Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) has reached high prevalence, paralleling the obesity pandemic. The aggressive form of the disease, nonalcoholic steatohepatitis (NASH), is characterized by fatty infiltration and inflammation of the liver, can progress to compensated cirrhosis (CC) and end-stage liver disease (ESLD: decompensated cirrhosis [DCC] and hepatocellular carcinoma [HCC]), and may ultimately require liver transplantation (LT). Real-world data on the burden of NAFLD/NASH are limited. This study aimed to evaluate the clinical and economic burden of NAFLD/NASH to the French hospital system.
Methods
This retrospective cohort study used data from the French PMSI-MCO database. Adults with NAFLD/NASH diagnosis identified between 2009 and 2015 were categorized into disease severity cohorts (NAFLD/NASH, CC, DCC, HCC, and LT). Demographic and clinical data were assessed at the index (diagnosis) date. Hospitalization resource utilization and costs were assessed in the pre- and post-index periods. Rates of liver disease progression and death were evaluated for each cohort.
Findings
During the median follow-up of 34.8 months, of the 131,656 patients included, 1491 patients developed CC (1.1%), 7846 developed DCC (5.9%), 1144 developed HCC (0.9%), and 52 required LT (0.04%). The diagnosis of NAFLD/NASH was associated with increasing annual costs: €7736 vs €5076 before the diagnosis. Rates of comorbidities, hospitalization resource utilization, and costs increased with disease progression. The rate of death at seven-year follow-up was 7.9% in NAFLD/NASH, CC: 18.0%, DCC: 34.9%, and HCC: 48.8%.
Interpretation
NAFLD/NASH is associated with high economic burden and imparts substantial risk of negative clinical outcomes and mortality at all stages of disease.
Keywords: Healthcare costs, Economic burden, Mortality, Real-world evidence, Disease progression, Fatty liver, Hepatic steatosis, Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis
Research in context.
Evidence before this study
The French population is ageing with an increasing prevalence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). However, the economic and clinical burdens associated with NAFLD/NASH and associated liver disease progression is not well characterised.
Added value of this study
We assessed the death rates and costs of hospitalization in diagnosed patients with NAFLD/NASH using records from a French National hospital database. All patients in the French hospital system with a diagnosis of NAFLD/NASH between 2009 – 2015 were captured. Patients diagnosed with these diseases had higher hospitalization costs after diagnosis compared with before, and costs increased with disease progression. Similarly, death rates and rates of accompanying diseases such as diabetes, hypertension and cardiovascular disease increased with disease progression.
Implications of all the available evidence
These results of high economic and clinical burdens have broad public health implications. They suggest that NALFD/ NASH will have a substantial and increasing future clinical and economic impact and the timely identification and effective treatment of NASH in the primary care setting prior to inpatient admission will be required to reduce the impact observed in this study to the French hospital system.
Alt-text: Unlabelled box
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) and its progressive form, nonalcoholic steatohepatitis (NASH), are chronic liver diseases recognized as the hepatic manifestation of metabolic syndrome [[1], [2]–3]. NASH can progress to advanced liver disease and lead to compensated cirrhosis (CC) followed by end-stage liver disease, which includes decompensated cirrhosis (DCC) and hepatocellular carcinoma (HCC). End-stage liver disease results in liver-related mortality, with liver transplantation (LT) providing the only curative option [4]. Modelling studies estimate that the prevalence of NASH in France will rise from 3.6% in 2016 (2.32 million cases) to 5.0% in 2030 (3.39 million cases) along with a rising prevalence of diabetes in an ageing population [5].
Emerging evidence suggests that advanced fibrosis in patients with NASH, including CC (fibrosis stage F4), is considered to be the most important predictor of clinical outcomes and overall and liver-related mortality [[6], [7], [8], [9], [10]–11]. A recent phase 2b randomized global clinical trial of 258 patients with NASH reported that 19.0% of those with CC at baseline experienced a clinical event (DCC, HCC or death) in a median follow-up of 30.9 months [12] and a recent systematic review and meta-analysis of 1495 patients with histologically confirmed fibrosis reported an exponential increase in the risk of liver-related mortality and an increase in all-cause mortality with increasing stage of fibrosis [13].
However, the risk of disease progression and mortality associated with NASH is not well understood due to inherent limitations in the designs of previous clinical trial and small cohort studies. Patient selection biases common in clinical trial design may have introduced a mismatch between trial populations and patients in the real-world. In addition, patients outside of a controlled trial setting are subject to routine clinical practice disease identification and management patterns which may differ from those dictated in clinical trial protocols. The well-designed systematic review of histologically confirmed patients evaluated findings from small cohort studies and study entry required both liver biopsy and referral by experienced hepatologists, which likely introduced selection bias towards patients with worse prognosis. Furthermore, while the gold-standard to assess liver disease severity stage is liver biopsy, it has low uptake in the real-world and is associated with sampling variability [14].
In addition to natural history, real-world data on the resource utilization and direct healthcare costs associated with NAFLD and NASH remain limited [15]. In one modeling study, the economic impact of NAFLD in France was estimated as €11.4 billion (€784 per patient) annually, with inpatient (hospitalization) costs as the primary cost driver [15]. This study was informed by limited real-world data and thus required several modeling assumptions.
With these limitations, data gaps regarding diagnosed NASH patients’ natural history and economic burden in the French population require further evaluation. Health Technology Assessment (HTA) organizations strive to improve medical decision making by leveraging real-world evidence from large health system, which generally provide data on large real-world populations over extended observation periods [16,17]. These database studies allow for an increased understanding beyond clinical trials and small cohort studies on patients’ health outcomes and healthcare utilization subject to real-world clinical practice patterns. Therefore, the objective of this study was to identify and longitudinally follow French hospitalized patients with NAFLD/NASH diagnoses to characterize their demographics, comorbidities, natural history including risk of mortality and disease progression, and economic burden by evaluating a national database of all hospital admissions in France over an extended time period.
2. Methods
2.1. Study design and data source
This was a retrospective, observational cohort study using data sourced from the French computerized hospital database for medicine, surgery and obstetrics (PMSI-MCO: Programme de Médicalisation des Systèmes d'Information – Médecine, Chirurgie et Obstétrique) from 1 January 2009 to 31 December 2015. The PMSI-MCO database is a comprehensive record of all hospitalizations in France (including public and private hospitalizations) [18,19] and includes over 30 million public and private admissions per annum (outpatient encounters not included). Discharge diagnoses are coded in the PMSI-MCO database using the International Classification of Diseases, 10th Revision (ICD-10), which was implemented in France starting in 1997. Medical procedures are coded using the national Common Classification of Medical Acts (Classification Commune des Actes Médicaux). Hospital inpatient date of death is also recorded in the PMSI-MCO database.
2.2. Study sample selection criteria
Previous studies have reported that NASH is underdiagnosed and thus under-coded, likely owing to a lack of available treatments. As such, both NAFLD- and NASH-diagnosed patients were included in the study in a combined cohort. Patients who were 18 years of age and older, with at least one claim with a diagnosis code for NASH and/or NAFLD (ICD-10 K75.8, K76.0), and who were identified in the PMSI-MCO database between 1 January 2009 and 31 December 2015 were included in the study. The first diagnosis of NAFLD/NASH marked the index date. At any point during the study period, patients with other causes of chronic liver disease were excluded as were patients infected with human immunodeficiency virus. Patients were required to have data available for at least 6 months preceding the index date. Patients were longitudinally followed from NAFLD/NASH diagnosis until death or the end of the study period (31 December 2015), whichever occurred first, as shown in the study schema (Supplemental Fig. 1). Included patients were split into four cohorts according to the level of liver disease severity at diagnosis (NAFLD/NASH, CC, DCC or HCC). For the hospitalization resource utilization and costs analyses the LT cohort was also characterized.
2.3. Study outcomes
Demographic data extracted from the database included age and sex. Medical data extracted from the database included number of hospital stays, the Diagnosis Related Group (DRG) for each hospital stay, length of stay and ICD-10 diagnosis. Comorbidities based on an ICD-10 diagnosis – HTN, hyperlipidaemia, obesity, CVD, sleep apnoea, DM, renal impairment – were also extracted from the database. Economic data included inpatient care costs (annual costs per patient) directly extracted from standard cost estimates completed in the PMSI-MCO database. Inpatient healthcare costs are reimbursed by the French national health insurance body to hospitals through a national DRG-based payment system. The DRG of a hospital stay is determined based on the recorded discharge diagnosis and classifying procedures performed during the stay.
Baseline demographics and clinical characteristics were assessed at the index date for each disease severity cohort. Presence of comorbidities was recorded in both the pre- and post-index periods (i.e., prior to and following diagnosis) for each disease severity cohort.
Post-index hospitalization resource utilization and costs for each cohort (NASH, CC, DCC, HCC and LT) were calculated to the earliest of (1) death, (2) end of study period, (3) 12 months after the index date, and (4) index for a new disease severity cohort. Pre-index resource utilization and costs were assessed in the period at least 6 months (up to 12 months) prior to the index date. Patients included in the analysis had a minimum of 1 month of follow-up following the index date. Annual resource utilization and costs were calculated from per-patient per-month utilization and costs within the > 1-month and ≤ 12-month index periods. Annual healthcare costs and resource utilization were evaluated separately for each disease state and no cumulation across multiple disease states occurred within a single patient. All costs are reported in €2015.
2.4. Statistical analysis
Absolute and relative frequencies of categorical variables are reported, and the mean, standard deviation median, and 95% confidence intervals are provided for quantitative variables. Student's t-test for comparison between two independent groups with the assumption of normal distributions was used for mean annual healthcare costs. Comparisons of the disease stages of NAFLD/NASH and CC, when medical and lifestyle interventions may be applied to slow or stop disease progression, were made to more severe disease stages.
The generalized linear model (log-link function; gamma distribution) was applied with incremental burdens of costs (mean annual cost) as the dependent variable. The purpose of the generalized linear model was to evaluate the adjusted effects associated with increased disease severity on incremental annual costs (cost ratios) after controlling for potential confounders, such as age, and certain comorbidities, such as DM and CVD, that are themselves associated with an increased cost burden. Variables for a disease severity model comprised of NAFLD/NASH (reference), CC, DCC, HCC and LT. Other variables applied to the model were age group (5-year increments), gender, HTN, DM, obesity, hyperlipidaemia, renal impairment, CVD and sleep apnoea.
In the disease progression analysis (Kaplan–Meier curves for progression), rates of liver disease progression were evaluated for NAFLD/NASH to CC, DCC, HCC and LT/death, as well as for CC to DCC, HCC and LT/death, and time to progression for different stages of liver disease.
In the survival analysis, Kaplan–Meier curves for mortality were generated for each patient cohort: NAFLD/NASH, CC, DCC, HCC and LT. Mortality as a variable was non-mutually exclusive (e.g. if a patient with CC moved to DCC and then death, they were counted in the CC mortality group and the DCC mortality group). Due to constraints associated with the PMSI database, only mortality events which occurred in the inpatient setting were evaluated. All statistical analyses were performed using SAS® version 9.4 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographic and clinical characteristics
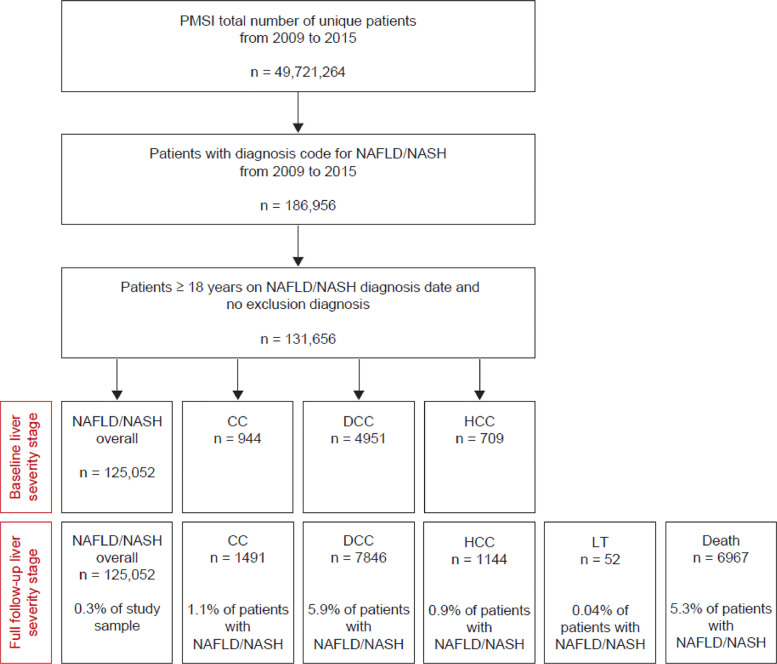
In total 49,721,264 unique patients were included in the PMSI-MCO database between 2009 and 2015 and 186,956 patients (0.4% of the population) had a diagnosis code for NAFLD/NASH. Of these patients, 131,656 met the inclusion criteria for the study (NAFLD/NASH patients without exclusionary diagnosis). Median follow-up was 34.8 months (interquartile range: 15.6–57.6). Over the full study period, 125,052 patients had a diagnosis of NAFLD/NASH and experienced no further progression; 1491 (1.1%) had a diagnosis of CC; 7846 (5.9%) had a diagnosis of DCC; 1144 (0.9%) had a diagnosis of HCC; and 52 (0.04%) underwent LT. A total of 6967 patients (5.3%) died within the study period (Fig. 1). The mean (standard deviation [SD]) age of the patients with NAFLD/NASH at index diagnosis was 55.9 (16.2) years and ranged from 51.7 (13.0) years (LT) to 70.1 (11.1) years (HCC) across all stages of severity (Table 1).
Fig. 1.
Flow diagram for selection of the study cohort. NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplant.
Table 1.
Demographic and clinical characteristics of patients by severity of liver disease.
| Severity of liver disease | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD/NASH (n = 125,052) | 95% CI | CC (n = 1491) | 95% CI | DCC (n = 7846) | 95% CI | HCC (n = 1144) | 95% CI | LT (n = 52) | 95% CI | |
| Demographics | ||||||||||
| Age, years | ||||||||||
| Mean (SD) | 55.9 (16.2) | 55.8 - 56.0 | 62.2 (12.9) | 61.6 - 62.9 | 65.0 (16.4) | 64.7 - 65.4 | 70.1 (11.1) | 69.5 - 70.7 | 51.7 (13.0) | 48.1 - 55.3 |
| Sex, n (%) | ||||||||||
| Female | 67,293 (53.8) | 53.5% - 54.1% | 675 (45.3) | 42.7% - 47.8% | 3900 (49.7) | 48.6% - 50.8% | 315 (27.5) | 25.0% - 30.2% | 25 (48.1) | 34.0% - 62.4% |
| Male | 57,759 (46.2) | 45.9% - 46.5% | 816 (54.7) | 52.2% - 57.3% | 3946 (50.3) | 49.2% - 51.4% | 829 (72.5) | 69.8% - 75.0% | 27 (51.9) | 37.6% - 66.0% |
| Comorbidities, n (%) | ||||||||||
| CVD | 50,990 (40.8) | 40.5% - 41.0% | 777 (52.1) | 49.5% - 54.7% | 5582 (71.1) | 70.1% - 72.1% | 767 (67.0) | 64.2% - 69.8% | 36 (69.2) | 54.9% - 81.3% |
| Diabetes mellitus | 57,979 (46.4) | 46.1% - 46.6% | 1011 (67.8) | 65.4% - 70.2% | 3559 (45.4) | 44.3% - 46.5% | 634 (55.4) | 52.5% - 58.3% | 25 (48.1) | 34.0% - 62.4% |
| Hyperlipidaemia | 46,919 (37.5) | 37.3% - 37.8% | 603 (40.4) | 37.9% - 43.0% | 2726 (34.7) | 33.7% - 35.8% | 366 (32.0) | 29.3% - 34.8% | 11 (21.2) | 11.1% - 34.7% |
| Hypertension | 65,225 (52.2) | 51.9% - 52.4% | 1001 (67.1) | 64.7% - 69.5% | 5073 (64.7) | 63.6% - 65.7% | 797 (69.7) | 66.9% - 72.3% | 28 (53.8) | 39.5% - 67.8% |
| Obesity | 69,267 (55.4) | 55.1% - 55.7% | 848 (56.9) | 54.3% - 59.4% | 3285 (41.9) | 40.8% - 43.0% | 429 (37.5) | 34.7% - 40.4% | 22 (42.3) | 28.7% - 56.8% |
| Sleep apnoea | 24,511 (19.6) | 19.4% - 19.8% | 327 (21.9) | 19.9% - 24.1% | 1338 (17.1) | 16.2% - 17.9% | 146 (12.8) | 10.9% - 14.8% | 4 (7.7) | 2.1% - 18.5% |
| Renal impairment | 27,173 (21.7) | 21.5% - 22.0% | 432 (29.0) | 26.7% - 31.3% | 3098 (39.5) | 38.4% - 40.6% | 337 (29.5) | 26.8% - 32.2% | 31 (59.6) | 45.1% - 73.0% |
| Diabetes mellitus OR hyperlipidaemia OR hypertension | 87,252 (69.8) | 69.5% - 70.0% | 1239 (83.1) | 81.1% - 85.0% | 5895 (75.1) | 74.2% - 76.1% | 940 (82.2) | 79.8% - 84.3% | 38 (73.1) | 59.0% - 84.4% |
| Diabetes mellitus AND hyperlipidaemia AND hypertension | 26,773 (21.4) | 21.2% - 21.6% | 460 (30.9) | 28.5% - 33.3% | 1688 (21.5) | 20.6% - 22.4% | 236 (20.6) | 18.3% - 23.1% | 6 (11.5) | 4.4% - 23.4% |
Demographic and clinical characteristics measured in the pre- and post-index (diagnosis) periods for each liver disease severity group. As patients can progress through the liver disease stages, groups were not mutually exclusive.
CC, compensated cirrhosis; CVD, cardiovascular disease; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CI, confidence interval; SD, standard deviation.
The majority (82.0%) of patients with cirrhosis (CC or DCC) were first identified with a decompensation event (7657 patients first identified with DCC out of a total of 9337 identified patients with cirrhosis) (Supplemental Table 1).
3.2. Comorbidities
Patients with NAFLD/NASH across all stages of disease severity had high comorbidity burdens, with the comorbid burden generally higher in patients with more advanced liver disease (Table 1). Rates of CVD were higher in patients with CC (52.1%), DCC (71.1%), HCC (67.0%) or patients requiring LT (69.2%) than in those with NAFLD/NASH (40.8%). Additionally, the combination of the comorbidities of DM, hyperlipidaemia and HTN was reported in 21.4% of patients with NAFLD/NASH and 30.9% of patients with CC.
3.3. Hospitalization resource utilization and costs
The annual number of hospital visits following diagnosis was higher in patients with more severe liver disease (CC, 4.2 [SD, 8.6]; DCC, 7.0 [SD, 11.1]; HCC, 8.7 [SD, 10.3]; LT, 10.9 [SD, 24.6]) than in patients with NAFLD/NASH (3.1 [SD, 5.6]) (Table 2). Patients with NAFLD/NASH had a higher annual number of visits (3.1 vs 2.4) following diagnosis compared with before.
Table 2.
Pre- and post-diagnosis annual mean all-cause hospitalization resource utilization and costs by severity of liver disease.
| Severity of liver disease | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD/NASH overall (n = 125,052) |
CC (n = 1491) |
DCC (n = 7846) |
HCC (n = 1144) |
LT (n = 52) |
||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Annual number of visits (n) | ||||||||||
| Mean (SD) | 2.4 (5.1) | 3.1 (5.6) | 2.8 (8.0) | 4.2 (8.6) | 4.7 (8.7) | 7.0 (11.1) | 3.0 (4.4) | 8.7 (10.3) | 11.6 (32.8) | 10.9 (24.6) |
| Median | 1.0 | 2.0 | 2.0 | 2.0 | 2.0 | 3.4 | 2.0 | 5.0 | 4.0 | 6.5 |
| 95% CI | 2.4 - 2.4 | 3.1 - 3.1 | 2.3 - 3.4 | 3.7 - 4.6 | 4.5 - 5.0 | 6.7 - 7.2 | 2.7 - 3.3 | 8.1 - 9.3 | 1.1 - 22.0 | 3.6 - 18.3 |
| Length per stay (days) | ||||||||||
| Mean (SD) | 2.7 (10.2) | 4.1 (7.4) | 3.2 (5.5) | 3.5 (5.6) | 5.2 (16.6) | 9.1 (15.4) | 3.8 (13.6) | 5.6 (6.3) | 5.2 (10.1) | 9.0 (7.6) |
| Median | 1.0 | 3.0 | 1.5 | 2.0 | 3.0 | 6.1 | 1.7 | 4.0 | 2.8 | 6.5 |
| 95% CI | 2.6 - 2.8 | 4.0 - 4.1 | 2.8 - 3.5 | 3.2 - 3.8 | 4.8 - 5.7 | 8.7 - 9.4 | 2.8 - 4.7 | 5.2 - 6.0 | 2.0 - 8.5 | 6.7 - 11.2 |
| Annual cost per patient (€) | ||||||||||
| Mean (SD) | 5075.9 (8387.8) | 7736.3 (13,870.1) | 5828.2 (7933.9) | 10,001.5 (19,933.3) | 11,892.0 (14,864.6) | 25,620.6 (35,101.6) | 7241.0 (8882.3) | 26,171.7 (23,461.5) | 19,844.4 (25,363.5) | 82,978.5 (64,774.0) |
| Median | 2482.8 | 4121.3 | 3252.2 | 4468.9 | 6711.2 | 14,109.9 | 4405.8 | 19,422.8 | 9478.2 | 61,534.3 |
| 95% CI | 5001.7 - 5150.2 | 7659.2 - 7813.5 | 5283.0 - 6373.4 | 8980.6 - 11,022.4 | 11,475.1 - 12,308.9 | 24,806.0 - 26,435.3 | 6602.1 - 7879.8 | 24,762.4 - 27,581.0 | 11,732.7 - 27,956.0 | 63,743.0 - 102,214.0 |
CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CI, confidence interval; SD, standard deviation.
Similarly, the length of stay per visit was longer in patients with DCC (9.1 [SD, 15.4] days), those with HCC (5.6 [SD, 6.3] days) and those who underwent LT (9.0 [SD, 7.6] days) compared with patients with NAFLD/NASH (4.1 [SD, 7.4] days), although the length of stay was shorter for patients with CC (3.5 [SD, 5.6] days). Diagnosis of NAFLD/NASH lead to an increase in length of stay per visit (4.1 post-index vs 2.7 days pre-index).
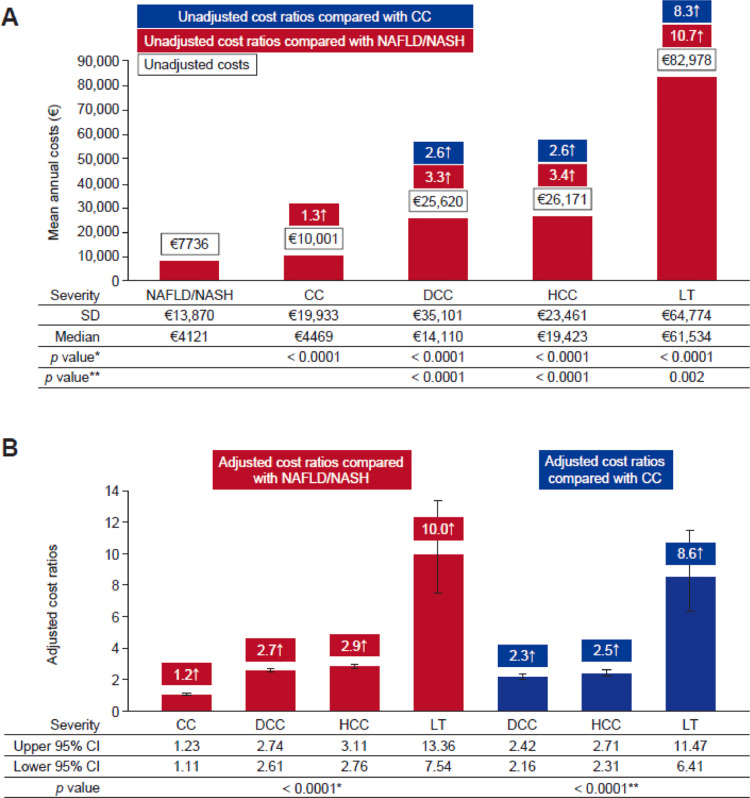
Annual hospitalization costs were generally higher for patients with more advanced liver disease compared with those with less severe disease, increasing from €7736.3 (SD, 13,870.1) in patients with NAFLD/NASH to €10,001.5 (SD, 19,933.3) with CC, €25,620.6 (SD, 35,101.6) with DCC, €26,171.7 (SD, 23,461.5) with HCC, and rising markedly to €82,978.5 (SD, 64,774.0) when patients require LT (p < 0.0001 for all comparisons with NAFLD/NASH, Fig. 2).
Fig. 2.
Incremental annual costs by liver disease severity: (A) unadjusted mean annual hospitalization costs per patient in 2015€ and (B) adjusted cost ratios (generalized linear model, gamma distribution, log-link function; adjusted for patient demographic and clinical characteristics). *P-values compared to NAFLD/NASH; **P-values compared to CC. NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplant; CI, confidence interval; SD, standard deviation.
3.4. Model for healthcare costs
An adjusted multivariable model demonstrated that age, gender, certain comorbid conditions and increased disease severity were significant predictors of higher hospitalization costs (all p < 0.0001; Table 3). Similar to the unadjusted model, adjusted cost ratios for patients with NAFLD/NASH (controlling for patient demographics and comorbidities) significantly increased with liver disease progression (Fig. 2). This result indicates that the rise in unadjusted costs observed with increased NAFLD disease severity are primarily driven by liver disease. Compared with patients with NAFLD/NASH, adjusted cost ratios were 1.2 (95% confidence interval [CI], 1.11–1.23) times higher for patients with CC, 2.7 (95% CI, 2.61–2.74) times higher for those with DCC, 2.9 (95% CI, 2.76–3.11) times higher for those with HCC, and 10.0 (95% CI, 7.54–13.36) times higher for those who underwent LT (p < 0.0001, Fig. 2b). Comorbidities including CVD, renal impairment, HTN and sleep apnoea were also significant predictors of higher healthcare costs (all p < 0.0001; Table 3).
Table 3.
Adjusted multivariable model for incremental burden of hospitalization costs in patients with liver disease.
| Parameter | Relative increase | 95% CI | p value |
|---|---|---|---|
| Demographics | |||
| Aged < 65 years | Reference | ||
| Aged 65–69 years | 1.13 | 1.11–1.15 | < 0.0001 |
| Aged 70–74 years | 1.26 | 1.24–1.29 | < 0.0001 |
| Aged 75–79 years | 1.32 | 1.29–1.35 | < 0.0001 |
| Aged ≥ 80 years | 1.50 | 1.47–1.53 | < 0.0001 |
| Female | Reference | ||
| Male | 0.94 | 0.93–0.95 | < 0.0001 |
| Disease severity | |||
| NAFLD/NASH overall | Reference | ||
| CC | 1.17 | 1.11–1.23 | < 0.0001 |
| DCC | 2.68 | 2.61–2.74 | < 0.0001 |
| HCC | 2.93 | 2.76–3.11 | < 0.0001 |
| LT | 10.04 | 7.54–13.36 | < 0.0001 |
| Disease severity | |||
| CC | Reference | ||
| DCC | 2.29 | 2.16–2.42 | < 0.0001 |
| HCC | 2.50 | 2.31–2.71 | < 0.0001 |
| LT | 8.58 | 6.41–11.47 | < 0.0001 |
| Comorbidities | |||
| CVD | 1.65 | 1.63–1.67 | < 0.0001 |
| Renal impairment | 1.32 | 1.30–1.34 | < 0.0001 |
| Diabetes | 0.88 | 0.87–0.89 | < 0.0001 |
| Hyperlipidaemia | 0.89 | 0.88–0.90 | < 0.0001 |
| Hypertension | 1.05 | 1.03–1.06 | < 0.0001 |
| Obesity | 1.01 | 1.00–1.02 | 0.2121 |
| Sleep apnoea | 1.18 | 1.17–1.20 | < 0.0001 |
Generalized linear model (gamma distribution; log-link function) adjusted for patient demographic and clinical characteristics and severity of liver disease.
CC, compensated cirrhosis; CI, confidence interval; CVD, cardiovascular disease; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplantation; NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
3.5. Disease progression
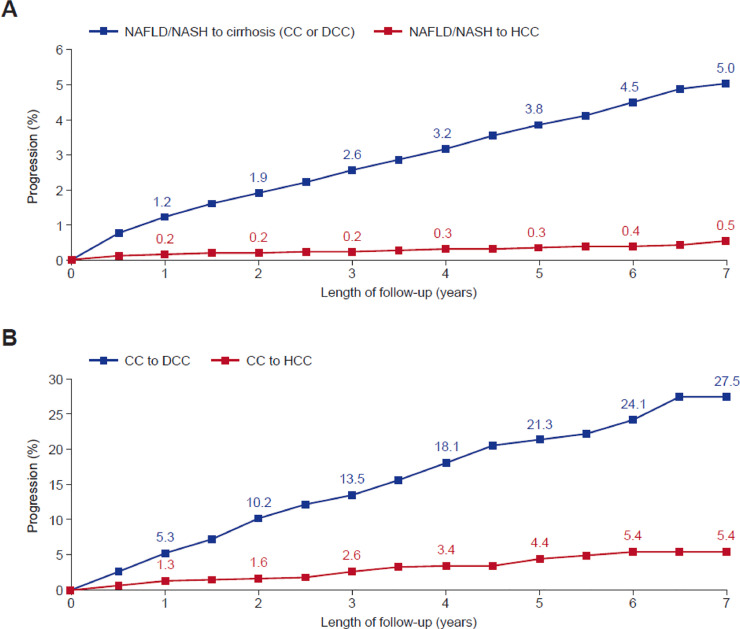
A Kaplan–Meier analysis of patients diagnosed with NAFLD/NASH over the 7 years of study follow-up found that 5.0% of patients progressed to cirrhosis (CC or DCC) and 0.5% of patients progressed to HCC (Fig. 3a). Furthermore, of patients diagnosed with CC, 27.5% progressed to DCC and 5.4% to HCC (Fig. 3b). Patients diagnosed with NAFLD/NASH at baseline were over 5 times more likely to subsequently be diagnosed with DCC (2.2%) than CC (0.4%) (Supplemental Fig. 2).
Fig. 3.
Kaplan–Meier Analysis of Progressive Liver Disease in Patients with NAFLD/NASH (A) or CC (B). NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; NAFLD/NASH N = 125,052, CC N = 1491.
3.6. Mortality
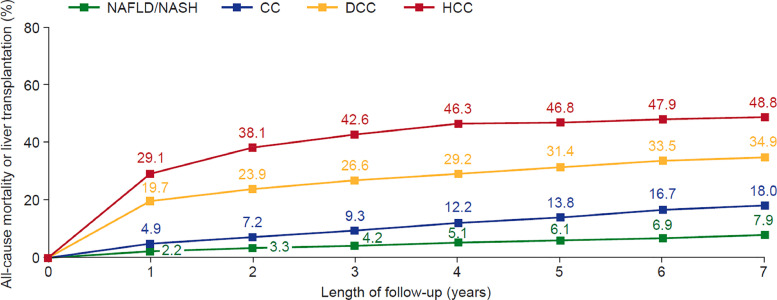
Kaplan–Meier analysis of mortality found that mortality increased with liver disease progression (Fig. 4), starting in the first year following diagnosis. After 1 year, mortality was more than twice as high in patients with CC than in those with NAFLD/NASH (4.9% vs 2.2%), more than 4 times higher in patients with DCC than in those with CC (19.7% vs 4.9%). Overall mortality after 7 years was 7.9% in patients with NAFLD/NASH, 18.0% in patients with CC, 34.9% in patients with DCC and 48.8% in patients with HCC.
Fig. 4.
Kaplan–Meier survival analysis of NAFLD/NASH patients to mortality or liver transplant by liver disease severity. Note: Patient groups are not mutually exclusive. NAFLD, non-alcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; NAFLD/NASH N = 125,052, CC N = 1491, DCC N = 7846, HCC N = 1144.
4. Discussion
By characterizing and longitudinally following for up to 7 years over 100,000 patients in the PMSI database, which has been used extensively to provide real-world evidence to HTAs and medical decision makers [20], this retrospective, observational cohort study of all hospitalized patients with a NAFLD/NASH diagnosis in France between 2009 and 2015 addresses important data gaps in our understanding of the disease. Specifically, this study provides important evidence on patient identification patterns in real-world clinical practice, natural history of the disease in diagnosed patients, and the substantial impact of NASH and its progression to severe liver disease on patients’ hospitalization resource utilization and costs. Unlike other French healthcare databases, which provided data on a limited sample of a population to inform generalizations to a broad population, the PMSI records every inpatient admission in the country and thus extrapolation of our findings from a sample to a population is not needed. Previous NAFLD studies report a substantial increase in the burden of the disease late in its course and driven by inpatient admissions [21,22]. Based on these factors, the PMSI provided an ideal research setting for an evaluation of French NAFLD disease burden.
This study provides evidence regarding the clinical practice identification patterns of patients with NAFLD/NASH in the real-world setting. While the NAFLD prevalence rate in France is estimated to be greater than 20%, [5] only 0.4% of the French hospital inpatient population had a diagnosis for NAFLD, which indicates that many hospitalized NAFLD patients are not correctly identified and diagnosed in the French hospital system. Furthermore, 82% of patients with cirrhosis in the study were first identified at decompensation stage. These findings are in alignment with previous studies which note that NAFLD/NASH is frequently identified late, as the majority of patients have non-specific symptoms in the early disease stages that would not routinely be identified in clinical practice [21,[23], [24], [25], [26]]. Moreover, it has been reported that reliable and validated non-invasive tests, which can distinguish between mild/moderate (stage F0–2) and advanced (stage F3–4) liver fibrosis in NAFLD and avoid the morbidity risk associated with liver biopsy, are not yet widely used [24,27]. Screening for NAFLD/NASH is currently not recommend by practice guidelines due to the lack of treatment options [28]. However, with consistent findings of vast underdiagnosis of NAFLD, along with the current development and review of promising NAFLD pharmaceutical interventions, [29] compelling evidence exists to support implementation of broad NAFLD screening to identify patients at earlier stages in their disease course.
The finding of the late diagnosis of NAFLD implies that this study's sample of NAFLD patients is skewed towards those with more advanced disease as these are the patients most likely to be identified in clinical practice; therefore, this study's natural history estimates likely overestimate the true risks. However, given this study's strict exclusion criteria and that all hospitalized patients with a diagnosis for NAFLD have been collected, this study provides valuable information to practitioners and decision makers regarding diagnosed NAFLD patients’ risks of progression and mortality for the foreseeable future and until a time when all NAFLD patients entering the French hospital system are correctly identified and diagnosed.
Kaplan–Meier survival analysis of patients diagnosed with NAFLD/NASH demonstrated that mortality increased substantially with liver disease progression and after 7 years, mortality was more than doubled in patients with CC (18.0%) compared with those with NAFLD/NASH (7.9%), and doubled again in patients with DCC (34.9%) compared with those with CC. These findings build on earlier studies of patients with NAFLD and histologically confirmed fibrosis, which found that mortality was substantially increased in patients with advanced fibrosis (fibrosis stage F3 and F4 [CC]) compared with patients with no fibrosis [13]. Furthermore, mortality was high for all patients with NAFLD/NASH, as the overall mortality in the NAFLD/NASH cohort of 7.9% was higher than the expected rate of 5.2% for the general French population of a similar age [30].
This study also found that patients diagnosed with NAFLD/NASH are at a high risk of progression to more advanced stages of liver disease. The real-world progression rate of patients diagnosed with CC to clinical events (DCC, HCC or death) found over 24 months in this study (19%) further builds on a recent clinical trial showing that 19% of patients with CC progressed to a clinical event in a median time of 30.9 months [12]. The findings provide evidence of the need for clinical management to screen and diagnose NAFLD/NASH at earlier stages of the disease, when interventions to potentially halt progression to cirrhosis or reverse liver disease progression can be applied [31].
NAFLD/NASH is one component of metabolic disorder, and a high incidence of comorbidities associated with metabolic disease have been observed in previous studies of patients with NASH [1,[32], [33], [34]–35]. Similarly, this study of patients with NAFLD/NASH found a high comorbidity burden: at least 40% of patients diagnosed with NAFLD/NASH had comorbidities of DM, CVD and HTN. Rates of comorbidities in this study increased with NAFLD/NASH disease progression to CC and DCC.
Our results show that hospitalization resource utilization increases with diagnosis of NAFLD/NASH and with liver disease progression, with an increase in the annual number of hospital visits and increased length of stay per visit. As resource utilization increased, unadjusted and adjusted hospitalization costs also increased with disease progression. While NAFLD is only one component of metabolic disease, which is associated with many costly comorbidities, similar significant increases in unadjusted and adjusted cost ratios (controlling for patient demographics and comorbidities) indicate that liver disease was the primary driver of increased costs seen in the NAFLD population. A study on patients with hepatitis-C also using data from the PMSI reported substantially lower mean annual costs with disease progression of €2104 (CC), €10,400 (DCC), and €11,739 (HCC) (all €2013) than found in this study [36]. Thus, progression to severe liver disease may have a markedly higher economic burden in NAFLD/NASH compared with hepatitis-C, possibly due to the high burden of costly metabolic comorbidities associated with NAFLD/NASH patients. Additional evaluations of the burden of NAFLD may consider building on the results of our adjusted model by establishing a cohort of non-NAFLD matched patients for comparison with NAFLD patients to further elucidate the incremental resource utilization burden associated with NAFLD diagnosis.
The substantial number of patients with DCC identified over the time period of this study, along with the high cost burden associated with DCC diagnosis, indicates a high economic burden associated with French NASH patients’ progression to DCC. Specifically, over 7500 individual patients diagnosed with DCC and an annual hospitalization cost associated with DCC diagnosis of over €25,000 suggest an estimated total cost burden to the French hospital system associated of over €200 million over the course of the study.
Certain comorbid conditions in patients with NAFLD/NASH were also associated with increased costs when controlling for patient demographics and liver disease severities, indicating that the cost burden associated with NASH also extends to extrahepatic conditions. Specifically, CVD and renal impairment were associated with the largest cost increases. However, an unexpected finding in the study was that DM and hyperlipidaemia were associated with a negative impact on costs. Previous research has suggested that treatments for these conditions, such as insulin sensitizers (e.g. pioglitazone) and statins, may have extended treatment effects on the liver, leading to a slowing of NASH disease progression [[37], [38], [39], [40]–41].
A key strength of this study is the large population-based, longitudinal dataset that has been utilized, which includes all hospitalized French patients during the study period. To our knowledge, this study represents the first assessment of NAFLD/NASH patients’ real-world economic and clinical outcome in France. Longitudinally following patients for up to 7 years provided sufficient time to evaluate disease severity progression and associated changes in economic burden. Certain limitations should be acknowledged that were inherent in the PMSI database. Calculated resource utilization and costs are also all-cause figures, rather than relating to a specific diagnosis, treatment, and management of comorbidities. In addition, costs incurred outside a hospital setting, including all outpatient costs, were not captured and patients with NAFLD/NASH concomitant with other aetiologies of liver disease were excluded from the study; therefore, the hospitalization resource utilization and costs estimated in this study are likely to be an underestimation of the true economic burden. Further evaluations of the NAFLD diagnosis patterns in routine clinical practice and characterizations of the clinical and economic burdens of the disease outside of the hospital setting are needed to fully elucidate the impact of the disease to the French health system. The data may be affected by database entry errors and misclassification. Patients with NAFLD/NASH alone who were included in our analysis might include F0-F3 patients as well as undiagnosed F4 (compensated cirrhosis) patients due to under-coding and the lack of specific ICD-10-CM codes for F0-F3. We thus could not granulize rates of progression by fibrosis stage and variations may exist in the composition of fibrosis score distribution by database. The apparent under-diagnosis of NAFLD likely led to this study providing overestimations of the true risks of progression and mortality. Furthermore, the observation of NAFLD underdiagnosis highlights the need for treatment guidelines to consider a recommendation of broad NAFLD screening with validated identification algorithms. Such screening algorithms, if based on age and combinations of obesity and metabolic commodities observed in this study to be associated with NAFLD, may also be utilized in future database evolutions to identify and characterize undiagnosed NAFLD patients and cryptogenic cirrhotic patients with likely NAFLD etiology of liver disease. In addition, owing to the nature of the PMSI database, patient mortality that occurred outside of the inpatient setting was not recorded leading to the potential for underestimation of the true risk of mortality by disease stage.
In conclusion, this study of the French hospitalized population found a high comorbidity and economic burden associated with NAFLD/NASH and a high risk of disease progression and mortality. These results, along with the model estimates of increasing prevalence of NASH among the French population, [5,15] likely have broad public health implications. They suggest that NASH will have a substantial and increasing future clinical and economic impact and the timely identification and effective treatment of NASH in the primary care setting prior to inpatient admission will be required to reduce the impact observed in this study to the French hospital system.
Financial support
This study was financially supported by Gilead Sciences.
Declaration of Competing Interest
Jérôme Boursier has received research grants, honoraria and fees from the following companies Abbvie, Allergan, Bio-Rad, Diafir, Echosens, Gilead, Intercept, Novo Nordisk, Pfizer, Siemens
Jeremy Fraysse and Sanatan Shreay are employees of Gilead Sciences
Cecile Fabron and Elodie Torreton are employees of CEMKA and worked as consultants for Gilead Sciences
Acknowledgment
Writing Assistance Obaro Evuaherhe, PhD.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100445.
Appendix. Supplementary materials
References
- 1.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 2.Wainwright P., Byrne C.D. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci. 2016;17(3):367. doi: 10.3390/ijms17030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z., Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150(8):1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Estes C., Anstee Q.M., Teresa Arias-Loste M. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Adams L.A., Lymp J.F., St Sauver J. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654. doi: 10.1016/j.cgh.2014.04.014. e641-649; quiz e639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekstedt M., Hagstrom H., Nasr P. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 11.Younossi Z.M., Stepanova M., Rafiq N. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal A.J., Harrison S.A., Ratziu V. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70:1913–1927. doi: 10.1002/hep.30664. [DOI] [PubMed] [Google Scholar]
- 13.Dulai P.S., Singh S., Patel J. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung A., Figueredo C., Rinella M.E. Nonalcoholic fatty liver disease: identification and management of high-risk patients. Am. J. Gastroenterol. 2019;114(4):579–590. doi: 10.14309/ajg.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 15.Younossi Z.M., Blissett D., Blissett R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 16.Berger M.L., Sox H., Willke R.J. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26(9):1033–1039. doi: 10.1002/pds.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motheral B., Brooks J., Clark M.A. A checklist for retrospective database studies—report of the ISPOR task force on retrospective databases. Value Health. 2003;6(2):90–97. doi: 10.1046/j.1524-4733.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 18.Moulis G., Lapeyre-Mestre M., Palmaro A., Pugnet G., Montastruc J.L., Sailler L. French health insurance databases: what interest for medical research? Rev Med Intern. 2015;36(6):411–417. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Tuppin P., Rudant J., Constantinou P. Value of a national administrative database to guide public decisions: from the systeme national d'information interregimes de l'Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Tuppin P., Rudant J., Constantinou P. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65:S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Patton H.M., Nyberg A.H., Caparosa S. Tu1459 - healthcare resource utilization, demographics, and comorbidities in non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) and progressive stages in a large, integrated healthcare delivery system. Gastroenterology. 2018;154(6, Supplement 1):S1223–S1224. [Google Scholar]
- 22.Gordon S., Fraysse J., Li S., Ozbay A.B., Wong R.J. Disease severity is associated with higher healthcare utilization in nonalcoholic steatohepatitis medicare patients. Am. J. Gastroenterol. 2020;115:562–574. doi: 10.14309/ajg.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 23.Liberal R., Grant C.R. Cirrhosis and autoimmune liver disease: current understanding. World J Hepatol. 2016;8(28):1157–1168. doi: 10.4254/wjh.v8.i28.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 25.Shah N.L., Banaei Y.P., Hojnowski K.L., Cornella S.L. Management options in decompensated cirrhosis. Hepat Med. 2015;7:43–50. doi: 10.2147/HMER.S62463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuppalanchi R., Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Association for the Study of the L European association for the study of D, European association for the study of O. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 29.Rotman Y., Sanyal A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66(1):180–190. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 30.Institut national d'études démographiques, 2018. Mortality rates by sex and age: https://www.ined.fr/en/everything_about_population/data/france/deaths-causes-mortality/mortality-rates-sex-age/.
- 31.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005. e365; quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A., Wong R.J., Harrison S.A. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13(12):2062–2070. doi: 10.1016/j.cgh.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Davis G.L., Roberts W.L. The healthcare burden imposed by liver disease in aging baby boomers. Curr Gastroenterol Rep. 2010;12(1):1–6. doi: 10.1007/s11894-009-0087-2. [DOI] [PubMed] [Google Scholar]
- 34.Lazo M., Hernaez R., Eberhardt M.S. Prevalence of nonalcoholic fatty liver disease in the United States: the third national health and nutrition examination survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayiner M., Otgonsuren M., Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254–260. doi: 10.1097/MCG.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abergel A., Rotily M., Gaudin A.F. Mean annual Cost of patients hospitalized for chronic hepatitis C In France: the hepc-lone study. Value Health. 2014;17(7):A364. doi: 10.1016/j.jval.2014.08.809. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell S. NASH Therapy: omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin Mol Hepatol. 2017;23(2):103–108. doi: 10.3350/cmh.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59(6):1112–1120. doi: 10.1007/s00125-016-3952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazlehurst J.M., Woods C., Marjot T., Cobbold J.F., Tomlinson J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65(8):1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maroni L., Guasti L., Castiglioni L. Lipid targets during statin treatment in dyslipidemic patients affected by nonalcoholic fatty liver disease. Am J Med Sci. 2011;342(5):383–387. doi: 10.1097/MAJ.0b013e318213e526. [DOI] [PubMed] [Google Scholar]
- 41.Musso G., Cassader M., Cohney S. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care. 2016;39(10):1830–1845. doi: 10.2337/dc15-1182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.