for therapy-resistant PNETs. NAD is a critical redox coenzyme that is essential for multiple physiological processes including DNA repair, oncogenic signal transduction, transcription, genomic integrity, and apoptosis[129]. Three different pathways govern the biosynthesis of NAD in mammals. The essential amino acid tryptophan is the precursor of the de novo pathway that includes 9 steps in which the tryptophan is converted into quinolinic acid that is further metabolized into NAD+[130]. This de novo pathway of NAD synthesis includes multiple steps and requires more energy; thus, most cancers cells rely on the alternative pathway of NAD synthesis. The alternative pathways of NAD biosynthesis are termed NAD salvage pathway and the Preiss-Handler pathway[131]. Nicotinate phosphoribosyltransferase (NAPRT1) is the rate-limiting enzyme in the Preiss-Handler pathway. In this pathway, Niacin (also known as Nicotinic acid or Vitamin B3) is converted into Nicotinic acid mononucleotide (NMN) by the nicotinate phosphoribosyltransferase (NAPRT), and then NMN is converted into Nicotinic acid adenine dinucleotide (NAAD) that is finally converted into NAD by the enzyme NAD synthetase[132]. NAPRT1 is often lost in cancer; thus, the salvage pathway, governed by NAMPT is preferably used in cancer; making NAMPT a potential therapeutic target for the management of cancers. In the salvage pathway, Nicotinamide (an additional form of vitamin B3) is converted into Nicotinamide mononucleotide by the rate-limiting enzyme NAMPT in the presence of the phosphoribosyl pyrophosphate (PRPP); next, the nuclear Nicotinamide Mononucleotide Adenylyltransferase (NMNAT) further converts the NMN into NAD[131]. NAMPT biological function is not limited to the regulation of total cellular and mitochondrial levels of NAD necessary for cell survival. NAMPT also exhibits growth factor activity in this regard, it is called Pre-B cell colony enhancer factor (PBEF)[133]. Evidence has also shown that NAMPT has a hormonal activity, it’s cad Visfatin (therefore named Visaftin)[134]. NAMPT could also be an adipocytokine and called Insulin-mimetic hormone; however, this adipocytokine function is the object of controversy hitherto. When located in the cytoplasm (intracellular milieu) iNAMPT has an enzymatic function mainly the catalysis of the salvage pathway of NAD[129]. Outside the cell (extracellular milieu or circulating in the plasma), eNAMPT presumably plays the role of growth factor, and hormone (PBEF, Visfatin respectively) and allegedly adipocytokine (Insulin-mimetic hormone)[132,134]. NAMPT is a 52-kDa molecule with a length of 36.908 base pairs encoded by the NAMPT gene located at the 7q22[129]. Human NAMPT’s crystal structure alone or in complex with nicotinamide was determined at 2.1 Å resolution by the selenomethionyl SAD method[135]. Tao Wang et al[136] described the crystal structure of NAMPT as a dimeric type II pho-sphoribosyltransferase homolog of NAPRT1. NAMPT comprises 491 (including initial methionine) amino acids and its active site includes an Asp 219 that forms a hydrogen bond with Nicotinamide[131]. Over the past three decades, multiple studies have illustrated the involvement of NAMPT in numerous malignancies[137-138]. An important number of cancers including PNETs[139] show increased expression of NAMPT; however, the mechanism associated with NAMPT upregulation is unknown. In a recent study, Alvarez M.J. and colleagues evaluated more than 200 patient cohort of GEP-NETs[140]. They showed that NAMPT is one of the mechanistic dependencies of neuroendocrine tumors. This paper looked at the responsiveness of GEP-NET cell lines to different agents and found that NAMPT inhibition can impact their proliferation. The findings of this comprehensive study support the fact that NAMPT is critical for GEP-NET survival. Additionally, Michael Ohanna and colleagues have shown that NAMPT regulates drug resistance and invasive phenotype in melanoma[141]. In the forthcoming passages we will discuss the utility of targeting NAMPT in PNET using small molecule drugs (Figure 2).
Figure 2.
Nicotinamide phosphoribosyltransferas’s biological functions. A: Structure of nicotinamide phosphoribosyltransferas (NAMPT). Structure obtained from RSCB Protein Data Bank, Deposited: 2008-06-15 Released: 2009-08-18. Deposition Author(s): Ho, M., Burgos, E.S., Almo, S.C., Schramm, V.L.; B: NAMPT and immune signaling; C: NAMPT signaling in NAD biosynthesis. NAD: Nicotinamide adenine dinucleotide; NAMPT: Nicotinamide phosphoribosyltransferas; eNAMPT: Extracellular Nicotinamide phosphoribosyltransferase; iNAMPT: Intracellular nicotinamide phosphoribosyltransferase; NMNAT: Nicotinamide mononucleotide adenylyltransferase; TNFa: Tumor necrosis factor alpha; IL: Interleukin; MCP-1: Monocyte chemoattractant protein-1; PARP: Poly(ADP-ribose) polymerase.
P21-activated kinase 4
P21-activated kinase 4 (PAK4) is a member of a family of serine-threonine kinases that play a role in both oncogenesis and cancer progression[142,143]. PAK family members are key effectors of the Rho family of GTPases (a sub family of the Ras superfamily), which act as regulatory switches that control critical cellular processes such as motility, proliferation, and survival[144]. The latter indicates that PAK4 is the downstream effector of Ras activity that promotes growth and proliferation in PNETs; thus making PAK4 a relevant target for this disease. P21-Activated kinase name arose following their identification as effectors of Rho GTPases (e.g., CDC42 and Rac), each of which is 21 kDa in size. Upon activation by mutation or overexpression, the majority of Pak isoforms (Group I PAK 1,2,3 or Group II PAK 4,5,6) have oncogenic signaling effects. As previously mentioned, PAK4 is a key effector of Cdc42 (cell division control protein 42 homolog) and Rac1 (Ras-related C3 botulinum toxin substrate 1); thus, acts as a critical mediator of the RhoA family of GTPases[145]. Pertinent to pancreatic neoplasia, earlier studies have shown that copy number alteration analyses illustrate increased expression of PAK4 in pancreatic ductal adenocarcinoma (PDAC) patients[146]. Hyperactivity of PAK4 has been implicated in cancer progression by activating oncogenic signaling pathways, such as RAF/MEK/ERK and PI3K/AKT[147-149]. Additionally, other investigations have also linked PAK4 overexpression to cell migration, cell adhesion, and anchorage-independent growth[150]. PAK amplification can cause the activation of markers associated with drug resistance in PNETs including Akt, ERK, mTORC1, mTORC2[151], β-catenin, and IGF-1[152]. PAKs have also been shown to promote FAK (additional drug resistant molecule in PNETs) by this means it enhances cell migration and metastasis in breast carcinoma models[153]. Our group has demonstrated that PAK4 knockdown by means of siRNA inhibits the growth of PNETs cellular models (QGP-1 and BON-1)[139].
NAMPT and PAK4 inhibition
For decades, PAK4 and NAMPT have remained non-drugable targets. The adenosine-triphosphate (ATP) binding cleft in PAK4 is a flexible hinge structure, which does not allow the development of effective inhibitors[154]. The first PAK4 small molecule inhibitor PF3578309 (IC50: 1.3nm in cell-free assay) is an ATP competitive Type I and pyrrolopyrazole inhibitor of PAK4 failed to move in advanced clinical trials for cancer management. PF3578309 failed clinical study because it happened to be a PGP substrate. Among all NAMPT inhibitors, only two: APO866/FK866, and GMX1777 (GMX1778/CHS828), were evaluated in phase I clinical trials. Unfortunately, further evaluations were discontinued predominantly due to undesired dose-limiting toxicities. APO866 is the first developed NAMPT inhibitor with an IC50 varying between (0.09 and 27.2 nm in cell-free based assay[155]. It had been well established that APO866 inhibits proliferation and growth in a wide variety of human cancers in vitro and in vivo. For instance, in 2003 Hasmann et al[156] showed that inhibition of NAMPT using APO866 is a novel mechanism to induce apoptosis in leukemia. At exactly the same time, Drevs et al[157] were the first to illustrate the antiangiogenic properties of APO866. These two pilot studies lead to a phase I/II trial (NCT00435084) opened in the United Kingdom to investigate the safety and tolerability of APO866 for the treatment of refractory chronic lymphocytic leukemia ( https://clinicaltrials.gov/ ct2/archive/NCT00435084). Phase II study (NCT00432107; and NCT00431912) of APO866 were opened at four locations (Austria, France, Germany, and Switzerland) to define its efficacy and safety for the treatment of melanoma and cutaneous T cell lymphoma https://clinicaltrials.gov/ct2/show/NCT00432107, and https:// clinicaltrials.gov/ ct2/show/NCT00431912, respectively. The primary outcome measure of APO866 in these studies lacked objective responses (pharmacodynamics) and the dose limit toxicity was found to be thrombocytopenia[158]. GMX1777 (EB1627) is a water-soluble prodrug of the GMX1778 a cyanoguanidine compound that selectively inhibits NAMPT with an IC50 of less than 100 nm in cell-free assay[159]. Two trials conducted by Gemin X pharmaceutical had investigated this drug for anticancer therapy. Firstly, GMX1777 was evaluated for safety and efficacy in phase I clinical trial (NCT00457574) for the treatment of refractory solid tumors and lymphomas https:// clinicaltrials.gov/ct2/show/NCT00457574. Secondly, GMX1777 was evaluated in phase I/II study in combination with Temozolomide (an oral chemotherapy drug) for the treatment of metastatic melanoma https://clinicaltrials.gov/ct2/show/ NCT00724841.
KPT-9274: Available PAK4-NAMPT dual inhibitor
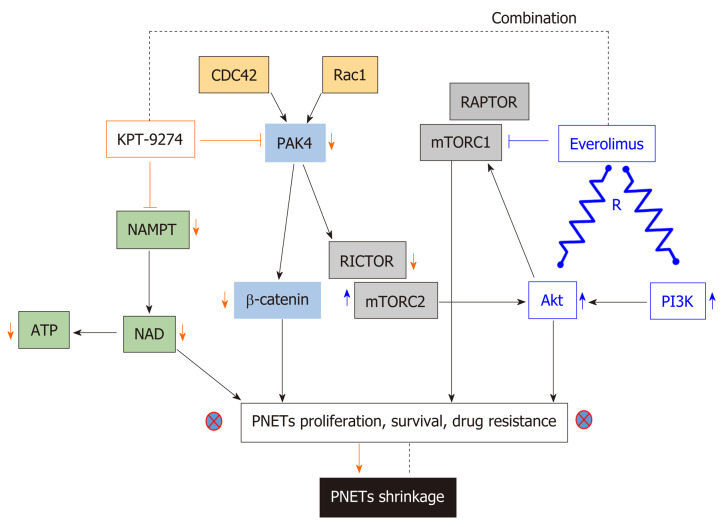
Recently, Karyopharm Therapeutics Inc. developed KPT-9274 a first in class orally bioavailable small molecule inhibitor which targets PAK4 and NAMPT[139]. KPT-9274 is a distinct class of allosteric modulator that binds to the kinase domain of PAK4. Most importantly, the latter investigational drug is not a PGP substrate. It is important to know that the drug KPT-9274 has been established to be a dual inhibitor of PAK4 and NAMPT[160,161]. Senapedis et al used stable isotope labeling of amino acids in cells (SILAC) to illustrate that PAK4 is a target of KPT-9274. In a very recent paper, Neggers and colleagues used CRISPRres, “a CRISPR-Cas-based genetic screening approach to rapidly derive and identify drug resistance mutations in essential genes”, to identify the targets of KPT-9274. They showed that NAMPT is the principal target of this investigational compound[162]. KPT-9274 remains the only agent in Phase I studies that target both PAK4 and NAMPT and KPT-9274 has demonstrated evaluable response in patients with solid tumor and hematological malignancies[163,164]. Our laboratory has recently shown that KPT-9274 is effective against PNET models in vitro and in vivo[139]. The drug blocks PAK4 signaling leading to inhibition of mTOR pathway molecules. We also demonstrated that KPT-9274 causes metabolic alterations in PNET cell that is reflective of its NAMPT targeted effects. More significantly, the drug synergized with everolimus and other commonly used therapies for PNETs. Based on these findings, it is anticipated that this agent will be evaluated in Phase 1b/II clinical study for advanced PNETs. The mechanism of action of KPT-9274 is illustrated in Figure 3.
Figure 3.
Mechanism of action of available PAK4-NAMPT dual inhibitor. Dual inhibition of PAK4 and NAMPT using KPT-9274 single agent or in combination with everolimus results in lower case tumor shrinkage. KPT-9274 inhibits NAMPT causing downregulation of NAD and ATP and alteration of cell metabolism. Additionally, KPT-9274 Inhibits PAK4 resulting in downregulation of β-catenin and RICTOR. Downregulation of RICTOR causes the inhibition of mTORC2 implicated in everolimus resistance. R: Resistance; PAK4: p21-activated kinase 4; NAMPT: Nicotinamide Phosphoribosyltransferase; KPT-9274: Available PAK4-NAMPT dual inhibitor; NAD: Nicotine adenine dinucleotide; ATP: Adenosine-triphosphate; RICTOR: Rapamycin-insensitive companion of Tor; mTORC2: Mammalian target of rapamycincomplex 2.
CONCLUSION
The incidence of PNETs is vastly increasing worldwide; therefore, novel strategies to manage this specific neoplasia are urgently needed. Several factors contribute to the management failure of PNETs in the clinic. PNET is characterized by significant heterogeneity that is the major challenge associated with the management of this neoplasia. Also, the majority of PNET therapeutics only stabilizes the disease with minimal benefits for patients. Lack of specific biomarkers inhibits early diagnosis and the selection of effective drugs in the clinic. The absence of preclinical models, mainly cellular models, limits effective anticancer examination and a better understanding of the biology of PNETs in the laboratory. Immunotherapy does not work in this patient population. Nevertheless, several molecules are emerging as new therapeutic targets for the management of PNETs. FOXM1 that is involved in all the hallmarks of cancer has been identified as a new target to effectively manage tumorigenicity, growth and proliferation in gastroenteropancreatic neuroendocrine tumors. The matricellular proteins CYR61 has also been identified as tumor-promoting gene in PNETs. Finally, overexpression of PAK4 and NAMPT in PNET patients’ biopsies suggests that inhibition of these two targets could become a feasible strategy for therapy resistant PNETs.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Corresponding Author's Membership in Professional Societies: AACR 157900
Peer-review started: April 31, 2020
First decision: July 8, 2020
Article in press: July 16, 2020
P-Reviewer: Fazio PD S-Editor: Wang DM L-Editor: A E-Editor: Zhang YL
Contributor Information
Gabriel Benyomo Mpilla, Department of Oncology, Wayne State University School of Medicine, Detroit, MI 48201, United States.
Philip Agop Philip, Department of Oncology, Wayne State University School of Medicine, Detroit, MI 48201, United States.
Bassel El-Rayes, Department of Hematology Oncology, Emory Winship Institute, Atlanta, GA 30322, United States.
Asfar Sohail Azmi, Department of Oncology, Wayne State University School of Medicine, Detroit, MI 48201, United States. azmia@karmanos.org.
References
- 1.Garcia-Segura LM, McCarthy MM. Minireview: Role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 2.Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med. 2017;33:324–330. doi: 10.1159/000481390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra R, Liddle RA. Modulation of pancreatic exocrine and endocrine secretion. Curr Opin Gastroenterol. 2013;29:517–522. doi: 10.1097/MOG.0b013e3283639326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pea A, Hruban RH, Wood LD. Genetics of pancreatic neuroendocrine tumors: implications for the clinic. Expert Rev Gastroenterol Hepatol. 2015;9:1407–1419. doi: 10.1586/17474124.2015.1092383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orditura M, Petrillo A, Ventriglia J, Diana A, Laterza MM, Fabozzi A, Savastano B, Franzese E, Conzo G, Santini L, Ciardiello F, De Vita F. Pancreatic neuroendocrine tumors: Nosography, management and treatment. Int J Surg. 2016;28 Suppl 1:S156–S162. doi: 10.1016/j.ijsu.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Dumlu EG, Karakoç D, Özdemir A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int Surg. 2015;100:1089–1097. doi: 10.9738/INTSURG-D-14-00204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Kim MK, Kim HG. Diagnosis of Pancreatic Neuroendocrine Tumors. Clin Endosc. 2017;50:537–545. doi: 10.5946/ce.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrio M, Ceppa EP. Diagnosing microscopic pancreatic neuroendocrine tumor using 68-Ga-DOTATATE PET/CT: case series. J Surg Case Rep. 2018;2018:rjy237. doi: 10.1093/jscr/rjy237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partelli S, Giannone F, Schiavo Lena M, Muffatti F, Andreasi V, Crippa S, Tamburrino D, Zamboni G, Rubini C, Doglioni C, Falconi M. Is the Real Prevalence of Pancreatic Neuroendocrine Tumors Underestimated? A Retrospective Study on a Large Series of Pancreatic Specimens. Neuroendocrinology. 2019;109:165–170. doi: 10.1159/000499606. [DOI] [PubMed] [Google Scholar]
- 11.Sonbol MB, Halfdanarson TR. Management of Well-Differentiated High-Grade (G3) Neuroendocrine Tumors. Curr Treat Options Oncol. 2019;20:74. doi: 10.1007/s11864-019-0670-1. [DOI] [PubMed] [Google Scholar]
- 12.Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Wang X. Deep learning for World Health Organization grades of pancreatic neuroendocrine tumors on contrast-enhanced magnetic resonance images: a preliminary study. Int J Comput Assist Radiol Surg. 2019;14:1981–1991. doi: 10.1007/s11548-019-02070-5. [DOI] [PubMed] [Google Scholar]
- 14.Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, Willinek W, Biersack HJ, Sabet A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 15.Sallinen VJ, Le Large TYS, Tieftrunk E, Galeev S, Kovalenko Z, Haugvik SP, Antila A, Franklin O, Martinez-Moneo E, Robinson SM, Panzuto F, Regenet N, Muffatti F, Partelli S, Wiese D, Ruszniewski P, Dousset B, Edwin B, Bartsch DK, Sauvanet A, Falconi M, Ceyhan GO, Gaujoux S Pancreas 2000 research group. Prognosis of sporadic resected small (≤ 2 cm) nonfunctional pancreatic neuroendocrine tumors - a multi-institutional study. HPB (Oxford) 2018;20:251–259. doi: 10.1016/j.hpb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 16.de Mestier L, Cros J. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) Ann Endocrinol (Paris) 2019;80:172–173. doi: 10.1016/j.ando.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 17.de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412–425. doi: 10.1159/000475527. [DOI] [PubMed] [Google Scholar]
- 18.Sadanandam A, Wullschleger S, Lyssiotis CA, Grötzinger C, Barbi S, Bersani S, Körner J, Wafy I, Mafficini A, Lawlor RT, Simbolo M, Asara JM, Bläker H, Cantley LC, Wiedenmann B, Scarpa A, Hanahan D. A Cross-Species Analysis in Pancreatic Neuroendocrine Tumors Reveals Molecular Subtypes with Distinctive Clinical, Metastatic, Developmental, and Metabolic Characteristics. Cancer Discov. 2015;5:1296–1313. doi: 10.1158/2159-8290.CD-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pipinikas CP, Berner AM, Sposito T, Thirlwell C. The evolving (epi)genetic landscape of pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2019;26:R519–R544. doi: 10.1530/ERC-19-0175. [DOI] [PubMed] [Google Scholar]
- 20.Grillo F, Florio T, Ferraù F, Kara E, Fanciulli G, Faggiano A, Colao A NIKE Group. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer. 2018;25:R453–R466. doi: 10.1530/ERC-17-0531. [DOI] [PubMed] [Google Scholar]
- 21.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Tedesco L, Lucia K, Schlitter AM, Garcia JM, Esposito I, Auernhammer CJ, Theodoropoulou M, Arzt E, Renner U, Stalla GK. RSUME is implicated in tumorigenesis and metastasis of pancreatic neuroendocrine tumors. Oncotarget. 2016;7:57878–57893. doi: 10.18632/oncotarget.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HL, Yang KC, Shen Y, Zhao EY, Loree JM, Kennecke HF, Kalloger SE, Karasinska JM, Lim HJ, Mungall AJ, Feng X, Davies JM, Schrader K, Zhou C, Karsan A, Jones SJM, Laskin J, Marra MA, Schaeffer DF, Gorski SM, Renouf DJ. Molecular characterization of metastatic pancreatic neuroendocrine tumors (PNETs) using whole-genome and transcriptome sequencing. Cold Spring Harb Mol Case Stud. 2018;4 doi: 10.1101/mcs.a002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L, Natov NS, Daly KP, Masud F, Chaudhry S, Sterling MJ, Saif MW. An update on the management of pancreatic neuroendocrine tumors. Anticancer Drugs. 2018;29:597–612. doi: 10.1097/CAD.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 25.Uri I, Grozinsky-Glasberg S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs) Clin Diabetes Endocrinol. 2018;4:16. doi: 10.1186/s40842-018-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain CE, German MS, Yang K, Wang J, VanBrocklin H, Regan M, Shokat KM, Ducker GS, Kim GE, Hann B, Donner DB, Warren RS, Venook AP, Bergsland EK, Lee D, Wang Y, Nakakura EK. A Patient-derived Xenograft Model of Pancreatic Neuroendocrine Tumors Identifies Sapanisertib as a Possible New Treatment for Everolimus-resistant Tumors. Mol Cancer Ther. 2018;17:2702–2709. doi: 10.1158/1535-7163.MCT-17-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274–281. doi: 10.1002/ags3.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillarisetty VG. The pancreatic cancer microenvironment: an immunologic battleground. Oncoimmunology. 2014;3:e950171. doi: 10.4161/21624011.2014.950171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32:312–324. doi: 10.5732/cjc.012.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigri G, Petrucciani N, Debs T, Mangogna LM, Crovetto A, Moschetta G, Persechino R, Aurello P, Ramacciato G. Treatment options for PNET liver metastases: a systematic review. World J Surg Oncol. 2018;16:142. doi: 10.1186/s12957-018-1446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 32.Stec-Michalska K, Peczek L, Krakowiak A, Michalski B, Chojnacki J, Knopik-Dabrowicz A, Klupinska G, Nawrot B. Expression of somatostatin receptor subtype 3 in the gastric mucosa of dyspeptic patients in relation to Helicobacter pylori infection and a family history of gastric cancer. J Gastroenterol Hepatol. 2008;23:424–429. doi: 10.1111/j.1440-1746.2007.05094.x. [DOI] [PubMed] [Google Scholar]
- 33.Zitzer H, Honck HH, Bachner D, Richter D, Kreienkam HJ. Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain. J Biol Chem. 1999;274:32997–33001. doi: 10.1074/jbc.274.46.32997. [DOI] [PubMed] [Google Scholar]
- 34.Kemm MH, Manly CD, Hoang TD, Mai VQ, Shakir MKM. Octreotide Use in a Patient with MEN-1 Syndrome and Multifocal Pancreatic Neuroendocrine Tumors: A Case Report and Review of the Literature. Case Rep Gastrointest Med. 2019;2019:9462942. doi: 10.1155/2019/9462942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19. doi: 10.1186/1756-9966-29-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yau H, Kinaan M, Quinn SL, Moraitis AG. Octreotide long-acting repeatable in the treatment of neuroendocrine tumors: patient selection and perspectives. Biologics. 2017;11:115–122. doi: 10.2147/BTT.S108818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 38.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Mestier L, Walter T, Evrard C, de Boissieu P, Hentic O, Cros J, Tougeron D, Lombard-Bohas C, Rebours V, Hammel P, Ruszniewski P. Temozolomide Alone or Combined with Capecitabine for the Treatment of Advanced Pancreatic Neuroendocrine Tumor. Neuroendocrinology. 2020;110:83–91. doi: 10.1159/000500862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunz PL, Lagunes DR, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC, North American Neuroendocrine Tumor Society Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandamme T, Peeters M, Dogan F, Pauwels P, Assche MB, Mortier G, Vandeweyer G, Herder WD, Camp GV, Hofland LJ, Beeck KOD. Whole-exome characterization of pancreatic neuroendocrine tumor cell lines BON-1 and QGP-1. J Mol Endocrinol. 2015;54:137–147. doi: 10.1530/JME-14-0304. [DOI] [PubMed] [Google Scholar]
- 42.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34:3906–3913. doi: 10.1200/JCO.2016.68.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, Vinik A, Van Cutsem E, Bang YJ, Lee SH, Borbath I, Lombard-Bohas C, Metrakos P, Smith D, Chen JS, Ruszniewski P, Seitz JF, Patyna S, Lu DR, Ishak KJ, Raymond E. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. 2017;28:339–343. doi: 10.1093/annonc/mdw561. [DOI] [PubMed] [Google Scholar]
- 45.Vinik AI, Raymond E. Pancreatic neuroendocrine tumors: approach to treatment with focus on sunitinib. Therap Adv Gastroenterol. 2013;6:396–411. doi: 10.1177/1756283X13493878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 47.Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556–1557. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 48.Ozdemir N, Yazici O, Zengin N. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1555–1556. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 49.Yang F, Jin C, Fu D. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 50.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 51.Exner S, Prasad V, Wiedenmann B, Grötzinger C. Octreotide Does Not Inhibit Proliferation in Five Neuroendocrine Tumor Cell Lines. Front Endocrinol (Lausanne) 2018;9:146. doi: 10.3389/fendo.2018.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The Role of Capecitabine/Temozolomide in Metastatic Neuroendocrine Tumors. Oncologist. 2016;21:671–675. doi: 10.1634/theoncologist.2015-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dilz LM, Denecke T, Steffen IG, Prasad V, von Weikersthal LF, Pape UF, Wiedenmann B, Pavel M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur J Cancer. 2015;51:1253–1262. doi: 10.1016/j.ejca.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, Cwikla J, Baum RP, Kwekkeboom DJ, Paganelli G, Krenning EP, Modlin IM. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging. 2018;45:1155–1169. doi: 10.1007/s00259-018-3967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma N, Naraev BG, Engelman EG, Zimmerman MB, Bushnell DL, Jr, O’Dorisio TM, O’Dorisio MS, Menda Y, Müller-Brand J, Howe JR, Halfdanarson TR. Peptide Receptor Radionuclide Therapy Outcomes in a North American Cohort With Metastatic Well-Differentiated Neuroendocrine Tumors. Pancreas. 2017;46:151–156. doi: 10.1097/MPA.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, Krenning EP, de Herder WW. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46:704–717. doi: 10.1007/s00259-018-4158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel P, Galoian K. Molecular challenges of neuroendocrine tumors. Oncol Lett. 2018;15:2715–2725. doi: 10.3892/ol.2017.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbara Nuñez-Valdovinos, Alberto Carmona-Bayonas, Paula Jimenez-Fonseca, Jaume Capdevila, Ángel Castaño-Pascual, Marta Benavent, Jose Javier Pi Barrio, Alex Teule, Vicente Alonso, Ana Custodio, Monica Marazuela, Ángel Segura, Adolfo Beguiristain, Marta Llanos, Maria Purificacion Martinez del Prado, Jose Angel Diaz-Perez, Daniel Castellano, Isabel Sevilla, Carlos Lopez, Teresa Alonso, Rocio Garcia-Carbonero. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE) Oncologist. 2018;Apr; 23:422–432. doi: 10.1634/theoncologist.2017-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol. 2015;21:9512–9525. doi: 10.3748/wjg.v21.i32.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J, Sun C, Li E, Wang J, He X, Yuan R, Yi C, Liao W, Wu L. Non-functional pancreatic neuroendocrine tumours: emerging trends in incidence and mortality. BMC Cancer. 2019;19:334. doi: 10.1186/s12885-019-5543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19:829–837. doi: 10.3748/wjg.v19.i6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu J, Ping F, Zhang H, Li W, Yuan T, Fu Y, Feng K, Xia W, Xu L, Li Y. Clinical Management of Malignant Insulinoma: a single Institution's experience over three decades. BMC Endocr Disord. 2018;18:92. doi: 10.1186/s12902-018-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hain E, Coriat R, Dousset B, Gaujoux S. [Management of gastrinoma] Presse Med. 2016;45:986–991. doi: 10.1016/j.lpm.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Krainev AA, Mathavan VK, Klink DF, Saxe JM, Ong GKB, Murphy JK. Pancreatic neuroendocrine microadenomatosis presenting as a functional VIPoma. J Surg Case Rep. 2019;2019:rjz196. doi: 10.1093/jscr/rjz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nigri G, Petrucciani N, Debs T, Mangogna LM, Crovetto A, Moschetta G, Persechino R, Aurello P, Ramacciato G. Treatment options for PNET liver metastases: a systematic review. World J Surg Oncol. 2018;16:142. doi: 10.1186/s12957-018-1446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gleeson FC, Voss JS, Kipp BR, Kerr SE, Van Arnam JS, Mills JR, Marcou CA, Schneider AR, Tu ZJ, Henry MR, Levy MJ. Assessment of pancreatic neuroendocrine tumor cytologic genotype diversity to guide personalized medicine using a custom gastroenteropancreatic next-generation sequencing panel. Oncotarget. 2017;8:93464–93475. doi: 10.18632/oncotarget.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crona J, Skogseid B. GEP- NETS UPDATE: Genetics of neuroendocrine tumors. Eur J Endocrinol. 2016;174:R275–R290. doi: 10.1530/EJE-15-0972. [DOI] [PubMed] [Google Scholar]
- 68.Dreijerink KM, Derks JL, Cataldo I, Scarpa A, Valk GD, Speel EJ. Genetics and Epigenetics of Pancreatic Neuroendocrine Tumors and Pulmonary Carcinoids. Front Horm Res. 2015;44:115–138. doi: 10.1159/000382138. [DOI] [PubMed] [Google Scholar]
- 69.Jensen RT, Norton JA. Treatment of Pancreatic Neuroendocrine Tumors in Multiple Endocrine Neoplasia Type 1: Some Clarity But Continued Controversy. Pancreas. 2017;46:589–594. doi: 10.1097/MPA.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Treijen MJC, van Beek DJ, van Leeuwaarde RS, Vriens MR, Valk GD. Diagnosing Nonfunctional Pancreatic NETs in MEN1: The Evidence Base. J Endocr Soc. 2018;2:1067–1088. doi: 10.1210/js.2018-00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karlikova M, Topolcan O, Wolfe OT, Barak V, Zima T. Optimal Use of Biomarkers in Oncology. Biomed Res Int. 2015;2015:423159. doi: 10.1155/2015/423159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang Y, Qiao G, Xu E, Xuan Y, Liao M, Yin G. Biomarkers for early diagnosis, prognosis, prediction, and recurrence monitoring of non-small cell lung cancer. Onco Targets Ther. 2017;10:4527–4534. doi: 10.2147/OTT.S142149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng L, Xu Y, Xu C, Zhang W. Biomarker discovery to improve prediction of breast cancer survival: using gene expression profiling, meta-analysis, and tissue validation. Onco Targets Ther. 2016;9:6177–6185. doi: 10.2147/OTT.S113855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulvirenti A, Rao D, Mcintyre CA, Gonen M, Tang LH, Klimstra DS, Fleisher M, Ramanathan LV, Reidy-Lagunes D, Allen PJ. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB (Oxford) 2019;21:612–618. doi: 10.1016/j.hpb.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lv Y, Han X, Zhang C, Fang Y, Pu N, Ji Y, Wang D, Xuefeng X, Lou W. Combined test of serum CgA and NSE improved the power of prognosis prediction of NF-pNETs. Endocr Connect. 2018;7:169–178. doi: 10.1530/EC-17-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12 Suppl 2:S69–S72. doi: 10.1093/annonc/12.suppl_2.s69. [DOI] [PubMed] [Google Scholar]
- 77.Wang YH, Yang QC, Lin Y, Xue L, Chen MH, Chen J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltimore) 2014;93:e247. doi: 10.1097/MD.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Risi ES, Al-Essry FS, Mula-Abed WS. Chromogranin A as a Biochemical Marker for Neuroendocrine Tumors: A Single Center Experience at Royal Hospital, Oman. Oman Med J. 2017;32:365–370. doi: 10.5001/omj.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto VD, Annibale B, Buonadonna A, Pederzoli P, Delle Fave G. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6–11. doi: 10.1007/BF03350903. [DOI] [PubMed] [Google Scholar]
- 80.Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435–e446. doi: 10.1016/S1470-2045(15)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, Bidart JM, Cailleux AF, Bonacci R, Ruffié P, Schlumberger M. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78:1102–1107. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajetta E, Ferrari L, Martinetti A, Celio L, Procopio G, Artale S, Zilembo N, Di Bartolomeo M, Seregni E, Bombardieri E. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86:858–865. doi: 10.1002/(sici)1097-0142(19990901)86:5<858::aid-cncr23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 83.Gao S, Pu N, Liu L, Li C, Xu X, Wang X, Lou W. The latest exploration of staging and prognostic classification for pancreatic neuroendocrine tumors: a large population-based study. J Cancer. 2018;9:1698–1706. doi: 10.7150/jca.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 85.Gut P, Czarnywojtek A, Fischbach J, Bączyk M, Ziemnicka K, Wrotkowska E, Gryczyńska M, Ruchała M. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016;12:1–9. doi: 10.5114/aoms.2016.57577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thelin EP, Jeppsson E, Frostell A, Svensson M, Mondello S, Bellander BM, Nelson DW. Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Crit Care. 2016;20:285. doi: 10.1186/s13054-016-1450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corsello A, Di Filippo L, Massironi S, Sileo F, Dolcetta Capuzzo A, Gemma M, Carlucci C, Cusini C, Colombo B, Dallatomasina A, Franchi GM, Corti A, Manzoni MF. Vasostatin-1: A novel circulating biomarker for ileal and pancreatic neuroendocrine neoplasms. PLoS One. 2018;13:e0196858. doi: 10.1371/journal.pone.0196858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chamberlain CE, German MS, Yang K, Wang J, VanBrocklin H, Regan M, Shokat KM, Ducker GS, Kim GE, Hann B, Donner DB, Warren RS, Venook AP, Bergsland EK, Lee D, Wang Y, Nakakura EK. A Patient-derived Xenograft Model of Pancreatic Neuroendocrine Tumors Identifies Sapanisertib as a Possible New Treatment for Everolimus-resistant Tumors. Mol Cancer Ther. 2018;17:2702–2709. doi: 10.1158/1535-7163.MCT-17-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benten D, Behrang Y, Unrau L, Weissmann V, Wolters-Eisfeld G, Burdak-Rothkamm S, Stahl FR, Anlauf M, Grabowski P, Möbs M, Dieckhoff J, Sipos B, Fahl M, Eggers C, Perez D, Bockhorn M, Izbicki JR, Lohse AW, Schrader J. Establishment of the First Well-differentiated Human Pancreatic Neuroendocrine Tumor Model. Mol Cancer Res. 2018;16:496–507. doi: 10.1158/1541-7786.MCR-17-0163. [DOI] [PubMed] [Google Scholar]
- 90.Grozinsky-Glasberg S, Shimon I, Rubinfeld H. The role of cell lines in the study of neuroendocrine tumors. Neuroendocrinology. 2012;96:173–187. doi: 10.1159/000338793. [DOI] [PubMed] [Google Scholar]
- 91.Babu V, Paul N, Yu R. Animal models and cell lines of pancreatic neuroendocrine tumors. Pancreas. 2013;42:912–923. doi: 10.1097/MPA.0b013e31827ae993. [DOI] [PubMed] [Google Scholar]
- 92.Townsend CM, Jr, Ishizuka J, Thompson JC. Studies of growth regulation in a neuroendocrine cell line. Acta Oncol. 1993;32:125–130. doi: 10.3109/02841869309083900. [DOI] [PubMed] [Google Scholar]
- 93.Buicko JL, Finnerty BM, Zhang T, Kim BJ, Fahey TJ, 3rd, Nancy Du YC. Insights into the biology and treatment strategies of pancreatic neuroendocrine tumors. Ann Pancreat Cancer. 2019;2 doi: 10.21037/apc.2019.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young K, Starling N, Sadanandam A. The molecular biology of pancreatic neuroendocrine neoplasms: Challenges and translational opportunities. Semin Cancer Biol. 2020;61:132–138. doi: 10.1016/j.semcancer.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 95.Hofving T, Arvidsson Y, Almobarak B, Inge L, Pfragner R, Persson M, Stenman G, Kristiansson E, Johanson V, Nilsson O. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocr Relat Cancer. 2018;25:367–380. doi: 10.1530/ERC-17-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Babu V, Paul N, Yu R. Animal models and cell lines of pancreatic neuroendocrine tumors. Pancreas. 2013;42:912–923. doi: 10.1097/MPA.0b013e31827ae993. [DOI] [PubMed] [Google Scholar]
- 97.Luley KB, Biedermann SB, Künstner A, Busch H, Franzenburg S, Schrader J, Grabowski P, Wellner UF, Keck T, Brabant G, Schmid SM, Lehnert H, Ungefroren H. A Comprehensive Molecular Characterization of the Pancreatic Neuroendocrine Tumor Cell Lines BON-1 and QGP-1. Cancers (Basel) 2020;12 doi: 10.3390/cancers12030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benten D, Behrang Y, Unrau L, Weissmann V, Wolters-Eisfeld G, Burdak-Rothkamm S, Stahl FR, Anlauf M, Grabowski P, Möbs M, Dieckhoff J, Sipos B, Fahl M, Eggers C, Perez D, Bockhorn M, Izbicki JR, Lohse AW, Schrader J. Establishment of the First Well-differentiated Human Pancreatic Neuroendocrine Tumor Model. Mol Cancer Res. 2018;16:496–507. doi: 10.1158/1541-7786.MCR-17-0163. [DOI] [PubMed] [Google Scholar]
- 99.Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol. 2003;17:1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 100.Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, Grover AC, Ylaya K, Hewitt SM, Marx SJ, Spiegel AM, Libutti SK. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009;69:1858–1866. doi: 10.1158/0008-5472.CAN-08-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li F, Su Y, Cheng Y, Jiang X, Peng Y, Li Y, Lu J, Gu Y, Zhang C, Cao Y, Wang W, Ning G. Conditional deletion of Men1 in the pancreatic β-cell leads to glucagon-expressing tumor development. Endocrinology. 2015;156:48–57. doi: 10.1210/en.2014-1433. [DOI] [PubMed] [Google Scholar]
- 102.Wong C, Tang LH, Davidson C, Vosburgh E, Chen W, Foran DJ, Notterman DA, Levine AJ, Xu EY. Two well-differentiated pancreatic neuroendocrine tumor mouse models. Cell Death Differ. 2020;27:269–283. doi: 10.1038/s41418-019-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeger H, Perbal B. CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal. 2016;10:229–240. doi: 10.1007/s12079-016-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang R, Chen Y, Chen D. Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review) Mol Med Rep. 2018;17:3–10. doi: 10.3892/mmr.2017.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jay P, Bergé-Lefranc JL, Marsollier C, Méjean C, Taviaux S, Berta P. The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p. Oncogene. 1997;14:1753–1757. doi: 10.1038/sj.onc.1200986. [DOI] [PubMed] [Google Scholar]
- 106.Huang YT, Lan Q, Lorusso G, Duffey N, Rüegg C. The matricellular protein CYR61 promotes breast cancer lung metastasis by facilitating tumor cell extravasation and suppressing anoikis. Oncotarget. 2017;8:9200–9215. doi: 10.18632/oncotarget.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Habel N, Stefanovska B, Carène D, Patiño-Garcia A, Lecanda F, Fromigué O. CYR61 triggers osteosarcoma metastatic spreading via an IGF1Rβ-dependent EMT-like process. BMC Cancer. 2019;19:62. doi: 10.1186/s12885-019-5282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haque I, Mehta S, Majumder M, Dhar K, De A, McGregor D, Van Veldhuizen PJ, Banerjee SK, Banerjee S. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol Cancer. 2011;10:8. doi: 10.1186/1476-4598-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maity G, Ghosh A, Gupta V, Haque I, Sarkar S, Das A, Dhar K, Bhavanasi S, Gunewardena SS, Von Hoff DD, Mallik S, Kambhampati S, Banerjee SK, Banerjee S. CYR61/CCN1 Regulates dCK and CTGF and Causes Gemcitabine-resistant Phenotype in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2019;18:788–800. doi: 10.1158/1535-7163.MCT-18-0899. [DOI] [PubMed] [Google Scholar]
- 110.Huang YT, Lan Q, Ponsonnet L, Blanquet M, Christofori G, Zaric J, Rüegg C. The matricellular protein CYR61 interferes with normal pancreatic islets architecture and promotes pancreatic neuroendocrine tumor progression. Oncotarget. 2016;7:1663–1674. doi: 10.18632/oncotarget.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Wu F, Tan Q, Guo M, Ma P, Wang X, Zhang S, Xu J, Luo P, Jin Y. The multifaceted roles of FOXM1 in pulmonary disease. Cell Commun Signal. 2019;17:35. doi: 10.1186/s12964-019-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WK, Medema RH, He X, Huang S. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwok CT, Leung MH, Qin J, Qin Y, Wang J, Lee YL, Yao KM. The Forkhead box transcription factor FOXM1 is required for the maintenance of cell proliferation and protection against oxidative stress in human embryonic stem cells. Stem Cell Res. 2016;16:651–661. doi: 10.1016/j.scr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Yao S, Fan LY, Lam EW. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin Cancer Biol. 2018;50:77–89. doi: 10.1016/j.semcancer.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong S, Liang J, Zhai W, Yu Z. Common and distinct features of potentially predictive biomarkers in small cell lung carcinoma and large cell neuroendocrine carcinoma of the lung by systematic and integrated analysis. Mol Genet Genomic Med. 2020;8:e1126. doi: 10.1002/mgg3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, Qiao WB, Shan L. Expression and functional characterization of FOXM1 in non-small cell lung cancer. Onco Targets Ther. 2018;11:3385–3393. doi: 10.2147/OTT.S162523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Briest F, Berg E, Grass I, Freitag H, Kaemmerer D, Lewens F, Christen F, Arsenic R, Altendorf-Hofmann A, Kunze A, Sänger J, Knösel T, Siegmund B, Hummel M, Grabowski P. FOXM1: A novel drug target in gastroenteropancreatic neuroendocrine tumors. Oncotarget. 2015;6:8185–8199. doi: 10.18632/oncotarget.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andersson E, Arvidsson Y, Swärd C, Hofving T, Wängberg B, Kristiansson E, Nilsson O. Expression profiling of small intestinal neuroendocrine tumors identifies subgroups with clinical relevance, prognostic markers and therapeutic targets. Mod Pathol. 2016;29:616–629. doi: 10.1038/modpathol.2016.48. [DOI] [PubMed] [Google Scholar]
- 119.De Rycke O, Raffenne J, Lacombe C, Hentic O, Gounant V, Sauvanet A, Zalcman G, Paradis V, Ruszniewski P, Couvelard A, de Mestier L, Cros J. Role of FOXM1 in Aggressive Pancreatic/Pulmonary Neuroendocrine Carcinomas and Anti-Tumor Effect of the FOXM1 Inhibitor Thiostrepton. Abstract # 2874 17th Annual ENETS Conference 2020 (2020) [Google Scholar]
- 120.Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adams CJ, Kopp MC, Larburu N, Nowak PR, Ali MMU. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front Mol Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Croft A, Tay KH, Boyd SC, Guo ST, Jiang CC, Lai F, Tseng HY, Jin L, Rizos H, Hersey P, Zhang XD. Oncogenic activation of MEK/ERK primes melanoma cells for adaptation to endoplasmic reticulum stress. J Invest Dermatol. 2014;134:488–497. doi: 10.1038/jid.2013.325. [DOI] [PubMed] [Google Scholar]
- 123.Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blazanin N, Son J, Craig-Lucas AB, John CL, Breech KJ, Podolsky MA, Glick AB. ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras. Proc Natl Acad Sci USA. 2017;114:9900–9905. doi: 10.1073/pnas.1701757114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodvold JJ, Chiu KT, Hiramatsu N, Nussbacher JK, Galimberti V, Mahadevan NR, Willert K, Lin JH, Zanetti M. Intercellular transmission of the unfolded protein response promotes survival and drug resistance in cancer cells. Sci Signal. 2017;10 doi: 10.1126/scisignal.aah7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klieser E, Illig R, Státtner S, Primavesi F, Jáger T, Swierczynski S, Kiesslich T, Kemmerling R, Bollmann C, Di Fazio P, Neureiter D. Endoplasmic Reticulum Stress in Pancreatic Neuroendocrine Tumors is Linked to Clinicopathological Parameters and Possible Epigenetic Regulations. Anticancer Res. 2015;35:6127–6136. [PubMed] [Google Scholar]
- 127.Huang YT, Lan Q, Ponsonnet L, Blanquet M, Christofori G, Zaric J, Rüegg C. The matricellular protein CYR61 interferes with normal pancreatic islets architecture and promotes pancreatic neuroendocrine tumor progression. Oncotarget. 2016;7:1663–1674. doi: 10.18632/oncotarget.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy JP, Giacomantonio MA, Paulo JA, Everley RA, Kennedy BE, Pathak GP, Clements DR, Kim Y, Dai C, Sharif T, Gygi SP, Gujar S. The NAD+ Salvage Pathway Supports PHGDH-Driven Serine Biosynthesis. Cell Rep. 2018;24:2381–2391.e5. doi: 10.1016/j.celrep.2018.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dalamaga M, Christodoulatos GS, Mantzoros CS. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: Diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. doi: 10.1016/j.metabol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 131.Liu L, Su X, Quinn WJ, 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, Rabinowitz JD. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018;27:1067–1080.e5. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Travelli C, Colombo G, Mola S, Genazzani AA, Porta C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharmacol Res. 2018;135:25–36. doi: 10.1016/j.phrs.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 135.Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- 136.Wang W, Hu Y, Yang C, Zhu S, Wang X, Zhang Z, Deng H. Decreased NAD Activates STAT3 and Integrin Pathways to Drive Epithelial-Mesenchymal Transition. Mol Cell Proteomics. 2018;17:2005–2017. doi: 10.1074/mcp.RA118.000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kraus D, Reckenbeil J, Veit N, Kuerpig S, Meisenheimer M, Beier I, Stark H, Winter J, Probstmeier R. Targeting glucose transport and the NAD pathway in tumor cells with STF-31: a re-evaluation. Cell Oncol (Dordr) 2018;41:485–494. doi: 10.1007/s13402-018-0385-5. [DOI] [PubMed] [Google Scholar]
- 138.Zhang SL, Xu TY, Yang ZL, Han S, Zhao Q, Miao CY. Crystal structure-based comparison of two NAMPT inhibitors. Acta Pharmacol Sin. 2018;39:294–301. doi: 10.1038/aps.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mpilla G, Aboukameel A, Muqbil I, Kim S, Beydoun R, Philip PA, Mohammad RM, Kamgar M, Shidham V, Senapedis W, Baloglu E, Li J, Dyson G, Xue Y, El-Rayes B, Azmi AS. PAK4-NAMPT Dual Inhibition as a Novel Strategy for Therapy Resistant Pancreatic Neuroendocrine Tumors. Cancers (Basel) 2019;11 doi: 10.3390/cancers11121902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alvarez MJ, Subramaniam PS, Tang LH, Grunn A, Aburi M, Rieckhof G, Komissarova EV, Hagan EA, Bodei L, Clemons PA, Dela Cruz FS, Dhall D, Diolaiti D, Fraker DA, Ghavami A, Kaemmerer D, Karan C, Kidd M, Kim KM, Kim HC, Kunju LP, Langel Ü, Li Z, Lee J, Li H, LiVolsi V, Pfragner R, Rainey AR, Realubit RB, Remotti H, Regberg J, Roses R, Rustgi A, Sepulveda AR, Serra S, Shi C, Yuan X, Barberis M, Bergamaschi R, Chinnaiyan AM, Detre T, Ezzat S, Frilling A, Hommann M, Jaeger D, Kim MK, Knudsen BS, Kung AL, Leahy E, Metz DC, Milsom JW, Park YS, Reidy-Lagunes D, Schreiber S, Washington K, Wiedenmann B, Modlin I, Califano A. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat Genet. 2018;50:979–989. doi: 10.1038/s41588-018-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohanna M, Cerezo M, Nottet N, Bille K, Didier R, Beranger G, Mograbi B, Rocchi S, Yvan-Charvet L, Ballotti R, Bertolotto C. Pivotal role of NAMPT in the switch of melanoma cells toward an invasive and drug-resistant phenotype. Genes Dev. 2018;32:448–461. doi: 10.1101/gad.305854.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong LE, Chen N, Karantza V, Minden A. The Pak4 protein kinase is required for oncogenic transformation of MDA-MB-231 breast cancer cells. Oncogenesis. 2013;2:e50. doi: 10.1038/oncsis.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.King H, Thillai K, Whale A, Arumugam P, Eldaly H, Kocher HM, Wells CM. PAK4 interacts with p85 alpha: implications for pancreatic cancer cell migration. Sci Rep. 2017;7:42575. doi: 10.1038/srep42575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dart AE, Wells CM. P21-activated kinase 4--not just one of the PAK. Eur J Cell Biol. 2013;92:129–138. doi: 10.1016/j.ejcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem. 2003;134:327–331. doi: 10.1093/jb/mvg149. [DOI] [PubMed] [Google Scholar]
- 146.Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, Chu GC, Zhang JX, Sahin E, Yeo G, Ponugoti A, Nabioullin R, Deroo S, Yang S, Wang X, McGrath JP, Protopopova M, Ivanova E, Zhang J, Feng B, Tsao MS, Redston M, Protopopov A, Xiao Y, Futreal PA, Hahn WC, Klimstra DS, Chin L, DePinho RA. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci USA. 2008;105:19372–19377. doi: 10.1073/pnas.0809966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–116. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, Tsao SW, Strömblad S, Cheung AN. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107:18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tabusa H, Brooks T, Massey AJ. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res. 2013;11:109–121. doi: 10.1158/1541-7786.MCR-12-0466. [DOI] [PubMed] [Google Scholar]
- 150.Mahlamäki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–439. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beauchamp RL, James MF, DeSouza PA, Wagh V, Zhao WN, Jordan JT, Stemmer-Rachamimov A, Plotkin SR, Gusella JF, Haggarty SJ, Ramesh V. A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget. 2015;6:16981–16997. doi: 10.18632/oncotarget.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, Kim EG. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J Invest Dermatol. 2015;135:1385–1394. doi: 10.1038/jid.2014.548. [DOI] [PubMed] [Google Scholar]
- 153.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rudolph J, Crawford JJ, Hoeflich KP, Wang W. Inhibitors of p21-activated kinases (PAKs) J Med Chem. 2015;58:111–129. doi: 10.1021/jm501613q. [DOI] [PubMed] [Google Scholar]
- 155.Shi KL, Qian JY, Qi L, Mao DB, Chen Y, Zhu Y, Guo XG. Atorvastatin antagonizes the visfatin-induced expression of inflammatory mediators via the upregulation of NF-κB activation in HCAECs. Oncol Lett. 2016;12:1438–1444. doi: 10.3892/ol.2016.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 157.Drevs J, Löser R, Rattel B, Esser N. Antiangiogenic potency of FK866/K22.175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Res. 2003;23:4853–4858. [PubMed] [Google Scholar]
- 158.Holen K, Saltz LB, Hollywood E, Burk K, Hanauske AR. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest New Drugs. 2008;26:45–51. doi: 10.1007/s10637-007-9083-2. [DOI] [PubMed] [Google Scholar]
- 159.Frost BM, Lönnerholm G, Nygren P, Larsson R, Lindhagen E. In vitro activity of the novel cytotoxic agent CHS 828 in childhood acute leukemia. Anticancer Drugs. 2002;13:735–742. doi: 10.1097/00001813-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 160.Aboukameel A, Muqbil I, Senapedis W, Baloglu E, Landesman Y, Shacham S, Kauffman M, Philip PA, Mohammad RM, Azmi AS. Novel p21-Activated Kinase 4 (PAK4) Allosteric Modulators Overcome Drug Resistance and Stemness in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2017;16:76–87. doi: 10.1158/1535-7163.MCT-16-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fulciniti M, Martinez-Lopez J, Senapedis W, Oliva S, Lakshmi Bandi R, Amodio N, Xu Y, Szalat R, Gulla A, Samur MK, Roccaro A, Linares M, Cea M, Baloglu E, Argueta C, Landesman Y, Shacham S, Liu S, Schenone M, Wu SL, Karger B, Prabhala R, Anderson KC, Munshi NC. Functional role and therapeutic targeting of p21-activated kinase 4 in multiple myeloma. Blood. 2017;129:2233–2245. doi: 10.1182/blood-2016-06-724831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Neggers JE, Kwanten B, Dierckx T, Noguchi H, Voet A, Bral L, Minner K, Massant B, Kint N, Delforge M, Vercruysse T, Baloglu E, Senapedis W, Jacquemyn M, Daelemans D. Target identification of small molecules using large-scale CRISPR-Cas mutagenesis scanning of essential genes. Nat Commun. 2018;9:502. doi: 10.1038/s41467-017-02349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rane C, Senapedis W, Baloglu E, Landesman Y, Crochiere M, Das-Gupta S, Minden A. A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci Rep. 2017;7:42555. doi: 10.1038/srep42555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mitchell SR, Larkin K, Grieselhuber NR, Lai TH, Cannon M, Orwick S, Sharma P, Asemelash Y, Zhang P, Goettl VM, Beaver L, Mims A, Puduvalli VK, Blachly JS, Lehman A, Harrington B, Henderson S, Breitbach JT, Williams KE, Dong S, Baloglu E, Senapedis W, Kirschner K, Sampath D, Lapalombella R, Byrd JC. Selective targeting of NAMPT by KPT-9274 in acute myeloid leukemia. Blood Adv. 2019;3:242–255. doi: 10.1182/bloodadvances.2018024182. [DOI] [PMC free article] [PubMed] [Google Scholar]