Abstract
Myocardial dysfunction and coronary artery dilation have been reported in the acute setting of severe acute respiratory syndrome coronavirus disease-2-related multisystem inflammatory syndrome in children. Through a longitudinal echocardiographic single-center study of 15 children, we report the short-term outcomes of cardiac dysfunction and coronary artery dilation in severe acute respiratory syndrome coronavirus disease-2-related multisystem inflammatory syndrome in children.
Keywords: coronary dilation, coronary aneurysm, systolic dysfunction, COVID-19, pediatrics
Abbreviations: COVID-19, Coronavirus disease-2019; MIS-C, Multisystem inflammatory syndrome in children; SARS-CoV-2, Severe acute respiratory syndrome coronavirus disease-2
Coronavirus disease-2019 (COVID-19), a disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has accounted for more than 460 000 deaths globally as of June 2020. Children of all ages have been affected, although the clinical manifestations are less severe than in adults. In early May, reports emerged from Europe and North America describing a hyperinflammatory condition in children related to SARS-CoV-2 and presenting with some features similar to Kawasaki disease and toxic shock syndrome.1 , 2 , 3 On May 14, 2020, the Centers for Disease Control and Prevention recognized this clinical complex as multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 and released a case definition based on clinical and laboratory criteria.4
Patients with MIS-C come to medical attention with fever, elevated inflammatory markers, multisystem organ involvement (renal, gastrointestinal, neurologic, dermatologic, cardiac), with evidence of a current or recent SARS-CoV-2 infection or recent close contact with a known or suspected case of COVID-19. In a report describing the experience of MIS-C in New York, 80% of diagnosed children were admitted to the intensive care unit. Of the hospitalized children, 52% had myocardial dysfunction and 9% had coronary artery aneurysms.5 Management of MIS-C has involved intravenous immunoglobulins, vasoactive agents, anticoagulants, and immune-modulating drugs with varied response.6 To date, reports are limited to description of cardiac findings during hospitalization; however, there is a paucity of data on the longitudinal follow-up and sequelae in this population. We sought to describe the echocardiographic manifestations of MIS-C, including the evolution of abnormalities of coronary artery dilation and ventricular systolic function, over short-term follow-up after discharge from the hospital.
Methods
A single-center retrospective review was conducted on all pediatric patients (<21 years of age) admitted with MIS-C to Mount Sinai Kravis Children's Hospital between April 24 (first admission) and May 16, 2020, who had at least 1 echocardiogram performed during hospitalization. The determination of diagnosis of MIS-C was based on Centers for Disease Control and Prevention criteria.4 Only patients with confirmed SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction and/or serology during hospitalization were included.
All of the patients in our cohort were described previously in three recent multicenter publications that focused on acute presentation and inpatient outcomes.5 , 7 , 8 Additionally, 4 patients from this cohort were described in a recent case series.9
Clinical data including hospital course and laboratory results were collected. Serial echocardiograms performed from admission to most recent outpatient follow-up visit (until June 18, 2020) were reviewed. The timing of follow-up echocardiograms was based on the findings of initial echocardiogram and ongoing clinical status. Absolute dimensions (millimeters) and z-scores of proximal coronary arteries including the left main coronary artery, left anterior descending coronary artery, left circumflex coronary artery, and right coronary artery were recorded. Coronary aneurysms were defined using z-scores as per American Heart Association Kawasaki guidelines (small aneurysm ≥2.5 to <5.0 mm, medium aneurysm ≥5 to <10 mm, and large aneurysm ≥10 or absolute dimension ≥8 mm).10 The Boston Children's Hospital Z-score system was utilized for all the echocardiographic measures.11 Ventricular function, effusion, valvar function, and coronary dimensions were tracked serially from the date of admission to the most recent outpatient follow-up visit.
Results
Fifteen pediatric patients (mean age, 11.5 years; range, 3-20 years; 60% male) met the criteria for MIS-C in the study timeframe. Six patients (40%) had a positive nasopharyngeal SARS-CoV-2 PCR test (SARS-CoV-2 test, Cobas 6800 system, Roche Diagnostics, Basel, Switzerland) and all had a positive COVID-19 antibody test (Mt. Sinai Laboratory COVID-19 ELISA antibody test, approved by the US Food and Drug Administration under emergency use authorization).12 The average length of stay in the hospital was 7.6 ± 2.3 days. There was 1 death in a patient who required extracorporeal membrane oxygenation support. The average follow-up period of the cohort from admission was 28.1 days (range, 5-50 days). Detailed clinical information is depicted in the Table (available at www.jpeds.com).
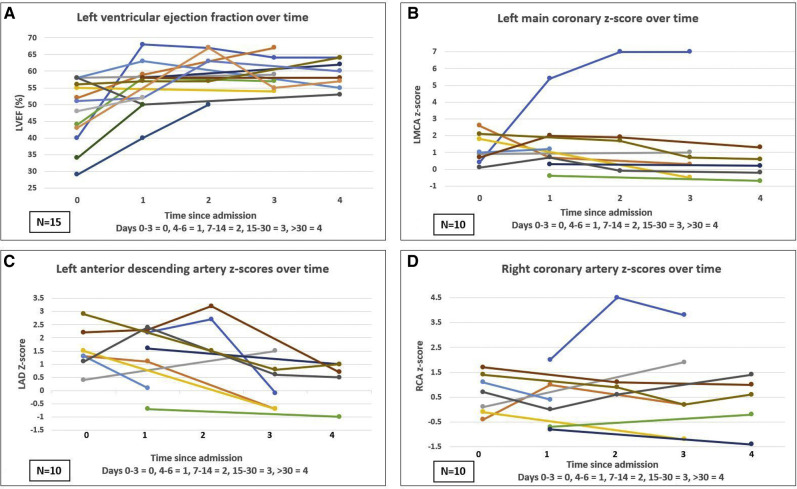
The average left ventricular ejection fraction at first echocardiogram was 49.5% (range, 29%-58%). Eight patients (53%) presented with left ventricular dysfunction (ejection fraction of <55%) ranging from mild to severe (n = 2 with an ejection fraction of 50%-54%; n = 4 with ejection fraction of 40%-49%, and n = 2 with and ejection fraction of <35%). Ten of the 13 children (excluding 1 lost to follow-up and 1 deceased patient) had recovery of cardiac function within 1 month of the initial study. Three patients continue to have low-normal systolic function (ejection fraction, 50%-55%) and are in ongoing follow-up (Figure 1, A). Of the 4 patients (27%) who had right ventricular dysfunction on the initial echocardiogram, all but 1 (deceased) had complete recovery of right ventricular function on follow-up imaging. Two patients had pericardial effusions (small) on initial echocardiograms that resolved. Approximately one-half (n = 8 [53%]) of the cohort had mitral regurgitation (mild), all but 2 resolved over the study period. All patients received therapeutic anticoagulation. Aspirin was started in patients with coronary artery dilation and/or after discontinuation of enoxaparin in patients without dilation.
Figure 1.
Longitudinal echocardiographic data plotted on a line chart demonstrating the trajectory of each individual patient's findings over the study period. Time is depicted on the x-axis and is divided into intervals based on days since admission as described in the figures. A, Left ventricular ejection fraction over time. B, Left main coronary artery (LMCA) dimensions over time. C, Left anterior descending coronary artery (LAD) artery dimensions over time. D, Right coronary artery (RCA) dimensions over time.
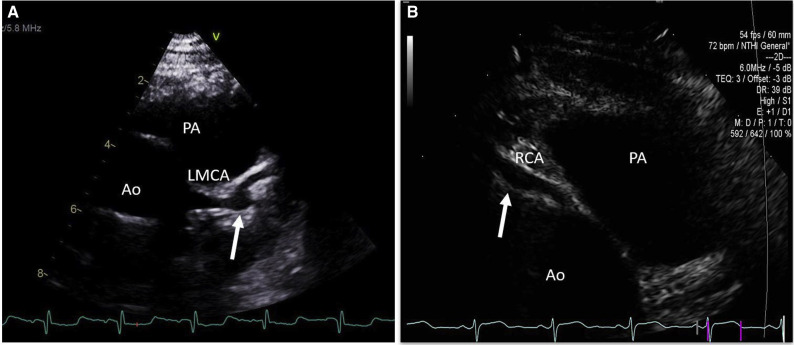
Coronary artery evaluation was performed in 12 patients (80%) and 10 of these 12 had more than 1 echocardiogram performed in the study period. Three patients did not have coronary arteries assessing during echocardiography, 2 of the 3 were due to hemodynamically instability, and 1 patient was the first admission with MIS-C, before the importance of coronary artery assessment was recognized. Of the patients with coronary artery evaluation, one-third (n = 4) had coronary involvement (z-score >2.5). Left main coronary artery aneurysms (1 small, 1 medium) were seen in 2 patients; 1 resolved and 1 remains persistent at follow-up. (Figure 1, B and Figure 2, A). Left anterior descending coronary artery aneurysms were seen in three patients (2 small, 1 medium aneurysm). Within 30 days, all patients with left anterior descending coronary artery involvement had normal z-scores (Figure 1, C). Only 1 patient had an aneurysm in the right coronary artery (small) that has remained aneurysmal, albeit slightly smaller on recent echocardiogram (Figure 1, D and Figure 2, B).
Figure 2.
A, Parasternal short-axis view demonstrates an aneurysm (arrow) at the bifurcation of left main coronary artery (LMCA) into left anterior descending and circumflex coronaries. B, Parasternal short axis image shows a fusiform aneurysm (arrow) of the right coronary artery. Ao, aorta, PA, pulmonary artery.
Discussion
This longitudinal study provides information on short-term echocardiographic outcomes of 15 patients with MIS-C. Although left ventricular dysfunction was common at the initial evaluation (53%), significant improvement was seen within a 30-day follow-up period. However, some patients had residual low-normal function at 4-6 weeks of follow-up. Our findings are congruent with 2 larger multicenter studies from New York that showed 53%-65% of patients had evidence of myocardial dysfunction during hospitalization. Follow-up outpatient data on systolic function was not reported.5 , 7
We found that coronary artery abnormalities can be evident in the first 2 weeks and tend to have a rapid resolution, with only 1 patient in this study demonstrating persistence of aneurysms. One-third (33%) of our patients (4/12) had coronary aneurysms. This is higher than a recent report by Capone et al, in which 5 of 33 patients (15%) with MIS-C showed evidence of coronary aneurysms during in-patient evaluation.13 It is our practice, as with patients with Kawasaki disease, that children with MIS-C have serial coronary evaluations at 1-2 weeks with a follow-up at 4-6 weeks at minimum.10 The majority of our patients (80%) received intravenous immunoglobulin therapy, akin to the treatment for Kawasaki disease. None of our patients showed evidence of coronary thrombosis, although all patients received therapeutic anticoagulation based on their hyper-inflammatory state and elevated d-dimer levels. Given the small sample size, clinical distinctions between patients with and without coronary aneurysms was not within the scope of this study.
This study provides short-term longitudinal cardiac information for a small number of patients with MIS-C that may aid in clinical management and counseling of families. Medium and long-term follow-up using echocardiography as well as advanced imaging modalities such as cardiac magnetic resonance and computed tomography will be important to provide comprehensive data.
Footnotes
The authors declare no conflicts of interest.
Appendix.
Table.
Descriptive clinical data of the study population
| Clinical variables | Patients with coronary artery involvement (n = 4) | Patients without coronary artery involvement (n = 8) | All patients (n = 15) |
|---|---|---|---|
| Age, years | 10.25 ± 3.59 | 11.63 ± 3.81 | 11.53 ± 4.49 |
| Sex, Male | 75 (3) | 50 | 60 (9) |
| BMI, kg/m2 | 26.85 ± 8.35 | 20.45 ± 4.23 | 22.27 ± 6.62 |
| BSA, m2 | 1.24 ± 0.37 | 1.42 ± 0.39 | 1.38 ± 0.43 |
| Duration of symptoms at admission, days | 4.25 ± 2.06 | 5 ± 1 | 4.53 ± 1.41 |
| Pressors administered | 75 (3) | 25 (2) | 53.3 (8) |
| Peak d-dimer, μg/mL | 4.37 ± 2.09 | 4.67 ± 3.25 | 5.47 ± 4.82 |
| Peak troponin, ng/mL | 0.98 ± 1.63 | 5.84 ± 10.23 | 3.73 ± 8.15 |
| Peak pro-BNP, pg/mL | 839.59 ± 634.55 | 1308.30 ± 1226.23 | 1487.64 ± 1774.84 |
| IVIG administered | 100 (4) | 87.5 (7) | 80 (12) |
| Corticosteroids administered | 25 (1) | 12.5 (1) | 20 (3) |
| Tocilizumab administered | 100 (4) | 62.5 (5) | 60 (9) |
| Anakinra administered | 25 (1) | 0 (0) | 13.3 (2) |
| Therapeutic anticoagulation | 100 (4) | 100 (8) | 100 (15) |
| Remdesivir administered | 25 (1) | 12.5 (1) | 20 (3) |
| Plasma therapy administered | 0 | 0 (0) | 6.7 (1) |
| ASA during admission | 50 (2) | 0 (0) | 13.3 (2) |
| Site of coronary artery involvement (individual patient) | |||
| Mid LAD | N/A | N/A | |
| LMCA | N/A | N/A | |
| Mid RCA | N/A | N/A | |
| LAD | N/A | N/A | |
| LAD | N/A | N/A | |
| LMCA | N/A | N/A | |
ASA, aspirin; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; IVIG, intravenous immunoglobulin; LMCA, left main coronary artery, LAD, left anterior descending coronary artery; N/A, not applicable; RCA, right coronary artery.
Values are mean ± SD or % (n).
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;6736:1–8. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J. COVID-19 and Kawasaki Disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Health Alert Network 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 5.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;6736:2019–2020. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–336. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waltuch T., Gill P., Zinns L.E., Whitney R., Tokarski J., Tsung J.W. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.058. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 11.Colan S.D. Normal echocardiographic values for cardiovascular structures. In: Lai W.W., Mertens L.L., editors. Echocardiography in pediatric congenital heart disease: from fetus to adult. 2nd ed. John Wiley & Sons, Ltd; New York: 2016. pp. 883–901. [Google Scholar]
- 12.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capone C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020;53:1–9. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]