Abstract
Objective
To develop a more comprehensive description of multisystem inflammatory syndrome in children (MIS-C), a novel syndrome linked to severe acute respiratory syndrome coronavirus 2, by conducting a systematic analysis of studies from different settings that used various inclusion criteria.
Study design
MIS-C studies were identified by searching PubMed and Embase as well as preprint repositories and article references to identify studies of MIS-C cases published from April 25, 2020, through June 29, 2020. MIS-C study metadata were assessed and information on case demographics, clinical symptoms, laboratory measurements, treatments, and outcomes were summarized and contrasted between studies.
Results
Eight studies were identified representing a total of 440 MIS-C cases. Inclusion criteria varied by study: 3 studies selected patients diagnosed with Kawasaki disease, 2 required cardiovascular involvement, and 3 had broader multisystem inclusion criteria. Median age of patients by study ranged from 7.3 to 10 years, and 59% of patients were male. Across all studies, the proportion of patients with positive results for severe acute respiratory syndrome coronavirus 2 reverse transcriptase-polymerase chain reaction tests ranged from 13% to 69% and for serology, from 75% to 100%. Patients with MIS-C had high prevalence of gastrointestinal (87%), dermatologic/mucocutaneous (73%), and cardiovascular (71%) symptoms. Prevalence of cardiovascular, neurologic, and respiratory system involvement significantly differed by study inclusion criteria. All studies reported elevated C-reactive protein, interleukin-6, and fibrinogen levels for at least 75% of patients in each study.
Conclusions
This systematic review of MIS-C studies assists with understanding this newly identified syndrome and may be useful in developing a refined, universal case definition of MIS-C.
Abbreviations: COVID-19, Coronavirus disease 2019; IVIG, Intravenous immunoglobulin; MIS-C, Multisystem inflammatory syndrome in children; RT-PCR, Reverse transcriptase-polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
The World Health Organization (WHO) was first notified of a cluster of unexplained pneumonia cases in Wuhan, China, on December 31, 2019. Over the first 6 months of 2020, coronavirus disease 2019 (COVID-19) became a global pandemic, with more than 10 million confirmed cases and more than 500 000 deaths by the end of June.1 Although the causative agent of COVID-19—severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—has proven difficult to contain, infection in children and adolescents has generally resulted in more mild or asymptomatic cases compared with adults.2, 3, 4
On April 25, 2020, the United Kingdom's National Health Service alerted physicians of a newly recognized syndrome with severe multisystem inflammation in children with clinical features similar to those found in Kawasaki disease and toxic shock syndrome.5 The new syndrome was temporally associated with COVID-19, with the development of symptoms typically about 3-4 weeks after escalation of geographically local COVID-19 incidence. Subsequent reports from other European countries and the US identified additional cases of children exhibiting a hyperinflammatory syndrome with multisystem involvement; many of the patients had laboratory confirmation of current or previous SARS-CoV-2 infection through reverse transcriptase-polymerase chain reaction (RT-PCR) or serologic tests.6 The first published study of multisystem inflammatory syndrome in children (MIS-C) described 8 children from the United Kingdom with hyperinflammatory shock7; since then, several additional descriptive studies have contributed a wider breadth of knowledge about the clinical picture for the syndrome, now referred to as MIS-C.8, 9, 10
Health officials have adjusted the clinical and epidemiologic criteria used to identify cases as the understanding of MIS-C has evolved. MIS-C is a novel syndrome for which no confirmatory diagnostic test exists. Clinical practice, including diagnostic criteria, for MIS-C have varied across time and setting. Therefore, studies of MIS-C may substantially differ in case inclusion criteria, laboratory values tested, and treatments administered. The purpose of this systematic review is to compare and summarize currently published studies to provide an overview of the clinical picture of MIS-C that accounts for these different settings and case inclusion criteria from different settings.
Methods
Literature searches were conducted using PubMed and Embase for papers published from April 25, 2020, through June 29, 2020. Given the novelty of MIS-C, preprint manuscripts uploaded to medRxiv and bioRxiv servers during the same time frame were also searched. Due to the rapidly evolving understanding of MIS-C, a variety of search terms were used to identify reports of MIS-C cases. The search terms included the following phrases or combinations of phrases “multisystem inflammatory syndrome”; “Kawasaki disease”; “hyperinflammatory”; “hyperinflammation”; “toxic shock syndrome”; “shock” with “children” or “pediatric”; “cytokine” with “children” or “pediatric”; “inflammation” or “inflammatory” with “syndrome” and “children” or “pediatric.”
Studies on MIS-C cases have used different names for the syndrome and used different criteria to identify cases. To ensure that no studies were missed through key word searches, Google Scholar was used to search through any studies that cited 2 early, impactful publications on MIS-C.7 , 10
Papers identified through all searches were examined to determine whether they were studies describing patients with clinical and epidemiologic characteristics consistent with MIS-C as defined by the UK Royal College of Paediatrics and Child Health, New York State Department of Health, the US Centers for Disease Control and Prevention,11 or the WHO.12 Studies describing patients fitting the following criteria were included in the analysis: (1) young age (typically <21 years old); (2) severe cardiovascular or multisystem clinical manifestations; (3) laboratory evidence of inflammation; and (4) either laboratory evidence of SARS-CoV-2 infection or epidemiologic association with COVID-19 (including temporal association with periods of high local COVID-19 transmission). In addition, given the substantial clinical overlap between MIS-C and Kawasaki disease, another syndrome without a diagnostic test, studies describing patients with Kawasaki disease with laboratory or epidemiologic links to SARS-CoV-2 were included in this systematic review.
Only studies that described at least 5 MIS-C cases were included, and studies with MIS-C patients largely or entirely described in other publications were excluded. We used information on setting (dates of case identification and health care setting) to identify potential duplication of patients between studies, following up with study authors for additional information. Data collected from the papers included case inclusion criteria, demographic information, clinical signs/symptoms and outcomes, laboratory markers of inflammation and cardiovascular function, and treatments administered. Studies that did not sufficiently describe the aforementioned data were excluded. Median and IQR for laboratory values were captured if reported by each study, along with the proportion of patients with abnormal values using reference ranges reported for each study. For studies that reported median and IQR but not the proportion with abnormal values, the range of possible proportions was reported based on which percentile values fell outside reference ranges (for example, if the reported 25th percentile was within the reference range but the median was above it, we know that between 50% and 75% of patients in the study had elevated values for that marker).
Studies were divided into 3 categories based on inclusion criteria; studies with cardiovascular involvement as a requirement for inclusion; patients meeting Kawasaki disease clinical case definition13 as a requirement for inclusion; and broader criteria that captured patients with multisystem involvement along with evidence of inflammation and links to COVID-19. Generalized linear mixed models with double arcsine-transformed proportions14 were used to assess differences in prevalence of clinical symptoms and treatments administered by study category. The mixed model approach allowed for the comparison of proportions by inclusion criteria while accounting for study heterogeneity.15 Statistical analyses were performed using R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria, 2020).
Results
A total of 1470 records (440 from PubMed, 734 from Embase, 65 from medRxiv and bioRxiv, and record 231 from subsequent citations) were reviewed for possible inclusion (Figure 1; available at www.jpeds.com). After review, 20 studies were identified that described cohorts of at least 5 MIS-C cases.7, 8, 9, 10 , 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Ten of the studies7 , 17, 18, 19, 20 , 23, 24, 25, 26 , 30 were excluded due to likely substantial overlap of patients with other studies; this included the report of patients with MIS-C from Riphagen et al whose patients were all included in a subsequent larger study.7 , 31 An additional 2 studies (describing n = 116 patients) were excluded due to insufficient data on clinical features and laboratory markers.16 , 27 A study from Toubiana et al9 was not excluded despite possibly having patients in common with a larger multi-institutional study,8 as due to the brief period of overlap (the study time periods were 15 days and 40 days, and the overlap was only 4 days) as well as different inclusion criteria, the number of patients in common was likely minimal (the authors could not be reached for comment).
Figure 1.
Flowchart of literature search and study identification.
Eight published studies were included in this systematic review that captured a total of 440 patients.8, 9, 10 , 21 , 22 , 28 , 29 , 31 (Table I ). The total number of patients included in the studies ranged from 10 to 186. The 2 largest studies were from the US, and the other 6 studies were from European countries. Primary inclusion criteria for 3 of the European studies (1 from Italy and 2 from France) was a diagnosis of Kawasaki disease.9 , 10 , 28 Two studies required cardiovascular system involvement: one study from the United Kingdom29 selected children referred for cardiovascular evaluation and who met the UK Royal College of Paediatrics and Child Health criteria for MIS-C32 and another study in France and Switzerland identified children with acute left ventricular systolic dysfunction or cardiogenic shock along with an associated multisystem inflammatory state.8 Two studies from the US21 , 22 and one from the United Kingdom31 used broader inclusion criteria that included fever, multisystem involvement, and laboratory evidence of inflammation; the US studies also required laboratory or epidemiologic link to COVID-19. Case definitions used for the studies are detailed in the Appendix (available at www.jpeds.com).
Table I.
Overview of studies included in the systematic review
| Studies | Whittaker et al31 | 2020 | Verdoni et al10 | 2020 | Belhadjer et al8 | 2020 | Toubiana et al9 | 2020 | Dufort et al21 | 2020 | Feldstein et al22 | 2020 | Ramcharan et al29 | 2020 | Pouletty et al28 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | United Kingdom (8 hospitals) | Bergamo, Italy (Papa Giovanni XXIII Hospital) | 12 hospitals in France and 1 in Switzerland | Paris, France (Necker-Enfants-Malades Hospital) | New York state, US | 53 sites in 27 US states | Birmingham, England (Birmingham Children's Hospital) | 5 hospitals in the Paris area | ||||||||
| N | 58 | 10 | 35 | 21 | 99 | 186 | 15 | 16 | ||||||||
| Case hospitalization date range | March 23 to May 16 | February 18 to April 20 | March 22 to April 30 | April 27 to May 11 | March 1 to May 10 | March 15 to May 20 | April 10 to May 9 | April 7 to April 30 | ||||||||
| Other inclusion criteria | Children meeting United Kingdom, WHO, or CDC criteria (without requiring proof of SARS-CoV-2 exposure) | Patients diagnosed with Kawasaki disease (complete or incomplete) | Children with acute left ventricular systolic dysfunction or cardiogenic shock and associated multisystem inflammatory state | Children ≤18 y who met criteria for Kawasaki disease (complete or incomplete) | New York state case definition: clinical and lab and/or epi criteria (includes positive SARS-CoV-2 test or reported exposure)50 | CDC case definition: clinical and lab and/or epi criteria (includes positive SARS-CoV-2 test or reported exposure) | All patients referred for cardiovascular evaluation as confirmed PIMS-TS |
<18 y, complete or incomplete Kawasaki disease, SARS-CoV-2 PCR+ or serology+ and/or close contact |
||||||||
| Median age, y (IQR) | 9 (5.7-14) | 7.3 (5.4-8.5) | 10 (IQR NA) | 7.9 (3.7-16.6) | NA | 8.3 (3.3-12.5) | 8.8 (6.4-11.2) | 10 (4.7-12.5) | ||||||||
| Sex (percent male) | 66% | 70% | 51% | 43% | 54% | 62% | 73% | 50% | ||||||||
| Race/ethnicity | 38% black 31% Asian 21% white 10% other |
NA | NA | 57% Afro-Caribbean 29% European 10% Asian 5% Middle Eastern |
40% black 37% white 5% Asian 18% Other; 36% Hispanic∗ |
39% Hispanic 31% black non-Hispanic 24% white non-Hispanic 6% other† |
40% Afro-Caribbean 40% South Asian 13% mixed 7% other |
62% Afro-Caribbean 25% European 12% Middle Eastern |
||||||||
| SARS-CoV-2 PCR, % | 26% | 26% | 20% | 34% | 51% | 40% | 13% | 69% | ||||||||
| SARS-CoV-2 serology, % | 87% | 83% | 80% | 86% | 99% | 75% | 100% | 100% | ||||||||
| Length of hospital stay: median d (IQR) | NA | NA | 8 | 8 | 6 (4 - 9) | 7 (4 - 10) | 12 (9 - 13) | NA | ||||||||
| Died | 2% | 0% | 0% | 0% | 2% | 2% | 0% | 0% | ||||||||
CDC, Centers for Disease Control and Prevention; NA, not available; PIMS-TS, pediatric multisystem inflammatory syndrome.
Hispanic ethnicity listed separately from race.
Among patients with known race/ethnicity.
Median age of patients by study ranged from 7.3 to 10 years, and 59% of all patients (43%-73% across studies) were male. In the 6 studies that reported race/ethnicity data, the proportion of black or Afro-Caribbean patients ranged from 31% to 62%. The 2 US studies reported Hispanic ethnicity, which comprised 36%-39% of patients. Across all studies, the proportion of patients with positive SARS-CoV-2 RT-PCR test results ranged from 13% to 69%, and the proportion with positive serology tests ranged from 75% to 100%. Seven (2%) of the 440 patients with MIS-C died.
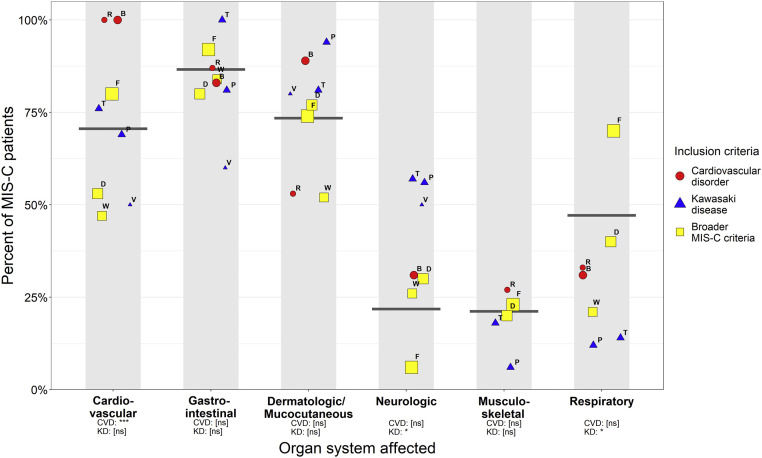
Figure 2 shows the proportion of patients in each study with clinical manifestation by different organ systems. Overall, the greatest proportion of patients had gastrointestinal symptoms (87%) followed by dermatologic/mucocutaneous symptoms (73%) and cardiovascular symptoms (71%), with fewer patients reporting respiratory (47%), neurologic (22%), and musculoskeletal (21%) symptoms. The proportion reporting cardiovascular symptoms, neurologic symptoms, and respiratory symptoms differed by inclusion criteria. The studies that used cardiovascular inclusion criteria had a prevalence of cardiovascular symptoms of 100%, and studies selecting patients with Kawasaki disease included higher prevalence of neurologic symptoms (55%) but lower prevalence of respiratory symptoms (14%).
Figure 2.
Proportion of patients with clinical symptoms in different organ systems by study. Size of circles is relative to study sample size, and shapes represent categories of inclusion criteria. Gray bars represent the prevalence of organ system involvement in patients in all studies. Not all studies reported involvement for each organ system. Point labels denote studies: W = Whittaker, V = Verdoni, B = Belhadjer, T = Toubiana, D = Dufort, F = Feldstein, R = Ramcharan, and P = Pouletty. Studies with inclusion criteria of CVD and KD were assessed for differences in proportions compared with studies with broader inclusion criteria:, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. CVD, cardiovascular disorder; KD, Kawasaki disease; ns, not significant.
Figure 3 shows the proportion of patients in each study receiving different therapies. Of listed therapies, intravenous immunoglobulin (IVIG) was the most commonly administered (76% of patients) compared with vasoactive medications (53%), steroids (52%), and immune modulators (18%). In addition, 26% of patients received intubation, and 6% required extracorporeal membrane oxygenation. IVIG was given to 98% of patients from the studies selecting patients with Kawasaki disease. Studies using cardiovascular inclusion criteria had lower prevalence of treatment with steroids (34%) but greater prevalence of use of extracorporeal membrane oxygenation (extracorporeal membrane oxygenation, 20%).
Figure 3.
Proportion of patients receiving different types of treatment by study. Size of circles is relative to study sample size, and shapes represent categories of inclusion criteria. Gray bars represent the proportion of patients receiving treatments in all studies. Not all studies reported each type of treatment. Point labels denote studies: W = Whittaker, V = Verdoni, B = Belhadjer, T = Toubiana, D = Dufort, F = Feldstein, R = Ramcharan, and P = Pouletty. Studies with inclusion criteria of CVD and KD were assessed for differences in proportions compared with studies with broader inclusion criteria. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Table II summarizes laboratory test results reported by each study. In every study for which the proportion of patients with abnormal levels could be ascertained, ferritin and d-dimer levels were elevated in at least 50% of patients, and C-reactive protein, interleukin-6, and fibrinogen were elevated in each study in at least 75% of patients. Decreased platelet levels were also commonly observed in many studies. Highly elevated levels for markers of cardiac damage—troponin, brain natriuretic peptide, and N-terminal pro b-type natriuretic peptide—were seen in the majority of patients across studies and were observed in potentially 100% of patients for the studies with cardiovascular inclusion criteria.
Table II.
Laboratory test results by study
| Studies | Whittaker et al31 | 2020 | Verdoni et al10 | 2020 | Belhadjer et al8 | 2020 | Toubiana et al9 | 2020 | Dufort et al21 | 2020 | Feldstein et al22 | 2020 | Ramcharan et al29 | 2020 | Pouletty et al28 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White blood cells, 1000/μL | ||||||||||||||||
| Median | 17 | – | 16 | 17.4 | 10.4 | – | – | 11.5 | ||||||||
| IQR | (12-22) | – | (12-23) | (5.4-42.8) | (6.7-14.5) | – | – | (9-14.4) | ||||||||
| % abnormal | 50%-75%∗(>13.5) | – | ∼75% (>12) | – | – | – | ||||||||||
| Neutrophils, 1000/μL | ||||||||||||||||
| Median | 13 | 84† | 13 | 13.6 | 82† | – | – | 9.2 | ||||||||
| IQR | (10-19) | (79-90)† | (8-19) | (3.3-36.4) | (76.0-89.0)† | – | – | (7.6-10.7) | ||||||||
| % Abnormal | 75%-100%∗ (>7) | 70% (>80)† | 50-75%∗(>8.5) | – | – | 68% (>7.7) | ||||||||||
| Lymphocytes, 1000/μL | ||||||||||||||||
| Median | 0.8 | 0.8 | – | 1.1 | 10† | – | – | 1.15 | ||||||||
| IQR | (0.5-1.5) | (0.5-1.0) | – | (0.4-5.6) | (5.0-16.0)† | – | – | (0.8-1.7) | ||||||||
| % Abnormal | ∼75% (<1.5) | 90% (<1.5) | 81%‡ | 66%§ | 80%¶ | |||||||||||
| Platelets, 1000/μL | ||||||||||||||||
| Median | 151 | 130 | – | – | 155 | 133 | – | 188 | ||||||||
| IQR | (104-210) | (118-144) | – | – | (105-233) | (88-235) | – | (164-244) | ||||||||
| % Abnormal | 50%-75%∗(<200) | 100% (≤300) | 11% (<80) | 55% (<150) | ||||||||||||
| Erythrocyte sedimentation rate, mm/h | ||||||||||||||||
| Median | – | 71 | – | – | 61.5 | 65 | 75 | – | ||||||||
| IQR | – | (52-94) | – | – | (43.0-77.5) | (42-91) | (45-90) | – | ||||||||
| % Abnormal | 77% (>40) | 77% (>40) | 75%-100%∗(>9) | |||||||||||||
| Ferritin, ng/mL | ||||||||||||||||
| Median | 610 | 893 | – | – | 522 | 639 | 558 | 1067 | ||||||||
| IQR | (359-1280) | (324-2000) | – | – | (305-820) | (333-1178) | (364-1325) | (272-1709) | ||||||||
| % Abnormal | 75%-100%∗(>140) | 56% (>684) | – | – | 75% (>300) | 61% (>500) | 75%-100%∗(>79) | 50% (>500) | ||||||||
| CRP, mg/L | ||||||||||||||||
| Median | 229 | 241 | 241 | 253 | 219 | 178 | 154 | 207 | ||||||||
| IQR | (156-338) | (110-353) | (150-311) | (89-363) | (150-300) | (128-259) | (129-231) | (162-236) | ||||||||
| % Abnormal | 75%-100%∗(>5) | 80% (>100) | 100% (>6) | – | 87% (>100) | 91% (>30) | 75%-100%∗(>10) | 100%‡ | ||||||||
| Albumin, g/L | ||||||||||||||||
| Median | 24 | – | – | 21 | 31 | 25 | – | 21 | ||||||||
| IQR | (21-27) | – | – | (16-37) | (25-36) | (20-29) | – | (19-23) | ||||||||
| % Abnormal | 75%-100%∗(<35) | – | – | 95% (<32) | 48% (<30) | 80% (<30) | 43%‡ | |||||||||
| Interleukin-6, pg/mL | ||||||||||||||||
| Median | – | – | 135 | 170 | 116.3 | – | – | – | ||||||||
| IQR | – | – | (87-175) | (4-1366) | (37.0-315.0) | – | – | – | ||||||||
| % Abnormal | – | 75%-100%∗(>8.5) | – | 97% (>5) | – | |||||||||||
| Troponin, ng/L | ||||||||||||||||
| Median | 45 | 111 | 347 | 282 | – | – | 396 | 58 | ||||||||
| IQR | (8-294) | (18-1879) | (186-1267) | (10-6900) | – | – | (100-1280) | (36-165) | ||||||||
| % Abnormal | 68% (>15) | 56% (>53) | 75-100%∗(>26) | 81% (>26) | 71%‡ | 50%∗∗ | 100% (>35) | |||||||||
| Fibrinogen, mg/dL | ||||||||||||||||
| Median | 570 | 618 | – | 499 | 624 | – | – | – | ||||||||
| IQR | (440-700) | (483-759) | – | (78-838) | (506-764) | – | – | – | ||||||||
| % Abnormal | 75%-100%∗(>409) | 90% (>360) | 86% (>400) | 80% (>400) | ||||||||||||
| D-dimers, ng/mL | ||||||||||||||||
| Median | 3578 | – | 5284 | 4025 | 2400 | 4090 | 2060 | – | ||||||||
| IQR | (2085-8235) | – | (4069-9095) | (350-19 330) | (1200-3700) | (2240-8405) | (1160-2610) | – | ||||||||
| % Abnormal | 75%-100%∗(>560) | – | 100% (>500) | 95% (>500) | 91% (>550) | 67% (>3000) | 75%-100%∗(>300) | |||||||||
| BNP, pg/mL | ||||||||||||||||
| Median | – | – | 5743 | 3354 | – | 1195 | – | – | ||||||||
| IQR | – | – | (2648-11 909) | (16-16 017) | – | (391-4833) | – | – | ||||||||
| % Abnormal | 100% (>100) | – | – | 73% (>400) | ||||||||||||
| proBNP, ng/L | ||||||||||||||||
| Median | 788 | 1236 | 41 484 | – | – | – | 24 470 | – | ||||||||
| IQR | (174-10 548) | (295-1921) | (35 811-52 475) | – | – | – | (17 212-26 655) | – | ||||||||
| % Abnormal | 83% (>100) | 100% (>100) | 100% (>100) | – | 90%†† | – | 100% (>400) | |||||||||
BNP, brain natriuretic peptide; proBNP, pro b-type natriuretic peptide; CRP, C-reactive protein.
The threshold for abnormal values is determined by study. Results in bold text describe abnormal values in at least 50% of patients. Results with ranges for % abnormal signify that these percentages were estimated by comparing the IQR with the reference range.
Values shown are range; IQR was not reported in the publication.
Values given in percent white blood cells.
Threshold not specified.
Classified as lymphocytes (%): (<2.5) under 1 month, (<4.0) 1-12 months, (<3) 1-2 years, (<2) 2-4 years, (<1.5) 4-10 years, (<1.2) 10-16 years, (<1.0) 16-21 years.
Lymphocyte count <1500/μL in those >8 months old and <4500/μL in children younger than 8 months of age.
Troponin categorized dichotomously as high or normal based on each laboratory's reference range.
Elevated proBNP classified as (ng/L): (>1121) 1 month to 1 year, (>675) 1-2 years, (>391) 6-14 years, (>363) 14-21 years.
Discussion
Our review summarizes and compares data from 8 separate studies that describe patients with MIS-C identified in various settings using different inclusion criteria. Patients with MIS-C predominantly had fever with gastrointestinal, cardiovascular, and mucocutaneous manifestations, which was consistent across studies despite the different inclusion criteria. Respiratory manifestations, which are predominant manifestations in COVID-19, were described in a minority of patients with MIS-C. All studies reported highly elevated levels of C-reactive protein and other laboratory markers of inflammation suggesting that hyperinflammatory state is a primary hallmark of MIS-C. The studies represent an evolving understanding of the clinical manifestations and management of this novel condition. Because the UK National Health Service's initial alert described cases similar to atypical Kawasaki disease or toxic shock syndrome, the early published studies focused their analyses either on patients diagnosed with Kawasaki disease or patients with shock and other cardiovascular disorders.7, 8, 9, 10 Many of the more recently published studies allowed for the inclusion of patients with more encompassing multisystem involvement along with laboratory evidence of inflammation.21 , 22 , 31
The studies describing patients diagnosed as Kawasaki disease or with required cardiovascular inclusion criteria most likely represent subsets of MIS-C as defined by the broader case definitions used by the UK, New York State, Centers for Disease Control and Prevention, and WHO. The patients described by Riphagen et al all had multisystem involvement, laboratory evidence of inflammation, and positive serological tests for SARS-CoV-2, and they all would have met the aforementioned case definitions.7 Similarly, the patients in other studies8, 9, 10 , 28 , 29 also overwhelmingly had multisystem involvement, laboratory evidence of inflammation, and positive tests for SARS-CoV-2. Conversely, substantial proportions of patients in the studies with broader case definitions would have met inclusion criteria for the other studies: many patients in other cohorts21 , 22 , 31 received diagnoses of Kawasaki disease (22%, 36%, and 40%, respectively) or had evidence of shock (50%, 10%, and 48%, respectively).
Importantly, although many MIS-C cases share clinical features similar to Kawasaki disease, increasing evidence suggests that they are 2 separate syndromes. Although MIS-C and Kawasaki disease present with some phenotypic similarities, there are several epidemiologic and clinical features distinguishing the 2 diseases. Approximately 80% of patients with Kawasaki disease are younger than 5 years old with a median age of 2 years,33 , 34 and the median age for patients reported in all 8 MIS-C studies was at least 7 years. The incidence of Kawasaki disease is dramatically greater among Japanese children and other children of East Asian descent. In contrast, the MIS-C studies do not describe a preponderance among children of East Asian descent, and despite extensive spread of COVID-19 in some East Asian countries, reports of MIS-C in that region are largely absent.34 Verdoni et al reported that patients meeting Kawasaki disease diagnostic criteria associated with COVID-19 had lower white blood cell count and lower platelet levels but greater levels of C-reactive protein and ferritin compared with cases of Kawasaki disease from before the COVID-19 pandemic.10 Whittaker et al reported that MIS-C cases had greater levels of neutrophils, C-reactive protein, ferritin, troponin, and d-dimers compared with a historic Kawasaki disease cohort, but lower levels of lymphocytes and platelets.31 MIS-C cases exhibit greater prevalence of multisystem involvement, notably cardiac abnormalities such as myocarditis and shock.34 Kawasaki disease is a clinically diagnosed syndrome; thus, given overlap in clinical features with MIS-C, a substantial proportion of MIS-C cases may continue to be classified as Kawasaki disease as more children are exposed to SARS-CoV-2. Early reports support the use of IVIG, which is the standard treatment for Kawasaki disease, for use in treating MIS-C patients7 , 9 , 23; corticosteroids also have an important role in the treatment of Kawasaki disease and the same may prove true for MIS-C.19 Despite this, there may be key etiological and pathophysiologic differences between Kawasaki disease and MIS-C, and distinguishing the 2 syndromes may be important for establishing best practices for MIS-C treatment and prevention.
In all studies, the proportion of patients with positive SARS-CoV-2 serology testing was substantially greater than of those with positive SARS-CoV-2 RT-PCR tests. These findings as well as increased markers of hyperinflammation are consistent with the hypothesis that MIS-C results from a postinfectious inflammatory process.21 , 34 , 35 Multiple reports21 , 22 , 36 describe a 4- to 6-week delay between the proliferation of local COVID-19 cases and the surge in MIS-C cases, whereas Feldstein et al describe a median of 22 days between onset of COVID-19 symptoms and MIS-C hospitalization.22 In the study with the greatest prevalence of RT-PCR positivity (69%),28 the authors noted that the viral load reported by cycle threshold was low in almost all patients and that all patients with serologic assessments were IgG positive, suggesting a substantial delay between the timing of SARS-CoV-2 infection and onset of MIS-C symptoms and subsequent RT-PCR testing. One interesting note is that the 2 large studies with the broader inclusion criteria21 , 22 had relatively large proportions of patients who tested positive by PCR (51% and 40%, respectively); they also had greater proportions of patients with respiratory symptoms (40% and 70%, respectively). The inclusion criteria for these studies may have captured a greater number of cases with severe acute COVID-19, and the etiology of those cases may be different from that of postinfectious MIS-C cases.
Fifty-nine percent of patients with MIS-C across all studies were male. All but one study reported at least 50% of patients as males. In contrast, boys represent just less than 50% of all laboratory-confirmed COVID-19 cases younger than 20 years in both the US and the United Kingdom.37 , 38 This suggests that male patients infected with SARS-CoV-2 may be more likely to develop MIS-C, similar to Kawasaki disease in which studies have reported a male to female ratio of ∼1.5 to 1 across many countries.39
Certain racial and ethnic groups may also be disproportionately affected by MIS-C. The proportions of Hispanic patients in the studies of Dufort et al and Feldstein et al (36% and 39%) are greater than the proportions of all Hispanic residents in New York state and in the entire US (19.3% and 18.5% respectively).21 , 22 , 40 However, the proportion of patients with MIS-C of Hispanic origin may not differ substantially from the proportion Hispanic reported in SARS-CoV-2 seroprevalence surveys among adults in several areas of New York State (36.6%)41 and the proportion of US COVID-19 cases that are Hispanic (33.4%).40 , 42 Conversely, the proportions of patients with MIS-C reported as black or Afro-Caribbean may be greater than proportions in both the underlying populations and in reported COVID-19 cases. The proportion of black patients in the study by Feldstein et al (31%) is greater than the proportions of black residents in the US (13.4%) and the proportion of all US patients with COVID-19 who are black (16%).22 , 40 , 42 , 43 Similarly, the proportion of black patients in the study of Dufort et al (40%) is greater than the proportions of black residents in New York State (17.6%) and in SARS-CoV-2 seroprevalence surveys among adults in several areas in New York State (20.2%).21 , 40 , 41 The proportion of patients with MIS-C who are black (38% and 40%) or Asian (31% and 40%) in the 2 studies from the United Kingdom29 , 31 (specifically, England) are greater than the proportions of Black or Asian residents in England (3.5% and 8.0%, respectively)44 and black and Asian proportions of all English COVID-19 cases (4.6% and 9.6%, respectively). Although the exact cause of these disparities may be unknown, conditions in the places where people live, learn, work, and play affect a wide range of health risks and outcomes.45 The link between social determinants of health, including social, economic, and environmental conditions, and health outcomes is widely recognized in the public health literature. Moreover, it is increasingly recognized that inequitable distribution of these conditions across various populations is a significant contributor to persistent and pervasive health disparities.46 Factors affecting disadvantaged groups such as living in more crowded conditions, employment in occupations not suitable to physical distancing, greater prevalence of underlying medical conditions, and reduced healthcare access have been implicated in the greater incidence and severity of COVID-19 observed in racial/ethnic minorities.47 , 48 Addressing social determinants of health that disproportionately affect certain racial/ethnic groups, while improving access to healthcare, is crucial to reducing the additional burden of MIS-C.
This study has several important limitations. Data for some variables of interest were either not reported for all studies or not reported in a consistent fashion, making direct comparisons between studies difficult. Potential issues included different criteria available to assess organ system involvement, lack of information on imaging studies, timing of laboratory tests, and different thresholds for defining abnormal laboratory values. Overall proportions for variables of interest were calculated by excluding studies that did not report those variables. It is possible that those variables were excluded from these studies due to their involvement being less prominent. In addition, the lack of a diagnostic test or universally used case inclusion criteria could have led to missed diagnosis or nonreporting of some MIS-C cases, particularly patients without a positive SARS-CoV-2 test and patients with less-severe symptoms. Exclusion of those patients may lead to an incomplete picture of the overall spectrum of disease, and further investigations would help to expand upon this preliminary characterization of MIS-C. Finally, although most patients improved with treatment or by supportive care alone,31 these descriptive studies are not sufficient for assessing the efficacy of different treatments. Efforts to better investigate treatments are currently under way,49 and ultimately clinical trials will be necessary to determine optimal clinical management of patients with MIS-C.
Data from the systematic review indicate that MIS-C cases from different studies across different countries have similar manifestations with a strong temporal, geographic, and laboratory link with SARS-CoV-2 infection. Clinical, laboratory, and epidemiologic characteristics of MIS-C appear to be different from those of Kawasaki disease. Future studies will hopefully shed additional light on MIS-C and improve prospects for prevention and treatment of this severe pediatric condition.
Data Statement
Data sharing statement available at www.jpeds.com.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors declare no conflicts of interest.
Supplementary Data
Appendix
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard World Health Organization: World Health Organization. 2020. https://covid19.who.int/
- 2.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus Disease 2019 in Children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PICS Statement Increased number of reported cases of novel presentation of multi-system inflammatory disease. Paediatric Intensive Care Society: Paediatric Intensive Care Society. 2020. https://picsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf Accessed July 27, 2020.
- 6.Schroeder A.R., Wilson K.M., Ralston S.L. COVID-19 and Kawasaki disease: finding the signal in the noise. Hosp Pediatr. 2020 doi: 10.1542/hpeds.2020-000356. in press. [DOI] [PubMed] [Google Scholar]
- 7.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. in press. [DOI] [PubMed] [Google Scholar]
- 9.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention Health Alert Network. 2020. [Google Scholar]
- 12.Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. World Health Organization; 2020. [Google Scholar]
- 13.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 14.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann Math Statist. 1950:607–611. [Google Scholar]
- 15.Stijnen T., Hamza T.H., Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 16.Belot A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capone C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020 doi: 10.1001/jama.2020.10374. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiotos K., Bassiri H., Behrens E.M., Blatz A.M., Chang J., Diorio C. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020;223:199–203.e1. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hameed S., Elbaaly H., Reid C.E., Santos R.M., Shivamurthy V., Wong J. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202543. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R. Multisystem inflammatory syndrome in shildren associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Peditar. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K.J.G. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Toledo M., Faustini S.E., Jossi S.E., Shields A.M., Kanthimathinathan H.K., Allen J.D. Serology confirms SARS-CoV-2 infection in PCR-negative children presenting with paediatric inflammatory multi-system syndrome. medRxiv. 2020 in press. [Google Scholar]
- 28.Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020 doi: 10.1007/s00246-020-02391-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riollano-Cruz M., Akkoyun E., Briceno-Brito E., Kowalsky S., Posada R., Sordillo E.M. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. 2020 doi: 10.1002/jmv.26224. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royal College of Paediatrics and Child Health Guidance—Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 2020. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf Accessed June 3, 2020.
- 33.Newburger J.W., Takahashi M., Burns J.C. Kawasaki disease. J Am Coll Cardiol. 2016;67:1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 34.Shulman S.T. Pediatric COVID-2019-associated multi-system inflammatory syndrome (PMIS) J Pediatr Infect Dis Soc. 2020;9:285–286. doi: 10.1093/jpids/piaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahase E. Covid-19: cases of inflammatory syndrome in children surge after urgent alert. BMJ. 2020;369:m1990. doi: 10.1136/bmj.m1990. [DOI] [PubMed] [Google Scholar]
- 37.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S. Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weekly Coronavirus Disease 2019 (COVID-19) Surveillance Report: Week 26. Public Health England; 2020. [Google Scholar]
- 39.Rowley A.H., Shulman S.T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. https://www.census.gov/quickfacts/fact/table/US/PST045219 Accessed July 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Population and Housing Unit Estimates. US Census Bureau, Population Estimates Program; Washington (DC): 2019. [Google Scholar]
- 41.Rosenberg E.S. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–29.e4. doi: 10.1016/j.annepidem.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC COVID Data Tracker. Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/covid-data-tracker/index.html#demographics Accessed July 6, 2020. [Google Scholar]
- 43.Foundation KF . Kaiser Family Foundation; 2020. COVID-19 Cases by Race/Ethnicity.https://www.kff.org/other/state-indicator/covid-19-cases-by-race-ethnicity Accessed July 1, 2020. [Google Scholar]
- 44.Research report on population estimates by ethnic group and religion. UK Office for National Statistics; 2020. p. 2019. [Google Scholar]
- 45.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2020. Social Determinants of Health: Know What Affects Health.https://www.cdc.gov/socialdeterminants/ Accessed July 1, 2020. [Google Scholar]
- 46.Brennan Ramirez L.K.B.E., Metzler M. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta (GA): 2008. Promoting Health Equity: A Resource to Help Communities Address Social Determinants of Health. [Google Scholar]
- 47.Hooper M.W., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khunti K., Singh A.K., Pareek M., Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 49.Best available treatment study for inflammatory conditions associated with COVID-19. ISRCTN Registry. Imperial College London; 2020. Accessed July 27, 2020. [DOI] [Google Scholar]
- 50.Health advisory: pediatric multi-system inflammatory syndrome temporally associated with COVID-19 interim case definition in New York State . Department of Health; 2020. New York State.https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf Accessed June 26, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.