Key Points
Question
Are differences in risk-adjusted mortality rates between critical access hospitals (CAHs) and non-CAHs related to differences in diagnosis coding practices?
Findings
In this serial cross-sectional study of 4 094 720 rural Medicare beneficiaries hospitalized from 2007 to 2017, combined in-hospital and 30-day postdischarge mortality rates were higher for CAHs than non-CAHs when risk adjustment included both preexisting conditions and in-hospital comorbidity measures, but were not significantly different for CAHs vs non-CAHs when adjusting only for preexisting conditions.
Meaning
The findings suggest that short-term mortality outcomes at CAHs may not differ from those of non-CAHs when risk adjustment is based on preexisting conditions that are not influenced by coding practices for in-hospital comorbidities.
Abstract
Importance
Critical access hospitals (CAHs) provide care to rural communities. Increasing mortality rates have been reported for CAHs relative to non-CAHs. Because Medicare reimburses CAHs at cost, CAHs may report fewer diagnoses than non-CAHs, which may affect risk-adjusted comparisons of outcomes.
Objective
To assess serial differences in risk-adjusted mortality rates between CAHs and non-CAHs after accounting for differences in diagnosis coding.
Design, Setting, and Participants
Serial cross-sectional study of rural Medicare Fee-for-Service beneficiaries admitted to US CAHs and non-CAHs for pneumonia, heart failure, chronic obstructive pulmonary disease, arrhythmia, urinary tract infection, septicemia, and stroke from 2007 to 2017. The final date of follow-up was December 31, 2017.
Exposure
Admission to a CAH vs non-CAH.
Main Outcomes and Measures
Discharge diagnosis count including trends from 2010 to 2011 when Medicare expanded the allowable number of billing codes for hospitalizations, and combined in-hospital and 30-day postdischarge mortality adjusted for demographics, primary diagnosis, preexisting conditions, and with vs without further adjustment for Hierarchical Condition Category (HCC) score to understand the contribution of in-hospital secondary diagnoses.
Results
There were 4 094 720 hospitalizations (17% CAH) for 2 850 194 unique Medicare beneficiaries (mean [SD] age, 76.3 [11.7] years; 55.5% women). Patients in CAHs were older (median age, 80.1 vs 76.8 years) and more likely to be female (58% vs 55%). In 2010, the adjusted mean discharge diagnosis count was 7.52 for CAHs vs 8.53 for non-CAHs (difference, −0.99 [95% CI, −1.08 to −0.90]; P < .001). In 2011, the CAH vs non-CAH difference in diagnoses coded increased (P < .001 for interaction between CAH and year) to 9.27 vs 12.23 (difference, −2.96 [95% CI, −3.19 to −2.73]; P < .001). Adjusted mortality rates from the model with HCC were 13.52% for CAHs vs 11.44% for non-CAHs (percentage point difference, 2.08 [95% CI, 1.74 to 2.42]; P < .001) in 2007 and increased to 15.97% vs 12.46% (difference, 3.52 [95% CI, 3.09 to 3.94]; P < .001) in 2017 (P < .001 for interaction). Adjusted mortality rates from the model without HCC were not significantly different between CAHs and non-CAHs in all years except 2007 (12.19% vs 11.74%; difference, 0.45 [95% CI, 0.12 to 0.79]; P = .008) and 2010 (12.71% vs 12.28%; difference, 0.42 [95% CI, 0.07 to 0.77]; P = .02).
Conclusions and Relevance
For rural Medicare beneficiaries hospitalized from 2007 to 2017, CAHs submitted significantly fewer hospital diagnosis codes than non-CAHs, and short-term mortality rates adjusted for preexisting conditions but not in-hospital comorbidity measures were not significantly different by hospital type in most years. The findings suggest that short-term mortality outcomes at CAHs may not differ from those of non-CAHs after accounting for different coding practices for in-hospital comorbidities.
This study uses Medicare data to estimate mortality differences for common medical conditions (pneumonia, heart failure, chronic obstructive pulmonary disease, urinary tract infection, others) at US critical access vs non–critical access hospitals between 2007 and 2017 with vs without adjustment for discharge diagnosis counts to assess the extent to which coding practices rather than illness severity might account for observed mortality differences.
Introduction
Critical access hospitals (CAHs) provide care to Americans living in remote rural areas. Ensuring that rural communities have adequate health care access is critically important given that they are disproportionately older, impoverished, and burdened by chronic disease.1,2,3 As such, the CAH program was established in 1997 to increase the viability of small rural hospitals, which were increasingly under financial strain and closing in large numbers. Small (<26-bed), geographically isolated hospitals are eligible for CAH designation, which allows them to receive cost-based reimbursements rather than prospective diagnosis-related group (DRG)–based reimbursements from Medicare.
Evidence pertaining to the quality of care provided by CAHs is mixed. For patients who underwent common surgical procedures, CAHs demonstrated lower complication and mortality rates compared with non-CAHs; however, among patients hospitalized for common medical conditions, CAHs demonstrated higher mortality rates.4,5,6,7,8 While higher mortality at CAHs may reflect lower quality of care, other factors may account for mortality differences between CAHs and non-CAHs. In particular, CAHs and non-CAHs may report diagnoses differently, and patients in non-CAHs may appear artificially sicker than patients in CAHs.9,10 This is because cost-based reimbursements do not incentivize CAHs to code diagnoses completely, while DRG-based reimbursements may incentivize overcoding at non-CAHs.11 Thus, risk-adjusted mortality differences between CAHs and non-CAHs may be exaggerated due to under- or over-reporting of illnesses, especially after 2011 when the Centers for Medicare and Medicaid Services (CMS) expanded the number of diagnosis codes hospitals could report.12
Assessing differences in CAH and non-CAH mortality rates, and whether they are related to diagnosis coding practices, has important implications for the CAH program and the vulnerable communities CAHs serve. Thus, the goal of this study was to examine serial differences in risk-adjusted mortality rates between CAHs and non-CAHs after accounting for differences in diagnosis coding practices between the 2 hospital types.
Methods
This study was approved by the Brown University institutional review board, which waived the requirement for participant informed consent.
Data Sources
We used 2007-2017 data from 100% Medicare Provider Analysis and Review (MedPAR) files and the Medicare Beneficiary Summary File (MBSF). MedPAR includes finalized claims for all Medicare-reimbursed inpatient services used by Medicare Fee-for-Service beneficiaries. The MBSF data include sociodemographic characteristics, chronic conditions, Medicare Advantage and Medicaid enrollment, residential location, and dates of death for all current and previously enrolled Medicare beneficiaries. We linked county of residence from the MBSF with Rural-Urban Continuum Codes to classify beneficiaries as rural or urban.13
Study Population
Our sample consisted of rural Medicare Fee-for-Service beneficiaries admitted to CAHs and non-CAHs between January 1, 2007, and November 30, 2017, with a primary diagnosis of pneumonia, congestive heart failure, chronic obstructive pulmonary disease, stroke, septicemia, urinary tract infection, and arrhythmia (identified with International Classification of Diseases codes; eTable 1 in the Supplement). These conditions were selected for being common causes for admission to both CAHs and non-CAHs (see eMethods and eTable 2 in the Supplement). Analyses were limited to rural beneficiaries because CAHs are generally not an alternative site of care for urban beneficiaries (eFigure 1 in the Supplement). Additionally, non-CAHs where urban beneficiaries receive care are likely to be inherently different from non-CAHs where rural beneficiaries receive care. Medicare Advantage enrollees were excluded given evidence that their diagnoses are overcoded due to different payment mechanisms that are not the focus of this study.14 We retained all hospitalizations in our analysis that met the CMS definition of index hospitalization (eMethods in the Supplement).15 Per CMS criteria, contiguous hospitalizations (involving transfers) were combined into a single episode of care and the patient outcome was attributed to the first hospital.
Study Variables
Main Exposure
The main exposure was admission to a CAH vs non-CAH, which was ascertained from the hospital claim. Specifically, the last 4 digits of the billing hospital’s 6-digit CMS Certification Number indicate whether a patient was hospitalized at a CAH (1300-1399) or a non-CAH providing short-term acute care (0001-0879).
Outcome
The primary outcomes of this study were discharge diagnosis count and patient mortality occurring from the time of hospital admission up until 30 days after discharge. In-hospital mortality and 30-day postdischarge mortality, combined for this analysis, have been examined in several previous analyses.5,16,17
Sociodemographic Covariates
From the MBSF, we determined age, age-squared, sex, race/ethnicity, Medicaid enrollment, and reason for Medicare entitlement (Old-Age and Survivor’s Insurance vs disability or end-stage kidney disease) at the time of hospitalization. Medicare collects self-reported race/ethnicity data at the time of enrollment using predefined categories. We controlled for race/ethnicity because prior studies have demonstrated an association between race and mortality and because the racial/ethnic composition of CAHs and non-CAHs may systematically differ.18
Clinical Characteristics
Prehospitalization illness burden was derived from the MBSF Chronic Conditions Segment, which includes indicators for the presence of 27 chronic conditions (Table), and for those identified, the dates of first occurrence.19 The dates of first occurrence are derived from previous Part A and B claims (since 1999), and were used to identify chronic conditions that were preexisting at the time of hospitalization, as done in other studies.20,21 Discharge diagnoses from the MedPAR claims were used to derive the Hierarchical Condition Category (HCC) risk score, which is severity-weighted by age, sex, Medicaid enrollment, and Medicare entitlement status.22
Table. Characteristics of Rural Medicare Beneficiaries Admitted to Critical Access Hospitals and Non–Critical Access Hospitals.
| Characteristic | Hospitalizations, % | |
|---|---|---|
| Critical access hospitals (n = 707 203) | Non–critical access hospitals (n = 3 387 517) | |
| Age, median (IQR), y | 80.1 (71.7-87.0) | 76.8 (68.8-84.4) |
| Sex | ||
| Male | 42 | 45 |
| Female | 58 | 55 |
| Race/ethnicitya | ||
| White | 94 | 89 |
| Black | 3 | 8 |
| Other | 3 | 3 |
| Medicaid enrollment | 25 | 24 |
| OASI Medicare entitlement | 90 | 85 |
| Preexisting chronic condition count, mean (SD)b | 8.8 (3.7) | 8.9 (3.8) |
| Preexisting chronic conditions | ||
| Hypertension | 87 | 88 |
| Cataracts | 73 | 66 |
| Hyperlipidemia | 70 | 75 |
| Ischemic heart disease | 64 | 67 |
| Anemia | 63 | 64 |
| Rheumatoid arthritis/osteoarthritis | 63 | 61 |
| COPD | 57 | 54 |
| Congestive heart failure | 56 | 55 |
| Depression | 44 | 43 |
| Diabetes | 42 | 47 |
| Chronic kidney disease | 34 | 39 |
| Atrial fibrillation | 29 | 30 |
| Acquired hypothyroidism | 28 | 28 |
| Alzheimer and related dementias | 27 | 24 |
| Osteoporosis | 25 | 23 |
| Stroke/transient ischemic attack | 23 | 25 |
| Asthma | 23 | 23 |
| Glaucoma | 20 | 19 |
| Benign prostatic hyperplasia | 19 | 20 |
| Acute myocardial infarction | 10 | 11 |
| Hip fracture | 8 | 7 |
| Prostate cancer | 6 | 5 |
| Breast cancer | 5 | 5 |
| Colorectal cancer | 4 | 4 |
| Lung cancer | 3 | 3 |
| Endometrial cancer | 1 | 1 |
| Discharge diagnosis count, mean (SD) | 9.2 (4.5) | 11.8 (5.4) |
| Primary diagnosis | ||
| Pneumonia | 33 | 18 |
| COPD | 19 | 15 |
| Heart failure | 15 | 16 |
| UTI | 11 | 7 |
| Arrhythmia | 8 | 14 |
| Septicemia | 7 | 18 |
| Stroke | 7 | 12 |
| HCC score, median (IQR)c | 1.3 (0.9-1.8) | 1.6 (1.1-2.3) |
Abbreviations: COPD, chronic obstructive pulmonary disease; HCC, Hierarchal Condition Category; IQR, interquartile range; OASI, Old Age and Survivor’s Insurance; UTI, urinary tract infection.
Medicare collects self-reported race/ethnicity data at the time of enrollment using predefined categories.
Count of 27 common conditions present before hospitalization, based on the date of first occurrence listed in the Medicare Master Beneficiary Summary File. Alzheimer disease is recorded separately from other dementias, but was not included because it is already captured by Alzheimer disease and related dementias.
HCC scores approximate illness burden and were derived from a combination of hospital discharge diagnoses (severity-weighted), age, sex, Medicaid enrollment, and Medicare entitlement status. Scores were normalized to a value of 1.0, with higher scores indicating greater illness.
Statistical Analysis
To examine differences in diagnosis coding between CAHs and non-CAHs, we fit linear regression models estimating the count of discharge diagnoses as a function of CAH status, sociodemographic characteristics, preexisting chronic conditions, and hospital referral region (HRR, health care market) fixed effects.23 Separate models were fit for each year of the study period. To formally assess changes over time and any trend breaks that may be associated with the 2011 CMS rule change that expanded the number of allowable diagnosis codes, we analyzed data pooled across all years and included an interaction term between calendar year dummy variables and CAH status, with 2010 as the reference year.
We then fit linear regression models, separately for each year, estimating mortality as function of CAH status, sociodemographic characteristics, preexisting chronic conditions, primary diagnosis fixed effects, HRR, and HCC score. To assess the contribution of diagnoses gathered during hospitalization, we fit a second set of models excluding the HCC score. Because primary diagnoses and components of the HCC score are included as covariates in the 2 models, the difference in mortality estimates produced effectively reflects the contribution of the secondary diagnoses coded during the inpatient stay. Changes over time were again assessed by including an interaction term between calendar year indicator and CAH status in an analysis including all years. Although mortality is a binary outcome, we used a linear probability model because our analyses include high dimensional fixed effects, which may bias a nonlinear approach such as logistic regression.24
In all analyses, standard errors were clustered at the hospital level to account for potential correlation between patient outcomes within the same hospital. Adjusted discharge diagnosis counts and mortality rates for CAHs and non-CAHs were calculated using marginal standardization.
Supplementary Analyses
We conducted several supplementary analyses. First, we examined changes in mean HCC scores for patients in CAHs and non-CAHs, given that they are frequently used in risk-adjustment methods and are included in our primary analysis. Second, we examined patients who were transferred to non-CAHs (eTable 3 in the Supplement), and compared discharge diagnosis counts at the original and final hospital. Third, we examined whether our mortality models were robust to the exclusion of transferred patients and using county fixed effects in place of HRR. Fourth, to further gauge the sensitivity of mortality differentials to diagnoses gathered during hospitalization, we estimated models with HCC scores derived from 2, 3, and 9 secondary discharge diagnoses.
Records with data missing on any covariate were excluded. Because of the potential for type I error due to multiple comparisons, findings for secondary analyses and outcomes should be interpreted as exploratory. Data were analyzed with Stata MP version 15.1 (StataCorp). Null hypotheses were tested assuming a 2-sided type I error probability of 0.05.
Results
Study Population
We identified 4 094 720 hospital admissions among rural Medicare Fee-for-Service beneficiaries who met our study inclusion criteria, including 707 203 (17%) for CAHs and 3 387 517 (83%) for non-CAHs. Data were missing on 1 or more covariates for 1653 excluded records (0.0004%). Among the overall cohort of 2 850 194 unique Medicare beneficiaries, the mean (SD) age was 76.3 (11.7) years, and 55.5% were women. Compared with patients in non-CAHs, patients in CAHs were older (median age, 80.1 years [interquartile age {IQR}, 71.7-87.0] vs 76.8 years [IQR, 68.8-84.4]) and more likely to be female (58% vs 55%), White (94% vs 89%), and eligible for Medicare due to old age vs disability or end-stage kidney disease (90% vs 85%) (Table). Patients in CAHs were more likely to have a primary diagnosis of pneumonia (33% vs 18%), chronic obstructive pulmonary disease (19% vs 15%), and urinary tract infection (11 vs 7%), and were less likely to have a primary diagnosis of arrhythmia (8% vs 14%), septicemia (7% vs 18%), or stroke (7% vs 12%). The number of discharge diagnoses for patients in CAHs was lower compared with patients in non-CAHs (mean [SD], 9.2 [4.5] vs 11.8 [5.4]).
Trends in Diagnosis Coding at CAHs vs Non-CAHs
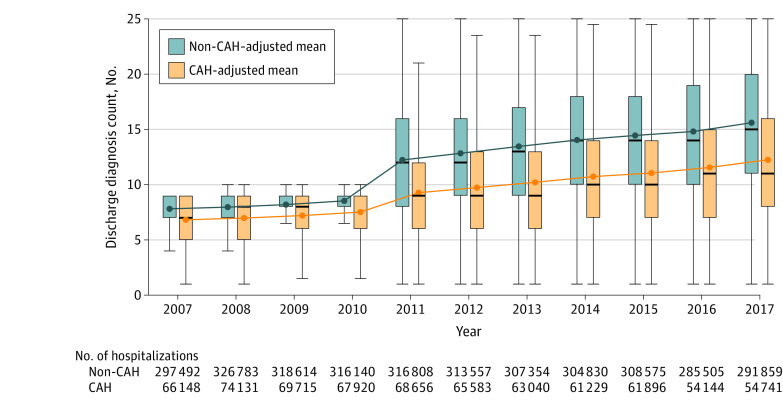
Figure 1 shows the adjusted mean count of discharge diagnoses at CAHs and non-CAHs over time. All point estimates and unadjusted results are listed in eTable 4 and eTable 5 in the Supplement. In all years, the number of discharge diagnoses submitted was significantly lower for patients in CAHs compared with patients in non-CAHs (P < .001; eTable 4 in the Supplement). In 2007, the adjusted mean number of discharge diagnoses was 6.82 for patients in CAHs and 7.81 for patients in non-CAHs (adjusted difference, −0.99 [95% CI, −1.08 to −0.90]; P < .001). In 2011, after billing code expansion, the difference in the number of diagnoses coded between CAHs and non-CAHs increased (P < .001 for interaction between CAH and year), and the adjusted mean number of discharge diagnoses became 9.27 for patients in CAHs and 12.23 for patients in non-CAHs (adjusted difference, −2.96 [95% CI, −3.19 to −2.73]; P < .001). By 2017, the adjusted mean number of discharge diagnoses was 12.25 for patients in CAHs and 15.60 for patients in non-CAHs (adjusted difference, −3.35 [95% CI, −3.65 to −3.06]; P < .001).
Figure 1. Adjusted Mean Count of Discharge Diagnoses for Critical Access Hospitals (CAHs) and Non-CAHs, 2007-2017.
The figure shows crude discharge diagnosis counts (box plots) and adjusted discharge diagnosis counts (blue and yellow lines). The box plot lines indicate the 25th percentile, median, and 75th percentile. The whiskers represent the furthest observed points that are within 1.5 times the interquartile range. Covariates used to estimate adjusted discharge diagnosis count were age, age-squared, sex, race/ethnicity (White, Black, or other), Medicaid enrollment, Medicare entitlement reason (Old Age and Survivor’s insurance vs all else), 27 chronic conditions present prior to the index admission (Table), primary diagnosis, and hospital referral region fixed effects.
Trends in the mean HCC score for CAH and non-CAH discharges are shown in eFigure 2 in the Supplement. These were significantly lower for CAHs compared with non-CAHs in all years (P < .001; eTable 6 in the Supplement). Discharge diagnosis counts for patients who were transferred, before and after 2011, are shown in eTable 7 in the Supplement. Compared with patients in non-CAHs who were transferred to other non-CAHs, patients in CAHs who were transferred to non-CAHs had a larger increase in the number of discharge diagnoses reported following transfer in the years prior to 2011 (pre-post transfer change, 1.34 for CAHs vs 0.45 for non-CAHs; difference, 0.89 [95% CI, 0.79 to 0.98]; P < .001) and afterwards (pre-post change, 5.20 for CAHs vs 2.56 for non-CAHs; difference, 2.63 [95% CI, 2.37 to 2.90]; P < .001).
Risk Adjustment and Differences in Mortality at CAHs and Non-CAHs
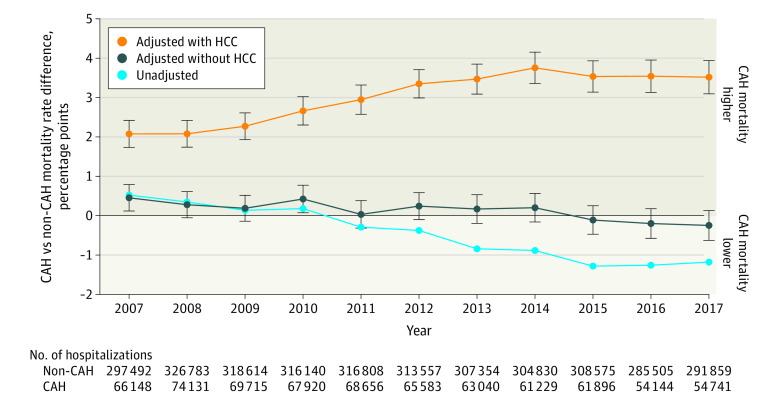
Figure 2 illustrates differences in combined in-hospital and 30-day postdischarge mortality rates between individuals admitted to CAHs and non-CAHs over time. Mortality event counts are listed in eTable 8 in the Supplement. Point estimates are listed in eTable 9 and eTable 10 in the Supplement. In analyses adjusted for sociodemographic characteristics, preexisting chronic conditions, primary diagnosis, and HCC score, mortality rates were significantly higher for CAHs in all years (P < .001; eTable 9 in the Supplement) and increased over time (P < .001 for interaction between CAH and year). In 2007, the adjusted mortality rate was 13.52% for CAHs and 11.44% for non-CAHs (adjusted percentage point difference, 2.08 [95% CI, 1.74 to 2.42]; P < .001). By 2017, the adjusted mortality rate was 15.97% for CAHs and 12.46% for non-CAHs (difference, 3.52 [95% CI, 3.09 to 3.94]; P < .001). When the HCC score was excluded from the risk-adjustment model, adjusted mortality rates were not significantly different between CAHs and non-CAHs in all years except for 2007 (12.19% for CAHs vs 11.74% for non-CAHs; difference, 0.45 [95% CI, 0.12 to 0.79]; P = .008) and 2010 (12.71% for CAHs vs 12.28% for non-CAHs; difference, 0.42 [95% CI, 0.07 to 0.77]; P = .02). Mortality rate point estimates produced from models excluding patients who were transferred (eTable 11 in the Supplement) and including county fixed effects (eTable 12 in the Supplement) were consistent with our primary results. In eFigure 3 in the Supplement, we demonstrated that mortality differentials were sensitive to the number of codes being used to calculate the HCC scores.
Figure 2. Percentage Point Difference in Mortality Between Critical Access Hospitals (CAHs) and Non-CAHs, 2007-2017.
The figure shows crude and adjusted differences between CAHs and non-CAHs in combined in-hospital and 30-day postdischarge mortality. The light blue curve depicts unadjusted differences, while the 2 other curves depict adjusted differences estimated from 2 different risk-adjustment models. In one model (yellow curve), the Hierarchical Condition Category (HCC) score is included as a covariate, and in the other model (dark blue curve), the HCC score is excluded. Both adjusted models include the following base set of covariates: age, age-squared, sex, race/ethnicity (White, Black, or other), Medicaid enrollment, Medicare entitlement reason (Old Age and Survivor’s insurance vs all else), 27 chronic conditions present prior to the index admission (Table), primary diagnosis, and hospital referral region fixed effects. Bars represent 95% CIs for each adjusted point estimate.
Discussion
In this study of rural Medicare beneficiaries hospitalized from 2007 to 2017, combined in-hospital and 30-day postdischarge mortality rates, adjusted for baseline characteristics, primary diagnosis, and preexisting conditions, but not in-hospital comorbidity measures, were not significantly different between CAHs and non-CAHs in most years.
Compared with patients in non-CAHs, patients in CAHs were on average 3 years older but had fewer diagnoses coded when hospitalized. Differences in the number of diagnoses coded for patients in CAHs and non-CAHs increased after 2011 when CMS expanded the allowable number of diagnosis billing codes from 10 to 25, resulting in about 3 more diagnoses per hospitalization at non-CAHs, on average. These differences in coding were related to differences in the adjusted short-term mortality rates calculated for CAHs and non-CAHs. Combined in-hospital and 30-day postdischarge mortality was estimated to be 2 percentage points higher for CAHs and increased over time when the risk-adjustment model included the HCC risk score, which was derived from diagnoses gathered during the inpatient stay. In contrast, risk-adjusted mortality rates for CAHs and non-CAHs were not significantly different in most years when the risk-adjustment model excluded the HCC score.
Supply-and-demand–side factors may explain the lower frequency of diagnoses coded at CAHs. On the demand side, rural residents are known to “bypass” local health care facilities or clinicians and travel greater distances to receive care elsewhere.25,26 If sicker patients bypass CAHs to receive care at non-CAHs, this may explain the lower frequency of diagnosis coding at CAHs. However, a previous study of bypass behavior found no association between self-reported health and the likelihood of bypassing a CAH.26 On the supply side, CAHs may select healthier patients for treatment. Yet this study provides evidence supporting a more rudimentary explanation for the differences in diagnoses coding between CAHs and non-CAHs, which is the differing sets of financial incentives.
Non-CAHs, unlike CAHs, are not exempt from quality reporting initiatives. Because publicly reported quality measures are risk-standardized, non-CAHs are incentivized to code diagnoses completely. In addition, non-CAHs are paid based on the DRG assigned to the discharge, which is determined from the set of diagnoses that are documented and eventually coded. DRGs are distinguished according to a base condition and a 2- or 3-level severity subclass. Higher severity subclasses yield greater reimbursements but require more documentation and coding. Therefore, non-CAHs are more likely to have responded to CMS’s 2011 rule change allowing for more codes to be submitted, whereas this change would have been less relevant to CAHs, which are paid outside of the DRG system.
Other studies have reduced the maximum number of diagnosis codes used in risk adjustment to the pre-2011 level for serial analyses of Medicare claims.27 However, this approach may not be an adequate solution for comparisons involving CAHs because even prior to 2011 coding incentives for CAHs and non-CAHs were different. The supplementary analysis, showing that patients in CAHs who were transferred to non-CAHs acquired more diagnoses compared with patients in non-CAHs who were transferred to other non-CAHs, further supports this notion. The underlying issue is that claims diagnoses are primarily recorded for billing purposes, not for measuring health status or illness severity. When payment mechanisms differ, the use of billing codes in risk adjustment may complicate analyses of patient outcomes.
This study’s finding, that CAH and non-CAH mortality rates may not differ after adjustment for preexisting conditions rather than in-hospital comorbidity measures, contrasts with those from previous studies that reported higher mortality rates for patients in CAHs admitted for stroke, pneumonia, acute myocardial infarction, and congestive heart failure.6,7,8 While the results of this study may differ from previous findings due to differences in risk-adjustment methods, the study population also included patients with a different set of principal diagnoses. Additionally, this analysis was exclusively limited to rural residents who are the primary patients of CAHs.
Rural Medicare beneficiaries are disproportionately older, poorer, and burdened by chronic illnesses.1,2,3 As rural hospitals close, CAHs become a site of care for many vulnerable patients.28 Given that there have been many closures in recent years, understanding CAH quality of care has become increasingly important.29 Mortality prevention is an essential function of CAHs because in their absence, many acutely ill patients would not have timely access to care. The results of this study suggest that the performance of CAHs on short-term mortality outcomes may not differ from that of non-CAHs after accounting for differential coding for in-hospital comorbidities. However, mortality is a single, crude dimension of quality of care and further research is needed to understand other outcomes.
Limitations
This study has several limitations. First, given the observational design of the study, the association between CAH status and mortality may be biased due to residual confounding. For example, while including the HCC score (or any claims-based measure of in-hospital comorbidities) in risk adjustment may bias comparisons between CAHs and non-CAHs, not doing so may ignore real differences in illness severity at the time of hospitalization.
Second, this study’s administrative data lacked several factors that may be related to mortality differentials between CAHs and non-CAHs such as smoking status, obesity, functional status, living environment, and local economy. Future studies may circumvent limitations due to unobserved data by using quasiexperimental approaches for comparing the outcomes of patients in CAHs and non-CAHs. For example, there may be factors unrelated to patient characteristics, such as geographic boundaries or ambulance routes, which may drive treatment at CAHs vs non-CAHs and which may be used to facilitate comparisons.
Third, while patients discharged from CAHs to non-CAHs (and vice versa) were identifiable using MedPAR, patients who were transferred from the emergency department or an observation unit could not be distinguished. Given that triage is an essential function of CAHs, estimates may be affected by differential misclassification bias if mortality rates differ for nonadmitted patients transferred from CAHs.30 Fourth, the findings cannot be generalized beyond the Medicare Fee-for-Service population.
Conclusions
For rural Medicare beneficiaries hospitalized from 2007 to 2017, CAHs submitted significantly fewer hospital diagnosis codes than non-CAHs, and short-term mortality rates adjusted for preexisting conditions but not in-hospital comorbidity measures were not significantly different by hospital type in most years. These findings suggest that short-term mortality outcomes at CAHs may not differ from those of non-CAHs after accounting for different coding practices for in-hospital comorbidities.
eMethods
eFigure 1. Proportion of Hospitalizations Among Traditional Medicare Beneficiaries Occurring at Critical Access Hospitals
eFigure 2. HCC Scores Among Patients Discharged From Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017.
eFigure 3. Sensitivity of Mortality Differences Between Critical Access Hospitals and Non-Critical Access Hospitals to Diagnosis Coding, 2007-2017.
eTable 1. List of Inclusionary Diagnoses and International Classification of Diseases Codes
eTable 2. Top 10 Most Prevalent Diagnosis Related Groups for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 3. Distribution of Transfers at Critical Hospitals and Non-Critical Access Hospitals
eTable 4. Count of Discharge Diagnoses Among Patients Admitted to Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 5. Assessment of Change in Diagnosis Coding Differences Between Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 6. Mean HCC Scores Among Patients Admitted to Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 7. Adjusted Mean Discharge Diagnosis Count Before and After Transfer by Critical Access Hospital Status
eTable 8. Mortality Event Counts at Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 9. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 10. Assessment of Change in Mortality Differentials for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 11. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals Excluding Transferred Patients, 2007-2017
eTable 12. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals Derived From a County Fixed Effects Model, 2007-2017
References
- 1.Meit M, Knudson A, Gilbert T, et al. The 2014 Update of the Rural-Urban Chartbook. North Dakota and NORC Rural Health Reform Policy Research Network; 2014. [Google Scholar]
- 2.Rural Health Research Gateway Rural communities: age, income, and health status. Accessed July 15, 2019. https://www.ruralhealthresearch.org/assets/2200-8536/rural-communities-age-income-health-status-recap.pdf
- 3.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, US, 1969-2009. Am J Prev Med. 2014;46(2):e19-e29. doi: 10.1016/j.amepre.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim AM, Hughes TG, Thumma JR, Dimick JB. Association of hospital critical access status with surgical outcomes and expenditures among Medicare beneficiaries. JAMA. 2016;315(19):2095-2103. doi: 10.1001/jama.2016.5618 [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim AM, Regenbogen SE, Thumma JR, Dimick JB. Emergency surgery for Medicare beneficiaries admitted to critical access hospitals. Ann Surg. 2018;267(3):473-477. doi: 10.1097/SLA.0000000000002216 [DOI] [PubMed] [Google Scholar]
- 6.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. doi: 10.1001/jama.2011.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. 30-Day risk-standardized mortality and readmission rates after ischemic stroke in critical access hospitals. Stroke. 2012;43(10):2741-2747. doi: 10.1161/STROKEAHA.112.665646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joynt KE, Orav EJ, Jha AK. Mortality rates for Medicare beneficiaries admitted to critical access and non-critical access hospitals, 2002-2010. JAMA. 2013;309(13):1379-1387. doi: 10.1001/jama.2013.2366 [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis JP. Are mortality differences detected by administrative data reliable and actionable? JAMA. 2013;309(13):1410-1411. doi: 10.1001/jama.2013.3150 [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim AM, Dimick JB, Sinha SS, Hollingsworth JM, Nuliyalu U, Ryan AM. Association of coded severity with readmission reduction after the Hospital Readmissions Reduction Program. JAMA Intern Med. 2018;178(2):290-292. doi: 10.1001/jamainternmed.2017.6148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowrisankaran G, Joiner KA, Lin J. Does Health IT Adoption Lead to Better Information or Worse Incentives? NBER Working Paper 22873. National Bureau of Economic Research; 2016. doi: 10.3386/w22873 [DOI] [Google Scholar]
- 12.Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases in readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38(1):36-43. doi: 10.1377/hlthaff.2018.05178 [DOI] [PubMed] [Google Scholar]
- 13.US Department of Agriculture Rural-Urban Continuum Codes. Accessed July 15, 2019. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/
- 14.Geruso M, Layton T. Upcoding: evidence from Medicare on squishy risk adjustment. J Polit Econ. 2020;128(3):984-1026. doi: 10.1086/704756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation 2013 Measure updates and specifications: acute myocardial infarction, heart failure, and pneumonia 30-day risk standardized mortality measure (version 7.0). Accessed July 15, 2019. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Mortality_AMI-HF-PN_Measures_Updates_Report_FINAL_06-13-2013.pdf
- 16.Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318(3):270-278. doi: 10.1001/jama.2017.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sloan FA, Ayyagari P, Salm M, Grossman D. The longevity gap between black and white men in the United States at the beginning and end of the 20th century. Am J Public Health. 2010;100(2):357-363. doi: 10.2105/AJPH.2008.158188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse: condition categories. Accessed July 15, 2019. https://www.ccwdata.org/web/guest/condition-categories
- 20.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts ET, Zaslavsky AM, Barnett ML, Landon BE, Ding L, McWilliams JM. Assessment of the effect of adjustment for patient characteristics on hospital readmission rates. JAMA Intern Med. 2018;178(11):1498-1507. doi: 10.1001/jamainternmed.2018.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope G, Kautter J, Ingber M, Freeman S, Sekar R, Newhart C. Evaluation of the CMS-HCC Risk Adjustment Model. RTI International; 2011. [Google Scholar]
- 23.The Dartmouth Atlas of Health Care Research methods. Accessed March 2020. http://www.dartmouthatlas.org/downloads/methods/research_methods.pdf
- 24.Greene W. The behaviour of the maximum likelihood estimator of limited dependent variable models in the presence of fixed effects. Econom J. 2004;7(1):98-119. doi: 10.1111/j.1368-423X.2004.00123.x [DOI] [Google Scholar]
- 25.Basu J, Cooper J. Out-of-area travel from rural and urban counties. J Rural Health. 2000;16(2):129-138. doi: 10.1111/j.1748-0361.2000.tb00446.x [DOI] [PubMed] [Google Scholar]
- 26.Liu JJ, Bellamy GR, McCormick M. Patient bypass behavior and critical access hospitals. J Rural Health. 2007;23(1):17-24. doi: 10.1111/j.1748-0361.2006.00063.x [DOI] [PubMed] [Google Scholar]
- 27.Tsugawa Y, Figueroa JF, Papanicolas I, Orav EJ, Jha AK. Assessment of strategies for managing expansion of diagnosis coding using risk-adjustment methods for Medicare data. JAMA Intern Med. 2019;179(9):1287-1290. doi: 10.1001/jamainternmed.2019.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll CE. Impeding access or promoting efficiency? Paper presented at: American Society of Health Economists Annual Meeting; June 25, 2019; Washington, DC. [Google Scholar]
- 29.Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. doi: 10.1111/jrh.12128 [DOI] [PubMed] [Google Scholar]
- 30.Moscovice IS, Casey MM. Quality of care in critical access hospitals. JAMA. 2011;306(15):1653-1655. doi: 10.1001/jama.2011.1487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Proportion of Hospitalizations Among Traditional Medicare Beneficiaries Occurring at Critical Access Hospitals
eFigure 2. HCC Scores Among Patients Discharged From Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017.
eFigure 3. Sensitivity of Mortality Differences Between Critical Access Hospitals and Non-Critical Access Hospitals to Diagnosis Coding, 2007-2017.
eTable 1. List of Inclusionary Diagnoses and International Classification of Diseases Codes
eTable 2. Top 10 Most Prevalent Diagnosis Related Groups for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 3. Distribution of Transfers at Critical Hospitals and Non-Critical Access Hospitals
eTable 4. Count of Discharge Diagnoses Among Patients Admitted to Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 5. Assessment of Change in Diagnosis Coding Differences Between Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 6. Mean HCC Scores Among Patients Admitted to Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 7. Adjusted Mean Discharge Diagnosis Count Before and After Transfer by Critical Access Hospital Status
eTable 8. Mortality Event Counts at Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 9. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 10. Assessment of Change in Mortality Differentials for Critical Access Hospitals and Non-Critical Access Hospitals, 2007-2017
eTable 11. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals Excluding Transferred Patients, 2007-2017
eTable 12. Estimated Mortality Rates for Critical Access Hospitals and Non-Critical Access Hospitals Derived From a County Fixed Effects Model, 2007-2017