Abstract
Objective: This review aimed to identify proper respiratory-related sample types for adult and pediatric pulmonary tuberculosis (PTB), respectively, by comparing performance of Xpert MTB/RIF when using bronchoalveolar lavage (BAL), induced sputum (IS), expectorated sputum (ES), nasopharyngeal aspirates (NPAs), and gastric aspiration (GA) as sample.
Methods: Articles were searched in Web of Science, PubMed, and Ovid from inception up to 29 June 2020. Pooled sensitivity and specificity were calculated, each with a 95% confidence interval (CI). Quality assessment and heterogeneity evaluation across included studies were performed.
Results: A total of 50 articles were included. The respective sensitivity and specificity were 87% (95% CI: 0.84–0.89), 91% (95% CI: 0.90–0.92) and 95% (95% CI: 0.93–0.97) in the adult BAL group; 90% (95% CI: 0.88–0.91), 98% (95% CI: 0.97–0.98) and 97% (95% CI: 0.95–0.99) in the adult ES group; 86% (95% CI: 0.84–0.89) and 97% (95% CI: 0.96–0.98) in the adult IS group. Xpert MTB/RIF showed the sensitivity and specificity of 14% (95% CI: 0.10–0.19) and 99% (95% CI: 0.97–1.00) in the pediatric ES group; 80% (95% CI: 0.72–0.87) and 94% (95% CI: 0.92–0.95) in the pediatric GA group; 67% (95% CI: 0.62–0.72) and 99% (95% CI: 0.98–0.99) in the pediatric IS group; and 54% (95% CI: 0.43–0.64) and 99% (95% CI: 0.97–0.99) in the pediatric NPA group. The heterogeneity across included studies was deemed acceptable.
Conclusion: Considering diagnostic accuracy, cost and sampling process, ES was a better choice than other sample types for diagnosing adult PTB, especially HIV-associated PTB. GA might be more suitable than other sample types for diagnosing pediatric PTB. The actual choice of sample types should also consider the needs of specific situations.
Keywords: diagnostic performance, pulmonary tuberculosis, sample types, Xpert MTB/RIF
Introduction
Globally, there were 10 million new tuberculosis (TB) cases and 1.24 million deaths in 2018 alone [1]. A body of studies have confirmed that early diagnosis and treatment can prevent most TB deaths [2–4], and thus excellent diagnostic tools need to be developed. The Xpert MTB/RIF (Cepheid, Sunnyvale, CA, United States) was endorsed as a diagnostic test for use in TB endemic countries by World Health Organization (WHO) in 2010 [5]. A systematic review of Xpert MTB/RIF studies has reported different sensitivities of Xpert MTB/RIF, ranging from 25 to 100% [6]. A study has shown the sensitivity of Xpert MTB/RIF varies with the sample types, samples quality, and bacterial load of samples [7]. Thus, choosing proper sample types is critical to improve the diagnostic performance of Xpert MTB/RIF. Given the high prevalence of pulmonary TB (PTB) [8], we paid attention to the selection of respiratory-related sample types to better diagnose PTB.
The application of urine and stool for diagnosing PTB have been reported [9,10], however, considering high TB burden areas which are usually economically underdeveloped, high cost of Xpert MTB/RIF and relatively low detection rate in non-respiratory samples [11], respiratory-related sample become the first choice for detecting Mycobacterium tuberculosis (MTB) in clinical practice, at least for now. Expectorated sputum (ES), induced sputum (IS), bronchoalveolar lavage (BAL) fluid, gastric aspiration (GA), and nasopharyngeal aspirates (NPAs) are considered suitable samples to detect PTB and are regularly collected in clinical practice. ES is readily available, however, has low bacterial load. IS, sputum induced by the inhalation of hypertonic saline, usually has large sample volume and higher quality than ES [12]. BAL has been widely accepted as the most specific sample type used for accurate and timely diagnosis of lung diseases [13,14]. The WHO supports the collection of BAL to diagnose PTB when possible [15]. However, high cost, invasive sampling and the potential risk of hemorrhage, pneumothorax, laryngospasm and other adverse reactions limit its application [16]. It is also worth noting that in clinical practice, bronchoscopy and lavage are not considered as feasible in young children due to the potential risk for anesthesia and need of specialized procedural expertise, unless otherwise specified [17]. NPA is sampled after stimulation of the cough reflex via inserting a small feeding catheter into the nasopharynx [18]. Low operational requirements, less invasive sampling, and ready access enable NPA to become an alternative to BAL for pediatric PTB, but not for adult PTB due to significantly different airway microbial composition between NPA and BAL [17]. Young children, particularly who under 5 years, are unable to expectorate sputum and always swallow sputum in their stomach by mistake, thus GA is collected by a nasogastric tube during night in three consecutive mornings to detect MTB for pediatric PTB [19,20]. Nonetheless, MTB is less likely to be detected in GA than smear in adults, and therefore, and thus GA is not regarded as an option for detecting MTB for adult PTB [21]. Enough volume and consecutive samples of GA support repeat tests to improve the rate of detection [22].
Although there are many samples types to choose from, different samples vary greatly in cost, requirement for operators and operating environment, the degree of sampling invasiveness, quality, volume, and others. It is hard to assess which is the best choice for diagnosing PTB. In addition, few studies pay attention to assess the impact of different sample types on the performance of Xpert MTB/RIF when diagnosing PTB. Therefore, we undertook this systematic review and meta-analysis to compare the detection capacity of Xpert MTB/RIF when using ES, IS, and BAL as samples for diagnosing adult PTB, as well as ES, IS, GA, and NPA for diagnosing pediatric PTB. We aimed to summarize and curate reliable evidence for choosing the proper sample types to diagnose PTB patients at different ages.
Materials and methods
Search strategy
We conducted a full search in Web of Science, PubMed, and Ovid on 29 June 2020, with the following terms: ‘tuberculosis’, ‘mycobacterium tuberculosis’, ‘TB’, ‘MTB’ AND ‘GeneXpert’, ‘Xpert’, ‘Xpert MTB/RIF’, ‘GX’ AND ‘gastric aspirate’, ‘gastric aspiration’, ‘gastric specimen’, ‘gastric lavage aspiration’, ‘GA’, ‘GLA’, ‘GS’, ‘bronchoalveolar lavage’, ‘bronchoalveolar lavage fluid’, ‘bronchoalveolar washing’, ‘BAL’, ‘BALF’, ‘BW’, ‘induced sputum’, ‘IS’, ‘expectorated sputum’, ‘ES’, ‘nasopharyngeal aspirates’, ‘NPA’. Potential studies were manually identified from reference lists of relevant articles. Any disagreements would be discussed by two independent authors and, if necessary, a third reviewer would arbitrate.
Inclusion and exclusion criteria
Included studies needed to: (i) be cross-sectional studies, cohort studies, or randomized controlled trials; (ii) focus on PTB patients with or without other diseases; (iii) use two diagnostic methods—Xpert MTB/RIF and culture, and take culture as the reference standard; (iv) collect GA, NPA from children, or BAL from adults, or IS or ES from both; (v) give clear information that participants were adults or children. Exclusion criteria were: (i) reviews, case reports, letters, conference abstracts, and animal experiments; (ii) articles not written in English; (iii) articles with incomplete basic data.
Quality assessment
Two independent reviewers assessed the quality of included studies according to the Revised Tool for Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 [23]. Both risk of bias and applicability concerns would be evaluated as ‘high’, ‘unclear’ or ‘low’. Advice of a third reviewer would be referred if necessary.
Data extraction and management
All data were collected and entered into Microsoft Excel version 2016. The basic information mainly comprised the first author, publication year, study population’s characteristics, and so on. Diagnostic information included true-positive, false-positive, true-negative, and false-negative. If necessary, we contacted the authors for more essential information, otherwise the trials with incomplete information would be excluded.
Heterogeneity
The threshold effect and non-threshold effect could result in heterogeneity. The heterogeneity caused by the threshold effect could be explored by performing the Spearman correlation coefficient or plotting summary receiver operating characteristic curves (sROCs). The threshold effect would exist if P-value of the Spearman correlation coefficient was less than 0.05 or the points in the plots had a curvilinear (shoulder arm) pattern. If Chi-square P-values were less than 0.10, heterogeneity might be caused by a non-threshold effect and a random-effects model would be chosen.
Sensitivity analysis
Sensitivity analysis was performed to determine the robustness of any treatment effect by removing low-quality studies.
Statistical analysis
The diagnostic performance of Xpert MTB/RIF was assessed by calculating the pooled sensitivity, specificity, and area under the curve (AUC), with a 95% confidence interval (CI). The forest plots and sROC were also plotted. We performed statistical analysis with Meta-DiSc 1.4 (XI Cochrane Colloquium, Barcelona, Spain) and Review Manager V.5.3 (The Cochrane Collaboration, Software Update, Oxford, U.K.).
Results
Studies’ characteristics
We identified 50 articles: 17 [7,24–39] in the adult BAL group, 14 [35,40–52] in the adult ES group, 7 [35,45,50,53–56] in the adult IS group, 2 [57,58] in the pediatric ES group, 6 [57,59–63] in the pediatric GA group, and 9 [59,64–71] in the pediatric IS group from 1791 studies for the meta-analysis. A total of 2864 BAL samples, 18176 ES samples, and 3876 IS samples were collected from adults. A total of 593 ES samples, 1595 GA samples, 2759 IS samples, and 780 NPA samples were collected from children. More than 80% of all samples were preprocessed, including neutralization, centrifugation, or other processing. The volume of BAL, ES, and IS were 1.0–5 ml, ≥1.0 ml, and 0.5–2.0 ml, respectively. The volume of GA varied from 0.1 ml to more than 5 ml, while 0.5–1.5 ml IS was collected from children.
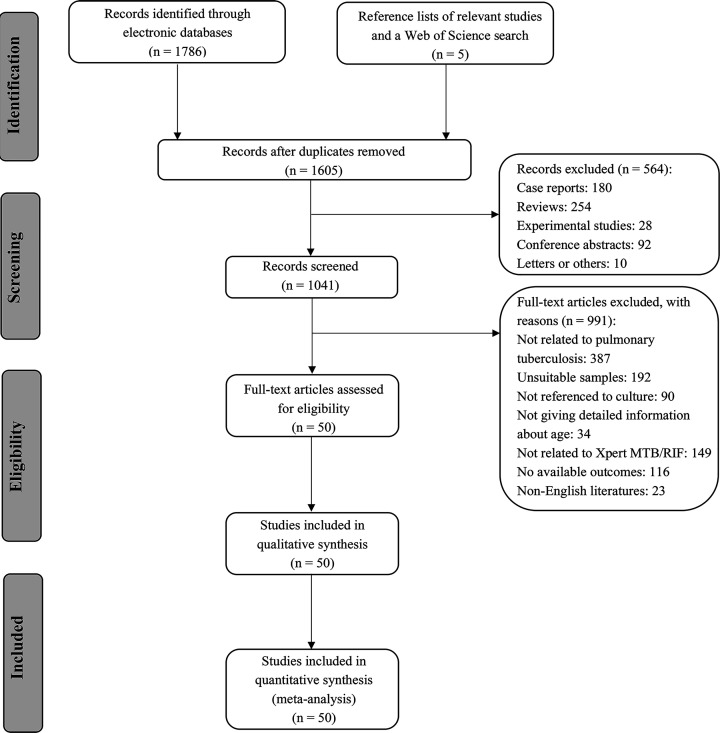
The flow diagram of articles included in this meta-analysis is presented in Figure 1. The main characteristics of 43 included articles are summarized in Table 1.
Figure 1. The flow diagram of articles included in this meta-analysis.
Table 1. The main characteristics of included trials.
| Study (year) | Country or Area | Patient recruitment | Sample size | Sample processing | Smear- positive rate (%) | HIV- positive rate (%) | Culture type |
|---|---|---|---|---|---|---|---|
| Adult BAL1 group | |||||||
| Barnard, 2015 | South Africa | NA2 | 112 | Decontamination and centrifugation | 15 | NA | MGIT |
| Gowda, 2018 | India | NA | 60 | Unprocessed | 12 | 7 | MGIT |
| Jo, 2016 | South Korea | NA | 320 | Centrifugation | 8 | 0 | MGIT and Ogawa |
| Khalil, 2015 | Pakistan | NA | 93 | NA | 37 | NA | LJ3 |
| Kilaru, 2019 | India | Inpatients and outpatients | 56 | Decontamination and centrifugation | 38 | 0 | MGIT |
| Ko, 2016 | Korea | NA | 249 | NA | NA | 0 | MGIT and Ogawa |
| Lee, 2013 | Korea | NA | 132 | Decontamination and centrifugation | 5 | 0 | MGIT and Ogawa |
| Lu, 2018 | China | NA | 238 | Decontamination | 9 | NA | MGIT and LJ |
| Mok, 2016 | Singapore | Inpatients and outpatients | 158 | NA | 6 | NA | MGIT |
| Pan, 2018 | China | NA | 190 | Decontamination | 19 | NA | MGIT |
| Prakash, 2018 | Six countries | Inpatients and outpatients | 383 | NA | 6 | NA | MGIT and LJ |
| Sánchez-Cabral, 2020 | Brazil | NA | 54 | NA | NA | 41 | MGIT and LJ |
| Sharma, 2015* | India | NA | 131 | Decontamination and centrifugation | NA | NA | MGIT and LJ |
| Theron, 2013 | South Africa | NA | 154 | Centrifugation | 10 | 30 | MGIT |
| To, 2018 | China | NA | 237 | NA | NA | 2 | NA |
| Ullah, 2017 | Pakistan | NA | 98 | Decontamination | 0 | 100 | NA |
| Silva, 2019 | Brazil | NA | 199 | NA | NA | NA | MGIT and LJ |
| Adult ES4 group | |||||||
| Al-Darraji, 2013 | Malaysia | Prison | 125 | Decontamination and centrifugation | NA | 100 | MGIT |
| Boum, 2016 | Uganda | NA | 1016 | NA | 20 | 66 | MGIT |
| Friedrich, 2011 | South Africa | NA | 292 | Decontamination and centrifugation | 97 | 42 | MGIT |
| Geleta, 2015 | Somali | NA | 227 | Decontamination | 26 | NA | MGIT and LJ |
| LaCourse, 2016 | Kenya | NA | 288 | Decontamination | NA | 100 | MGIT |
| Luetkemeyer, 2016* | American, Brazil, and Rio de Janeiro | Inpatients and outpatients | 992 | Unprocessed | 15 | NA | MGIT and solid media |
| Madico, 2016 | Mbarara and Uganda | Inpatients | 261 | Decontamination | 15 | 100 | MGIT |
| Pinyopornpanish, 2015 | Thailand | NA | 57 | NA | 14 | 46 | MGIT |
| Rachow, 2011 | Tanzania | NA | 126 | Decontamination | NA | NA | MGIT and LJ |
| Rasheed, 2019 | Pakistan | NA | 225 | Unprocessed | 0 | NA | LJ |
| Rice, 2017* | American | NA | 637 | Decontamination and centrifugation | 30 | NA | MGIT and LJ |
| Sahle, 2019 | Ethiopia | Prison | 13803 | NA | 0.1 | 0.6 | MGIT |
| Sharma, 2015* | India | NA | 71 | Decontamination and centrifugation | NA | NA | MGIT and LJ |
| Yeong, 2020 | Australia | Inpatients | 56 | Decontamination | 11 | 0 | MGIT |
| Adult IS5 group | |||||||
| Acuna, 2017 | Uganda | Inpatients and outpatients | 860 | Decontamination | 89 | 69 | MGIT |
| Luetkemeyer, 2016* | America and Brazil | NA | 324 | NA | NA | NA | MGIT and solid media |
| Mishra, 2020 | South Africa | NA | 585 | Decontamination | 12 | 20 | MGIT and LJ |
| O’Grady, 2012 | Zambia | Inpatients | 881 | Centrifugation | 18 | 71 | MGIT |
| Rice, 2017* | America | NA | 653 | Decontamination and centrifugation | NA | NA | MGIT and LJ |
| Sharma, 2015* | India | NA | 71 | Decontamination and centrifugation | NA | NA | MGIT and LJ |
| Sohn, 2014 | Canada | NA | 502 | Decontamination | 3 | 2 | MGIT |
| Pediatric ES group | |||||||
| Bates, 2013* | Zambia | Inpatients | 142 | Decontamination and centrifugation | 7 | 31 | MGIT |
| Reither, 2015 | Tanzania and Uganda | NA | 451 | Decontamination | NA | 44 | MGIT and LJ |
| Pediatric GA6 group | |||||||
| Bates, 2013* | Zambia | Inpatients | 788 | Decontamination and centrifugation | 4 | 30 | MGIT |
| Das, 2018* | India and adjoining areas of Nepal | Inpatients and outpatients | 106 | NA | NA | NA | LJ |
| Hasan, 2017 | Pakistan | NA | 49 | Decontamination and centrifugation | NA | NA | MGIT |
| Myo, 2018 | Myanmar | NA | 231 | Decontamination and centrifugation | 5 | 13 | LJ |
| Pang, 2014 | China | NA | 211 | Decontamination and centrifugation | 0 | NA | MGIT |
| Sharma 2020 | India | Outpatients | 210 | Decontamination | 7 | NA | MGIT |
| Pediatric IS group | |||||||
| Das, 2018* | India and adjoining areas of Nepal | Inpatients and outpatients | 8 | Nebulization | NA | NA | LJ |
| LaCourse, 2014 | Malawi | NA | 300 | NA | 0 | 17 | MGIT |
| Nicol, 2011 | South Africa | NA | 452 | Decontamination and centrifugation | 6 | 24 | MGIT |
| Nicol, 2013 | South Africa | NA | 115 | Decontamination and centrifugation | 3 | 15 | MGIT |
| Nicol, 2018 | South Africa | NA | 367 | Decontamination and centrifugation | NA | 19 | MGIT and LJ |
| Sekadde, 2013 | Uganda | Mixed | 250 | Digestion and decontamination | 6 | 42 | MGIT and LJ |
| Togun, 2015 | Gambia | Outpatients | 487 | Digestion, decontamination, and centrifugation | 1 | 0 | MGIT and LJ |
| Zar, 2012* | South Africa | Inpatients | 396 | Decontamination and centrifugation | 5 | 20 | MGIT |
| Zar, 2013* | South Africa | NA | 384 | Decontamination and centrifugation | 1 | 8 | MGIT |
| Pediatric NPA7 group | |||||||
| Zar, 2012* | South Africa | Inpatients | 396 | Decontamination and centrifugation | 4 | 20 | MGIT |
| Zar, 2013* | South Africa | NA | 384 | Decontamination and centrifugation | NA | NA | MGIT |
These studies were divided into at least two groups.
a, BAL.
b, Not available.
c, Löwenstein–Jensen medium.
d, ES.
e, IS.
f, GA.
g, NPAs.
Risk of bias
The risk of bias of the included studies is shown in Table 2.
Table 2. The risk of bias of the included studies.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Adult BAL1 group | |||||||
| Barnard, 2015 | unclear | unclear | low | low | low | low | low |
| Gowda, 2018 | unclear | low | low | low | low | low | low |
| Jo, 2016 | unclear | low | low | low | unclear | low | low |
| Khalil, 2015 | unclear | unclear | unclear | unclear | low | low | low |
| Kilaru, 2019 | low | low | low | low | low | low | low |
| Ko, 2016 | unclear | unclear | unclear | unclear | low | low | low |
| Lee, 2013 | unclear | low | low | low | low | low | low |
| Lu, 2018 | low | low | low | low | low | low | low |
| Mok, 2016 | unclear | low | low | low | low | low | low |
| Pan, 2018 | unclear | low | low | low | low | low | low |
| Prakash, 2018 | low | low | low | low | low | low | high |
| Sánchez-Cabral, 2020 | low | low | low | low | low | low | low |
| Sharma, 2015* | low | low | low | low | low | low | low |
| Silva, 2019 | unclear | unclear | low | low | low | low | low |
| Theron, 2013 | unclear | low | low | low | low | low | low |
| To, 2018 | unclear | low | low | low | unclear | low | low |
| Ullah, 2017 | unclear | low | low | low | high | low | low |
| Adult ES2 group | |||||||
| Al-Darraji, 2013 | high | low | low | low | low | low | unclear |
| Boum, 2016 | low | low | low | low | low | low | low |
| Friedrich, 2011 | unclear | low | low | low | low | low | low |
| Geleta, 2015 | low | low | low | low | low | low | low |
| LaCourse, 2016 | high | low | low | low | low | low | low |
| Luetkemeyer, 2016* | low | low | low | low | low | low | low |
| Madico, 2016 | high | low | low | low | low | low | low |
| Pinyopornpanish, 2015 | low | low | low | low | low | low | low |
| Rachow, 2011 | low | low | low | low | low | low | low |
| Rasheed, 2019 | low | low | low | low | low | low | low |
| Rice, 2017* | low | low | low | low | low | low | low |
| Sahle, 2019 | low | low | low | low | low | low | unclear |
| Sharma, 2015* | low | low | low | low | low | low | low |
| Yeong, 2020 | low | low | low | low | low | low | low |
| Adult IS3 group | |||||||
| Acuna, 2017 | high | low | low | low | low | low | low |
| Luetkemeyer, 2016* | unclear | low | unclear | low | low | low | low |
| Mishra, 2020 | low | low | low | low | low | low | low |
| O’Grady, 2012 | unclear | low | low | low | low | low | low |
| Rice, 2017* | unclear | unclear | unclear | low | low | low | low |
| Sharma, 2015* | low | low | low | low | low | low | low |
| Sohn, 2014 | low | unclear | unclear | low | low | low | low |
| Pediatric ES group | |||||||
| Bates, 2013* | low | low | low | low | low | low | low |
| Reither, 2015 | low | low | low | low | low | low | low |
| Pediatric GA4 group | |||||||
| Bates, 2013* | unclear | low | low | low | low | low | low |
| Das, 2018* | unclear | unclear | unclear | low | low | low | low |
| Hasan, 2017 | unclear | low | low | low | low | low | low |
| Myo, 2018 | unclear | low | low | low | low | low | low |
| Pang, 2014 | low | low | low | low | high | low | low |
| Sharma, 2020 | low | low | low | low | low | low | low |
| Pediatric IS group | |||||||
| Das, 2018* | unclear | unclear | unclear | low | low | low | low |
| LaCourse, 2014 | low | unclear | unclear | low | high | low | low |
| Nicol, 2011 | low | low | low | low | low | low | low |
| Nicol, 2013 | low | unclear | unclear | low | low | low | low |
| Nicol, 2018 | low | low | unclear | low | low | low | low |
| Sekadde, 2013 | high | low | low | low | low | low | low |
| Togun, 2015 | low | low | low | low | high | low | low |
| Zar, 2012* | low | low | low | low | low | low | low |
| Zar, 2013* | low | low | low | low | low | low | low |
| Pediatric NPA5 group | |||||||
| Zar, 2012* | low | low | low | low | low | low | low |
| Zar, 2013* | low | low | low | low | low | low | low |
The studies of these authors were divided into at least two groups.
a, BAL.
b, ES.
c, IS.
d, GA.
e, NPAs.
Among 17 studies in the adult BAL group, 5 articles were assessed as low risk and the rest had an unclear risk on the patient selection. For the index test, 13 publications had a low risk and 4 publications had an unclear risk. In the aspect of reference standard and flow and timing, all trials gave clear description about trial details, except for two [27,29]. A total of 14 studies had low concern for applicability, however, the risk of patient selection of 2 studies [37,72] were unclear and that of Ullah et al. [38] was high.
For 14 studies in the adult ES group, 10 studies were assessed as low risk on the patient selection, 3/14 were considered as high risk and the remaining was identified as unclear risk. All of them showed low risk on the index test, reference standard and flow, and timing. Low concern of patient selection and index test appeared in all 14 publications. All studies were evaluated as low risk on reference standard except for two [40,51].
For seven studies in the adult IS group, three articles had low risk of patient selection, three articles had unclear risk of patient selection and only one had high risk of patient selection. Two studies [50,56], which did not state whether the results from two methods were double-blind, assessed as unclear risk on the index test and reference standard. Besides, Luetkemeyer et al. [45] also had an unclear risk on reference standard. All articles in this group were considered as low risk on applicability concerns.
In the pediatric ES group, two studies showed low risk in risk of bias and applicability concerns.
In the pediatric GA group, two articles had low risk of patient selection while others were of unclear risk. Unclear risk of the index test and reference standard only appeared in one publication. Five trials had low risk of flow and timing and applicability concerns, and one [62] was evaluated as high risk for patient selection.
In the pediatric IS group, all studies had low risk of patient selection except two [59,68]. Das et al. [59], LaCourse et al. [64], and Nicol et al. [66] had unclear risk of the index test and reference standard. Five articles had low concern of applicability, while another two studies [64,69] were assessed as high risk of patient selection.
In the pediatric NPA group, two studies showed low risk of bias and applicability concerns.
Heterogeneity
The value of Spearman correlation coefficient showed that the threshold effects did not exist in the adult BAL group (Spearman correlation coefficient: 0.158; P=0.727), adult ES group (Spearman correlation coefficient: 0.251; P=0.387), adult IS group (Spearman correlation coefficient: 0.321; P=0.482), pediatric GA group (Spearman correlation coefficient: −0.257; P=0.623), and pediatric IS group (Spearman correlation coefficient: 0.200; P=0.606). Besides, the distribution of points in sROC curve also confirmed this. Due to the limited articles in the pediatric ES group and pediatric NPA group, Spearman correlation coefficient and sROC were unavailable.
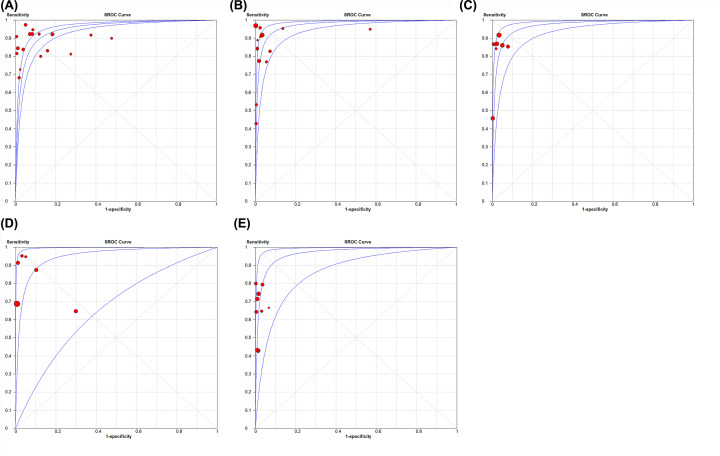
The value of Chi-square P-values indicted heterogeneity was caused by non-threshold effects in the adult BAL group (Chi-square: 42.33; P<0.001), adult ES group (Chi-square: 52.15; P<0.001), adult IS group (Chi-square: 18.95; P=0.004), pediatric ES group (Chi-square: 13.89; P=0.002) and pediatric GA group (Chi-square: 47.45; P<0.001). However, non-threshold effects were not detected in the pediatric IS group (Chi-square: 8.40; P=0.396), and pediatric NPA group (Chi-square: 1.57; P=0.211). The sROC curves for Xpert MTB/RIF of five groups (adult BAL group, adult ES group, adult IS group, pediatric GA group, and pediatric IS group) are shown in Figure 2.
Figure 2. sROC curve for Xpert MTB/RIF of five groups.
(A) sROC of adult BAL group; (B) sROC of adult ES group; (C) sROC of adult IS group; (D) sROC of pediatric GA group; (E) sROC of pediatric IS group.
Results on diagnostic accuracy
The diagnostic performance of Xpert MTB/RIF was calculated, compared with culture. Xpert MTB/RIF showed the pooled sensitivity, pooled specificity, and AUC were 87% (95% CI: 0.84–0.89), 91% (95% CI: 0.90–0.92) and 95% (95% CI: 0.93–0.97) in the adult BAL group; 90% (95% CI: 0.88–0.91), 98% (95% CI: 0.97–0.98) and 97% (95% CI: 0.95–0.99) in the adult ES group; 86% (95% CI: 0.84–0.89), 97% (95% CI: 0.96–0.98) and 98% (95% CI: 0.94–0.99) in the adult IS group; 80% (95% CI: 0.72–0.87), 94% (95% CI: 0.92–0.95) and 95% (95% CI: 0.87–1.00) in the pediatric GA group; 67% (95% CI: 0.62–0.72), 99% (95% CI: 0.98–0.99) and 97% (95% CI: 0.92–1.00) in the pediatric IS group, respectively. The pooled sensitivity and pooled specificity were 14% (95% CI: 0.10–0.19) and 99% (95% CI: 0.97–1.00) in the pediatric ES group and 54% (95% CI: 0.43–0.64) and 99% (95% CI: 0.97–0.99) in the pediatric NPA group, respectively. Due to the limited articles in the pediatric ES group and pediatric NPA group, the AUC was unavailable.
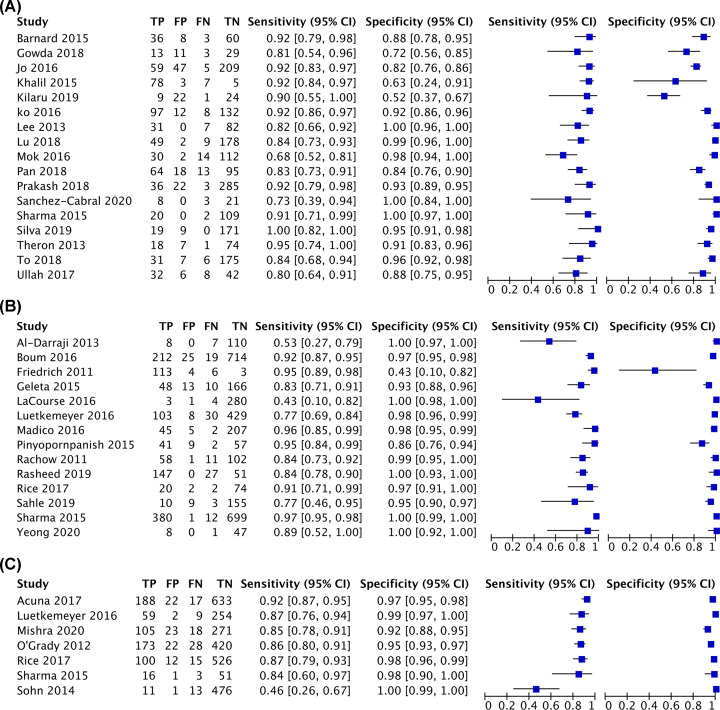
The summary plots of Xpert MTB/RIF for adult and children are presented in Figures 3 and 4, respectively.
Figure 3. The summary plots of adult PTB.
(A) Summary plots of adult BAL group; (B) summary plots of adult ES group; (C) summary plots of adult IS group. Abbreviations: FN, false-negative; FP, false-positive; TP, true-positive; TN, true-negative.
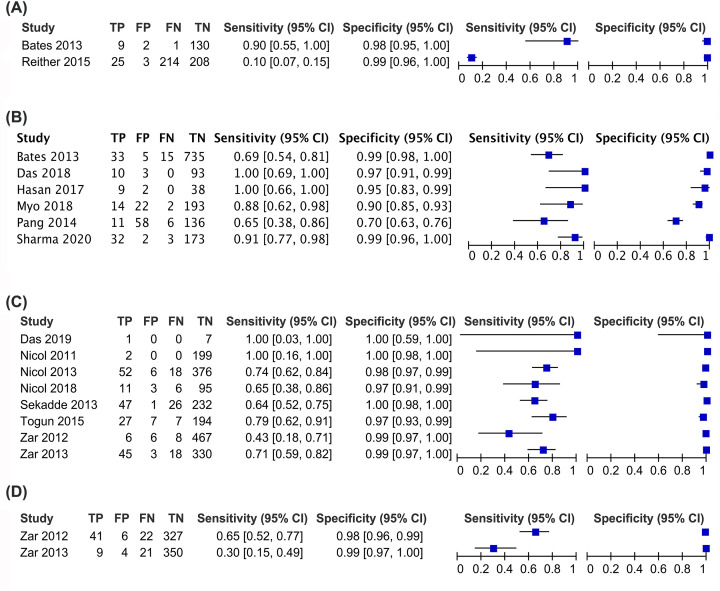
Figure 4. The summary plots of pediatric PTB.
(A) Summary plots of pediatric ES group; (B) summary plots of pediatric GA group; summary plots of pediatric IS group; (D) summary plots of pediatric NPA group. Abbreviations: FN, false-negative; FP, false-positive; TN, true-negative; TP, true-positive.
Subgroup analysis was carried out to determine the potential source of heterogeneity among included articles. The detailed information is presented in Supplementary Table S1.
Discussion
Since we only included studies using Xpert MTB/RIF, all comparisons made were between the types of biological samples used. For adults, choosing ES as sample provided better diagnostic performance than BAL or IS. For children, GA showed superior diagnostic capacity to ES, IS, or NPA. Of note, GA also has its own disadvantages including invasive sampling and needing approximately three consecutive days for collecting samples. The actual choice needs to be decided according to specific situations.
It is particularly notorious that microbiology methods (including sputum microscopy and culture), immunological methods [interferon-γ release assay (IGRA) and T-cell spot (T-SPOT)] and molecular methods [polymerase chain reaction (PCR)] are widely applied worldwide before the Xpert MTB/RIF came to market. The celerity and convenience of sputum microscopy make it the most commonly used method in clinical practice, nonetheless low sensitivity (20–40%) is the fatal defect of the sputum microscopy [73]. Culture, as the reference standard for the diagnosis of TB, requires too long detection time and a highly equipped laboratory. Thus, culture is greatly limited in some developing regions typically facing a high TB burden. IGRA and T-SPOT are effective ways to screen latent TB infection. However, inoculating Bacille Calmette–Guérin (BCG), prior infection and inhalation of non-pathogenic mycobacterium can result in false positivity of IGRA and T-SPOT [74]. Thus, the clinical value of these two diagnostic methods is somewhat discounted in TB endemic areas. Although PCR is a relatively sensitive method to detect MTB, high requirement of operator skills and a laboratory environment also restrict its application. The emergency of Xpert MTB/RIF meets the urgent demand for rapid, simple, integrated, and fully automated detective methods of MTB detection. A myriad of randomized controlled trials (RCTs) have been carried out to assess the diagnostic performance of Xpert MTB/RIF and found the detection rate of MTB is dramatically improved by this novel molecular tool [75]. Apparent benefit brought by Xpert MTB/RIF to PTB patient individually and public health enables this molecular tool to be rapidly accepted and applied all over the world.
However, Xpert MTB/RIF still faces some challenges of inadequate sensitivity when diagnosing smear-negative PTB, pediatric PTB, HIV-associated PTB, and extra-PTB [76,77]. In order to increase diagnostic yield, the Xpert MTB/RIF Ultra (Ultra; Cepheid) appeared. Based on the same platform as the Xpert MTB/RIF, the Ultra harbors some improvements including using two PCR amplification targets (IS6110 and IS1081) and a melt curve analysis [78]. These two changes confer Ultra’s lower limit of detection than Xpert MTB/RIF and culture [78]. The following clinical trials have found that improper sample types and poor sample quality still have an adverse influence on the sensitivity of Ultra [79,80]. Obviously, no matter how sensitive the detection method is, choosing proper sample type and ensuring the quality of sample are crucial.
In our systematic review, choosing ES as sample provided better diagnostic performance (sensitivity, specificity, and AUC) than BAL or IS for adult PTB. Since both BAL and IS had a higher bacterial load than ES [81,82], they should theoretically provide better diagnostic performance than ES. However, our study showed opposite results. One reason is the smaller volume of IS, ranging from 0.5 to 2 ml, whereas the volume of ES ranges from 1 ml to more than 2 ml. This inconsistency may also be explained by the ratio of processed samples. The ratio of processed samples in the adult ES group was higher than that in the adult BAL group. In addition, we also found there was a high agreement in diagnosing adult PTB when using IS and BAL as sample. Considering cost, sampling method, acceptance and other factors, IS might be a better choice than BAL to diagnose PTB. Blau et al. [83] gave the interpretation of this results that sampling from one or two parts of lung led to poor repeatability and limited detection rate when taking BAL as sample to diagnose lung infection diseases. Garcia et al. [84] found the gene transcriptional patterns of MTB in BAL and sputum were similar, and additionally, sputum could reflect the physiological state of MTB in the lower airway of PTB patients. Conde et al. [85] conducted a trial and reconfirmed the higher diagnostic yield of taking IS (relative to BAL) as sample to diagnose PTB, in line with our results.
For pediatric PTB, similar specificity and AUC appeared when using IS and GA as sample, nonetheless, Xpert MTB/RIF showed much higher sensitivity when using GA as sample to diagnose pediatric PTB. Although GA is sampled by invasive ways and need approximately three consecutive days for collecting samples, the characteristics of lower probability of contamination, easy sample collection, and sufficient sample volume still make GA become an ideal sample type to increase detection rate for pediatric PTB. Ruiz Jimenez et al. [86] may agree with our results that GA is an excellent sample for diagnosing PTB, and IS should be considered as a supplementary sample type. However, Zar et al. [87] drew an opposite conclusion by conducting a prospective study, with IS for diagnosing PTB and GA as supplementary. The differences may result from different diagnostic tools and the variation in sample collection. The culture, used in their study [87], was unable to detect dead MTB which was caused by the neutralization of GA [88], while Xpert MTB/RIF used in our included studies can detect both dead and live MTB and is not affected by sample neutralization. Furthermore, sample collection, technician skill level, the time from sampling to testing and choice of pre-processing methods also influence finial accuracy [89]. Thus, it is worthy for us to pay attention to the standardization of GA sampling.
Subgroup analysis showed that for adult PTB, superior diagnostic performance appeared in the smear-positive subgroup whether using BAL, ES, or IS as samples. Xpert MTB/RIF showed similar sensitivity whether using smear-positive BAL, smear-positive ES or smear-positive IS, while the highest AUC (90%) was identified when using BAL to diagnose smear-negative PTB. BAL was sampled directly from the lesion region, which was more suitable and reliable for detecting PTB patients with low bacterial load [90,91]. In addition, outperformed capacity of Xpert MTB/RIF appeared when detecting MTB in IS or ES than in BAL for HIV-positive patients. Singer-Leshinsky et al. [92] obtained consistent results with ours that collecting IS from HIV patients may be more beneficial than others. Sample pre-procession steps, the number of samples, the immune status of patient, and the degree of disease severity are the potential confounding factors. More sound evidence is still lacking to support the value of ES in diagnosing HIV-associated adult PTB, which need to be further confirmed.
For pediatric PTB, we reconfirmed the strong association between smear status, bacterial load, and the diagnostic performance of Xpert MTB/RIF. Besides, testing for MTB in IS from children provided a little additional diagnostic yield from the prospective of specificity and AUC for Xpert MTB/RIF when diagnosing smear-negative PTB, however, Xpert MTB/RIF had higher likelihood to detect MTB in GA from these smear-negative children who really suffered from PTB. Maynard-Smith et al. [6] reported that detecting MTB in GA for sputum-scarce PTB was a good choice. However, Maynard-Smith et al. did not analyze the diagnostic accuracy of Xpert MTB/RIF when detecting MTB in IS from children and make further comparisons. The diagnostic value of GA and IS for smear-negative PTB for children still need to be explored. In the pediatric IS group, subgroup analysis showed that the pooled sensitivity was very heterogeneous between HIV-positive and HIV-negative subgroups and higher sensitivity was in the HIV-positive subgroup. This result was contrary to Connell et al. [93], however, supported by Nicol et al. [65] The smear status, disease severity and samples’ pre-processing may affect the results, and a further research should be warranted.
Strengths and limitations
Our study made a rigorous and comprehensive analysis and comparison about the diagnostic performance of Xpert MTB/RIF using different sample types for PTB patients with different ages by including as many studies as possible. Furthermore, we performed reasonable subgroup analysis to identify the source of heterogeneity and potential factors. However, the present study also has some limitations. Limited articles in the pediatric ES group and pediatric NPA group restricted us from further analysis. Some factors including cost, the tolerance of patients and sample contamination rate should be taken into consideration for final choice of diagnostics. Most included articles only used one sample type to analyze the performance of Xpert MTB/RIF, and thus the comparison of different sample types may be influenced by a number of potential confounding factors such as patient characteristics and the process of sampling. Thus, more eligible trials are needed.
Conclusion
When considering diagnostic performance of Xpert MTB/RIF, sampling method and patients’ tolerance, using ES as sample might be a better choice to diagnose adult PTB than using BAL and IS. For pediatric PTB, GA was superior to other samples. However, the invasive sampling and relatively long time of collecting samples also should be considered. The actual choice of sample types needs to be decided according to specific situations.
Supplementary Material
Abbreviations
- AUC
area under the curve
- BAL
bronchoalveolar lavage
- CI
confidence interval
- ES
expectorated sputum
- GA
gastric aspiration
- IGRA
interferon-γ release assay
- IS
induced sputum
- MTB
Mycobacterium tuberculosis
- NPA
nasopharyngeal aspirate
- PCR
polymerase chain reaction
- PTB
pulmonary tuberculosis
- sROC
summary receiver operating characteristic
- TB
tuberculosis
- T-SPOT
T-cell spot
- WHO
World Health Organization
Data Availability
All data generated or analyzed in the present study are contained and presented in this document.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Science and Technology Major Project [grant number 2018ZX10715003-001].
Author Contribution
Mengyuan Lyu designed the present study, conducted literature search, extracted the data, made data analysis and interpretation, drafted and revised the manuscript. Jian Zhou designed the study, conducted literature search, extracted the data, drafted and revised the manuscript. Yuhui Cheng conducted literature search and analyzed the data. Weelic Chong, Kang Wu, Teng Fang and Tianbo Fu extracted data. Binwu Ying gave administrative support. All authors have read and approved the final manuscript.
References
- 1.WHO (2019) Global tuberculosis report 2019. https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Chee C.B.E., Reves R., Zhang Y. and Belknap R. (2018) Latent tuberculosis infection: opportunities and challenges. Respirology 23, 893–900 [DOI] [PubMed] [Google Scholar]
- 3.Lawn S.D., Mwaba P., Bates M., Piatek A., Alexander H., Marais B.J. et al. (2013) Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect. Dis. 13, 349–361 10.1016/S1473-3099(13)70008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yew W.W. and Yoshiyama T. (2018) Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology 23, 567–575 [DOI] [PubMed] [Google Scholar]
- 5.Walzl G., McNerney R., du Plessis N., Bates M., McHugh T.D., Chegou N.N. et al. (2018) Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect. Dis. 18, e199–e210 10.1016/S1473-3099(18)30111-7 [DOI] [PubMed] [Google Scholar]
- 6.Maynard-Smith L., Larke N., Peters J.A. and Lawn S.D. (2014) Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect. Dis. 14, 709 10.1186/s12879-014-0709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theron G., Peter J., Meldau R., Khalfey H., Gina P., Matinyena B. et al. (2013) Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax 68, 1043–1051 10.1136/thoraxjnl-2013-203485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goussard P. and Gie R. (2014) The role of bronchoscopy in the diagnosis and management of pediatric pulmonary tuberculosis. Expert Rev. Respir. Med. 8, 101–109 10.1586/17476348.2013.863712 [DOI] [PubMed] [Google Scholar]
- 9.Heydari A.A., Movahhede Danesh M.R. and Ghazvini K. (2014) Urine PCR evaluation to diagnose pulmonary tuberculosis. Jundishapur J. Microbiol. 7, e9311 10.5812/jjm.9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno A., Narumoto O., Matsui H., Takeda K., Hirano Y., Shinfuku K. et al. (2019) The benefit of stool mycobacterial examination to diagnose pulmonary tuberculosis for adult and elderly patients. J. Clin. Tuberc. Other Mycobact. Dis. 16, 100106 10.1016/j.jctube.2019.100106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters E., Demers A.M., van der Zalm M.M., Whitelaw A., Palmer M., Bosch C. et al. (2017) Stool culture for diagnosis of pulmonary tuberculosis in children. J. Clin. Microbiol. 55, 3355–3365 10.1128/JCM.00801-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldenhuys H.D., Kleynhans W., Buckerfield N., Tameris M., Gonzalez Y., Mahomed H. et al. (2012) Safety and tolerability of sputum induction in adolescents and adults with suspected pulmonary tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 31, 529–537 10.1007/s10096-011-1344-5 [DOI] [PubMed] [Google Scholar]
- 13.Heron M., Grutters J.C., ten Dam-Molenkamp K.M., Hijdra D., van Heugten-Roeling A., Claessen A.M. et al. (2012) Bronchoalveolar lavage cell pattern from healthy human lung. Clin. Exp. Immunol. 167, 523–531 10.1111/j.1365-2249.2011.04529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer K.C. (2007) Bronchoalveolar lavage as a diagnostic tool. Semin. Respir. Crit. Care Med. 28, 546–560 10.1055/s-2007-991527 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (2013) Laboratory testing for Middle East respiratory syndrome coronavirus;. http://www.who.int/csr/disease/coronavirus_infections/MERS_Lab_recos_16_Sept_2013.pdf?ua=1&ua=1 [Google Scholar]
- 16.Liu X., Hou X.F., Gao L., Deng G.F., Zhang M.X., Deng Q.Y. et al. (2018) Indicators for prediction of Mycobacterium tuberculosis positivity detected with bronchoalveolar lavage fluid. Infect. Dis. Poverty 7, 22 10.1186/s40249-018-0403-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloepfer K.M., Deschamp A.R., Ross S.E., Peterson-Carmichael S.L., Hemmerich C.M., Rusch D.B. et al. (2018) In children, the microbiota of the nasopharynx and bronchoalveolar lavage fluid are both similar and different. Pediatr. Pulmonol. 53, 475–482 10.1002/ppul.23953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu A.Z., Shi P., Wang L.B., Qian L.L. and Zhang X.B. (2017) Diagnostic value of nasopharyngeal aspirates in children with lower respiratory tract infections. Chin. Med. J. (Engl.) 130, 647–651 10.4103/0366-6999.201595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciel E.L., Brotto L.D., Sales C.M., Zandonade E. and Sant’anna C.C. (2010) Gastric lavage in the diagnosis of pulmonary tuberculosis in children: a systematic review. Rev. Saude Publica 44, 735–742 10.1590/S0034-89102010005000019 [DOI] [PubMed] [Google Scholar]
- 20.Stockdale A.J., Duke T., Graham S. and Kelly J. (2010) Evidence behind the WHO guidelines: hospital care for children: what is the diagnostic accuracy of gastric aspiration for the diagnosis of tuberculosis in children? J. Trop. Pediatr. 56, 291–298 10.1093/tropej/fmq081 [DOI] [PubMed] [Google Scholar]
- 21.Piccini P., Chiappini E., Tortoli E., de Martino M. and Galli L. (2014) Clinical peculiarities of tuberculosis. BMC Infect. Dis. 14, S4 10.1186/1471-2334-14-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shingadia D. and Novelli V. (2003) Diagnosis and treatment of tuberculosis in children. Lancet Infect. Dis. 3, 624–632 10.1016/S1473-3099(03)00771-0 [DOI] [PubMed] [Google Scholar]
- 23.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B. et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 24.Barnard D.A., Irusen E.M., Bruwer J.W., Plekker D., Whitelaw A.C., Deetlefs J.D. et al. (2015) The utility of Xpert MTB/RIF performed on bronchial washings obtained in patients with suspected pulmonary tuberculosis in a high prevalence setting. BMC Pulm. Med. 15, 103 10.1186/s12890-015-0086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gowda N.C., Ray A., Soneja M., Khanna A. and Sinha S. (2018) Evaluation of Xpert((R))Mycobacterium tuberculosis/rifampin in sputum-smear negative and sputum-scarce patients with pulmonary tuberculosis using bronchoalveolar lavage fluid. Lung India 35, 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo Y.S., Park J.H., Lee J.K., Heo E.Y., Chung H.S. and Kim D.K. (2016) Discordance between MTB/RIF and real-time tuberculosis-specific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PloS one 11, e0164923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil K.F. and Butt T. (2015) Diagnostic yield of Bronchoalveolar Lavage gene Xpert in smear-negative and sputum-scarce pulmonary tuberculosis. J. Coll. Phys. Surg. Pak. 25, 115–118 [PubMed] [Google Scholar]
- 28.Kilaru S.C., Chenimilla N.P., Syed U., Momin K., Kilaru H., Patil E. et al. (2019) Role of Xpert MTB/RIF in Bronchoalveolar lavage fluid of sputum-scarce, suspected Pulmonary TB patients. J. Clin. Tuberc. Other Mycobact. Dis. 14, 7–11 10.1016/j.jctube.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko Y., Lee H.K., Lee Y.S., Kim M.Y., Shin J.H., Shim E.J. et al. (2016) Accuracy of Xpert((R)) MTB/RIF assay compared with AdvanSure TB/NTM real-time PCR using bronchoscopy specimens. Int. J. Tuberc. Lung Dis. 20, 115–120 10.5588/ijtld.15.0227 [DOI] [PubMed] [Google Scholar]
- 30.Lee H.Y., Seong M.W., Park S.S., Hwang S.S., Lee J., Park Y.S. et al. (2013) Diagnostic accuracy of Xpert(R) MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 17, 917–921 10.5588/ijtld.12.0885 [DOI] [PubMed] [Google Scholar]
- 31.Lu Y., Zhu Y., Shen N., Tian L. and Sun Z. (2018) Evaluating the diagnostic accuracy of the Xpert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. Int. J. Infect. Dis. 71, 14–19 [DOI] [PubMed] [Google Scholar]
- 32.Mok Y., Tan T.Y., Tay T.R., Wong H.S., Tiew P.Y., Kam J.W. et al. (2016) Do we need transbronchial lung biopsy if we have bronchoalveolar lavage Xpert(R) MTB/RIF? Int. J. Tuberc. Lung Dis. 20, 619–624 10.5588/ijtld.15.0463 [DOI] [PubMed] [Google Scholar]
- 33.Pan X., Yang S., Deighton M.A., Qu Y., Hong L. and Su F. (2018) A comprehensive evaluation of Xpert MTB/RIF assay with bronchoalveolar lavage fluid as a single test or combined with conventional assays for diagnosis of pulmonary tuberculosis in China: a two-center prospective study. Front. Microbiol. 9, 444 10.3389/fmicb.2018.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Cabral O., Santillan-Diaz C., Paul Flores-Bello A., Ivannia Herrera-Ortega M., Luis Sandoval-Gutierrez J., Santillan-Doherty P. et al. (2020) GeneXpert (R) MTB/RIF assay with transbronchial lung cryobiopsy for Mycobacterium tuberculosis diagnosis. Ann. Transl. Med. 8, 351 10.21037/atm.2020.02.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S.K., Kohli M., Yadav R.N., Chaubey J., Bhasin D., Sreenivas V. et al. (2015) Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS ONE 10, e0141011 10.1371/journal.pone.0141011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva T.M.D., Soares V.M., Ramos M.G. and Santos A.D. (2019) Accuracy of a rapid molecular test for tuberculosis in sputum samples, bronchoalveolar lavage fluid, and tracheal aspirate obtained from patients with suspected pulmonary tuberculosis at a tertiary referral hospital. J. Bras. Pneumol. 45, e20170451 10.1590/1806-3713/e20170451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To K.W., Kam K.M., Chan D.P.C., Yip W.H., Chan K.P., Lo R. et al. (2018) Utility of GeneXpert in analysis of bronchoalveolar lavage samples from patients with suspected tuberculosis in an intermediate-burden setting. J. Infect. 77, 296–301 10.1016/j.jinf.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 38.Ullah I., Javaid A., Masud H., Ali M., Basit A., Ahmad W. et al. (2017) Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear-negative pulmonary suspects using Xpert MTB/RIF. J. Med. Microbiol. 66, 412–418 10.1099/jmm.0.000449 [DOI] [PubMed] [Google Scholar]
- 39.Prakash A.K., Datta B., Tripathy J.P., Kumar N., Chatterjee P. and Jaiswal A. (2018) The clinical utility of cycle of threshold value of GeneXpert MTB/RIF (CBNAAT) and its diagnostic accuracy in pulmonary and extra-pulmonary samples at a tertiary care center in India. Indian J. Tuberc. 65, 296–302 10.1016/j.ijtb.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 40.Al-Darraji H.A., Abd Razak H., Ng K.P., Altice F.L. and Kamarulzaman A. (2013) The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PLoS ONE 8, e73717 10.1371/journal.pone.0073717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boum Y. II, Kim S., Orikiriza P., Acuna-Villaorduna C., Vinhas S., Bonnet M. et al. (2016) Diagnostic accuracy of the small membrane filtration method for diagnosis of pulmonary tuberculosis in a high-HIV-prevalence setting. J. Clin. Microbiol. 54, 1520–1527 10.1128/JCM.00017-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedrich S.O., Venter A., Kayigire X.A., Dawson R., Donald P.R. and Diacon A.H. (2011) Suitability of Xpert MTB/RIF and genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J. Clin. Microbiol. 49, 2827–2831 10.1128/JCM.00138-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geleta D.A., Megerssa Y.C., Gudeta A.N., Akalu G.T., Debele M.T. and Tulu K.D. (2015) Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiol. 15, 220 10.1186/s12866-015-0566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaCourse S.M., Cranmer L.M., Matemo D., Kinuthia J., Richardson B.A., John-Stewart G. et al. (2016) Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J. Acquir. Immune Defic. Syndr. 71, 219–227 10.1097/QAI.0000000000000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luetkemeyer A.F., Firnhaber C., Kendall M.A., Wu X., Mazurek G.H., Benator D.A. et al. (2016) Evaluation of Xpert MTB/RIF versus AFB smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin. Infect. Dis. 62, 1081–1088 10.1093/cid/ciw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madico G., Mpeirwe M., White L., Vinhas S., Orr B., Orikiriza P. et al. (2016) Detection and quantification of Mycobacterium tuberculosis in the sputum of culture-negative HIV-infected pulmonary tuberculosis suspects: a proof-of-concept study. PLoS ONE 11, e0158371 10.1371/journal.pone.0158371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinyopornpanish K., Chaiwarith R., Pantip C., Keawvichit R., Wongworapat K., Khamnoi P. et al. (2015) Comparison of Xpert MTB/RIF assay and the conventional sputum microscopy in detecting Mycobacterium tuberculosis in Northern Thailand. Tuberc. Res. Treat. 2015, 571782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rachow A., Zumla A., Heinrich N., Rojas-Ponce G., Mtafya B., Reither K. et al. (2011) Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay–a clinical validation study. PLoS ONE 6, e20458 10.1371/journal.pone.0020458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasheed W., Rao N.A., Adel H., Baig M.S. and Adil S.O. (2019) Diagnostic accuracy of Xpert MTB/RIF in sputum smear-negative pulmonary tuberculosis. Cureus 11, e5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice J.P. and Seifert M. (2017) Performance of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis and rifampin resistance in a low-incidence, high-resource setting. PLoS One 12, e0186139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahle E.T., Blumenthal J., Jain S., Sun S., Young J., Manyazewal T. et al. (2019) Bacteriologically-confirmed pulmonary tuberculosis in an Ethiopian prison: prevalence from screening of entrant and resident prisoners. PLoS ONE 14, e0226160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeong C., Byrne A.L., Cho J.-G., Sintchenko V., Crighton T. and Marais B.J. (2020) Use of GeneXpert MTB/RIF on a single pooled sputum specimen to exclude pulmonary tuberculosis among hospital inpatients placed in respiratory isolation. Int. J. Infect. Dis. 92, 175–180 10.1016/j.ijid.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 53.Acuna-Villaorduna C., Orikiriza P., Nyehangane D., White L.F., Mwanga-Amumpaire J., Kim S. et al. (2017) Effect of previous treatment and sputum quality on diagnostic accuracy of Xpert((R)) MTB/RIF. Int. J. Tuberc. Lung Dis. 21, 389–397 10.5588/ijtld.16.0785 [DOI] [PubMed] [Google Scholar]
- 54.Mishra H., Reeve B.W.P., Palmer Z., Caldwell J., Dolby T., Naidoo C.C. et al. (2020) Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respir. Med. 8, 368–382 10.1016/S2213-2600(19)30370-4 [DOI] [PubMed] [Google Scholar]
- 55.O’Grady J., Bates M., Chilukutu L., Mzyece J., Cheelo B., Chilufya M. et al. (2012) Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin. Infect. Dis. 55, 1171–1178 10.1093/cid/cis631 [DOI] [PubMed] [Google Scholar]
- 56.Sohn H., Aero A.D., Menzies D., Behr M., Schwartzman K., Alvarez G.G. et al. (2014) Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin. Infect. Dis. 58, 970–976 10.1093/cid/ciu022 [DOI] [PubMed] [Google Scholar]
- 57.Bates M., O’Grady J., Maeurer M., Tembo J., Chilukutu L., Chabala C. et al. (2013) Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect. Dis. 13, 36–42 10.1016/S1473-3099(12)70245-1 [DOI] [PubMed] [Google Scholar]
- 58.Reither K., Manyama C., Clowes P., Rachow A., Mapamba D., Steiner A. et al. (2015) Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in children: a prospective, multi-centre evaluation. J. Infect. 70, 392–399 10.1016/j.jinf.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 59.Das A., Anupurba S., Mishra O.P., Banerjee T. and Tripathi R. (2019) Evaluation of Xpert MTB/RIF assay for diagnosis of tuberculosis in children. J. Trop. Pediatr. 65, 14–20 10.1093/tropej/fmy005 [DOI] [PubMed] [Google Scholar]
- 60.Hasan Z., Shakoor S., Arif F., Mehnaz A., Akber A., Haider M. et al. (2017) Evaluation of Xpert MTB/RIF testing for rapid diagnosis of childhood pulmonary tuberculosis in children by Xpert MTB/RIF testing of stool samples in a low resource setting. BMC Res. Notes 10, 473 10.1186/s13104-017-2806-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myo K., Zaw M., Swe T.L., Kyaw Y.Y., Thwin T., Myo T.T. et al. (2018) Evaluation of Xpert (R) MTB/RIF assay as a diagnostic test for pulmonary tuberculosis in children in Myanmar. Int. J. Tuberc. Lung Dis. 22, 1051 10.5588/ijtld.18.0024 [DOI] [PubMed] [Google Scholar]
- 62.Pang Y., Wang Y., Zhao S., Liu J., Zhao Y. and Li H. (2014) Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. Pediatr. Infect. Dis. J. 33, 1047–1051 10.1097/INF.0000000000000403 [DOI] [PubMed] [Google Scholar]
- 63.Sharma S., Shulania A., Achra A., Jeram H., Kansra S. and Duggal N. (2020) Diagnosis of pulmonary tuberculosis from gastric aspirate samples in nonexpectorating pediatric patients in a tertiary care hospital. Indian J. Pathol. Microbiol. 63, 210–213 [DOI] [PubMed] [Google Scholar]
- 64.LaCourse S.M., Chester F.M., Preidis G., McCrary L.M., Arscott-Mills T., Maliwichi M. et al. (2014) Use of Xpert for the diagnosis of pulmonary tuberculosis in severely malnourished hospitalized Malawian children. Pediatr. Infect. Dis. J. 33, 1200–1202 10.1097/INF.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicol M.P., Workman L., Isaacs W., Munro J., Black F., Eley B. et al. (2011) Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect. Dis. 11, 819–824 10.1016/S1473-3099(11)70167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicol M.P., Spiers K., Workman L., Isaacs W., Munro J., Black F. et al. (2013) Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin. Infect. Dis. 57, e18–e21 10.1093/cid/cit230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicol M.P., Workman L., Prins M., Bateman L., Ghebrekristos Y., Mbhele S. et al. (2018) Accuracy of Xpert Mtb/Rif ultra for the diagnosis of pulmonary tuberculosis in children. Pediatr. Infect. Dis. J. 37, E261–E263 10.1097/INF.0000000000001960 [DOI] [PubMed] [Google Scholar]
- 68.Sekadde M.P., Wobudeya E., Joloba M.L., Ssengooba W., Kisembo H., Bakeera-Kitaka S. et al. (2013) Evaluation of the Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: a cross-sectional diagnostic study. BMC Infect. Dis. 13, 133 10.1186/1471-2334-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Togun T.O., Egere U., Sillah A.K., Ayorinde A., Mendy F., Tientcheu L. et al. (2015) Contribution of Xpert(R) MTB/RIF to the diagnosis of pulmonary tuberculosis among TB-exposed children in The Gambia. Int. J. Tuberc. Lung Dis. 19, 1091–1097, i-ii 10.5588/ijtld.15.0228 [DOI] [PubMed] [Google Scholar]
- 70.Zar H.J., Workman L., Isaacs W., Munro J., Black F., Eley B. et al. (2012) Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin. Infect. Dis. 55, 1088–1095 10.1093/cid/cis598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zar H.J., Workman L., Isaacs W., Dheda K., Zemanay W. and Nicol M.P. (2013) Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet Global Health 1, e97–e104 10.1016/S2214-109X(13)70036-6 [DOI] [PubMed] [Google Scholar]
- 72.Jo Y.S., Park J.-H., Lee J.K., Heo E.Y., Chung H.S. and Kim D.K. (2016) Discordance between MTB/RIF and real-time tuberculosis-specific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PLoS ONE 11, e0164923 10.1371/journal.pone.0164923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steingart K.R., Ng V., Henry M., Hopewell P.C., Ramsay A., Cunningham J. et al. (2006) Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6, 664–674 10.1016/S1473-3099(06)70602-8 [DOI] [PubMed] [Google Scholar]
- 74.Hoft D.F., Brown R.M. and Belshe R.B. (2000) Mucosal bacille calmette-Guerin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin. Infect. Dis. 30, S217–S222 10.1086/313864 [DOI] [PubMed] [Google Scholar]
- 75.Albert H., Nathavitharana R.R., Isaacs C., Pai M., Denkinger C.M. and Boehme C.C. (2016) Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur. Respir. J. 48, 516–525 10.1183/13993003.00543-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dorman S.E., Schumacher S.G., Alland D., Nabeta P., Armstrong D.T., King B. et al. (2018) Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 18, 76–84 10.1016/S1473-3099(17)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Detjen A.K., DiNardo A.R., Leyden J., Steingart K.R., Menzies D., Schiller I. et al. (2015) Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir. Med. 3, 451–461 10.1016/S2213-2600(15)00095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakravorty S., Simmons A.M., Rowneki M., Parmar H., Cao Y., Ryan J. et al. (2017) The New Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 8, e00812–17 10.1128/mBio.00812-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osei Sekyere J., Maphalala N., Malinga L.A., Mbelle N.M. and Maningi N.E. (2019) A comparative evaluation of the new genexpert MTB/RIF ultra and other rapid diagnostic assays for detecting tuberculosis in pulmonary and extra pulmonary specimens. Sci. Rep. 9, 16587 10.1038/s41598-019-53086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meldau R., Randall P., Pooran A., Limberis J., Makambwa E., Dhansay M. et al. (2019) Same-day tools, including Xpert Ultra and IRISA-TB, for rapid diagnosis of pleural tuberculosis: a prospective observational study. J. Clin. Microbiol. 57, 10.1128/JCM.00614-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vashistha R., Somani K., Patel A., Maheshwari M. and Datey S. (2018) Clinical profile of abdominal tuberculosis in a tertiary care centre of central india-a descriptive observational study. J. Evol. Med. Dental Sci. 7, 3699–3702 10.14260/jemds/2018/830 [DOI] [Google Scholar]
- 82.Briceno R.K., Sergent S.R., Benites S.M. and Alocilja E.C. (2019) Nanoparticle-based biosensing assay for universally accessible low-cost TB detection with comparable sensitivity as culture. Diagnostics 9, 222 10.3390/diagnostics9040222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blau H., Linnane B., Carzino R., Tannenbaum E.L., Skoric B., Robinson P.J. et al. (2014) Induced sputum compared to bronchoalveolar lavage in young, non-expectorating cystic fibrosis children. J. Cyst. Fibros. 13, 106–110 10.1016/j.jcf.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 84.Garcia B.J., Loxton A.G., Dolganov G.M., Van T.T., Davis J.L., de Jong B.C. et al. (2016) Sputum is a surrogate for bronchoalveolar lavage for monitoring Mycobacterium tuberculosis transcriptional profiles in TB patients. Tuberculosis 100, 89–94 10.1016/j.tube.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conde M.B., Soares S.L., Mello F.C., Rezende V.M., Almeida L.L., Reingold A.L. et al. (2000) Comparison of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis: experience at an acquired immune deficiency syndrome reference center in Rio de Janeiro, Brazil. Am. J. Respir. Crit. Care Med. 162, 2238–2240 10.1164/ajrccm.162.6.2003125 [DOI] [PubMed] [Google Scholar]
- 86.Ruiz Jimenez M., Guillen Martin S., Prieto Tato L.M., Cacho Calvo J.B., Alvarez Garcia A., Soto Sanchez B. et al. (2013) Induced sputum versus gastric lavage for the diagnosis of pulmonary tuberculosis in children. BMC Infect. Dis. 13, 222 10.1186/1471-2334-13-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zar H.J., Hanslo D., Apolles P., Swingler G. and Hussey G. (2005) Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 365, 130–134 10.1016/S0140-6736(05)17702-2 [DOI] [PubMed] [Google Scholar]
- 88.Parashar D., Kabra S.K., Lodha R., Singh V., Mukherjee A., Arya T. et al. (2013) Does neutralization of gastric aspirates from children with suspected intrathoracic tuberculosis affect mycobacterial yields on MGIT culture? J. Clin. Microbiol. 51, 1753–1756 10.1128/JCM.00202-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mathew J.L., Vijayasekharan D. and Singh S. (2014) Is Xpert MTB/RIF assay in gastric lavage aspirate useful for diagnosis of smear-negative childhood pulmonary tuberculosis? Indian Pediatr. 51, 1007–1011 10.1007/s13312-014-0548-z [DOI] [PubMed] [Google Scholar]
- 90.Ramirez P., Valencia M. and Torres A. (2007) Bronchoalveolar lavage to diagnose respiratory infections. Semin Respir. Crit. Care Med. 28, 525–533 10.1055/s-2007-991524 [DOI] [PubMed] [Google Scholar]
- 91.Zhang G.P., Chen Q., Liu J.M., Zhou S.P., Yu X.J., Lu J. et al. (2012) Application of bacterial cultures of bronchoalveolar lavage fluids in children with pulmonary infection. Zhongguo Dang Dai Er Ke Za Zhi 14, 350–352 [PubMed] [Google Scholar]
- 92.Singer-Leshinsky S. (2016) Pulmonary tuberculosis: improving diagnosis and management. JAAPA 29, 20–25 10.1097/01.JAA.0000476207.96819.a7 [DOI] [PubMed] [Google Scholar]
- 93.Connell T.G., Zar H.J. and Nicol M.P. (2011) Advances in the diagnosis of pulmonary tuberculosis in HIV-infected and HIV-uninfected children. J. Infect. Dis. 204, S1151–S1158 10.1093/infdis/jir413 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in the present study are contained and presented in this document.