Abstract
Background: Ae. aegypti mosquitoes stably transfected with the intracellular bacterium Wolbachia pipientis ( wMel strain) have been deployed for biocontrol of dengue and related arboviral diseases in multiple countries. Field releases in northern Australia have previously demonstrated near elimination of local dengue transmission from Wolbachia-treated communities, and pilot studies in Indonesia have demonstrated the feasibility and acceptability of the method. We conducted a quasi-experimental trial to evaluate the impact of scaled Wolbachia releases on dengue incidence in an endemic setting in Indonesia.
Methods: In Yogyakarta City, Indonesia, following extensive community engagement, wMel Wolbachia-carrying mosquitoes were released every two weeks for 13–15 rounds over seven months in 2016–17, in a contiguous 5 km 2 area (population 65,000). A 3 km 2 area (population 34,000) on the opposite side of the city was selected a priori as an untreated control area. Passive surveillance data on notified hospitalised dengue patients was used to evaluate the epidemiological impact of Wolbachia deployments, using controlled interrupted time-series analysis.
Results: Rapid and sustained introgression of wMel Wolbachia into local Ae. aegypti populations was achieved. Thirty-four dengue cases were notified from the intervention area and 53 from the control area (incidence 26 vs 79 per 100,000 person-years) during 24 months following Wolbachia deployment. This corresponded in the regression model to a 73% reduction in dengue incidence (95% confidence interval 49%,86%) associated with the Wolbachia intervention. Exploratory analysis including 6 months additional post-intervention observations showed a small strengthening of this effect (30 vs 115 per 100,000 person-years; 76% reduction in incidence, 95%CI 60%,86%).
Conclusions: We demonstrate a significant reduction in dengue incidence following successful introgression of Wolbachia into local Ae. aegypti populations in an endemic setting in Indonesia. These findings are consistent with previous field trials in northern Australia, and support the effectiveness of this novel approach for dengue control.
Keywords: Wolbachia, Aedes aegypti, dengue, vector-borne disease, mosquito release, quasi-experimental study, interrupted time series analysis, Indonesia, World Mosquito Program
List of abbreviations
AWED: ‘Applying Wolbachia to eliminate dengue’ (randomised controlled trial currently ongoing in Yogyakarta)
BG trap: Biogents Sentinel Trap
CI: confidence intervals
CRCT: cluster randomised controlled trial
DENV: dengue virus
DF: dengue fever
DHF: dengue haemorrhagic fever
DHO: District Health Office
ICD-10: International Classification of Diseases, 10 th revision
IRR: incidence rate ratio
ITS: interrupted time series
MRC: mosquito release container
NS1: non-structural protein 1
QA: quality assurance
RDT: rapid diagnostic test
RT-PCR: reverse transcription polymerase chain reaction
WMP : World Mosquito Program
Background
Aedes aegypti mosquitoes are the primary vectors of dengue, Zika and chikungunya viruses. Of these, dengue is the most common arboviral infection of humans. Indonesia has among the highest dengue case burden, with an estimated 10 million clinical cases and 3000 deaths each year 1. The annual per capita incidence is estimated at 36–44 symptomatic cases per 1000 population 1, 2 with a resultant cost in excess of USD $2 billion per annum 3. Yogyakarta is typical of many cities in Indonesia; dengue is endemic with a seasonal peak between November and May. The high force of infection in Yogyakarta is evidenced by the hospitalised dengue case burden and high seroprevalence of dengue virus (DENV) neutralising antibodies (68%) in children 1–10 years 4.
The World Mosquito Program (WMP) is an international research collaboration deploying Wolbachia-infected Ae. aegypti mosquitoes for the biocontrol of dengue and other Ae. aegypti-borne viral infections. Wolbachia is an intracellular endosymbiotic bacterium present naturally in many insect species, but not present in Ae. aegypti until methods enabling stable transinfection emerged 5. Wolbachia is maintained in Ae. aegypti through maternal inheritance, and confers a reproductive advantage via cytoplasmic incompatibility which facilitates its introgression into mosquito populations following open field release. A second feature of wMel Wolbachia in Ae. aegypti is that it confers resistance to infection with all four DENV serotypes 6– 9 plus other medically important arboviruses like chikungunya, Zika and Yellow Fever 10– 14. Mathematical modelling predicts that the wMel strain of Wolbachia should eliminate DENV transmission in most endemic country epidemiological circumstances 7, 15, 16.
The first open releases of wMel Wolbachia-carrying Ae. aegypti were undertaken in northern Australia, in small isolated communities in 2011 and then into contiguous urban areas from 2013, resulting in the near elimination of local dengue transmission in northern Australia 17, 18. In 2014–15, WMP conducted small-scale field trials in four small peri-urban communities (0.18–0.61 km 2; populations of 1157–2681) in Sleman and Bantul districts in Yogyakarta Province, Indonesia 19. These proof-of-concept trials demonstrated successful introgression and long-term persistence of Wolbachia in local Ae. aegypti populations.
On the basis of these promising results, wMel Wolbachia-carrying mosquitoes were deployed in the city of Yogyakarta in 2016–2017 with the dual aims of optimising methods for deployment at scale and demonstrating a reduction in arboviral disease incidence. Two consecutive prospective studies were conducted to evaluate the public health impact of Wolbachia releases in non-overlapping areas of Yogyakarta city ( Figure 1). The first, reported here, was a quasi-experimental study in which interrupted time series analysis 20 of routine dengue surveillance data was used to evaluate the epidemiological impact of Wolbachia deployments in one contiguous area on the urban fringe, in comparison to a pre-specified untreated control area. The second study was a cluster randomised controlled trial (Applying Wolbachia to Eliminate Dengue (AWED); ClinicalTrials.gov # NCT03055585) 21. Participant enrolment in the AWED trial is ongoing until late 2020, and no results are reported here.
Figure 1. Map of intervention and control areas in the Yogyakarta quasi-experimental study (QES).
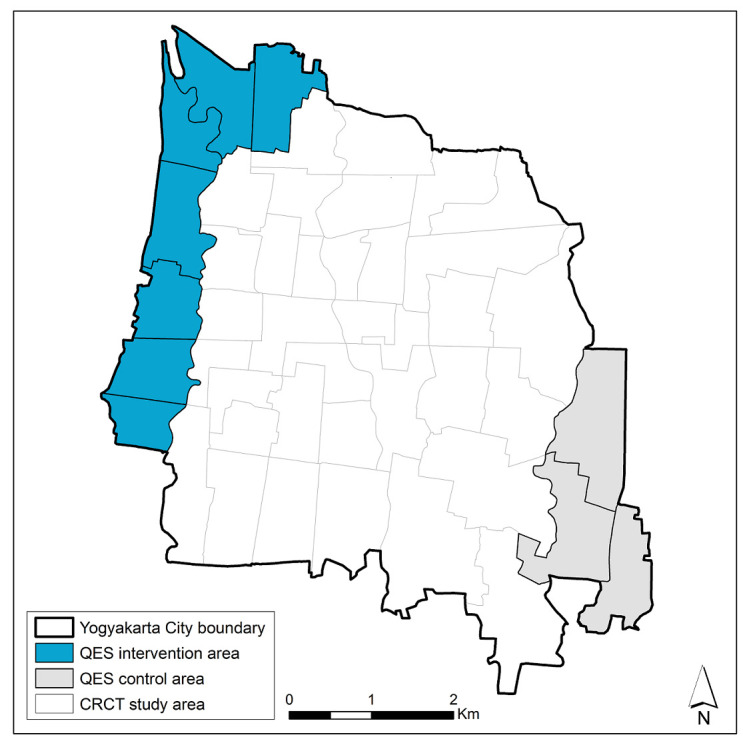
The study area for the ‘Applying Wolbachia to Eliminate Dengue (AWED)’ cluster randomised controlled trial (CRCT), ongoing until late 2020, is also indicated.
The entomological and epidemiological results of the quasi-experimental study, at the a priori defined two year time point after Wolbachia deployment and with six months additional observation time, are reported here. They demonstrate a significant and sustained reduction in notified dengue haemorrhagic fever (DHF) case incidence in Wolbachia-treated communities.
Methods
Study setting
Yogyakarta City in south-central Java, Indonesia, has a population of 422,732 in an area of 32 km 2, with 14 administrative districts comprising 45 kelurahans (urban villages). Seven kelurahans (total population 64,599; area 4.9 km 2) on the north-western perimeter of the city were selected as the site for scaled deployment of Wolbachia-carrying mosquitoes ( Figure 1), on the basis of logistical and operational feasibility. Three kelurahans (population 33,535; 3.1 km 2) on the south-eastern perimeter were selected a priori as an untreated control area, on the basis of comparable socio-demographic characteristics ( Table 1) and historical dengue incidence.
Table 1. Characteristics of intervention and control areas.
| Area | Total size (km 2) | Total population a | % completed high
school b |
% <15 years
of age a |
|---|---|---|---|---|
| Intervention
(7 kelurahans) |
4.90 | 64 599 | 50% | 22% |
| Control
(3 kelurahans) |
3.07 | 33 535 | 49% | 23% |
Source: aStatistics Indonesia (BPS) Yogyakarta City, 2017; bYogyakarta Province Population Bureau, 2017.
Community engagement
A Public Acceptance Model 17 was applied in engaging with the local community prior to Wolbachia deployments, and throughout the release and monitoring periods. Key elements of this approach included: meetings with key stakeholders and community leaders; meetings and ongoing regular communication with existing community reference groups at the village, city and provincial level; a communications campaign through social media, traditional media, mobile billboards, and community events; a household-based survey to evaluate awareness and acceptance prior to releases; and a ‘stakeholder enquiry system’ to receive and respond to any issues arising from stakeholders or community members.
A household survey of baseline community acceptance of WMP’s Wolbachia method was undertaken in November 2015 prior to intensive community engagement, followed by a second survey in June 2016 to evaluate acceptance prior to commencing releases. Two-stage cluster random sampling was used to select respondent households from across Yogyakarta City: n=587 in the 2015 survey (of which n=96 were from the seven urban villages in the quasi-experimental intervention area) and n=862 in the 2016 survey (n=180 from the intervention area). The sample size in the 2016 pre-release survey was sufficient to estimate community acceptance in the quasi-experimental intervention area with a margin of error of 10%, under conservative assumptions of a true community acceptance rate of 50% and a design effect of 2 due to the clustered sampling. Administrative wards were the primary sampling unit, and 12 households were randomly selected per ward using a list of households provided by the ward head as the sampling frame. The survey respondent was the head of household or another adult family member; there were no other inclusion or exclusion criteria. Respondents were asked about their level support for Wolbachia releases as a method for controlling dengue in their community. The survey participation rate was > 95% in each survey. During the 2015 baseline survey 67% of respondents from the quasi-experimental intervention area were supportive of the release of Wolbachia-infected Ae. aegypti as a new technology for controlling dengue, 14% were not supportive, and 20% were neutral or did not know. In the 2016 pre-release survey, acceptance in the intervention area had increased to 79%; 9% were not supportive and 11% were neutral or did not know.
The stakeholder enquiry system was managed by a dedicated staff member and enquiries could be logged by phone call, SMS text, WhatsApp, email or direct communication with WMP field staff. In total we had 216 enquiries during the release phase, relating to: details of release activities (location, timing, methods) (35%), requests for face-to-face meetings with community leaders (25%), expressions of support for the Wolbachia technology (18%), reports of dengue cases in the release area (7%), reports from householders on the condition of mosquito release containers (MRCs) (6%), and refusal to host an MRC (6%).
Mosquito rearing and egg production
An existing colony of local Ae. aegypti containing the wMel Wolbachia strain, created in 2013 for pilot releases in Bantul District of Yogyakarta Province 19, was used as the founder colony for the releases described here. It was backcrossed for three generations with wild-type males collected from the study intervention area. Four cages with 150 wMel-carrying female Ae. aegypti and 150 wild-type males were maintained for two gonotrophic cycles, and blood-fed by human volunteers. One hundred larvae were randomly selected from each cage and screened for Wolbachia, and only cages with 100% Wolbachia prevalence were maintained for the second backcrossing. An open colony was maintained by adding 10% of wild-type Aegypti after the second backcrossing. Insecticide resistance testing was conducted as described in 22, except that test specimens were adult females derived from egg, rather than larval, collections. This demonstrated equivalent insecticide resistance profiles between the colony and wild material ( Figure 2).
Figure 2. Insecticide susceptibility of the wMel Ae. aegypti colony and wild-type Ae. aegypti collected from the quasi-experimental study intervention area.
Bars show mean (st. dev.) % mortality across four replicate tests, each with 20 – 28 mosquitoes tested against each insecticide.
Mosquitoes were blood-fed by human volunteers once a week for two gonotrophic cycles, as per previous protocols 17. All volunteer blood-feeders were afebrile and free of clinical signs or symptoms of any arbovirus infection both at the time of blood-feeding and for three days thereafter. Four days after blood feeding, mosquito eggs were collected by placing oviposition strips in each cage for 3 days. Eggs were dried slowly and stored in sealed containers until releases.
Quality assurance of mosquito release material
For quality assurance (QA) of the mosquito colony, a sample of ~1% of eggs from the parent generation of the release material was screened for Wolbachia by qualitative PCR Taqman assay on a Roche LightCycler 480. The pre-specified minimum acceptable Wolbachia prevalence was 97%. In each gonotrophic cycle, nine blood-fed female mosquitoes per human volunteer were tested for infection with DENV-1–4, chikungunya and Zika virus by qRT-PCR as previously described 23.
For QA of the release material, eggs from 10 oviposition strips were hatched each release week, with a minimum threshold hatch rate of 80%. Each release week, 10% of all release containers were selected randomly for QA, to estimate numbers of mosquitoes released. Eggs from these QA containers were manually counted under a microscope prior to field deployment. Release success was evaluated in the same QA containers at the time of servicing two weeks later (see below). A container was classified as ‘failed’ if: 1) it was lost; 2) it was dry; 3) pupae skin n<25; 4) dead adult mosquitoes >10; 5) wing carcass >20; or 6) remaining larvae and pupae >30.
Deployment
Wolbachia-carrying mosquitoes were released as eggs using mosquito release containers (MRCs). These were 2-litre plastic buckets each containing one oviposition strip with 100–150 eggs, Tetra Pleco Wafers fish food (Tetra GmbH, Germany), and 1 litre of water. MRCs were covered and placed outside houses, protected from direct sun and rain. Holes drilled near the top of the bucket walls allowed adult mosquitoes to escape. Releases occurred between August 2016 and March 2017, with 13–15 rounds of releases in each kelurahan. Releases stopped in each kelurahan when the prevalence of Wolbachia in field-caught mosquitoes was >60% for three consecutive weeks releases ( Table 2). MRCs were reset every two weeks. An MRC was placed in 1–2 randomly selected locations within each 50×50 m grid square across the intervention area. Permission was obtained from property owners to place MRCs on private property.
Table 2. Summary of Wolbachia deployments in seven urban villages.
| Kelurahan
(urban village) |
Total area
(km 2) |
Release start and end
date |
# release
rounds |
Mean release
points per round (min, max) |
Mean estimated
mosquitoes released per round (st dev) a |
|---|---|---|---|---|---|
| Kricak | 0.84 | 15 Aug 2016 - 13 Feb 2017 | 15 | 315 (280, 362) | 23 756 (5337) |
| Pakuncen | 0.64 | 22 Aug 2016 - 6 Mar 2017 | 14 | 265 (193, 357) | 20 766 (9003) |
| Patangpuluhan | 0.45 | 24 Aug 2016 - 8 Mar 2017 | 14 | 189 (169, 233) | 14 401 (4440) |
| Tegalrejo | 0.82 | 7 Sept 2016 - 8 Mar 2017 | 13 | 260 (228, 312) | 18 786 (3852) |
| Bener | 0.59 | 5 Sept 2016 - 6 Mar 2017 | 13 | 126 (123, 133) | 9005 (1687) |
| Karangwaru | 0.77 | 15 Sept 2016 - 15 Mar 2017 | 13 | 269 (238, 314) | 21 564 (4426) |
| Wirobrajan | 0.79 | 18 Sept 2016 - 13 Mar 2017 | 13 | 260 (232, 320) | 19 691 (3949) |
a The number of mosquitoes released each round in each kelurahan was estimated from the mean number of eggs in the mosquito release containers (MRCs) randomly selected for quality assurance (QA), multiplied by the number of successful release containers in that kelurahan. Shown here is the mean number (and standard deviation) of mosquitoes released per release round, in each kelurahan.
Monitoring
Prevalence of Wolbachia in the local Ae. aegypti population was monitored by weekly collection of adult mosquitoes via a network of 89 BG Sentinel traps (Biogents, Germany). The median (range) trap density was 15.7 (13.0–20.0) BG/km 2 in the intervention area and 3.6 (2.0–4.0) BG/km 2 in the non-release area. Mosquitoes were demobilised at -20°C for ≥1 hour, then identified by morphological features. The number of mosquitoes caught in each BG trap was recorded by species, sex, and in total. Ae. aegypti were stored at -20°C in 80% ethanol until Wolbachia screening.
Diagnostics
Field-caught Ae. aegypti were screened for wMel Wolbachia by qualitative PCR Taqman assay on a Roche LightCycler 480. The qPCR conditions consisted of a denaturation step at 95°C for 5 minutes followed by 45 cycles of PCR (denaturation at 95 °C for 10 seconds, annealing at 60 °C for 15 seconds, and extension at 72 °C for 1 second with the single acquisition) followed by a cooling down step at 40°C for 10 seconds. Specific primers targeting the gene encoding Ae aegypti Rps17 and wMel WD0513 were used as previously described 24, but with replacement of the Cy5-BHQ3 fluorophore-quencher pair in the wMel probe with the fluorophore-quencher LC640-IowaBlack (Integrated DNA technologies) 25. Testing was weekly when Wolbachia prevalence was <80%; two-weekly when ≥80% and four-weekly when ≥90%.
Epidemiological data
Data on hospitalized dengue haemorrhagic fever (DHF) cases (ICD-10 code A91; International Classification of Diseases 10th revision) were obtained from the Yogyakarta District Health Office (DHO), for January 2006–September 2019. Data on hospitalised dengue fever (DF) cases (ICD-10 code A90) were also obtained for January 2017–September 2019, as DF notification only began in 2017. Data were monthly case counts by kelurahan of residence. Population data were obtained from the Indonesian Bureau of Statistics. Anonymised results of dengue rapid diagnostic tests (RDTs) (SD Bioline Dengue Duo, Abbott, USA) performed in 18 primary health care clinics across Yogyakarta City from March 2016–September 2019 were obtained directly from the clinics. RDT results were extracted for participants resident in the quasi-experimental study intervention or control area, based on the kelurahan of residence where available, otherwise the kelurahan of clinic location.
Statistical analysis
The crude dengue incidence rate ratio in the pre-intervention and post-intervention periods was calculated as the aggregate number of DHF cases divided by the aggregate person-months, in the intervention area versus the control area. Crude dengue incidence was similarly calculated using an endpoint that included both DF and DHF case notifications, but only in the post-intervention period due to the lack of DF reporting prior to January 2017. Spearman’s rank correlation test was used to evaluate the correlation in monthly notified dengue incidence between the intervention and control areas, in the pre-intervention period. The Wolbachia intervention effect was estimated using controlled interrupted time series analysis 17, 18, 20. Negative binomial regression was used to model monthly DHF case counts in the aggregate intervention and control areas, with an offset for population size. The primary analysis, defined a priori, included data from January 2006 until March 2019, two years post-release. A secondary analysis included an additional six months of data to September 2019. Annual population estimates were used for 2006–2013 and 2015–2017; 2013 population estimates were used for 2014 and 2017 estimates were used for 2018 and 2019, due to unavailability of data for those years. Seasonal variability in dengue incidence was controlled using 6-monthly flexible cubic splines. A binary ‘group’ variable indicated the study arm (intervention or control). A binary ‘treatment’ variable distinguished the pre-intervention period (up to the end of Wolbachia deployments in the last release area) and the post-intervention period, and the intervention effect was estimated from the interaction between the ‘group’ and ‘treatment’ variables. This allows explicitly for a level change in the outcome (dengue case incidence) in both intervention and control arms in the post-intervention period, for example in a scenario where other secular effects coincident with the Wolbachia deployments may have influenced dengue incidence independently of Wolbachia. The unavailability of DF data prior to January 2017 precluded an ITS analysis with a combined DF/DHF endpoint. Robust standard errors were used for all models. Fisher’s exact test was used to compare the proportion of patients with an NS1-positive RDT result between intervention and control areas, in the pre-intervention period and post- Wolbachia deploymnets. Analyses were performed using Stata® statistical software package version 14.2 (StataCorp, College Station, TX).
Power calculation
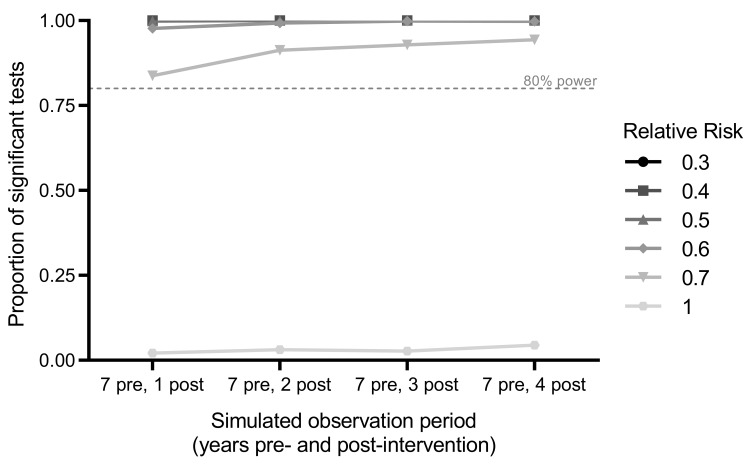
Power was estimated using 1000 simulated datasets drawn from a negative binomial distribution fitted to an 11-year time series (2006–2016) of monthly DHF case notifications from the intervention and control areas prior to Wolbachia deployment. Post-intervention time periods of 1, 2, 3 or 4 years were simulated, with the pre-intervention period fixed at 7 years (maximum total simulated time series of 11 years). Dengue case numbers were not modified for the untreated control area, and for the Wolbachia intervention area were either kept at baseline values (for the simulation at the null; i.e. relative risk (RR)=1) or reduced proportionately (for simulations of intervention effects of RR=0.7, 0.6, 0.5, 0.4, 0.3). For each of these five ‘true’ effect sizes, applied to each of the 1000 simulated time series, the ‘observed’ effect size was calculated from a negative binomial regression model of monthly case counts in the intervention and control areas, as described above. Power was estimated from the proportion of 1000 simulated scenarios in which a significant intervention effect (p<0.05) was observed ( Figure 3). With seven years of pre-intervention data and one year post-intervention follow-up, there was 80% power to detect a ≥30% reduction in dengue incidence (incidence rate ratio (IRR)=0.7). There was only a marginal gain in power from >1 year of follow-up, if the true reduction in dengue incidence is greater than 30%. Power calculations were performed using R version 3.5.0 (R Foundation for Statistical Computing, Austria) with the “MASS” package 26 used for estimation of the regression models.
Figure 3. Power estimation.
Power to detect a Wolbachia-associated reduction in dengue incidence using interrupted time series analysis was calculated as the proportion of significant results out of 1,000 simulations for varying post-intervention observation periods and relative risks.
Regulatory and ethics approval and consent
An independent risk assessment conducted by the Indonesia Ministry of Research, Technology and Higher Education in 2016 concluded that over the next 30 years, there is a negligible risk of harm as a result of releasing Wolbachia-infected Ae. aegypti 27.
Approval to release Wolbachia mosquitoes was obtained from the provincial and city governments of Yogyakarta prior to releases. Ethical approval was obtained from the Universitas Gadjah Mada Faculty of Medicine, Public Health and Nursing Ethics Committee (approval number KE/FK/105/EC/2016) to release Wolbachia mosquitoes, for blood-feeding the mosquito colony on human volunteers, and to access non-identifiable aggregate data on monthly notified DHF and DF case numbers from the Yogyakarta DHO. Approval to conduct the household surveys of community acceptance was also obtained from the UGM Ethics Committee (approval number KE/FK/1023/EX/2015). Written agreement to share dengue surveillance data and non-identifiable individual dengue RDT results from puskesmas clinics was obtained from Yogyakarta DHO.
Verbal and written consent was obtained from heads of households for participating in the baseline surveys, and for hosting a BG trap or MRC. BG hosts were compensated 50,000 IDR for the cost of powering traps.
Results
Wolbachia establishment
Longitudinal entomological monitoring demonstrated rapid establishment of Wolbachia in the intervention areas, a continuous increase in Wolbachia prevalence in trapped Ae. aegypti throughout the first year post-release, and persistence at a very high prevalence ever since ( Figure 4). The median Wolbachia prevalence was 73% (range 67–92%) one week after releases stopped, and 100% (96–100%) two years post-deployment. In the control areas, single Wolbachia-infected Ae. aegypti mosquitoes were detected on 11 occasions, but there has been no evidence of Wolbachia establishment.
Figure 4. Wolbachia infection prevalence in local Aedes aegypti mosquito populations.
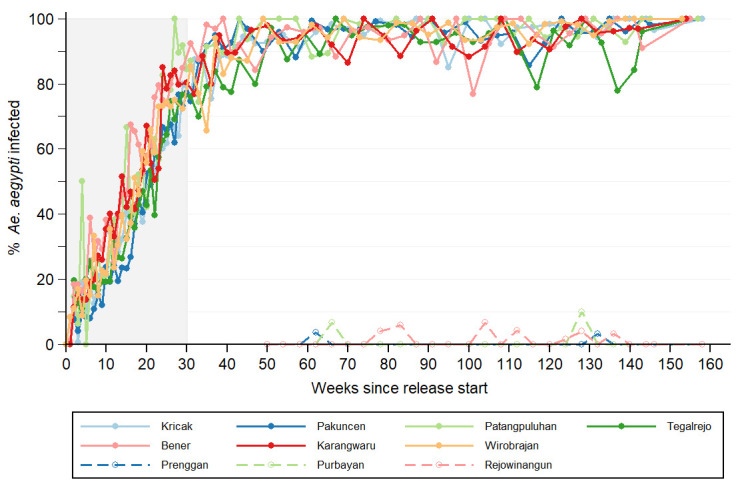
Lines show the percentage of Aedes aegypti collected from intervention areas (closed circles; solid line) and untreated control areas (open circles; dashed line) that were Wolbachia infected, each week since the start of deployments until September 2019. For the intervention areas, week 0 is the week in which deployment commenced (between 15 August and 18 September 2016, see Table 2). For the control areas, week 0 is the week in which the first deployments commenced in the intervention area (15 August 2016). Shaded area indicates release period.
Dengue incidence in intervention and control areas
In the decade prior to Wolbachia releases, dengue outbreaks occurred annually in both the intervention and control areas ( Figure 4). A median of 125 DHF cases were notified each year in the intervention area (range 65–308), corresponding to a median annual incidence of 169 per 100,000 population (range 98–477 per 100,000). In the control area, a median of 44 DHF cases were notified each year (range 19–159) corresponding to a median annual incidence of 130 per 100,000 (range 59–477 per 100,000). Per-capita dengue incidence in the intervention area was on average 15% higher than the control area during the pre-intervention period (crude IRR 1.15, p = 0.002). Monthly dengue incidence in the intervention and control areas was highly correlated over time (Spearman’s rho = 0.75, p<0.001), supporting the validity of the control area as a counterfactual for evaluating the epidemiological impact of Wolbachia releases ( Figure 5).
Figure 5. Dengue incidence in intervention and control areas, before and after the Wolbachia intervention.
Monthly notified dengue case incidence (per 100,000 population) in the intervention (solid line) and control (dashed line) areas before and after Wolbachia deployments, January 2006 - September 2019. Blue shading indicates the Wolbachia infection prevalence in Ae. aegypti collected from the intervention area.
Wolbachia-associated reduction in dengue incidence
During two years following the completion of Wolbachia deployments (April 2017–March 2019), 34 DHF cases were notified from the intervention area and 53 from the control area, corresponding to 67% lower crude incidence in the Wolbachia-treated area (26 vs 79 cases per 100,000 person-years; IRR = 0.33, p<0.001).
In an interrupted time series (ITS) analysis of monthly DHF case notifications January 2006 – March 2019, which adjusts for baseline differences between the intervention and control areas and seasonal and inter-annual time effects, this translates to a 73% reduction in notified DHF incidence (95% CI 49%,86%; p<0.001) associated with the Wolbachia intervention.
In an exploratory analysis including six months of additional post-intervention data to September 2019, the DHF incidence in intervention vs control areas was 30 vs 115 per 100,000 person-years (crude IRR = 0.26, p<0.001) which translates in the regression model to a 76% reduction in dengue incidence (95% CI 60%,86%; p<0.001) associated with the Wolbachia intervention
During the first 2.5 years post-intervention (April 2017–September 2019), 97 cases (DF + DHF) were notified from the intervention area and 137 from the control area (60 vs 163 per 100,000 person-years; IRR = 0.37, p<0.001) corresponding to 63% lower crude incidence in the Wolbachia-treated area, using a combined endpoint of DF and DHF case notifications. The lack of pre-release data on hospitalised DF cases precluded an ITS analysis with this combined endpoint.
Dengue diagnostic findings in intervention and control areas
Prior to Wolbachia deployments (March 2016 – March 2017), there was no difference between intervention and control areas in the proportion of tested patients with an NS1-positive RDT result (127/447 (28%) in intervention area vs 106/408 (26%) in control area; Fisher’s exact p=0.44; Figure 5; raw data available on Figshare 32). By comparison, in the post-intervention period (April 2017–September 2019) NS1 positivity was significantly lower in the intervention area than the control area (6/568 (1%) vs 61/429 (14%); Fisher’s exact p<0.001; Figure 6).
Figure 6. Laboratory-confirmed dengue cases in intervention and control areas, before and after the Wolbachia intervention.
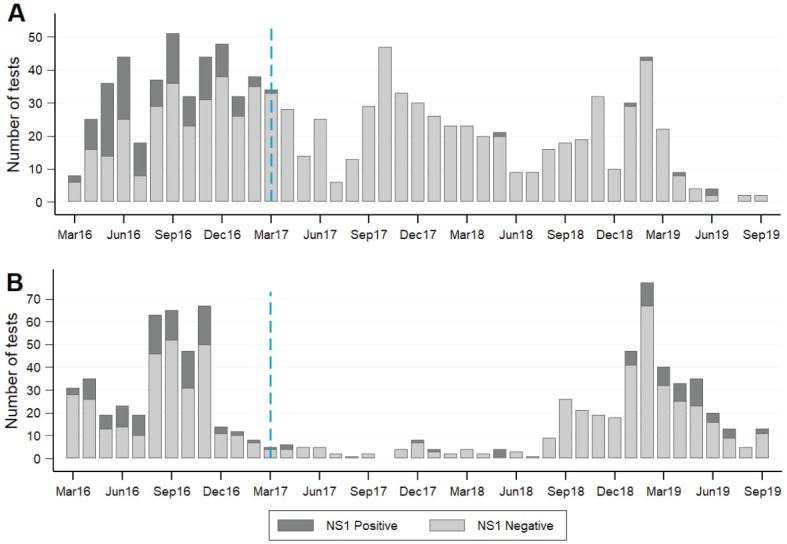
Dengue rapid diagnostic test results for patients presenting to primary care clinics in the intervention ( A) and control ( B) areas. Standard Diagnostics Dengue Duo rapid diagnostic kits for the detection of dengue virus NS1 antigen and IgM/IgG antibody were available in primary care clinics throughout Yogyakarta city from March 2016, and were used at the discretion of clinic staff as part of routine clinical care. Monthly counts of positive and negative results for DENV NS1 antigen, as recorded by clinic staff, were aggregated for all patients resident in the intervention area or control area. The blue dashed line indicates the completion of Wolbachia releases in the intervention area in March 2017.
Discussion
Enabled by a successful community engagement campaign, release of wMel Wolbachia-carrying mosquitoes throughout an urban community of 65,000 people in Yogyakarta City, Indonesia, resulted in rapid introgression and durable establishment of Wolbachia in the local Ae. aegypti population. Predefined analysis of public health surveillance data on dengue case notifications demonstrated a 73% reduction in DHF incidence in the intervention area during the 2 years after Wolbachia deployment, compared to a counterfactual untreated control area with a comparable historical incidence of disease. These epidemiological data from a dengue endemic setting are consistent with previous positive public health findings in Australia 17, 18.
Nazni et al. recently reported on wAlbB establishment in Ae. aegypti in Kuala Lumpur, Malaysia including results of an exploratory analysis suggestive of a modest reduction (40%, 95%CI 5–65%) in dengue incidence in release areas compared to several untreated control areas 28. Nazni et al. elected to use the wAlbB strain on the basis of a better thermostability profile than wMel in the mosquito larval life stages. However we found no evidence that wMel establishment was compromised in the climatic conditions of Yogyakarta. Elsewhere Ross et al. reported that heatwaves have only transient effects on wMel frequencies in Australia 29. Evidence from pilot field studies is likely the best method for selecting the optimal Wolbachia strain for deployment in a given setting.
By chance, the commencement of Wolbachia releases in the quasi-experimental study area in late 2016 coincided with the largest dengue epidemic on record in Yogyakarta City. Unsurprisingly, dengue case notifications to the District Health Office from across Yogyakarta City were then lower during 2017 and 2018 than in any two-year period in the previous 25 years, and it is notable that a highly significant Wolbachia-associated effect was observed even during this period of record low dengue transmission. A resurgence in dengue incidence was seen throughout southeast Asia in 2019 30, including in Indonesia. Our exploratory analysis including an additional six months of observations after the a priori analysis time point of March 2019 showed a small strengthening of the intervention effect and tighter confidence intervals around the point estimate. Evidence of field effectiveness will continue to accumulate throughout subsequent dengue epidemic seasons. Our a priori analysis plan includes re-estimation of the intervention effect every 12 months, until five years post-intervention.
The dengue case time series used to evaluate the effectiveness of wMel Wolbachia included only hospitalised patients with a clinical diagnosis of DHF, which was historically the case definition for mandatory notification in Indonesia. Using additional notifications data available since January 2017 for patients hospitalised with a clinical diagnosis of dengue fever (DF), we saw moderate attenuation of the intervention effect compared with the endpoint of DHF alone (63% vs 74% lower crude incidence in intervention area). Rather than a real interaction between the intervention effect and disease severity, we hypothesise that this reflects the reduced specificity of the DF clinical case definition compared with DHF, and the limited usage of confirmatory diagnostic tests; i.e. a greater proportion of notified DHF cases than DF cases represent true DENV infections. The hospitalised dengue patient population represents a more severe clinical subgroup of a much larger clinical burden, most of which is not counted in disease surveillance systems. Estimates of expansion factors from notified hospitalised cases to the true case burden in Indonesia range from 7–11 31. The AWED CRCT currently underway in Yogyakarta will address whether the incidence of ambulatory dengue cases, who are usually not detected by passive dengue case surveillance systems, is also reduced by Wolbachia.
The results reported here are consistent with mathematical modelling projections . Those predictions suggested that wMel introgression would eliminate DENV transmission in most endemic settings for over a decade 7, 32. A recent modelling study estimated that Indonesia had 7.8 million (95% uncertainty interval 1.8–17.7 million) symptomatic dengue cases in 2015, which were associated with 332,865 (94,175–754,203) lost disability-adjusted life years 16. The authors estimated that a nationwide wMel Wolbachia campaign could avert a large proportion of this burden (86.2%, 36.2–99.9%), with elimination predicted in low transmission settings. There are several reasons why elimination of DHF case notifications in the Wolbachia intervention area was not observed in the present study, and was likely infeasible. First, the geographic scale of the intervention area (~5 km 2) coupled with the mobility of the resident population means that some notified cases could have acquired their DENV infection at a location outside of the Wolbachia-treated area. Second, the specificity of DHF case reporting is likely to be imperfect, such that some DHF case notifications were not true dengue cases. Third, the intervention may not be entirely efficacious in blocking transmission of all DENV serotypes across the spectrum of transmission intensities that occurred during the period of observation.
Our study had limitations. The short-term, but open-label nature of releasing Wolbachia-infected mosquitoes means it is plausible that community awareness altered healthcare seeking behaviour, leading to differences in DHF notifications. However, we believe this is unlikely to explain such a large and prolonged difference in hospitalised DHF incidence. We also cannot exclude a concurrent change in dengue control practices between the intervention and control areas, although we are not aware of any such alterations to standard practice. Due to the pragmatic nature of the study we did not determine the DENV serotypes circulating during the study period nor the incidence of other Ae. aegypti-borne diseases like Zika and chikungunya. The AWED CRCT currently underway in Yogyakarta City 21 should provide further detailed evidence on the impact of wMel Wolbachia on the incidence of dengue, individual DENV serotypes and other arboviral diseases.
This study demonstrated the feasibility, acceptability and positive public health impact of the wMel Wolbachia introgression method in a community of 65,000 people in Yogyakarta, Indonesia. This represents the first field evidence from an endemic setting of the effectiveness of this novel strategy in reducing dengue incidence, and is consistent with previous results from northern Australia where Wolbachia deployments have resulted in the effective elimination of local dengue transmission. Additional epidemiological evidence from this quasi-experimental study, and also the AWED trial, will accumulate during 2020/21. Continued optimisation of Wolbachia deployments plus additional long-term safety and efficacy data from a range of ecological and transmission settings will further enhance the attractiveness of this approach for disease control efforts.
Data availability
Underlying data
Figshare:
Figure 2_IRtesting.xlsx https://doi.org/10.6084/m9.figshare.12026943.v3 33
Figure 3_PowerPlot.xlsx https://doi.org/10.6084/m9.figshare.12026937.v3 34
Figure 4_MosquitoCollections.xls https://doi.org/10.6084/m9.figshare.12026940.v3 35
Figure 5_6_DengueCaseData.xls https://doi.org/10.6084/m9.figshare.12199688.v3 36
Wolbachia community acceptance survey. https://doi.org/10.6084/m9.figshare.12199754.v1 37
The dengue case notification data and NS1 rapid diagnostic test results were provided to us by the Yogyakarta District Health Office and individual Yogyakarta puskesmas clinics for the purpose of the current study, and the conditions of the release of the raw data do not permit further sharing to a third party. Data aggregated by month and kelurahan is available from the Figshare link above. Applications for access to raw data for research purposes or data reanalysis can be made directly to the Head of the Yogyakarta City Health Office at kesehatan@jogjakota.go.id.
Data deposited with Figshare are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Reporting guidelines
STROBE checklist for 'Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis' https://doi.org/10.6084/m9.figshare.12026955.v2 38
Acknowledgements
We gratefully acknowledge the contributions of the staff in the Disease Control Division at Yogyakarta City Health Office for providing surveillance data; staff at the Yogyakarta puskesmas clinics for providing rapid diagnostic test result data; community members for their support and participation, particularly their contribution to mosquito releases and collections; provincial, district and kelurahan leaders for their support for this project; Iwan Dwi Prahasto for his contribution to the epidemiological study design; and all members of the World Mosquito Program (WMP) Yogyakarta and Global teams who contributed to the planning and implementation of the quasi experimental study.
Funding Statement
This work was funded by the Tahija Foundation, Indonesia. WMP Global is funded in part by the Bill and Melinda Gates Foundation (OPP1180815).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Stanaway JD, Shepard DS, Undurraga EA, et al. : The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–723. 10.1016/S1473-3099(16)00026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. L'Azou M, Moureau A, Sarti E, et al. : Symptomatic Dengue in Children in 10 Asian and Latin American Countries. N Engl J Med. 2016;374(12):1155–1166. 10.1056/NEJMoa1503877 [DOI] [PubMed] [Google Scholar]
- 3. Shepard DS, Undurraga EA, Halasa YA, et al. : The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–941. 10.1016/S1473-3099(16)00146-8 [DOI] [PubMed] [Google Scholar]
- 4. Indriani C, Ahmad RA, Wiratama BS, et al. : Baseline Characterization of Dengue Epidemiology in Yogyakarta City, Indonesia, before a Randomized Controlled Trial of Wolbachia for Arboviral Disease Control. Am J Trop Med Hyg. 2018;99(5):1299–1307. 10.4269/ajtmh.18-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xi Z, Khoo CC, Dobson SL: Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310(5746):326–328. 10.1126/science.1117607 [DOI] [PubMed] [Google Scholar]
- 6. Carrington LB, Tran BCN, Le NTH, et al. : Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2018;115(2):361–366. 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferguson NM, Kien DT, Clapham H, et al. : Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7(279):279ra237. 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, et al. : A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 9. Walker T, Johnson PH, Moreira LA, et al. : The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476(7361):450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 10. Aliota MT, Peinado SA, Velez ID, et al. : The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. 10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aliota MT, Walker EC, Uribe Yepes A, et al. : The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl Trop Dis. 2016;10(4):e0004677. 10.1371/journal.pntd.0004677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caragata EP, Dutra HL, Moreira LA: Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb Cell. 2016;3(7):293–295. 10.15698/mic2016.07.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutra HL, Rocha MN, Dias FB, et al. : Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19(6):771–774. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Hurk AF, Hall-Mendelin S, Pyke AT, et al. : Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6(11):e1892. 10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cattarino L, Rodriguez-Barraquer I, Imai N, et al. : Mapping global variation in dengue transmission intensity. Sci Transl Med. 2020;12(528): pii: eaax4144. 10.1126/scitranslmed.aax4144 [DOI] [PubMed] [Google Scholar]
- 16. O'Reilly KM, Hendrickx E, Kharisma DD, et al. : Estimating the burden of dengue and the impact of release of wMel Wolbachia-infected mosquitoes in Indonesia: a modelling study. BMC Med. 2019;17(1):172. 10.1186/s12916-019-1396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Neill SL, Ryan PA, Turley AP, et al. : Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2019;2:36. 10.12688/gatesopenres.12844.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryan PA, Turley AP, Wilson G, et al. : Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. [version 2; peer review: 2 approved] Gates Open Res. 2020;3:1547. 10.12688/gatesopenres.13061.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tantowijoyo W, Andari B, Arguni E, et al. : Stable establishment of wMel Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl Trop Dis. 2020;14(4):e0008157. 10.1371/journal.pntd.0008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernal JL, Cummins S, Gasparrini A: Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–355. 10.1093/ije/dyw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anders KL, Indriani C, Ahmad RA, et al. : The AWED trial (Applying Wolbachia. to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial. Trials. 2018;19(1):302. 10.1186/s13063-018-2670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization: Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: WHO;2013 Reference Source [Google Scholar]
- 23. Quyen NTH, Kien DTH, Rabaa M, et al. : Chikungunya and Zika Virus Cases Detected against a Backdrop of Endemic Dengue Transmission in Vietnam. Am J Trop Med Hyg. 2017;97(1):146–150. 10.4269/ajtmh.16-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeap HL, Axford JK, Popovici J, et al. : Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors. 2014;7:58. 10.1186/1756-3305-7-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dar M, Giesler T, Richardson R, et al. : Development of a novel ozone- and photo-stable HyPer5 red fluorescent dye for array CGH and microarray gene expression analysis with consistent performance irrespective of environmental conditions. BMC Biotechnol. 2008;8:86. 10.1186/1472-6750-8-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venables WN, Ripley BD: Modern Applied Statistics with S. Fourth edition ed. New York: Springer;2002. 10.1007/978-1-4757-3121-7 [DOI] [Google Scholar]
- 27. Ministry of Research Technology and Higher Education Indonesia: Risk assessment on the release of Wolbachia-infected Aedes Aegypti .2017. Reference Source [Google Scholar]
- 28. Nazni WA, Hoffmann AA, NoorAfizah A, et al. : Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr Biol. 2019;29(24):4241–4248.e5. 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ross PA, Axford JK, Yang Q, et al. : Heatwaves cause fluctuations in wMel Wolbachia. densities and frequencies in Aedes aegypti. PLoS Negl Trop Dis. 2020;14(1):e0007958. 10.1371/journal.pntd.0007958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European Centre for Disease Prevention and Control: Dengue worldwide overview. Situation update, 17 January2020. Reference Source [Google Scholar]
- 31. Nealon J, Taurel AF, Capeding MR, et al. : Symptomatic Dengue Disease in Five Southeast Asian Countries: Epidemiological Evidence from a Dengue Vaccine Trial. PLoS Negl Trop Dis. 2016;10(8):e0004918. 10.1371/journal.pntd.0004918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dorigatti I, McCormack C, Nedjati-Gilani G, et al. : Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol. 2018;34(2):102–113. 10.1016/j.pt.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tantowijoyo W: Figure 2_IRtesting.xlsx. figshare.Dataset.2020. 10.6084/m9.figshare.12026943.v3 [DOI] [Google Scholar]
- 34. Dufault S, Anders K: Figure 3_PowerPlot.xlsx. figshare.Dataset.2020. 10.6084/m9.figshare.12026937.v3 [DOI] [Google Scholar]
- 35. Tantowijoyo W: Figure 4_MosquitoCollections.xls. figshare.Dataset.2020. 10.6084/m9.figshare.12026940.v3 [DOI] [Google Scholar]
- 36. Indriani C, Anders K: Figure 5_6_DengueCaseData. figshare.Dataset.2020. 10.6084/m9.figshare.12199688.v3 [DOI] [Google Scholar]
- 37. Indriani C: Wolbachia community acceptance survey. figshare.Dataset.2020. 10.6084/m9.figshare.12199754.v1 [DOI] [Google Scholar]
- 38. Anders K: STROBE checklist for ‘Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis’. figshare.Figure.2020. 10.6084/m9.figshare.12026955.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]