Abstract
A 44-year-old gentleman with stage III (T4N1M0) unresectable pancreatic adenocarcinoma at the uncinate process underwent percutaneous image guided Irreversible Electroporation (IRE). At day-1 post IRE the patient developed severe abdominal pain and had computed tomography for assessment of his symptoms. Computed tomography showed severe duodenal wall thickening with local inflammatory changes and was reported as duodenal infarction based on imaging features. Following conservative management with better pain management, both the clinical symptoms and imaging features resolved uneventfully. This case has highlighted severe duodenal swelling seen in patients post IRE for locally advanced pancreatic cancer may mimic duodenal infarction and is an important differential diagnosis to ensure appropriate clinical management.
Keywords: Irreversible Electroporation, Locally Advanced Pancreatic Cancer (LAPC), Duodenal Thickening, Duodenal Infarction
Case report
Irreversible electroporation (IRE) is a non-thermal ablation modality that complements chemotherapy for patients with unresectable pancreatic tumor [1]. The technique fires short pulses of direct electrical current with high voltage, punching nanopores in cell membranes and consequently causing cell lysis [2]. Due to its nonthermal nature in contrast to other ablation techniques, IRE does not pose risk to important neighboring organs or vascular structures and its ablation effectiveness is not limited by heat sink effect. IRE is therefore the preferred ablation modality when tumors situates near structures such as blood vessels, bowel, bile duct, and ureter [3].
In this article, we report unusual radiological findings of severe duodenal thickening mimicking duodenal infarction post-IRE of Locally Advanced Pancreatic Cancer (LAPC) near the uncinate process, which resolved spontaneously with conservative management.
A 44-year-old gentleman diagnosed with stage III (T4N1M0) unresectable pancreatic adenocarcinoma in the uncinate process was referred for percutaneous IRE. He was unresponsive to FOLFIRINOX chemotherapy in an attempt to downstage the cancer and therefore, deemed unfit for surgical resection. Computed Tomography (CT) imaging revealed a 3 cm mass in the uncinate process with superior mesenteric artery and coeliac axis encasement. There was no biliary dilatation, no metastatic disease and the tumor size appears stable when compared with CT images at the time of treatment.
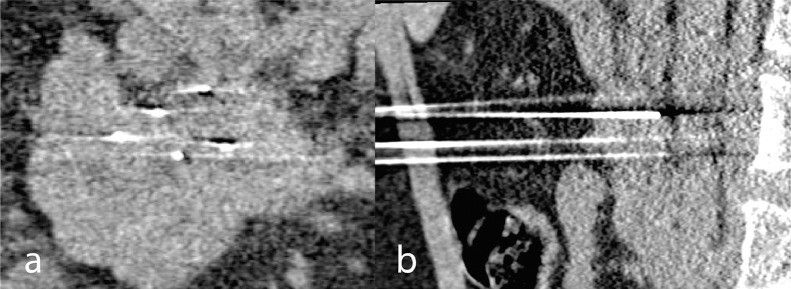
Patient underwent percutaneous image-guided IRE under general anesthesia with CT guidance. An IV antibiotics was provided. A contrast-enhanced CT was performed for pre-procedural planning to allow targeting of the LAPC with IRE treatment. The needle placement was performed under CT guidance. A total of 5 IRE electrodes – 1 activator and 4 standard electrodes – were placed around the tumor (aiming for around 1.8–2.2 cm interelectrode distance) with exposure tip of 1.5cm (Fig. 1A and B) to achieve 2 overlapping ablations. The IRE generator device (NanoKnife; AngioDynamics, Inc, Queensbury, New York) produced 70-ms high-voltage (1500–3000 V) of direct current (25–45 A) electrical pulses across 8 paired unipolar electrodes in 20 sets. A total of 2 sessions of 90 paired electrical pulses across the paired electrodes were fired with a pullback treatment of 1 cm. All activations were gated with the cardiac cycle with synchronization device to deliver the electrical pulse during the ventricular refractory period. Immediately post-IRE, the patient was monitored for as per standard care in the peri-operative recovery ward, with IV opiates given for pain relief. He was transferred to the normal ward after recovering from general anesthesia.
Fig. 1.
IRE electrode needle placement around tumor in A: coronal B: sagittal plane on CT.
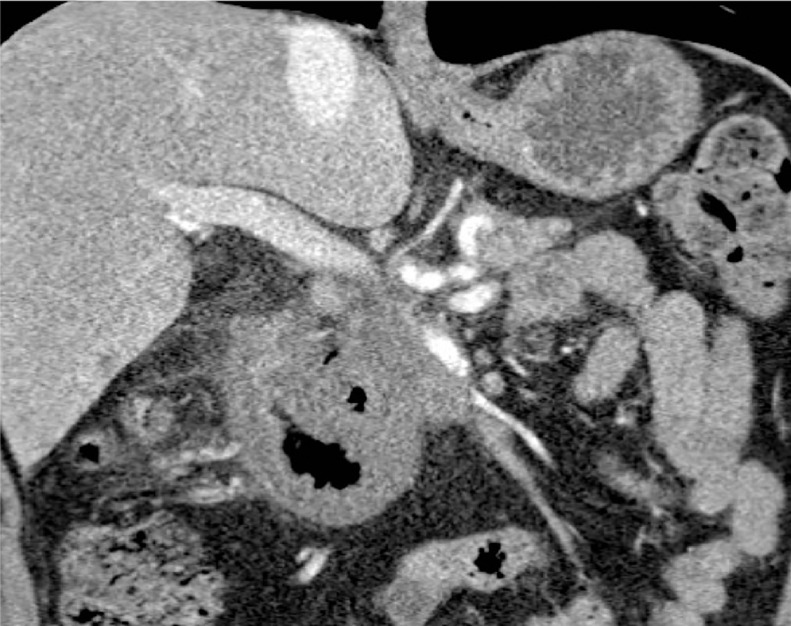
The patient developed severe abdominal pain mimicking peritonitis the following day and an urgent CT was performed to assess his symptoms. The post IRE CT scan when compared to baseline pre-IRE CT (Fig. 2) showed massive duodenal wall thickening with surrounding inflammatory changes and was reported as Duodenal Infarction (Fig. 3). The D2 and proximal half of D3 were circumferentially oedematous with no significant wall enhancement. Presence of gas within duodenal wall and within epicentre of pancreatic tumor and small volume of free gas within the mesentery was noted together with traces of pelvic fluid. When reviewed, the patient was clinically stable with white cell count pre- and post-IRE unchanged measuring at 16 × 109/L and CRP only mildly rose from 6.7 to 25. The radiological diagnosis of duodenal infarction was discussed with the patient and his family. Due to the advanced nature of the pancreatic tumor it was explained that there was no surgical option and we would treat him expectantly with analgesia and total parental nutrition (TPN). Preprocedure the patient was on a significant level of opiates, once his analgesia was optimized, his symptoms started to settle, and he improved clinically.
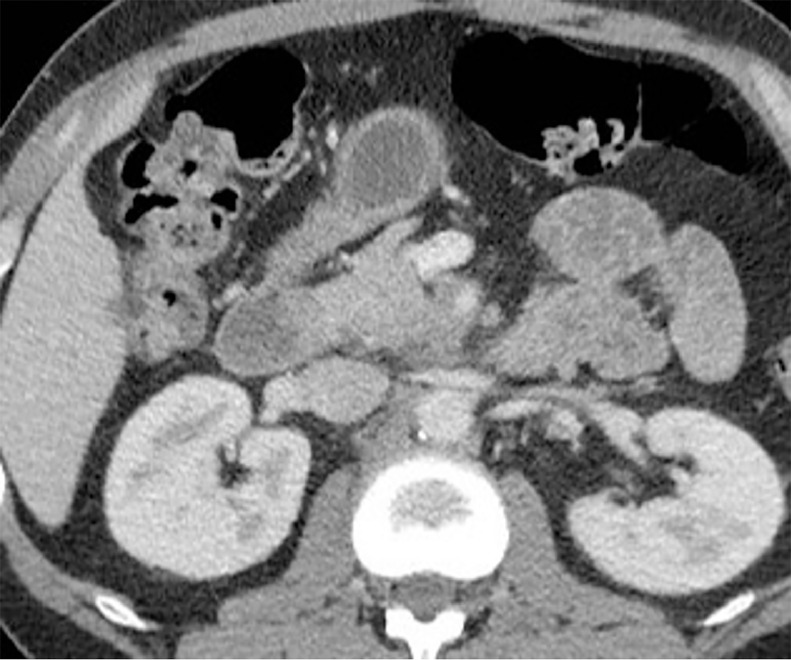
Fig. 2.
Pre IRE treatment axial CT showing normal thin walled duodenum.
Fig. 3.
1-day post IRE coronal CT scan showing duodenal thickening and signs mimicking duodenal infarction.
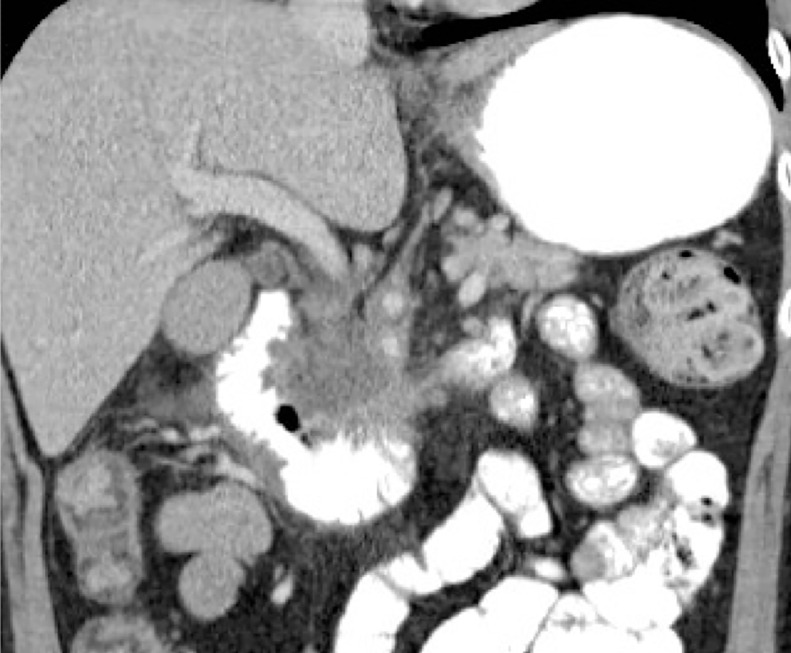
After a week of conservative management, he further improved. A repeat contrast-enhanced CT scan with oral contrast was performed, showing improved appearances of the duodenum with resolution of the duodenal thickening and oedema (Fig. 4). Subsequently the patient started oral intake with no adverse events. At 2-weeks, he had a transient rise in liver function tests, likely reflecting pancreatic inflammation. The patient was discharged home after.
Fig. 4.
1-week post IRE coronal CT scan showing resolution of duodenal wall thickening.
PET CT scan performed 1-month later suggested disease progression. The tumor within the uncinate process of pancreas increased in size and multiple new bone metastasis were identified. Unfortunately, the gentleman passed away 4 months after the initial IRE procedure, with an overall survival of 12 months following the initial diagnosis of LAPC.
Discussion
Reported complications of IRE of LAPC include duodenal leakage and pancreatic leakage [1], however, massive duodenal swelling mimicking duodenal infarction has not been reported previously. Duodenal infarction is a life-threatening condition with mortality rate ranging between 60% and 100% when attributed to acute mesenteric ischaemia. Acute mesenteric ischaemia is commonly caused by embolism in mesenteric arteries, most commonly related to interventional radiology procedure. Other causes include arterial thrombosis, arterial vasculitis, external compression of mesenteric arteries, venous thrombosis, and hypoperfusion induced non-occlusive ishcaemia. Patient normally presents with sudden onset severe abdominal pain, accompanied with nausea, vomitting, and diarrhoea. Laboratory test results would show leucocytosis, metabolic acidaemia, increased CRP, and serum amylase. Management of acute mesenteric ischaemia in general includes resuscitation, supportive measures, and appropriate enteric antibiotics. Depending on the underlying aetiology, additional treatments such as revascularisation, heparinisation, bowel resection, papaverine infusion, or corticosteriod therapy might be initiated [4].
In our case, the patient's CT scan 1-day post-op mimicked the radiological appearances of duodenal infarction [5]. Only conservative supportive measures were initiated as the patient was clinically stable. We hypothesize that IRE triggered inflammatory response in the duodenum which leads to duodenal wall thickening that mimics duodenal infarction. It is known that IRE post-op can trigger a pro-inflammatory environment [6]. Therefore, swollen duodenum post-IRE of LAP should be interpreted with caution as it could be due to inflammation rather than infarction as illustrated in our case (Fig. 3).
Conclusion
Severe duodenal thickening/swelling post-IRE of LAPC is an important differential of duodenal thickening mimicking duodenal infarction and this can be treated with conservative management.
Ethical approval
No ethics approval was required as the study was not classified as research under the United Kingdom National Health Service Health Research Authority.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent for publication was obtained for every individual person's data included in the study.
Footnotes
This study was not supported by any funding.
Acknowledgment: The authors made the following contributions to this study: guarantor of integrity of entire study: T.M. Wah; literature search: K. Wang, H. Ng, T.M. Wah; case acquisition: T.M. Wah; manuscript preparation and editing: K. Wang, H. Ng, T.M. Wah; and manuscript review: K. Wang, H. Ng, T.M. Wah.
Competing Interests: Professor Tze Min Wah receives Educational Grant from Angiodynamics and Research Grant from Boston Scientific.
References
- 1.Martin R.C.G., Kwon D., Chalikonda S., Sellers M., Kotz E., Scoggins C. Treatment of 200 locally advanced (Stage III) pancreatic adenocarcinoma patients with irreversible electroporation safety and efficacy. Ann. Surg. 2015;262::486–492. doi: 10.1097/SLA.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 2.Davalos R.V., Mir L.M., Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 3.Mann C.D., Metcalfe M.S., Lloyd D.M., Maddern G.J., Dennison A.R. The safety and efficacy of ablative techniques adjacent to the hepatic vasculature and biliary system. ANZ J Surg. 2010;80:41–49. doi: 10.1111/j.1445-2197.2009.05174.x. [DOI] [PubMed] [Google Scholar]
- 4.Roon A.von., Lewis J. Ischaemic bowel disease. BMJ Best Pract. 2020 https://bestpractice.bmj.com/topics/en-gb/818/pdf/818/Ischaemic bowel disease.pdf (accessed 3 May 2020) [Google Scholar]
- 5.Ansari D., Torén W., Lindberg S., Pyrhönen H.-S., Andersson R. Diagnosis and management of duodenal perforations: a narrative review. Scand J Gastroenterol. 2019;54:939–944. doi: 10.1080/00365521.2019.1647456. [DOI] [PubMed] [Google Scholar]
- 6.Brock R.M., White N.B., Ringel-Scaia V.M., Coutermarsh-Ott S.L., Eden K., Coutri J. Irreversible electroporation stimulates a pro-inflammatory tumor microenvironment in pancreatic cancer. J Immunol. 2019;202 [Google Scholar]