Abstract
Clinical trials represent an essential component of improving treatment for substance use disorders (SUD). The SARS coronavirus-2 pandemic disrupted our ongoing clinical trial of smoking cessation and forced us to rapidly implement changes to assure participants access to ongoing counseling and monitoring via telephone calls and/or video chat sessions. Our experiences suggest that this pandemic will lead to changes for both future clinical trial participants and project staff. While challenges remain, it will be important to assessing the impact of these changes with regard to participant experiences and treatment outcomes.
1. Background
Clinical trials focused on improving treatment for substance use disorders (SUD), including smoking cessation, are challenging and require substantial fiscal resources, extended follow-up time periods, and continued attention to recruitment, retention and staffing (Greenfield et al., 2014). For many seeking treatment, particularly vulnerable and underserved populations, clinical trials represent an essential source of assistance in their quit attempt. The rapid global spread of SARS coronavirus-2 (e.g., SARS CoV-2, also referred to as COVID19) in late 2019 internationally and early 2020 within the United States, resulted in the profound disruption of clinical research. A recent survey of 353 younger adult, mostly white, male dual users of combusted tobacco and electronic cigarettes noted high levels of concern regarding health risks associated with SARS-CoV-2 but mixed behavior changes with about 25% reporting decreased tobacco use and about 30% reporting increased tobacco use (Klemperer, West, Peasley-Miklus, & Villanti, 2020). Therefore, it is imperative that the research community adapt to this ‘new normal’ in order to continue providing treatment and advancing the science of behavior change.
Our on-going randomized clinical trial examines the impact of an alternative varenicline dosing strategy to enhance rates of cessation among treatment seeking adult cigarette smokers (https://clinicaltrials.gov/ct2/show/study/NCT03262662). The project involves prescreening by phone followed by a baseline visit to determine eligibility; enrolled subjects are randomized to one of two varenicline dosing regimens, complete 2 lab visits, and attend 6 clinic visits and 2 phone calls during which measures are completed and brief individual cessation counseling is delivered. Study outcomes include bio-verified continuous abstinence at end-of-treatment and at 6 months. As of February 2020, recruitment to this trial was slightly ahead of schedule.
2. Adjusting to the covid19 situation
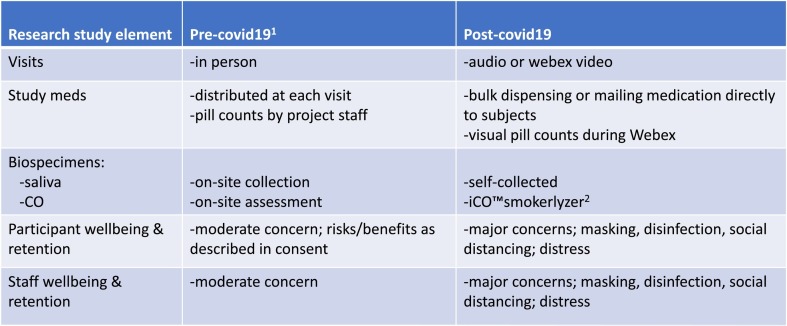
On 3/22/2020, the “New York State PAUSE” executive order, which closed nonessential businesses and decreed social distancing, was enacted. Thus, we were forced to implement a series of logistical changes to our project over an interval of 2 weeks in March 2020 following this executive order. Shortly after making these changes, all clinical trial recruitment was suspended by our academic health center. Following investigators' meetings, changes were implemented to assure active research participants had continued access to study resources such as study drug, safety monitoring, and counseling (Fig. 1 ).
Fig. 1.
Changes to an on-going smoking cessation clinical trial before and after start of viral pandemic.
Over a 2-week period, project staff updated data acquisition capabilities to support online completion of study measures using secure links sent to participants via email or text messages. Recognizing disparities in access to smartphones, laptops and desktop computers, each participant was provided with a series of labeled envelopes, each identified by clinic visit and scheduled date, which contained paper copies of study measures. These envelopes also contained any relevant counseling materials, study medications and kits for saliva self-collection (instructions, supplies, a pre-addressed and pre-stamped return mailer). In-person clinic visits were conducted via the zoom virtual meeting software application (audio and video) and by phone (audio only). Participants were provided with all of their study medications as we were uncertain whether we would have access to research buildings and mailrooms for periodic mailings. Pill counts of study medication are completed visually during video chat sessions (e.g., zoom) when possible and verbally when video in unavailable.
Fortunately, we were able to complete a final in-person clinic visit with each participant and provide ongoing counseling. Participants received a canvas bag containing envelopes, labeled by study visit and date, for pending visits. The contents of the bag were reviewed, saliva collection kits were introduced, and arrangements were made for either video chat or telephone follow-up with each participant.
3. Implications for future clinical research
The SARS CoV-2 pandemic will lead to long lasting changes in how clinical trials of smoking cessation and other SUDs are conducted. As noted in Fig. 1, both participants and project staff will have increased concerns regarding the safety of face-to-face interactions. Accordingly, the number of in-person visits is likely to substantially decrease and will be replaced with virtual visits. The “new normal” for in-person visits for the foreseeable future is likely to be “mask-to-mask” interactions, symptoms and temperature screening, frequent hand washing, continued attention to disinfection of hard surfaces, and social distancing.
Although some participants view virtual visits as less burdensome as it avoids time and travel to attend in-person visits, others may view visits from home as intrusive. Also, virtual visits may represent a potential barrier to subgroups without access to the technology including minority and lower income populations. In addition, smoking cessation studies generally enroll older persons who may be less comfortable with technology making it difficult to transition to a digital platform. It is unclear how moving from in-person to virtual counseling impacts the level of engagement and future research by our team will explore participant experiences.
Data collection and management for this project is based on REDCap, which allows for surveys to be sent to participants via emails and smartphones. Various data management platforms for clinical research are available; REDCap software is free, while others such as MedidataRave and qualtrex require a subscription. Moreover, our clinical trial has used REDCap from the beginning and we acknowledge that transitioning to a new data management during the SARS-CoV-2 pandemic would have been especially burdensome.
The experience of project staff interactions with research subjects is also likely to change; some staff may find a lower level of direct interaction to be less rewarding. Project staffing will have to accommodate the ability to work from remote locations. Staff on this project needed to be trained on revised methods of documenting counseling, collecting pill counts, observing saliva collection and bioverification of cessation, as well as learning protocols related to sanitizing and disinfection.
The impact of other changes is more difficulty to assess. Many SUD clinical trials make use of group visits for consenting, orientations, and/or counseling, as well as individual visits with clinicians to review medical histories. It is unclear how these more traditional approaches can be adapted to meet social distancing standards and/or transitioned to a video meeting format. Regulatory agencies have relaxed certain requirements (e.g., allowing e-consenting using Redcap to sign informed consent forms and video to enable consent discussions; shipping of medications to subjects) making these shifts possible. However, it remains unclear whether these statutes will be permanent.
Other clinical trials procedures appear to be less compatible with a shift to virtual platforms. For example, cognitive testing cannot transition to a new platform mid-study because of a lack of comparability to existing data collection and blood draws and other biospecimen collection remain problematic. Another ongoing challenge is the remote assessment of smoking status using carbon monoxide (CO) measurement. Due to the very limited time frame available, we were not able to transition to use of the iCO™ smokerlyzer (Bedfont, Ltd) for CO measurements via a smart phone connection. The need for flexible, scalable strategies is essential and highlights the potential role of mobile health (mHealth) interventions delivered via smartphone (Vilardaga, Casellas-Pujol, McClernon, & Garrison, 2019). It is likely that our own experiences, and those of others, will contribute to innovations in our ability to conduct clinical research, though we also believe this will require systematic assessment of these changes and whether the rigor of our research is maintained.
Based on our experiences, we offer several recommendations for others conducting SUD clinical trials: develop a protocol outlining the rapid transition from in-person to phone or internet-based follow-up visits ahead of time and make participants aware of this possibility, provide participants with smart phones from which on-line surveys and internet sessions can be conducted, train participants on use of Zoom, or other similar meeting platforms, train participants on completion of on-line surveys, train participants on biospecimen collection and provide hard copies of surveys along with pre-addressed post-paid mailers for the return of completed paper surveys and biospecimens.
In summary, the SARS CoV-2 pandemic forced us to implement changes in our ongoing smoking cessation clinical trial. While we are continuing to explore the impact of these changes, we are hopeful that we can incorporate many of these changes into our ongoing and future research activities. Moreover, randomized clinical trials are often criticized because they are rigid, inflexible, and do not reflect real-world practice, this experience suggests that it doesn't have to be that way. The SARS-CoV-2 pandemic highlights that clinical trials can change, and they can change rapidly – suggesting that if we're willing to put in the work there is more lemonade to be extracted from this situation.
Funding
Supported in part by National Cancer Institute grants R01CA206193 and P30CA016056.
References
- Greenfield S.F., Crisafulli M.A., Kaufman J.S., Freid C.M., Bailey G.L., Connery H.S.…Rodolico J. Implementing substance abuse group therapy clinical trials in real-world settings: Challenges and strategies for participant recruitment and therapist training in the Women’s Recovery Group Study. The American Journal on Addictions. 2014;23(3):197–204. doi: 10.1111/j.1521-0391.2014.12099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemperer E.M., West J.C., Peasley-Miklus C., Villanti A.C. Change in tobacco and electronic cigarette use and motivation to quit in response to COVID-19. Nicotine & Tobacco Research. 2020 doi: 10.1093/ntr/ntaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga R., Casellas-Pujol E., McClernon J.F., Garrison K.A. Mobile applications for the treatment of tobacco use and dependence. Current Addiction Reports. 2019;6(2):86–97. doi: 10.1007/s40429-019-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]